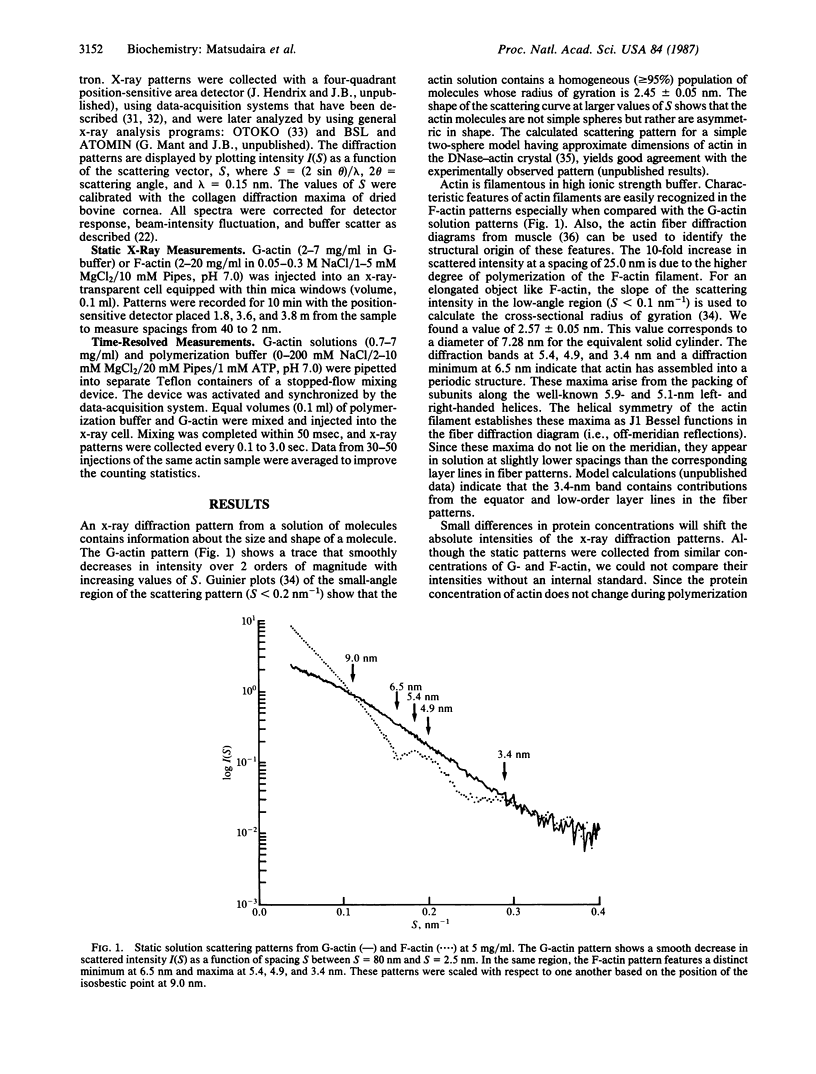
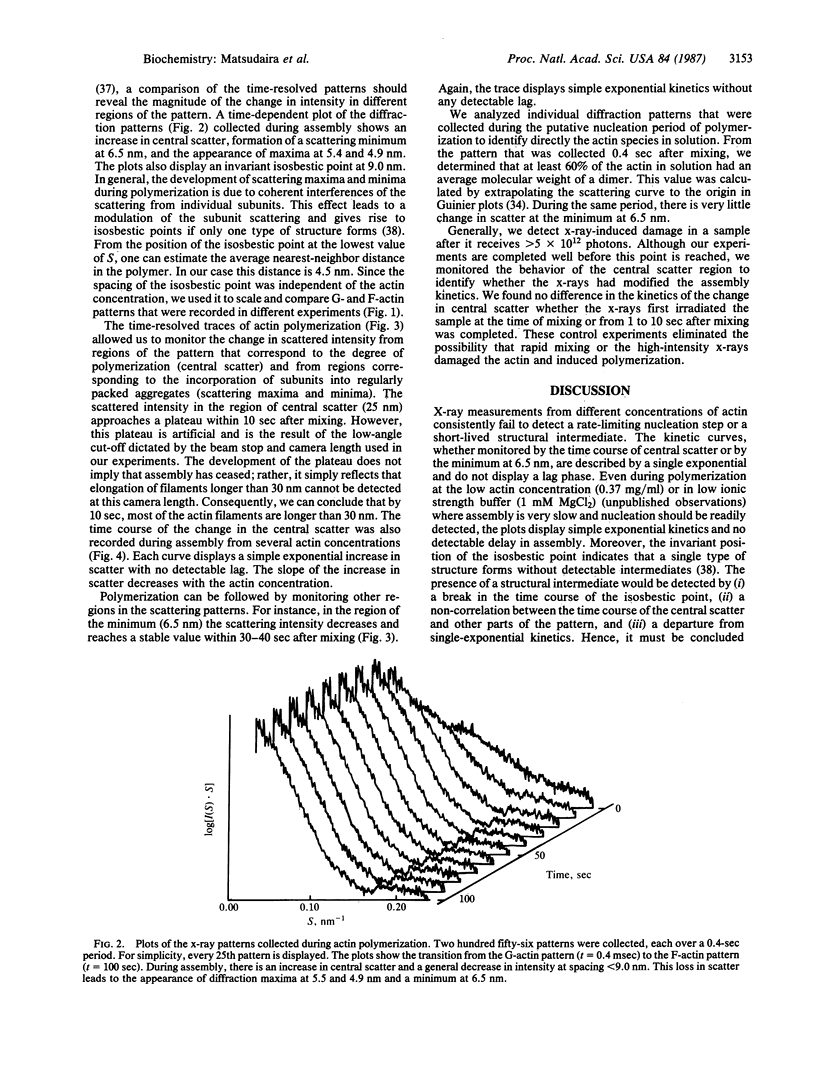
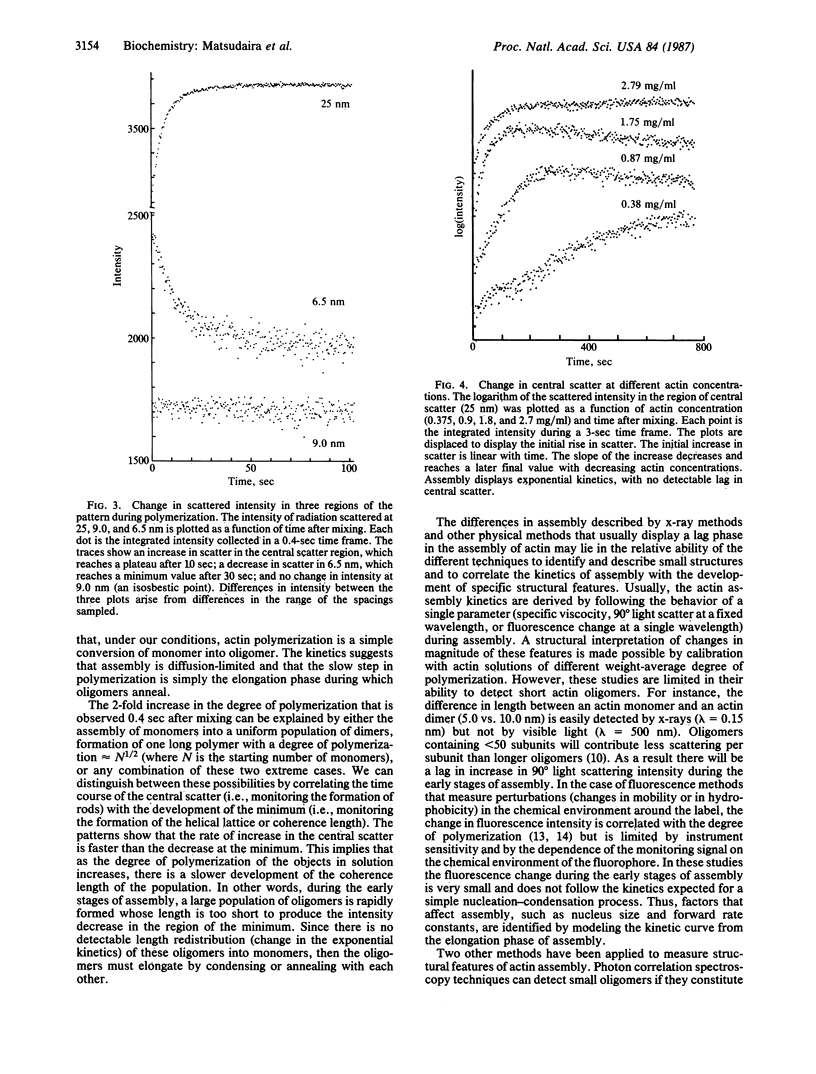
Abstract
Synchrotron x-ray diffraction was used to identify the oligomers that formed during the earliest stages of actin polymerization. Solution diffraction patterns from G-actin (monomer) and from F-actin (polymer) contain information about the size and shape of actin monomers and the length, width, and subunit organization of filaments. Comparison of patterns collected during polymerization reveals an increase in scatter at spacings greater than 9.0 nm; formation of scattering bands at 5.4,4.9, and 3.4 nm; formation of a scattering minimum at 6.5 nm; and the presence of an isosbestic point at 9.0 nm. These scattering bands arise from the formation of, and organization of subunits in, filaments. At various actin concentrations (0.37-5 mg/ml), the change in scatter in these regions follows simple exponential kinetics with no detectable lag. Our analysis of the x-ray patterns shows that by 0.4 sec after mixing, most of the actin has formed dimers, which then rapidly incorporate into oligomers. At 4 mg/ml the early oligomers increase in length to greater than 30.0 nm within 10 sec. These results suggest that under our conditions actin molecules condense into filaments without the rate-limiting formation of nuclei.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordas J., Mandelkow E. M., Mandelkow E. Stages of tubulin assembly and disassembly studied by time-resolved synchrotron X-ray scattering. J Mol Biol. 1983 Feb 15;164(1):89–135. doi: 10.1016/0022-2836(83)90089-x. [DOI] [PubMed] [Google Scholar]
- Bordas J., Perez-Grau L., Koch M. H., Vega M. C., Nave C. The superstructure of chromatin and its condensation mechanism. II. Theoretical analysis of the X-ray scattering patterns and model calculations. Eur Biophys J. 1986;13(3):175–185. doi: 10.1007/BF00542561. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D., Korn E. D. Polymerization of ADP-actin and ATP-actin under sonication and characteristics of the ATP-actin equilibrium polymer. J Biol Chem. 1985 Jun 10;260(11):6565–6571. [PubMed] [Google Scholar]
