Abstract
Background
Symptomatic multilevel cervical myelopathy is often addressed using posterior decompression using two-dimensional fluoroscopy. Intraoperative three-dimensional fluoroscopy provides more accurate information on the position of instrumentation to prevent screw-related complications.
Questions/purposes
We documented the incidence of hardware-related complications and evaluate cost-effectiveness when using intraoperative three-dimensional fluoroscopy (ISO-C CT) in posterior cervical spine surgery.
Methods
Records from 87 patients who underwent posterior cervical decompression and instrumented fusion for multilevel cervical spondylosis with myelopathy were retrospectively reviewed. Patients in whom a lateral mass, pars, or pedicle screw was removed or revised based on intraoperative ISO-C CT was recorded. Cost analysis was performed using 2008 Medicare reimbursements and was compared against cost estimates for ISO-C CT.
Results
Seven patients (8%) had screws changed based on the results of the three-dimensional fluoroscopy: 0.5% of lateral mass screws, 3.1% of thoracic pedicle screws, and 15% of C2 pars screws. No patients who had evaluation of hardware with the ISO-C CT required a return to surgery for complications secondary to hardware failure, malposition, or cutout.
Conclusions
Cost savings are achieved if use of intraoperative ISO-C CT prevents eight patients from requiring a return to the operating room. If every malpositioned screw has the potential to be symptomatic, then 240 patients must have screws placed to be cost-effective. ISO-C CT can safely replace postoperative CT as the standard of care in patients undergoing posterior cervical spinal fusion.
Level of Evidence
Level III, economic and decision analyses. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Cervical myeloradiculopathy is one of the most common indications for spinal surgical intervention in the United States and worldwide. Among spinal fusion procedures, cervical spine fusion is the most common with a reported incidence of seven to 38 per 100,000 people in the United States [17]. The total cost of spinal fusion surgery in the United States in 2004 was estimated at $4.8 billion [17]. Specifically, posterior cervical fusion surgery was estimated to cost $71,632 per patient [17]. This high cost necessitates that instrumentation be accurately placed and radiographically confirmed in a timely manner to prevent the need for a costly revision surgery secondary to malposition or poor placement leading to hardware cutout or failure.
Authors such as Magerl, Roy-Camille, Anderson, and An have developed different methods of placing lateral mass screws, but each method carries the risk of well-reported complications [1, 2, 19, 26, 32]. The most commonly reported complications of lateral mass screw placement include screw breakage or pullout, vertebral artery injury, nerve root injury, and spinal cord impingement. The incidence of symptomatic misplacement of hardware necessitating a return to the operating room for removal and/or replacement of the offending screw has been reported as 1.5% to 1.8% [4, 9, 14, 16, 22, 25, 27, 28, 35, 37].
Currently the standard of care in evaluating posterior cervical instrumentation is two-dimensional intraoperative fluoroscopy (or radiography) and/or postoperative CT scanning. The reported incidence of hardware complications when using fluoroscopic guidance alone is 0.4% to 1.4% for neurologic injury and 0% to 8.2% for vascular injury per screw placed [5, 6, 11, 13, 18, 21, 23, 24, 30, 31, 33, 35]. The availability of intraoperative three-dimensional imaging techniques such as isocentric C-arm three-dimensional CT (ISO-C CT) enables the surgeon to more accurately evaluate the placement of hardware in complex anatomic regions.
The purpose of our study was to document the incidence of hardware-related complications and evaluate cost-effectiveness when using intraoperative three-dimensional fluoroscopy in posterior cervical spine surgery.
Materials and Methods
We retrospectively reviewed inpatient and outpatient records of 87 patients who underwent posterior cervical laminectomy and instrumented fusion for multilevel cervical myeloradiculopathy between January 2005 and December 2008. The age distribution of patients who underwent posterior cervical decompression and instrumented fusion for multilevel cervical spondylosis with myelopathy ranged from 10 to 89 years with a mean of 58.6 years. Males and females constituted 63% and 37% of the cases, respectively (Table 1). Data were obtained from medical records. Additionally, all patients were directly contacted to ensure a revision procedure had not been performed at an outside institution.
Table 1.
