Abstract
Background
Chronic infections in TKA have been traditionally treated with a two-stage protocol incorporating a temporary antibiotic-loaded cement spacer. The use of a static as opposed to an articulating spacer is controversial. Some surgeons believe a static spacer results in a higher rate of infection eradication, whereas others believe an articulating spacer provides equivalent rates of infection control with improved function between stages and the potential for better eventual range of motion.
Questions/purposes
We determined the rates of infection control and postoperative function for an articulating all-cement antibiotic spacer fashioned intraoperatively from prefabricated silicone molds.
Methods
We retrospectively reviewed 60 patients with an infected TKA using the same cement-on-cement articulating spacer. A minimum of 4 g antibiotic per package of cement was used when making the spacer. Complications and pre- and postoperative knee flexion, extension, and Knee Society scores were recorded. Bone loss associated with the spacer was determined radiographically and by intraoperative inspection of the bony surfaces at the second stage. Minimum followup was 24 months (mean, 35 months; range, 24–51 months).
Results
Seven patients (12%) became reinfected, four with an organism different from that identified at the index resection arthroplasty. One spacer femoral component broke between stages but did not require any specific treatment. We identified no bone loss between stages and no complications related to the cement-on-cement articulation. The mean pretreatment Knee Society scores of 53 improved to 79. The mean preoperative flexion of 90.6º improved to 101.3º at final followup.
Conclusions
An articulating antibiotic spacer was associated with control of a deep periprosthetic infection in 88% of patients while allowing range of motion between stages.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Infection in TKA creates a difficult paradox for the treating surgeon, because patient function must be balanced with infection eradication. For chronic infections, Insall et al. [17] first proposed a two-stage exchange protocol with resection of the prosthesis without a spacer combined with parenteral antibiotics; this regimen controlled the infection in 91% of patients at 34 months. However, three patellectomies in 11 cases were performed with two of them being necessary for exposure at reimplantation. Furthermore, the patients were unable to weightbear during treatment. Wilde and Ruth [25] and Booth et al. [2] subsequently modified the technique to include the implantation of a static antibiotic-impregnated spacer block after the first-stage de´bridement and reported similar infection control rates of 80% and 96%, respectively, with improved function. Thus, a two-stage approach with the use of a temporary antibiotic spacer has become the treatment of choice in North America for chronically infected TKAs.
A variety of techniques have been proposed for fashioning a cement spacer after the first-stage débridement. Static spacers have been associated with potential problems, including difficulty in exposure at the time of the second-stage reimplantation, bone loss [3, 8], extensor mechanism contracture, decreased patient ambulatory function and satisfaction, and stiffness after the second-stage reimplantation [6, 8]. Thus, in recent years, there has been interest in mobile antibiotic spacers that allow for knee ROM. Multiple reports have described a number of different techniques that all show acceptable rates of infection control while facilitating mobilization during treatment and showing substantial improvements in ROM postoperatively [5, 7–9, 12, 13, 18, 21].
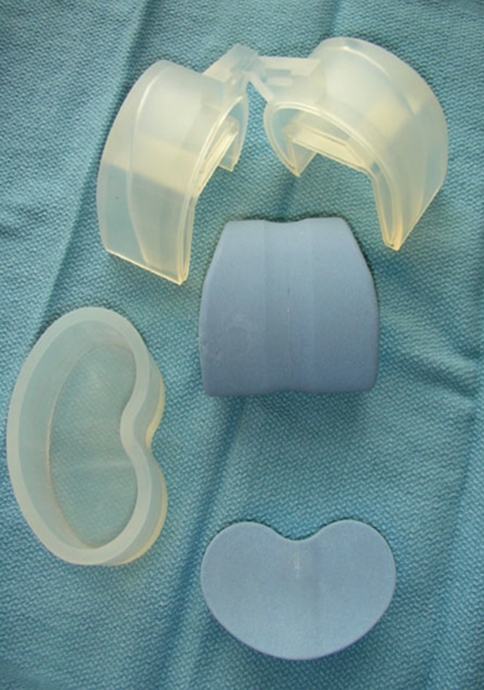
Recently, commercially available molds have become available (Stage One; Biomet, Warsaw, IN) that facilitate the production of an articulating spacer that is made from bone cement (Fig. 1). In addition to allowing for knee ROM between stages, benefits of these molds include the ability to mix the type and amount of antibiotics the surgeon believes is appropriate, sizing of the components is both independent and consistent, stability can be obtained by varying the thickness of the tibial component, and no metal or plastic is introduced into the joint that could act as a nidus for persistent infection. Concerns include potential complications related to the cement on cement articulation and the cost of the molds.
