Abstract
Background
Retention treatment is reportedly associated with lower infection control rates than two-stage revision. However, the studies on which this presumption are based depend on comparisons of historical rather than concurrent controls.
Questions/purposes
We (1) asked whether the infection control rates, number of additional procedures, length of hospital stay, and treatment duration differed between implant retention and two-stage revision treatment; and (2) identified risk factors that can contribute to failure of infection control.
Methods
We reviewed the records of 60 patients treated for 64 infected TKA from 2002 to 2007. Twenty-eight patients (32 knees) underwent débridement with retention of component, and 32 patients (32 knees) were treated with component removal and two-stage revision surgery. We determined patients’ demographics, type of infection, causative organisms, and outcome of treatment. Mean followup was 36 months (range, 12–84 months).
Results
Infection control rate was 31% in retention and 59% in the removal group after initial surgical treatment, and 81% and 91% at latest followup, respectively. Treatment duration was shorter in the retention group and there was no difference in number of additional surgeries and length of hospital stay. Type of treatment (retention versus removal) was the only factor associated with infection control; subgroup analysis in the retention group showed Staphylococcus aureus infection and polyethylene nonexchange as contributing factors for failure of infection control.
Conclusions
Although initial infection control rate was substantially lower in the retention group than the removal group, final results were comparable at latest followup. We believe retention treatment can be selectively considered for non-S. aureus infection, and when applied in selected patients, polyethylene exchange should be performed.
Level of Evidence
Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Periprosthetic joint infection is one of the most challenging complications after TKA. Despite the use of prophylactic antibiotics and strategies such as a body exhaust system, laminar flow, and improved surgical technique, periprosthetic infection of TKA still occurs in 1% to 2% of patients [3, 10, 13, 25].
Treatment options include chronic antibiotic suppression [10, 32], irrigation and débridement (I/D) with component retention [6, 14, 17, 19, 22, 26], one- [13, 28] or two-stage exchange arthroplasty [7, 12, 17, 24], and salvage procedures such as resection arthroplasty, arthrodesis, or amputation [8, 9, 15, 18, 35]. Of these treatment options, two-stage exchange arthroplasty using an antibiotic-laden cement spacer is considered the gold standard protocol with a high rate of infection control, which ranges from 85% to 100% [7, 12, 17, 18, 24, 36]. However, two-stage revision is associated with pain and difficult mobility between stages, financial ramifications, and can cause skeletal defects and soft tissue loss resulting in possible need for a constrained prosthesis [10, 18, 24]. As a result, less extensive approaches are being attempted, and I/D with retention of the prosthesis has been considered an alternative for treatment of TKA infection [6, 14, 17, 19, 22, 26].
Many surgeons prefer retention treatment in acute infections of less than 3- or 4-week symptom duration, although it is reportedly associated with lower infection control rates (ranging from 20% to 40% [4–6, 14, 19, 22, 26, 29, 30]) than removal treatment. However, direct comparison of infection control rates for different treatment options is difficult because of many confounding variables such as host condition, characteristics of the microorganism, implant state, operation history, type of surgery, surgeon’s ability in the various series [6, 14, 18, 23, 25, 26], and the absence of randomized, controlled, prospective comparison studies [17, 27, 33, 36]. Other risk factors include age, gender, diabetes, implant status (primary versus revision), type of infection (early postoperative, acute hematogenous, and chronic), type of treatment (retention versus removal), and polyethylene (PE) exchange at retention treatment (yes versus no). An investigation focused on less extensive treatment protocols that could yield infection control rates comparable to two-stage procedures would be valuable.
The purposes of this study were to (1) compare the infection control rates between implant retention and two-stage revision treatment with particular focus on the difference in their infection control rates after initial surgical treatment and at the latest followup; (2) compare the number of additional operative procedures after initial surgical failure for infection control, total length of hospital stay, and treatment duration between the two treatment approaches; (3) identify factors that can contribute to infection control; and (4) analyze retention subgroups to assess the influence of various factors retrospective to infection control in patients who underwent retention as the initial treatment.