Patient demographic data and hardware
| Patient and hardware | Number |
|---|---|
| Number of patients | 87 |
| Gender | 55 male, 32 female |
| Average age | 58.6 years (range, 10–89) |
| Average number of levels of instrumentation | 5 (range, 2–7) |
| Total number of screws placed | 713 |
| Lateral mass | 597 (81.3%) |
| Pedicle | 96 (13.1%) |
| C2 pars | 20 (2.7%) |
| Lateral mass screws/pedicle screws | |
| C3 | 116 (16.7%) |
| C4 | 139 (20.1%) |
| C5 | 160 (23%) |
| C6 | 140 (20.2%) |
| C7 | 42 (6.1%) |
| T1 | 96 (13.9%) |
| Average screw length | |
| Lateral mass | 11.2 mm |
| Pedicle | 22.4 mm |
Surgery was performed by the senior investigators (ACH, AJ) and all intraoperative three-dimensional fluoroscopic images were interpreted by the same surgeon. Intraoperative three-dimensional fluoroscopy was used in 67 of the 87 cases (77%) (Table 2). It was not used in 100% of cases because ACH began using intraoperative CT before AJ. Thus, the population had a control group including those who only received postoperative CT evaluation. We used intraoperative three-dimensional fluoroscopy (Siemens Siremobil Iso-C 3D, C-arm CT, Erlangen, Germany) for evaluation of subaxial cervical lateral mass, C2 pars, and upper thoracic (T1) pedicle screws. It is our preference to perform all posterior cervical spine surgery using a Jackson table with a chest board and hip and thigh pads. Intraoperative neuromonitoring is routinely used. The patient’s head is held on a face board using a face pillow and Gardner-Wells tongs are used to provide 20 lbs of traction during positioning and surgery. The arms are placed at the patient’s side and rest on arm boards. Traction is applied through the shoulders using silk tape to remove any redundancy in the skin over the posterior cervical spine. The Iso-C-CT scan is then draped sterilely and is used for its biplanar fluoroscopy capability during the initial portions of the surgery to localize the appropriate levels (Fig. 1). After all instrumentation is placed, the wound is completely sealed and a three-dimensional study, including axial, sagittal, and coronal views, is obtained intraoperatively to assess hardware. This study is immediately available for review by the operating surgeon. On satisfactory review, the wound is uncovered for completion of the procedure.
Table 2.
ISO-C use and screw demographics
| ISO-C use and screw demographics | Number |
|---|---|
| ISO-C CT used | 67 (77.0%) |
| Postoperative CT also used | 23 (26.4%) |
| Postoperative CT not used | 44 (50.6%) |
| ISO-C CT Not Used | 20 (23.0%) |
| Postoperative CT also used | 7 (8.0%) |
| Postoperative CT not used | 13 (14.9%) |
| Number of patients who had screws changed based on ISO-C image | 7 patients (8%) |
| Number of screws changed based on ISO-C image | 9 screws (1.2%) |
| Breakdown of changed screws | |
| Lateral mass | 3 screws (3.5% per patient) |
| Removed | 2 screws |
| RT C6—lateral breakout | |
| LT C6— lateral breakout | |
| Shortened | 1 screw |
| LT C4—shortened 2 mm to 10 mm | |
| Pedicle | 3 screws (4.8% per patient) |
| Repositioned | 3 screws |
| 2 T1—lateral breakout | |
| RT T1—lateral breakout | |
| Occipital | 0 screws |
| C2 Pars | 3 screws (20% per patient) |
| Removed | 2 screws |
| abutting vertebral arteries | |
| Repositioned | 1 screw |
| LT crossed facet joint | |
| Surgical revisions | |
| ISO-C was used | 0 revisions |
| ISO-C Not used | 1 revision |
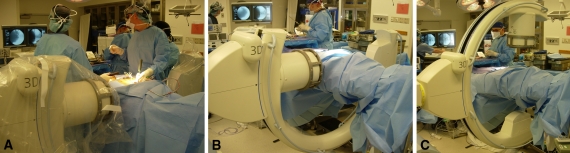
Fig. 1A–C.
(A) An image of patient draping during intraoperative use of the isocentric 3-dimensional C-arm is shown. (B) An image of sterile patient draping during fluoroscopy is shown. (C) An image of sterile patient draping during intraoperative CT is shown.