Fig. 1.
A photograph shows the molds used for fabrication of antibiotic cement articulating components
We therefore determined (1) the rate of infection control; (2) ease of the surgical exposure at the time of reimplantation; (3) ROM achieved after reimplantation; and (4) complications associated with articulating spacers made with these molds.
Patients and Methods
This is a retrospective case series that includes 65 consecutive patients with an infected TKA treated at three institutions with the same cement-on-cement articulating spacer between 2005 and 2007. Patients were included in the study if the arthroplasty was deemed to be infected based on the results of joint cultures, presence of a sinus tract or gross purulence seen intraoperatively, and histopathologic evidence of acute inflammation consistent with infection. Articulating spacers were used when the operating surgeon believed there was adequate bone to support an articulating spacer and that the soft-tissue envelope could tolerate postoperative ROM; no other technique for creation of an articulating spacer was used by the authors during the time period of the study. Five patients were lost to followup before the 2-year minimum, leaving 60 patients available for study. Minimum followup was 24 months (mean, 35 months; range, 24–51 months). The patients included 29 women and 31 men with a mean age of 66 years (range, 42–91 years). The mean number of prior TKAs was 2.0 (range, 1–7). Institutional Review Board approval was obtained at all three study sites.
The preoperative evaluation included determination of ROM and Knee Society scores [16]. Intraoperative culture results at the time of the first-stage procedure were available for 59 of the 60 patients (Table 1). The most common infecting organisms were methicillin-resistant Staphylococcus aureus (20%, 12 patients), methicillin-susceptible S. aureus (20%, 12 patients), coagulase-negative S. aureus (10%, seven patients), and α-hemolytic Streptococcus (5%, three patients). Operative cultures were negative in 18 cases; however, all of these cases had definitive evidence of infection with a draining sinus, gross purulence seen at the time of resection arthroplasty, or permanent histopathology consistent with infection (a mean of greater than 10 polymorphonuclear cells per high-power field). All patients were treated with organism-specific intravenous antibiotic therapy in consultation with an infectious disease specialist.
Table 1.
Microbiology results before spacer
| Organism | Number of patients |
|---|---|
| Methicillin-resistant Staphylococcus aureus | 12 |
| Methicillin-susceptible S. aureus | 12 |
| Coagulase-negative Staphylococcus epidermidis | 7 |
| α-hemolytic Streptococcus | 3 |
| Mycobacterium fortuitum | 1 |
| Group B Streptococcus | 1 |
| Serratia marcescens | 1 |
| Enterococcus | 1 |
| Trichophyton | 1 |
| Escherichia coli | 1 |
| Propionibacterium | 1 |
| Culture negative | 18 |
All surgery was performed by one of four surgeons (CDV, KRB, AVL, GRK). A similar surgical technique was used at all three centers. The initial procedure consisted of a thorough débridement of infected and devitalized tissue with hardware and cement removal. The residual bony surfaces of the femur and tibia were meticulously débrided and bony cuts freshened with a saw. After removal of the patellar component and any associated cement, the patella was left unresurfaced between stages. In all patients, we used an all-cement spacer fashioned intraoperatively using silicone molds (Stage One; Biomet, Inc). The femoral molds are available in four sizes in 5-mm increments and were selected intraoperatively based on a comparison to the femoral component removed and patient anatomy. The femoral mold is made first by injecting antibiotic-loaded cement into the mold and allowing it to harden. Tibial molds are similarly available in four sizes in 5-mm increments, and the appropriate size was selected to provide coverage for the cut surface of the tibia. Spacer blocks are then used to determine the size of the flexion and extension gaps and the tibial spacer was made to a thickness 2 to 4 mm thinner than the determined gap size to facilitate both knee ROM and a small cement mantle for fixation of the spacer while maintaining knee stability (Fig. 1).