- Cooper J. A., Buhle E. L., Jr, Walker S. B., Tsong T. Y., Pollard T. D. Kinetic evidence for a monomer activation step in actin polymerization. Biochemistry. 1983 Apr 26;22(9):2193–2202. doi: 10.1021/bi00278a021. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Pollard T. D. Effect of capping protein on the kinetics of actin polymerization. Biochemistry. 1985 Jan 29;24(3):793–799. doi: 10.1021/bi00324a039. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Walker S. B., Pollard T. D. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983 Apr;4(2):253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Egelman E. H. The structure of F-actin. J Muscle Res Cell Motil. 1985 Apr;6(2):129–151. doi: 10.1007/BF00713056. [DOI] [PubMed] [Google Scholar]
- Frieden C., Goddette D. W. Polymerization of actin and actin-like systems: evaluation of the time course of polymerization in relation to the mechanism. Biochemistry. 1983 Dec 6;22(25):5836–5843. doi: 10.1021/bi00294a023. [DOI] [PubMed] [Google Scholar]
- Frieden C. Polymerization of actin: mechanism of the Mg2+-induced process at pH 8 and 20 degrees C. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6513–6517. doi: 10.1073/pnas.80.21.6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Ikkai T., Ooi T., Noguchi H. Actin: volume change on transformation of G-form to F-form. Science. 1966 Jun 24;152(3730):1756–1757. doi: 10.1126/science.152.3730.1756. [DOI] [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The cooperative nature of G-F transformation of actin. Biochim Biophys Acta. 1962 Feb 12;57:22–31. doi: 10.1016/0006-3002(62)91073-9. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D. Three-dimensional structure of the complex of actin and DNase I at 4.5 A resolution. EMBO J. 1985 Aug;4(8):2113–2118. doi: 10.1002/j.1460-2075.1985.tb03900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai M. Thermodynamical aspect of G-F transformations of actin. Biochim Biophys Acta. 1969 Jun 24;180(2):399–409. doi: 10.1016/0005-2728(69)90124-8. [DOI] [PubMed] [Google Scholar]