A total of 713 screws were placed, 597 in the lateral masses of the subaxial cervical spine, 96 in the T1 pedicles, and 20 in the C2 pars region. Of the lateral mass screws placed, 116 (16.7%) were placed in C3, 139 (20.1%) were placed in C4, 160 (23%) were placed in C5, 140 (20.2%) were placed in C6, and 42 (6.1%) were placed in C7. All 96 upper thoracic pedicle screws (13.9%) were placed in T1. The average length of screw placed was 11.2 mm in the lateral mass and 22.4 mm in the pedicle or pars.
We recorded cases in which hardware was changed (screw removal, screw shortening/lengthening, screw redirection) based on the result of the intraoperative three-dimensional study. All inpatient and outpatient records were further reviewed to identify any patients whose surgery was complicated by a new radiculopathy, vascular injury, or hardware failure secondary to hardware malpositioning postoperatively despite the use of intraoperative three-dimensional fluoroscopy. All patients were directly contacted to ensure a revision procedure had not been performed at an outside institution.
We performed cost analysis using 2008 Medicare reimbursement for revision cervical spine surgery (Diagnosis Related Groups 471, 472, 473) and was compared against cost estimates for startup and incremental costs associated with isocentric C-arm three-dimensional fluoroscopy (obtained from industry sources) as well as the cost of postoperative CT.
Results
Nine screws (1.2%) in seven patients (8%) were changed based on the findings of intraoperative three-dimensional fluoroscopic evaluation. Two screws were removed during revision surgery from one of the 20 patients who was not evaluated with intraoperative three-dimensional fluoroscopy. In this patient, the screws were removed because the patient reported numbness and tingling in a C5/6 dermatome distribution. Of the nine screws changed based on ISO-C CT evaluation, four screws were removed secondary to malpositioning but not replaced as a result of insufficient bone stock or lateral cutout. Two screws were removed at C6 for lateral breakout and two C2 pars screws were removed for abutting the vertebral arteries. Four other screws (three T1 pedicle and one C2 pars) were repositioned and one screw was shortened (Table 2). The three T1 pedicle screws were repositioned as a result of lateral breakout. The C2 pars screw was repositioned because it crossed the facet joint. One C4 lateral mass screw was shorted from 12 mm to 10 mm as a result of violation of the anterior wall of the lateral mass. No patients who had evaluation of hardware with intraoperative three-dimensional fluoroscopy required a return to the operating room for complications of hardware.
The cost of purchasing the ISO-C CT is $300,000 (Table 3). Cost savings is achieved if use of intraoperative three-dimensional fluoroscopy prevents eight patients from requiring a return to the operating room. If every malpositioned screw has the potential to become symptomatic, and assuming malpositioning rates similar to ours (1.2% per screw and in 8% of patients), then 100 patients must have screws placed for cost-effectiveness to be achieved. When considering individual types of instrumentation, 299 patients must have lateral mass screws placed (assuming 6.86 screws/patient), 167 must have T1 pedicle screws placed, and 40 must have C2 pars screws placed for cost-effectiveness to be achieved.
Table 3.