A minimum of 4 g of antibiotics per package of cement (but up to 8 g in some cases) was used in all cases. Thirty-five patients received a mixture of tobramycin and vancomycin, whereas 25 patients received gentamicin and vancomycin. The femoral and tibial canals were opened, débrided, and subsequently filled with dowels of antibiotic-loaded cement in 55 of the 60 patients (92%). When present, these intramedullary dowels were routinely attached to the femoral and tibial components to both augment stability of the components and to provide high local concentrations of antibiotics. Once the cement spacers and dowels had fully hardened, one additional package of antibiotic-loaded cement was used to loosely cement the components in place with the tourniquet deflated to encourage a “poor” cement mantle with blood interposed between the cement and bone. This was performed in the later stages of hardening to facilitate removal at the second-stage procedure (Fig. 2).
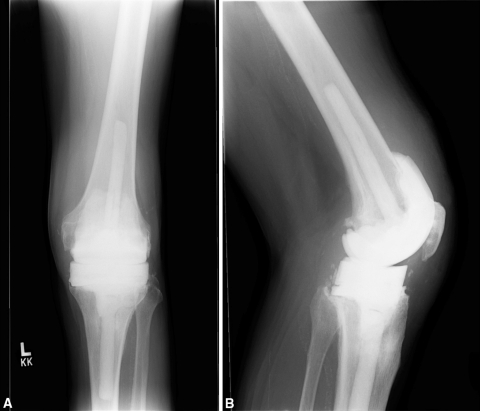
Fig. 2A–B.
(A) AP and (B) lateral radiographs show the articulating antibiotic spacer
The postoperative protocol after the first stage differed for each surgeon. Thirty-nine patients were braced and 21 patients were not braced postoperatively. Of the patients braced, 20 patients were placed in a hinged knee brace and allowed ROM from 0° to 60° or 90°; 14 patients were placed in a knee immobilizer worn only at night and five patients were placed in a knee immobilizer at all times. Thirty-nine patients were allowed to be touch-down or partial weightbearing, 16 were weightbearing as tolerated, and five were nonweightbearing.
The second stage was performed at a mean of 75 days after the first stage (range, 30–326 days) and included a reimplantation in 58 patients, a second débridement in one patient, and a knee fusion in one patient whose extensor mechanism was chronically disrupted. The definitive implants included 18 Zimmer LCCKs, 32 Vanguard SSKs, two Vanguard PSs, one Smith and Nephew Legion, one Vanguard OSS, three Link Endo RHs, and one DePuy MBT revision. In total, one distal femoral replacement, four rotating hinges, and 53 stemmed components were used. A medial gastrocnemius flap was performed concomitantly with the first-stage resection arthroplasty in two patients with complex wounds and a draining sinus. A third patient had a gastrocnemius flap at the time of a second débridement procedure.
After the second-stage procedure, patients were allowed to weightbear as tolerated and ROM exercises were initiated along with quadriceps strengthening exercises that were supervised by a physiotherapist for a minimum of 6 weeks. Physiotherapy was initially performed in the hospital and then on an outpatient basis a minimum of three times per week.
Patients at all three centers were evaluated at least once within the first 6 weeks and then at approximately 3 months, 1 year, and yearly thereafter. At each visit, the wound was assessed for healing or any overt signs of infection (such as a sinus tract), radiographs were obtained (including at a minimum a weightbearing AP, lateral, and patellar views), and a physical examination was performed that assessed knee ROM and stability. No specific laboratory testing for infection recurrence was performed in patients who were not symptomatic (eg, pain) or who did not have any overt signs of infection. Knee flexion, extension, and Knee Society scores were recorded at the most recent followup.
Patients were assessed for the presence of complications related to the spacer, including dislocation, breakage, reaction to the cement-on-cement articulation, and bone loss related to the spacer. Bone loss was assessed by comparing the radiographs obtained after the first stage with the radiographs obtained before the second stage and by intraoperatively assessing the bone behind the spacer at the time of their removal. The operative approach used at the time of reimplantation (standard medial parapatellar as opposed to the need for a more extensile approach such as quadriceps snip or a tibial tubercle osteotomy) was recorded as a proxy for exposure difficulty. Recurrent infection was defined as any additional surgery related to infection, a culture of the joint that showed bacterial growth, or the development of overt signs of infection such as a sinus tract.