- Keiser T., Schiller A., Wegner A. Nonlinear increase of elongation rate of actin filaments with actin monomer concentration. Biochemistry. 1986 Aug 26;25(17):4899–4906. doi: 10.1021/bi00365a026. [DOI] [PubMed] [Google Scholar]
- Koch M., Blumenthal W. Bedingungen und Ergebnisse der medizinisch-sozialen Rehabilitation im Rehabilitatinszentrum der Universität zu Köln. Rehabilitation (Stuttg) 1981 Feb;20(1):13–16. [PubMed] [Google Scholar]
- Kouyama T., Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114(1):33–38. [PubMed] [Google Scholar]
- Mandelkow E. M., Harmsen A., Mandelkow E., Bordas J. X-ray kinetic studies of microtubule assembly using synchrotron radiation. Nature. 1980 Oct 16;287(5783):595–599. doi: 10.1038/287595a0. [DOI] [PubMed] [Google Scholar]
- Mandelkow E., Mandelkow E. M., Bordas J. Structure of tubulin rings studied by X-ray scattering using synchrotron radiation. J Mol Biol. 1983 Jun 15;167(1):179–196. doi: 10.1016/s0022-2836(83)80040-0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P., Mandelkow E., Renner W., Hesterberg L. K., Weber K. Role of fimbrin and villin in determining the interfilament distances of actin bundles. Nature. 1983 Jan 20;301(5897):209–214. doi: 10.1038/301209a0. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Oosawa F. The flexibility of F-actin. Biophys Chem. 1980 Jun;11(3-4):443–446. doi: 10.1016/0301-4622(80)87021-9. [DOI] [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F., Korn E. D. The interaction between ATP-actin and ADP-actin. A tentative model for actin polymerization. J Biol Chem. 1985 Jun 10;260(11):6572–6578. [PubMed] [Google Scholar]
- Pardee J. D., Spudich J. A. Mechanism of K+-induced actin assembly. J Cell Biol. 1982 Jun;93(3):648–654. doi: 10.1083/jcb.93.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich S. A., Estes J. E. Detection of conformational changes in actin by proteolytic digestion: evidence for a new monomeric species. J Mol Biol. 1976 Jul 15;104(4):777–792. doi: 10.1016/0022-2836(76)90181-9. [DOI] [PubMed] [Google Scholar]
- Sayers Z., Koch M. H., Bordas J., Lindberg U. Time-resolved X-ray scattering study of actin polymerization from profilactin. Eur Biophys J. 1985;13(2):99–108. doi: 10.1007/BF00256530. [DOI] [PubMed] [Google Scholar]