Cost analysis of ISO-C CT
| Cost-effectiveness criteria | Cost/patients |
|---|---|
| Cost of purchasing ISO-C CT scanner | $300,000 |
| Cost of one use of ISO-C CT scanner | $0 |
| Cost of postoperative CT scan | $650 |
| Cost of revision spine surgery | $39,643 |
| Number of revision patients necessary to equal cost of ISO-C CT scanner | 8 patients |
| Number of patients receiving posterior cervical instrumentation and fusion necessary to justify ISO-C CT cost | 100 patients |
| Number of patients receiving posterior cervical instrumentation and fusion necessary to justify ISO-C CT cost (specific to type of instrumentation) | |
| Lateral mass screws only | 229 patients |
| Pedicle screws only | 167 patients |
| Pars screws only | 40 patients |
Illustrative Case
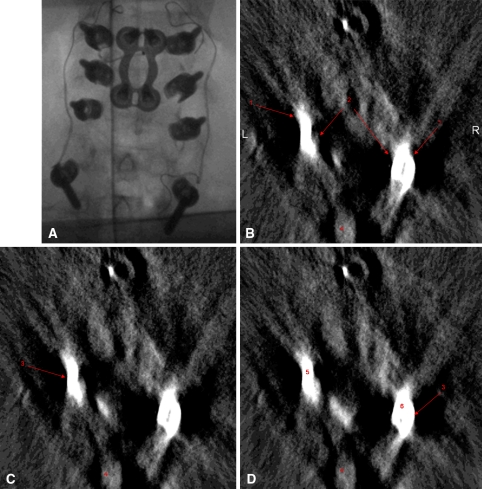
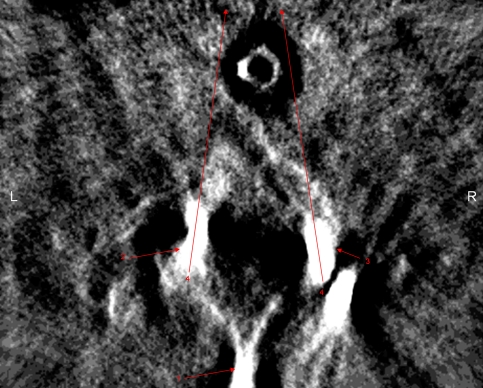
A 48-year-old man was indicated for operative management for the treatment of multilevel cervical pseudarthrosis after failing nonoperative treatment measures after symptomatic nonunion of a previously attempted anterior cervical fusion from C4 to C7. The patient also demonstrated advanced disc degeneration at the C7-T1 interspace. After positioning in the manner described previously, sterile prep and drape was applied and a posterior approach to the cervical spine was performed. After confirmation of the appropriate level of surgery, lateral mass screws were placed bilaterally at C4, C5, and C6 using the technique described by An et al. [1]. Pedicle screws were then placed bilaterally at T1 using anatomic landmarks as well as a laminoforaminotomy allowing tactile confirmation on the location of the pedicle. After placement of all screws, biplanar fluoroscopy was obtained, which appeared to show appropriate hardware position; however, the T1 screws were difficult to visualize (Fig. 2A). Intraoperative three-dimensional fluoroscopy was then performed, which demonstrated lateral trajectory of the T1 pedicle screws (Fig. 2B–D). Based on these findings, the screws were redirected in a more medial direction and repeat three-dimensional fluoroscopy confirmed a more appropriate screw track (Fig. 3).
Fig. 2A–D.
ISO-C CT images of the illustrative case are shown. (A) A two-dimensional fluoroscopy image before screw redirection is shown. (B) Intraoperative isocentric 3-dimensional C-arm axial images demonstrate bilateral T1 pedicle screws that were too lateral. The lateral border of the pedicles is shown. (C) The medial border of the pedicles is shown. (D) The bilateral lateral breakout by the pedicle screws shows 1—the spinous process of the T1 vertebrae; 2—the left pedicle screw; 3—the right pedicle screw. In addition to the lateral breakout, the other indication that the screws are too lateral is the divergent trajectory of the two screws.
Fig. 3.
Intraoperative isocentric 3-dimensional C-arm axial images demonstrating bilateral T1 pedicle screws redirected medially. 1 = the spinous process of the T1 vertebrae; 2 = the left pedicle screw redirected medially; 3 = the right pedicle screw redirected medially; 4 = the arrows demonstrate the convergent trajectory of the screws, indicating the screws have been medialized.
Discussion
Cervical myeloradiculopathy is one of the most common indications for spinal surgical intervention in the United States and worldwide. The high cost of cervical spine revision surgery necessitates that instrumentation be accurately placed and radiographically confirmed in a timely manner. Currently the standard of care in evaluating posterior cervical instrumentation is two-dimensional intraoperative fluoroscopy and/or postoperative CT scanning. The availability of intraoperative three-dimensional imaging techniques such as ISO-C CT enables the surgeon to more accurately evaluate the placement of hardware in complex anatomic regions and obviates the need for postoperative CT. The purpose of our study was to document the incidence of hardware-related complications and evaluate cost-effectiveness when using intraoperative three-dimensional fluoroscopy in posterior cervical spine surgery.