The most recent radiographs were reviewed by one of three observers (CDV, GVT, KRB) to evaluate for prosthetic loosening in the zones as defined by the Knee Society and modified by Whaley et al. [24] to allow for evaluation of stemmed revision components.
Continuous variables including comparisons of pre and postoperative knee extension, knee flexion and knee society scores were compared using a paired Student’s t test.
Results
At an average of 35 months, seven patients (12%) had become reinfected; reinfection occurred at a mean of 16.3 months after the reimplantation procedure (range, 2–30 months) (Table 2). Two of these patients were infected with the same initial organism, one patient was culture-negative despite gross purulence seen at the time of reoperation, and four patients were infected with a new organism. Four patients were managed with a second two-stage exchange with component removal and placement of a second antibiotic spacer. The remaining three patients were managed with an open débridement and polyethylene exchange; two of these were acute hematogenous infections and one was determined intraoperatively to be a superficial infection without deep extension. The reinfection subset of patients had a greater (p = 0.026) number of prior surgeries (mean, 3.4; range, 1–6) than the cohort overall (mean, 2.0; range, 1–7).
Table 2.
Failures with reinfection
| Patient | Number of prior TKAs | Initial culture | Time to reinfection (months) | Reinfection organism | Notes |
|---|---|---|---|---|---|
| 1 | 1 | Methicillin-susceptible Staphylococcus aureus | 20 | Methicillin-resistant S. aureus | Acute hematogenous infection; components retained |
| 2 | 3 | S. aureus | 13 | Streptococci agalactiae | Components removed and second spacer |
| 3 | 4 | Serratia marcescens | 30 | Streptococci | Components removed and second spacer |
| 4 | 6 | Methicillin-resistant S. aureus | 2 | Methicillin-resistant S. aureus | Superficial infection |
| 5 | 4 | Escherichia coli | 15 | E. coli | Components removed and second spacer |
| 6 | 1 | Staphylococcus epidermidis | 13 | No growth | Débridement with component retention |
| 7 | 5 | Culture negative | 21 | Methicillin-susceptible S. aureus | Components removed and second spacer |
A standard medial parapatellar approach was used for all patients except one in whom a quadriceps snip was performed; no patient had a tibial tubercle osteotomy.
The mean extension before placement of the spacer was 3.2° (range, 0°–30°) and a mean of 2.0° at final followup (range, 0°–10°); the mean pretreatment flexion of 90.6° (range, 10°–125°) improved to a mean of 101.3° (range, 0°–130°) at final followup (Table 3). The mean pretreatment Knee Society score of 53 (range, 10–100) improved to a mean of 79 (range, 37–100) at most recent followup.
Table 3.
Motion and function before and after two-stage revision using an articulating spacer
| Preoperative | Postoperative | ||||
|---|---|---|---|---|---|
| Parameter | Average | SD | Average | SD | Significance |
| Extension | −3.2º | ± 7.7 | −2.0º | ± 3.7 | p = 0.269 |
| Flexion | 90.6º | ± 31.2 | 101.3º | ± 18.9 | p = 0.025 |
| Knee Society score | 53.3 | ± 20.6 | 78.6 | ± 17.8 | p < 0.001 |
| Knee Society score | (90–100) 21 patients | (70–89) 23 patients | (60–69) 6 patients | (< 59) 10 patients | |
At final radiographic followup, none of the revision components were radiographically loose. The one mechanical complication identified was breakage of one of the antibiotic spacers (a femoral component fracture in the sagittal plane) that was noted radiographically between stages but required no specific treatment. There were no complications observed related to the cement-on-cement articulation and no evidence of bone loss associated with use of the spacer. As noted, one patient had a second débridement and a second articulating spacer before definitive reimplantation. That patient had a débridement with component retention attempted before the two-stage exchange and initially presented with a draining sinus. A medial gastrocnemius flap was performed at the time of the second articulating spacer and the wound healed uneventfully. A second patient with superficial skin compromise had a local advancement flap before definitive reimplantation while the spacer was in place.