Patients and Methods
We retrospectively reviewed 75 patients surgically treated for 79 infected TKAs from 2002 to 2007. Primary diagnoses for the initial TKAs were osteoarthritis in 66 knees, rheumatoid arthritis in seven knees, posttraumatic osteoarthritis in four knees, osteonecrosis in one knee, and congenital dislocation of the knee in one knee. There were 36 males and 39 females, and the mean age at initial treatment for infection was 66.6 years (range, 38–87 years). Primary TKA was performed at our institution in 29 knees and elsewhere in 50 knees. Periprosthetic infection was diagnosed at our hospital for the first time in 46 knees and 33 knees were diagnosed at an outside hospital and referred to us. We defined “postoperative infection index procedure (PIIP)” as the first operative treatment performed at our institution for periprosthetic infection after TKA. The interval from implantation of the prosthesis to time of initial diagnosis of infection averaged 54.5 months (range, 2 weeks to 20 years). We evaluated infection control rates at two different time points: (1) after the PIIP and (2) at the latest followup. The treatment was considered a failure when infection was not controlled or recurred after PIIP or when the patient had chronic infection with ongoing treatment or was on chronic antibiotic suppression at the latest followup. Of the 75 patients, seven died of medical problems unrelated to the orthopaedic treatment within 1 year of followup. Five patients were lost to followup, and three patients received only reimplantation surgery at our institution after they were diagnosed and treated for infection at an outside hospital. After excluding those 15 patients, we were left with 60 patients (64 knees). There were 29 males and 31 females; two of each gender had bilateral TKA infections. The mean age at the time of first surgery at our hospital was 65.2 years (range, 38–85 years) and minimum followup was 12 months (mean, 36 months; range, 12–84 months). No patients were recalled specifically for this study; all data were obtained from medical records and radiographs.
Infection was diagnosed by growth of the microorganism in pre- and intraoperative joint aspirates or intraoperative periprosthetic tissue specimen in conjunction with (1) purulence surrounding the prosthesis at the time of surgery; (2) acute inflammation consistent with infection on histopathologic examination; or (3) a sinus tract or abscess communicating with the prosthesis [4, 29]. When the patient presented with infection that did not meet these criteria, however, final diagnosis was made by surgeons’ clinical decision based on clinical symptoms, radiographic signs, and laboratory findings; erythrocyte sedimentation rate (> 25 mm/hr); C-reactive protein (> 8.0 mg/L); and joint aspirate white blood cell count (> 2000/μL) with polymorphonuclear differential count (> 65%) [31]. Fifty-seven of the 64 infections (89%) were microbiologically confirmed and seven (11%) were culture-negative. Organisms were considered the pathogens when grown from synovial fluid cultures or periprosthetic tissue or fluid. Additionally, patients who were bacteremic immediately before or at the time of development of periprosthetic TKA infection were also considered culture-positive. Staphylococcus aureus was the most common pathogen followed by coagulase-negative staphylococci and streptococci (Table 1). Ten percent were polybacterial. Of 26 S. aureus and 18 coagulase-negative staphylococcal infections (including polybacterial), six and 10 knees were by methicillin-resistant strain, respectively.
Table 1.
Microbiologic characteristics of 64 knees
| Type of microorganism | Number (%) |
|---|---|
| Gram-positive | |
| Staphylococcus aureus | 21 (33) |
| Staphylococcus lugdunensis | 1 |
| Coagulase-negative Staphylococcus | 16 (25) |
| Streptococcus species | 5 (8) |
| Peptostreptococcus | 2 |
| Propionibacterium acnes | 1 |
| Actinomyces | 1 |
| Gram-negative | |
| Escherichia coli | 2 |
| Haemophilus influenzae | 1 |
| Pseudomonas | 1 |
| Microorganism unknown | 7 (11) |
| Polybacterial | 6 (10) |
We applied the classification of McPherson et al. [20, 21] for the host condition. There were 26 uncompromised patients (27 knees, Group A), 24 compromised patients (26 knees, Group B), and 10 significantly compromised patients (11 knees, Group C). Thirteen retention and 14 removal treatments were applied for uncompromised patients, and 19 retention and 18 removal treatments were applied for compromised (Group B and C) patients. Infection type was classified based on the clinical scenario: early postoperative, acute hematogenous, and chronic infection [27, 33]. Early postoperative infection was diagnosed when patients presented infection symptom within 4 weeks from the initial arthroplasty surgery. Acute hematogenous infections were characterized by an acute presentation of symptoms in a previously well-functioning joint arthroplasty. Chronic infections are those that had an insidious clinical course 1 month or more after the initial arthroplasty or when treatment was delayed more than 4 weeks from symptom onset in acute hematogenous infection. There were six early postoperative infections, 29 acute hematogenous, and 29 chronic infections.