We draw the reader’s attention to two limitations. First, our cost-effectiveness calculation is sensitive to the assumption that all malpositioned hardware will result in postoperative symptoms necessitating a return to the operating room. Second, our calculation also does not include any medicolegal costs from revision surgery secondary to technical errors.
Many clinical and laboratory studies have focused on lateral mass screw trajectories and their complications. Ebraheim et al. examined neurovascular injury in cadavers and demonstrated a laterally directed screw will likely miss the vertebral artery [7, 8]. Xu et al. demonstrated the risk of nerve root violation is higher with the Magerl and Anderson techniques than with the An technique [38]. Pait et al. divided the lateral masses into quadrants and reported the upper outer quadrant has the lowest risk of vertebral artery or nerve root injury [29]. Roy-Camille et al. suggested the starting point for screw positioning be located in the midpoint of the lateral mass and oriented 10° lateral and perpendicular to the posterior aspect of the cervical spine [32]. Anderson recommended positioning the screw 1 mm medial to the midpoint of the lateral mass and angling the screw 30° to 40° cranial and 10° lateral [2]. Magerl advocated starting the trajectory 2 to 3 mm medial and superior to the midpoint of the lateral mass and oriented 30° upward and 25° outward [19]. Lastly, An et al. proposed starting 1 mm medial to the center of the lateral mass and angling 15° to 18° superiorly and 30° to 33° laterally [1].
To reduce potential complications, intraoperative imaging is routinely used, especially in difficult cases such as revision operations and whenever instrumentation is placed [10]. Intraoperative imaging helps to confirm the proper placement of instrumentation and is most commonly used in the form of biplanar fluoroscopy. Despite the routine use of intraoperative biplanar fluoroscopy, malpositioning rates of screws continue to remain substantial and often necessitate a return to the operating room [5, 6, 11, 13, 18, 21, 23, 24, 31, 32, 34, 35]. The use of intraoperative three-dimensional fluoroscopy allows for accurate assessment of complex anatomic regions. Its use is increasing in fracture surgery, especially when internal fixation is used for articular fractures. Specifically with regard to spinal surgery, authors have reported lower screw malpositioning rates when using three-dimensional imaging in the lumbar spine. Kim et al. found screw malpositioning rates of 7.5% using this method [21].
The reported rates of single level radiculopathy secondary to malpositioned hardware are between 1.4% and 1.8% [6, 15]. In Graham’s series, revision of misplaced screws was required in 14% of cases [15]. It is important to note not all malpositioned screws cause radiculopathy. In Graham’s series, 6.1% of screws were misplaced; however, the majority of these screws were asymptomatic. In a larger series reported by Katonis et al., 27% of screws were suboptimally placed and 2.4% breached the foramen tranversarium without vertebral artery injury [20]. Screw pullout can also occur and was noted in 2.9% of patients in the Katonis series.
We found malpositioning of hardware using isocentric three-dimensional CT scan in 1.2% of screws placed. Importantly, because malpositioning was immediately addressed, no patient required a return to the operating room for hardware-related complications nor did any patient sustain a neurovascular injury as a result of the position of the screw. This is in contrast to those patients in whom the ISO-C CT was not used and one patient required revision surgery to remove the C5/6 lateral mass screws (Table 2).
The unique use of the ISO-C CT is demonstrated in our illustrative case. Biplanar fluoroscopy was ineffective in identifying malpositioned screws and the ISO-C CT was instrumental in allowing for immediate correction.
Additionally, we recognize that real-time image-guided (sterotactic) surgery has gained popularity because its efficacy has been validated in the literature [3, 39]. It allows surgical instruments to be tracked in reference to the displayed anatomy in real time. The benefits of image-guided surgery with regard to safety and accuracy have been well documented. However, there are limitations such as the learning curve that results in longer operating room times and the time-consuming intraoperative point-to-point registration steps and preoperative planning. The ISO-C CT also provides three-dimensional reconstructed images of the patient. Furthermore, there is no risk of navigation inaccuracy as a result of intervertebral alignment differences between the preoperative CT data set and the intraoperative position. The need for a preoperative CT with a specific image-guided protocol is eliminated. The ISO-C CT can serve as a standard C-arm fluoroscope and allows a single device to serve as both a C-arm and CT. Most importantly, it obviates the need for postoperative CT and provides a method of intraoperative three-dimensional evaluation of hardware.