Discussion
Although the use of an interim articulating spacer is attractive, their use can only be justified if an acceptable rate of infection control can be realized. Like with any new surgical technique (such as we describe), theoretical advantages may not be realized and unanticipated complications can occur. Thus, we wanted to examine our early experience with this spacer to determine (1) the rate of infection control; (2) ease of the surgical exposure at the time of reimplantation; (3) ROM achieved after reimplantation; and (4) complications associated with the articulated spacer.
We recognize limitations to our study. First, we used only one technique and thus direct comparisons to other techniques or implants cannot be made. Second, this is a multicenter retrospective review and thus the surgical technique and postoperative management were not identical at the different sites. Third, we did not specifically test asymptomatic patients for infection recurrence (with testing such as blood work or aspiration of the joint) and thus we may have missed some infection recurrences that were low-grade or not clinically symptomatic. Finally, although not a limitation of our study per se, the spacer described may have a higher cost than the use of a static spacer or one that is made by hand; however, facilitation of the second stage with the potential for decreased operative time may balance the increased costs at the first-stage procedure.
The most essential outcome measure for any treatment protocol of a deep periprosthetic infection is eradication of infection (Table 4). We report an 88% rate of infection control in a relatively complex set of patients with a substantial number of multiply operated knees and infecting organisms that were resistant to standard antibiotics. Our rate is similar to that with other series [1, 12, 14, 15, 20]. Haddad et al. [10] reported 45 patients followed for an average of 48 months with the PROSTALAC® system, which includes femoral and tibial components made of antibiotic-loaded bone cement with a small metal-on-polyethylene articular surface (this device is not FDA-approved in the United States), and found that 91% of patients cleared the infection. Meek et al. [22] substantiated these results by reporting a 96% infection control rate at a minimum of 2 years in 58 patients. Durbhakula et al. [5] has also used prefabricated molds to create articulating antibiotic cement spacers and showed a 92% infection-free rate in 24 patients at an average of 33 months. Hofman et al. [12, 13] described an approach that differed from the fabrication of articulating spacers. It included a resterilization of the previously infected femoral component and cementing it in place loosely with high-dose antibiotic cement and a new polyethylene tibial insert. At an average of 73 months, the authors reported an 88% infection-free rate. Various authors have shown similar rates of infection control with this technique [1, 4, 6, 19]; however, some surgeons have concerns regarding the implantation of metal and polyethylene in the setting of an established infection.
Table 4.
Results of static and articulating antibiotic spacers
| Type of spacer | Study | Number of knees | Recurrent infection | Followup | Notes |
|---|---|---|---|---|---|
| Static | Booth and Lotke [2] | 25 | 1 | 25 months (6–59 months) | Average flexion = 100 |
| Emerson et al. [6] | 26 | 2 | 7.5 years (2.8–12.7 years) | ROM static = 94°, mobile = 108° | |
| Fehring et al. [8] | 25 | 3 | 36 months (24–72 months) | Bone loss seen in static group | |
| Flexion = 98° in static group and 105° in articulating | |||||
| Wilde and Ruth [25] | 15 | 2 | 2.9 years (1–6 years) | Average ROM 6°–81° | |
| Haleem et al. [11] | 96 | 9 | 7.2 yrs (2.5–13 years) | 34 knees required augments and 5 required allograft at replant. | |
| Average ROM = 90° | |||||
| Articulating spacers | |||||
| Cement-on-cement | Durbhakula et al. [5] | 24 | 2 | 33 months (28–51 months) | Average knee flexion = 104° |
| Fehring et al. [8] | 15 | 1 | 27 months (24–36 months) | Bone loss seen in static group | |
| Flexion = 98° in static group and 105° in articulating | |||||
| Pitto et al. [23] | 19 | 0 | 24 months (12–43 months) | Average ROM 94° | |
| Cement-on-polyethylene | Evans [7] | 31 | 2 | Minimum of 2 years | Average ROM 2-111° |
| Metal on polyethylene articulating spacers | Cuckler [4] | 44 | 1 | Minimum of 1 year | Average ROM 112° |
| Emerson et al. [6] | 22 | 2 | 3.8 years (2.8–6.4 years) | ROM static = 94°, mobile = 108° | |
| Haddad et al. [10] | 45 | 4 | 48 months (20–112 months) | PROSTALAC, average ROM 3°–95° | |
| Hofmann et al. [13] | 26 | 0 | 31 months (12–70 months) | Average ROM 5°–106° | |
| Hofmann et al. [12] | 50 | 6 | 73 months (24–150 months) | Average ROM 4°–104° | |
| Jämsen et al. [19] | 22 | 2 | 32 months (2–86 months) | Average ROM 104° | |
| Meek et al. [22] | 47 | 2 | Average 41 months | PROSTALAC, average ROM 87° | |
One of the purported benefits of an articulating spacer is that it allows ROM during the interval between stages. In our experience, this not only increases patient comfort and function, but is also associated with a straightforward exposure at the time of reimplantation when compared with a static spacer. In our study, one quadriceps snip exposure was necessary, whereas the remaining revisions were completed with a standard medial parapatellar approach. This is similar to the findings of Durbhakula et al. [5] and Fehring et al. [8] with only two of 24 and two of 30 patients, respectively, requiring an extensible exposure at the time of reimplantation when an articulating spacer was used.