As a treatment option, 32 of the 64 knees had I/D with retention of the prosthesis (retention group) and 32 knees had component removal and two-stage revision treatment (removal group). Retention treatment was chosen when the patient had presented within 4 weeks of symptom duration with no evidence of implant loosening in early postoperative or acute hematogenous infection. In patients whose symptoms were longer than 4 weeks or with signs of implant loosening, we considered two-stage revision arthroplasty as the primary treatment option. Of 29 acute hematogenous infections, 23 knees underwent retention and six knees received removal treatment. However, we could not identify rationale of removal treatment for those six acute infections. Of 29 patients with chronic infections, 26 had removal treatment and three underwent retention treatment. The general condition of one patient was compromised for extended removal treatment and two patients refused to have their implants removed and chose to undergo retention treatment. All six patients with early postoperative infections underwent retention treatment. Baseline characteristics showed differences only in the type of infection and treatment history between two groups (Table 2).
Table 2.
Comparison of baseline characteristics and initial infection control between retention and removal groups
| Variable | Retention (N = 32) | Removal (N = 32) | p Value (chi-square test |
|---|---|---|---|
| number (%) | number (%) | ||
| Age at PIIP (years) | 0.8 | ||
| ≤ 65 | 16 (50) | 17 (53) | |
| > 65 | 16 (50) | 15 (47) | |
| Gender | 0.45 | ||
| Female | 15 (47) | 18 (56) | |
| Male | 17 (53) | 14 (44) | |
| Host factor | 0.8 | ||
| Uncompromised | 13 (41) | 14 (44) | |
| Compromised | 19 (59) | 18 (56) | |
| Diabetes mellitus | 1 | ||
| Yes | 9 (28) | 9 (28) | |
| No | 23 (72) | 23 (72) | |
| Pre-PIIP number of operations | 0.21 | ||
| One | 18 (56) | 13 (41) | |
| Two or more | 14 (44) | 19 (59) | |
| Pre-PIIP treatment for infection | < 0.001* | ||
| Yes | 2 (6) | 19 (59) | |
| No | 30 (94) | 13 (41) | |
| Pre-PIIP implant | 0.77 | ||
| Primary | 25 (78) | 24 (75) | |
| Revision | 7 (22) | 8 (25) | |
| Type of infection | < 0.001* | ||
| Early postoperative | 6 (19) | 0 (0) | |
| Acute hematogenous | 23 (72) | 6 (19) | |
| Chronic | 3 (9) | 26 (81) | |
| Microorganism | 0.61 | ||
| Staphylococcus aureus | 14 (44) | 12 (38) | |
| Other | 18 (56) | 20 (62) | |
| Number of microorganisms | 1 | ||
| Polybacterial | 3 (9) | 3 (9) | |
| Monobacterial | 29 (91) | 29 (91) | |
| Infection control outcome | 0.02* | ||
| Controlled | 10 (31) | 19 (59) | |
| Not controlled | 22 (69) | 13 (41) | |
Percentages are shown in parentheses. PIIP = postoperative infection index procedure.
* Statistically significant difference.