There is no increased risk of radiation with the use of the ISO-C CT. Based on work performed by Villavicencio et al., the dose associated with the initial 2-minute spin for ISO-C fluoroscopy is 0.75× lower than for standard two-dimensional fluoroscopy [36]. So, the 120-second spin of the ISO-C CT is equivalent to 30 seconds of standard fluoroscopy. Furthermore, Gebhard et al. compared the dose of radiation exposure for surgical procedures performed with and without image guidance using CT-based navigation, conventional C-arm navigation, and ISO-C navigation [12]. The lowest emission of ionizing radiation was for navigation with the ISO-C method.
Despite the fact that most spine surgeons would not question the effectiveness of intraoperative three-dimensional CT, administrators and surgeons have questioned the cost-effectiveness of its routine use for spine surgery [22]. Our data demonstrate the cost-effectiveness of using ISO-C CT intraoperatively instead of conventional postoperative CT. Seven patients were spared potential revision surgeries because of the ISO-C CT, saving an estimated $500,000 in operating room costs. The ISO-C CT is a fixed cost of $300,000 with no per-patient costs; our surgeons do not bill for use of this modality. Thus, we believe the ISO-C CT provides adequate efficacy to precisely and accurately evaluate hardware placement and can safely replace postoperative CT evaluation as the standard of care in patients undergoing posterior cervical spinal fusion.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.An HS, Gordin R, Renner K. Anatomic considerations for plate-screw fixation of the cervical spine. Spine. 1991;16(Suppl):S548–S551. doi: 10.1097/00007632-199110001-00019. [DOI] [PubMed] [Google Scholar]
- 2.Anderson PA, Henley MB, Grady MS, Montesano PX, Winn HR. Posterior cervical arthrodesis with AO reconstruction plates and bone graft. Spine. 1991;16(Suppl):S72–S79. doi: 10.1097/00007632-199103001-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bloch O, Holly LT, Park J, Obasi C, Kim K, Johnson JP. Effect of frameless stereotaxy on the accuracy of C1–2 transarticular screw placement. J Neurosurg. 2001;95(Suppl):74–79. doi: 10.3171/spi.2001.95.1.0074. [DOI] [PubMed] [Google Scholar]
- 4.Burke JP, Gerstzen PC, Welch WC. Iatrogenic vertebral artery injury during anterior cervical spine surgery. Spine J. 2005;5:508–514. doi: 10.1016/j.spinee.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Castro WH, Halm H, Jerosch J, Malms J, Steinbeck J, Blasius S. Accuracy of pedicle screw placement in lumbar vertebrae. Spine. 1996;21:1320–1324. doi: 10.1097/00007632-199606010-00008. [DOI] [PubMed] [Google Scholar]
- 6.Deen H, Birch B, Wharen R, Reimer R. Lateral mass screw-rod fixation of the cervical spine: a prospective clinical series with 1-year follow-up. Spine J. 2003;3:489–495. [PubMed] [Google Scholar]
- 7.Ebraheim NA, Xu R, Stanescu S, Yeasting RA. Anatomic relationship of the cervical nerves to the lateral masses. Am J Orthop. 1999;28:39–42. [PubMed] [Google Scholar]
- 8.Ebraheim NA, Xu R, Yeasting RA. The location of the vertebral artery foramen and its relation to posterior lateral mass screw fixation. Spine. 1996;21:1291–1295. doi: 10.1097/00007632-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Farey ID, Nadkarni S, Smith N. Modified Gallie technique versus transarticular screw fixation in C1–C2 fusion. Clin Orthop Relat Res. 1999;359:126–135. doi: 10.1097/00003086-199902000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Freidberg SR, Pfeifer BA, Dempsey PK, Tarlov EC, Dube MA, Day JD, Machado DE. Intraoperative computerized tomography scanning to assess the adequacy of decompression in anterior cervical spine surgery. J Neurosurg. 2001;94:8–11. doi: 10.3171/spi.2001.94.1.0008. [DOI] [PubMed] [Google Scholar]