Immobilization during static spacer treatment can lead not only to soft tissue contractures, but also to permanent decreases in ROM. In a systematic review by Jamsen et al. [20], articulating spacers were associated with the greatest increase in final ROM. Emerson et al. [6] also showed in a direct comparison that articulating spacers resulted in a greater final ROM than static spacers. Similarly, we demonstrated an increase in ROM with patients increasing from 90.6º of flexion to 101.3° at final followup.
Among the potential problems with a static antibiotic-loaded spacer is periarticular bone loss. Calton et al. [3] reported a 40% rate of tibial and a 44% rate of femoral bone loss in 25 patients treated with a static spacer. Fehring et al. [8] substantiated these results in a retrospective comparison of handmade cement articulating spacers with a static spacer block technique. They found 15 of 25 patients (60%) in the static subset had bone loss directly related to the spacer with none identified in the articulating group. Our results are similar to those reported by Fehring et al. [8] because no bone loss was identified in association with the articulating spacer used in our series.
The spacer we used was not without problems; specifically, femoral component breakage occurred in one patient. Although this complication did not compromise treatment and was managed without specific surgical intervention, we believed this was related to implanting the spacer too tightly and thus we presently recommend fashioning a tibial component that is 2 to 4 mm smaller than the measured size of the flexion and extension gaps to avoid this potential problem. In addition, although tibiofemoral joint dislocation was not observed in the cohort described in this report, we have experienced this complication. This can be avoided with proper soft tissue balance and intraoperative trialing of the components combined with limiting patient flexion with a brace if deemed necessary intraoperatively. Finally, although the use of a cement-on-cement articulation is a potential concern, we were unable to identify any adverse events associated with the temporary use of this bearing surface.
The advantages of the articulating antibiotic spacer system described in this report lies in the ability to customize both the sizes of the femoral and tibial components, select an appropriate tibial component thickness to optimize stability and ROM, and select the amount and type of antibiotics used in the cement. No metal hardware is retained that could serve as a further nidus for infection, and the patient is allowed functional ROM during treatment. This ROM facilitates reimplantation (as evidenced by the need for a quadriceps snip in only one patient) through continued mobilization of soft tissues and the prevention of contractures. In addition, patient ambulation and mobilization during the spacer stage are theoretically improved because the knee has more motion and weightbearing is often allowed.
Footnotes
One of the authors (KRB) is a consultant for and has received research support and royalties for intellectual property from Biomet, Inc (Warsaw, IN) and is a consultant for Synvasive (Reno, NV) and Salient Surgical (Portsmouth, NH). One of the authors (GRK) is a consultant for Biomet. One of the authors (ACG) is a consultant for Biomet, Wright Medical Technology, Inc (Arlington, TN), DePuy Orthopaedics, Inc (Warsaw, IN), and BrainLab (Westchester, IL). One of the authors (AVL) is a consultant for and has received research support and royalties for intellectual property from Biomet and has received royalties from Innomed (Savannah, GA). One of the authors (CJDV) is a consultant for Biomet, Kinamed (Camarillo, CA), Smith and Nephew Inc (Memphis, TN), Angiotech (Vancouver, BC, Canada), and is on the advisory board for CD Diagnostics (Philadelphia, PA). They have also received research support from Zimmer (Warsaw, IN).