In the retention group, the knee was explored, débrided, and irrigated with saline-retaining components with or without PE exchange. We tried to pinpoint when surgeons exchanged PE, but it was difficult to know the rationale for choice of nonexchange except in five arthroscopic débridements. A complete synovectomy was performed as part of débridement in all cases. The two-stage procedure included removal of all components with thorough débridement, irrigation, and placement of an antibiotic-loaded cement spacer. For the spacer, 1.0 g of vancomycin and 2.4 g of tobramycin were mixed with 40 g of cement. A static spacer was inserted in 27 knees and an articulating spacer was inserted in five knees. Of five articulating spacers, four knees underwent reimplantation and one knee retained the spacer at latest followup of 37 months with no evidence of recurrent infection. Reimplantation of TKA was performed when the infection was judged under control based on clinical findings, negative aspiration results, and normalization of inflammatory markers with the same criteria as the diagnosis of infection. A frozen specimen was used for judgment of infection by consultation with the pathologist at the time of reimplantation. Our pathologic criterion of diagnosis was five neutrophils in five consecutive high-power fields. The mean time interval between stages was 4.4 months (range, 1.5–28 months). Organism-specific intravenous antibiotic therapy was administrated after PIIP for 6 weeks in both retention and removal groups. Two patients received 4 weeks of antibiotics. In one patient, the surgeon judged that the positive culture was contamination and discontinued further antibiotic treatment. The other patient was changed to oral antibiotics after 4 weeks of intravenous injection therapy, because the culture showed Haemophilus influenzae. There were two patients who received intravenous injection therapy for a total of 8 weeks. Both had adverse effects from vancomycin and were twice treated with a change of antibiotics, finally receiving 6 weeks of linezolid and cefepime treatment.
We recommended patients be routinely seen in our clinic at postoperative 2 weeks, 1 to 3 months, 1 year, and then every 2 to 3 years. Wound condition and clinical knee function were evaluated as well as a plain radiographic examination, including AP, lateral, axial, and 45° flexion view. The medical records and radiographs were reviewed by an orthopaedic surgeon (HRC) not associated with the treatment. Twenty-seven of the 60 patients were not available for followup visits and in those patients, we obtained an evaluation by questionnaire letter (n = 18) or telephone interview (n = 9). The questionnaire consisted of EQ5D, UCLA activity score, and Knee Injury and Osteoarthritis Outcome Score (KOOS). We also asked if patients were taking any oral antibiotics and if they had received any further surgical treatment for the infection after their treatment at our hospital. Questionnaire letters were handled by research nurses, and the telephone interview was performed by one of authors (HRC).
We initially used the chi-square test to determine the differences in proportions for each candidate predictor variable between the retention and removal groups and their initial infection control results. We used multivariate logistic regression (backward selection) to identify independent predictors of failure after PIIP and at final evaluation with the likelihood ratio test used to assess significance and the adjusted odds ratio and 95% confidence interval for risk factors [16]. Nonparametric Mann-Whitney U test [1] was used for comparison of other parameters. Statistical analysis was performed using SPSS Version 17.0 (SPSS Inc, Chicago, IL) and two-tailed tests. The Kaplan-Meier survival method was used to estimate postoperative survival free of infection.
Results
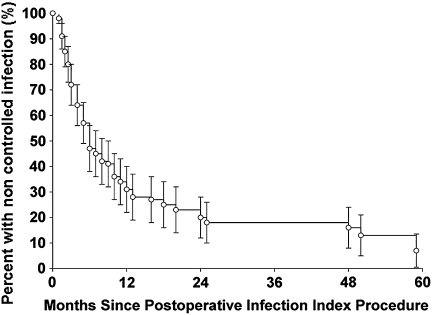
The infection control rate was 31% in the retention group and 59% in the removal group after PIIP; patients who underwent retention had lower (p = 0.02) early infection control rates (Table 2). These initial infection control rates improved 81% and 91% in each group, respectively, at latest followup. The estimated survival free of infection was 66% at 12 months and 80% at 24 months (Fig. 1). According to the type of infection, two of six early postoperative infections resulted in infection control by initial retention treatment. Of 29 hematogenous infections, seven of 23 retention treatments and three of six removal treatments achieved infection control. Of 29 chronic infections, 16 of 26 removal treatments and one of three retention treatments resulted in infection control. There was no association between type of infection and infection control (p = 0.15).