- 11.Fu TS, Chen LH, Wong CB, Lai PL, Tsai TT, Niu CC, Chen WJ. Computer-assisted fluoroscopic navigation of pedicle screw insertion. An in vivo feasibility study. Acta Orthop Scand. 2004;75:730–735. doi: 10.1080/00016470410004102. [DOI] [PubMed] [Google Scholar]
- 12.Gebhard F, Kraus M, Schneider E, et al. Radiation dosage in orthopedics—a comparison of computer-assisted procedures [in German] Unfallchirurg. 2003;106:492–497. doi: 10.1007/s00113-003-0606-9. [DOI] [PubMed] [Google Scholar]
- 13.Gertzbein SD, Robbins SE. Accuracy of pedicle screw placement in vivo. Spine. 1990;15:11–14. doi: 10.1097/00007632-199001000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Golfinos JG, Dickman CA, Zabramski JM, Sonntag VK, Spetzler RF. Repair of vertebral artery injury during anterior cervical decompression. Spine. 1994;19:2552–2556. doi: 10.1097/00007632-199411001-00010. [DOI] [PubMed] [Google Scholar]
- 15.Graham AW, Swank ML, Kinard RE, Lowery GL, Dials BE. Posterior cervical arthrodesis and stabilization with a lateral mass plate. Clinical and computed tomographic evaluation of lateral mass screw placement and associated complications. Spine. 1996;21:323–329. doi: 10.1097/00007632-199602010-00014. [DOI] [PubMed] [Google Scholar]
- 16.Grob D, Jeanneret B, Aebi M, Markwalder TM. Atlanto-axial fusion with transarticular screw fixation. J Bone Joint Surg Br. 1991;73:972–976. doi: 10.1302/0301-620X.73B6.1955447. [DOI] [PubMed] [Google Scholar]
- 17.HCUP Nationwide Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2003-2005. Rockville, MD: Agency for Healthcare Research and Quality. Available at: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed April 27, 2009.
- 18.Heller JG, Silcox DH, Sutterlin CE. Complications of posterior cervical plating. Spine. 1995;20:2442–2448. doi: 10.1097/00007632-199511001-00013. [DOI] [PubMed] [Google Scholar]
- 19.Jeanneret B, Magerl F, Ward EH, Ward JC. Posterior stabilization of the cervical spine with hook plates. Spine. 1991;16(Suppl):S56–S63. doi: 10.1097/00007632-199103001-00010. [DOI] [PubMed] [Google Scholar]
- 20.Katonis P, Papadopoulos CA, Muffoletto A, Papagelopoulos PJ, Hadjipavlou AG. Factors associated with good outcome using lateral mass plate fixation. Orthopedics. 2004;27:1080–1086. doi: 10.3928/0147-7447-20041001-18. [DOI] [PubMed] [Google Scholar]
- 21.Kim YJ, Lenke LG, Bridwell KH, Cho YS, Riew LD. Free hand pedicle screw placement in the thoracic spine: is it safe? Spine. 2004;29:333–342. doi: 10.1097/01.BRS.0000109983.12113.9B. [DOI] [PubMed] [Google Scholar]
- 22.Kotil K, Bilge T. Accuracy of pedicle and mass screw placement in the spine without using fluoroscopy: a prospective clinical study. Spine J. 2008;8:591–596. doi: 10.1016/j.spinee.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Laine T, Lund T, Ylikoski M, Lohikoski J, Schlenzka D. Accuracy of pedicle screw insertion with and without computer assistance: a randomized controlled clinical study in 100 consecutive patients. Eur Spine J. 2000;9:235–240. doi: 10.1007/s005860000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Learch TJ, Massie JB, Pathria MN, Ahlgren BA, Garfin SR. Assessment of pedicle screw placement utilizing conventional radiography and computed tomography: a proposed systematic approach to improve accuracy of interpretation. Spine. 2004;29:767–773. doi: 10.1097/01.BRS.0000112071.69448.A1. [DOI] [PubMed] [Google Scholar]