- Suarez G., Oronsky A. L., Bordas J., Koch M. H. Synchrotron radiation x-ray scattering in the early stages of in vitro collagen fibril formation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4693–4696. doi: 10.1073/pnas.82.14.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. L., Reidler J., Spudich J. A., Stryer L. Detection of actin assembly by fluorescence energy transfer. J Cell Biol. 1981 May;89(2):362–367. doi: 10.1083/jcb.89.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. S., Weeds A. G. The magnesium-ion-dependent adenosine triphosphatase of bovine cardiac Myosin and its subfragment-1. Biochem J. 1976 Nov;159(2):301–315. doi: 10.1042/bj1590301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellam R., Frieden C. Cytochalasin D and platelet gelsolin accelerate actin polymer formation. A model for regulation of the extent of actin polymer formation in vivo. Biochemistry. 1982 Jun 22;21(13):3207–3214. doi: 10.1021/bi00256a027. [DOI] [PubMed] [Google Scholar]
- Ware B. R., Klein J. W. Assembly of actin filaments studied by laser light scattering and fluorescence photobleaching recovery. Biophys J. 1986 Jan;49(1):147–149. doi: 10.1016/S0006-3495(86)83629-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A., Engel J. Kinetics of the cooperative association of actin to actin filaments. Biophys Chem. 1975 Jul;3(3):215–225. doi: 10.1016/0301-4622(75)80013-5. [DOI] [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]
- Wegner A., Isenberg G. 12-fold difference between the critical monomer concentrations of the two ends of actin filaments in physiological salt conditions. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4922–4925. doi: 10.1073/pnas.80.16.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner A., Savko P. Fragmentation of actin filaments. Biochemistry. 1982 Apr 13;21(8):1909–1913. doi: 10.1021/bi00537a032. [DOI] [PubMed] [Google Scholar]
- Wegner A. Spontaneous fragmentation of actin filaments in physiological conditions. Nature. 1982 Mar 18;296(5854):266–267. doi: 10.1038/296266a0. [DOI] [PubMed] [Google Scholar]