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in this study was not required.
Study data collected at Rush University Medical Center, Chicago, IL; Joint Implant Surgeons, New Albany, OH; and Hartzband Center for Hip and Knee Replacement, Paramus, NJ.
References
- 1.Anderson JA, Sculco PK, Heitkemper S, Mayman DJ, Bostrom MP, Sculco TP. An articulating spacer to treat and mobilize patients with infected total knee arthroplasty. J Arthroplasty. 2009;24:631–635. doi: 10.1016/j.arth.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Booth RE, Jr, Lotke PA. The results of spacer block technique in revision of infected total knee arthroplasty. Clin Orthop Relat Res. 1989;248:57–60. [PubMed] [Google Scholar]
- 3.Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res. 1997;345:148–154. doi: 10.1097/00003086-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Cuckler JM. The infected total knee: management options. J Arthroplasty. 2005;20(Suppl 2):33–36. doi: 10.1016/j.arth.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Durbhakula SM, Czajka J, Fuchs MD, Uhl RL. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768–774. doi: 10.1016/j.arth.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 6.Emerson RH, Jr, Muncie M, Tarbox TR, Higgins LL. Comparison of a static with a mobile spacer in total knee infection. Clin Orthop Relat Res. 2002;404:132–138. doi: 10.1097/00003086-200211000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 8.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis. The Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ha CW. A technique for intraoperative construction of antibiotic spacers. Clin Orthop Relat Res. 2006;445:204–209. doi: 10.1097/01.blo.0000201161.52196.c5. [DOI] [PubMed] [Google Scholar]
- 10.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 11.Haleem AA, Berry DJ, Hanssen AD. Mid-term to long-term followup of two-stage reimplantation for infected total knee arthroplasty. Clin Orthop Relat Res. 2004;428:35–39. doi: 10.1097/01.blo.0000147713.64235.73. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125–131. doi: 10.1097/01.blo.0000149241.77924.01. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 14.Hsu YC, Cheng HC, Ng TP, Chiu KY. Antibiotic-loaded cement articulating spacer for 2-stage reimplantation in infected total knee arthroplasty: a simple and economic method. J Arthroplasty. 2007;22:1060–1066. doi: 10.1016/j.arth.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 15.Huang HT, Su JY, Chen SK. The results of articulating spacer technique for infected total knee arthroplasty. J Arthroplasty. 2006;21:1163–1168. doi: 10.1016/j.arth.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 17.Insall JN, Thompson FM, Brause BD. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Joint Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 18.Jacobs C, Christensen CP, Berend ME. Static and mobile antibiotic-impregnated cement spacers for the management of prosthetic joint infection. J Am Acad Orthop Surg. 2009;17:356–368. doi: 10.5435/00124635-200906000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Jamsen E, Sheng P, Halonen P, Lehto MU, Moilanen T, Pajamaki J, Puolakka T, Konttinen YT. Spacer prostheses in two-stage revision of infected knee arthroplasty. Int Orthop. 2006;30:257–261. doi: 10.1007/s00264-006-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamsen E, Stogiannidis I, Malmivaara A, Pajamaki J, Puolakka T, Konttinen YT. Outcome of prosthesis exchange for infected knee arthroplasty: the effect of treatment approach. Acta Orthop. 2009;80:67–77. doi: 10.1080/17453670902805064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McPherson EJ, Lewonowski K, Dorr LD. Techniques in arthroplasty. Use of an articulated PMMA spacer in the infected total knee arthroplasty. J Arthroplasty. 1995;10:87–89. doi: 10.1016/S0883-5403(05)80105-6. [DOI] [PubMed] [Google Scholar]
- 22.Meek RM, Masri BA, Dunlop D, Garbuz DS, Greidanus NV, McGraw R, Duncan CP. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Joint Surg Am. 2003;85:1888–1892. doi: 10.1302/0301-620X.85B8.14214. [DOI] [PubMed] [Google Scholar]
- 23.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–308. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18:592–599. doi: 10.1016/S0883-5403(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 25.Wilde AH, Ruth JT. Two-stage reimplantation in infected total knee arthroplasty. Clin Orthop Relat Res. 1988;236:23–35. [PubMed] [Google Scholar]