- 25.Madawi AA, Casey AT, Solanki GA, Tuite G, Veres R, Crockard HA. Radiological and anatomical evaluation of the atlantoaxial transarticular screw fixation technique. J Neurosurg. 1997;86:961–968. doi: 10.3171/jns.1997.86.6.0961. [DOI] [PubMed] [Google Scholar]
- 26.Nazarian SM, Louis RP. Posterior internal fixation with screw plates in traumatic lesions of the cervical spine. Spine. 1991;16(Suppl):S64–S71. doi: 10.1097/00007632-199103001-00011. [DOI] [PubMed] [Google Scholar]
- 27.Neo M, Fujibayashi S, Miyata M, Takemoto M, Nakamura T. Vertebral artery injury during cervical spine surgery: a survey of more than 5600 operations. Spine. 2008;33:779–785. doi: 10.1097/BRS.0b013e31816957a7. [DOI] [PubMed] [Google Scholar]
- 28.Neo M, Sakamoto T, Fujibayashi S, Nakamura T. A safe trajectory for atlantoaxial transarticular screw fixation achieved using an aiming device. Spine. 2005;30:E236–E242. doi: 10.1097/01.brs.0000160998.53282.3f. [DOI] [PubMed] [Google Scholar]
- 29.Pait TG, McAllister PV, Kaufman HH. Quadrant anatomy of the articular pillars of the cervical spine. J Neurosurg. 1995;82:1011–1014. doi: 10.3171/jns.1995.82.6.1011. [DOI] [PubMed] [Google Scholar]
- 30.Pateder DB, Carbone JJ. Lateral mass screw fixation for cervical spine trauma: associated complications and efficacy in maintaining alignment. Spine J. 2006;6:40–43. doi: 10.1016/j.spinee.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Pihlamajaki H, Myllynen P, Bostman O. Complications of transpedicular lumbosacral fixation for non-traumatic disorders. J Bone Joint Surg Br. 1997;79:183–189. doi: 10.1302/0301-620X.79B2.7224. [DOI] [PubMed] [Google Scholar]
- 32.Roy-Camille R, Saillant G, Laville C, Benazet JP. Treatment of lower cervical spinal injuries—C3 to C7. Spine. 1992;17(Suppl):S442–S446. doi: 10.1097/00007632-199210001-00017. [DOI] [PubMed] [Google Scholar]
- 33.Schwarzenback O, Berleman NO, Jost B, Visarius H, Arm E, Langlotz F. Accuracy of computer-assisted pedicle screw placement. An in vivo computer tomography analysis. Spine. 1997;22:452–458. doi: 10.1097/00007632-199702150-00020. [DOI] [PubMed] [Google Scholar]
- 34.Sekhon LHS. Posterior cervical lateral mass screw fixation. J Spinal Disord Tech. 2005;18:297–303. doi: 10.1097/01.bsd.0000166640.23448.09. [DOI] [PubMed] [Google Scholar]
- 35.Smith MD, Emery SE, Dudley A, Murray KJ, Leventhal M. Vertebral artery injury during anterior decompression of the cervical spine. A retrospective review of ten patients. J Bone Joint Surg Br. 1993;75:410–415. doi: 10.1302/0301-620X.75B3.8496209. [DOI] [PubMed] [Google Scholar]
- 36.Villavicencio AT, Burneikiene S, Bulsara KR, Thramann JJ. Utility of computerized isocentric fluoroscopy for minimally invasive spinal surgical techniques. J Spinal Disord Tech. 2005;18:369–375. doi: 10.1097/01.bsd.0000168511.67189.64. [DOI] [PubMed] [Google Scholar]
- 37.Wright NM, Lauryssen C. Vertebral artery injury in C1–2 tranarticular screw fixation: results of a surgery of the AANS/CNS section on disorders of the spine and peripheral nerves. J Neurosurg. 1998;88:634–640. doi: 10.3171/jns.1998.88.4.0634. [DOI] [PubMed] [Google Scholar]
- 38.Xu R, Haman SP, Ebraheim NA, Yeasting RA. The anatomic relation of lateral mass screws to the spinal nerves. A comparison of the Magerl, Anderson, and An techniques. Spine. 1999;24:2057–2061. doi: 10.1097/00007632-199910010-00016. [DOI] [PubMed] [Google Scholar]
- 39.Youkilis AS, Quint DJ, McGillicuddy JE, Papadopoulos SM. Stereotactic navigation for placement of pedicle screws in the thoracic spine. Neurosurgery. 2001;48:771–779. doi: 10.1097/00006123-200104000-00015. [DOI] [PubMed] [Google Scholar]