Fig. 1.
The Kaplan-Meier survivorship curve shows estimated uncontrolled rates of infection were 47% at 6 months, 34% at 12 months, and 20% at 24 months.
After failure of infection control by PIIP, 35 knees (22 of the retention group and 13 of the removal group) underwent additional operative procedures. In the retention group, staged revision operation (11 knees), repeated I/D (11 knees), fusion (two knees), amputation (two knees), and resection (one knee) were performed. In the removal group, staged revision (eight knees), re-revision (two knees), repeated I/D (five knees), fusion (two knees), and amputation (three knees) were performed. The average number of additional procedures was 1.7 in both groups. There was no difference in the total length of hospital stay (sum of hospital stay for PIIP and additional procedures), but the retention group showed shorter total duration of treatment (time from admission to discharge in case of infection controlled by PIIP, or time from PIIP to the last operation for infection control in case of failed PIIP) than the removal group (p = 0.04) (Table 3).
Table 3.
Number of additional operations after PIIP, hospital length of stay, and treatment duration for retention and removal subgroups
| Variable | Retention group (N = 32) | Removal group (N = 32) | Mann-Whitney U test p value |
|---|---|---|---|
| Number of operations after PIIP | 2 (0–3) | 1 (1–2) | 0.65 |
| Total length of stay (days) | 16 (9–40) | 20 (16–29) | 0.73 |
| Treatment duration (months) | 4 (0–12) | 6 (4–12) | 0.04* |
* Statistically significant; data are median with interquartile range in parentheses; treatment duration indicates time from PIIP to discharge or last procedure.
PIIP = postoperative infection index procedure.
Multivariate logistic regression analysis identified only the type of initial procedure (PIIP) as correlated with infection control. The retention treatment clearly was associated with much lower (p = 0.02) rates of infection control than removal treatment, independent of the other 10 factors analyzed (Table 4). A power analysis indicated the 64 knees in this study would provide 80% power for detecting a minimum 35% difference in the infection control percentages between levels of the different variables using Fisher’s exact test for binomial proportions.
Table 4.
Factors associated with infection control after postoperative infection index procedure: entire cohort (N = 64 knees)
| Variable | Infection controlled (N = 29) | Infection not controlled (N = 35) | Multivariable p value (logistic regression) |
|---|---|---|---|
| Age at PIIP (years) | 0.64 | ||
| ≤ 65 | 16 (55) | 17 (49) | |
| > 65 | 13 (45) | 18 (51) | |
| Gender | 0.74 | ||
| Female | 16 (55) | 17 (49) | |
| Male | 13 (45) | 18 (51) | |
| Host factor | 0.16 | ||
| Uncompromised | 15 (52) | 12 (34) | |
| Compromised | 14 (48) | 23 (66) | |
| Diabetes mellitus | 0.62 | ||
| Yes | 9 (31) | 9 (26) | |
| No | 20 (69) | 26 (74) | |
| Pre-PIIP number of operations | 0.16 | ||
| One | 16 (55) | 15 (43) | |
| Two or more | 13 (45) | 20 (57) | |
| Pre-PIIP treatment for infection | 0.55 | ||
| Yes | 11 (38) | 10 (29) | |
| No | 18 (62) | 25 (71) | |
| Pre-PIIP implant | 0.07 | ||
| Primary | 25 (86) | 24 (69) | |
| Revision | 4 (14) | 11 (31) | |
| Type of infection | 0.88 | ||
| Early postoperative | 2 (7) | 4 (12) | |
| Acute hematogenous | 10 (34) | 19 (54) | |
| Chronic | 17 (59) | 12 (34) | |
| Microorganism | 0.18 | ||
| Staphylococcus aureus | 9 (31) | 17 (49) | |
| Other | 20 (69) | 18 (51) | |
| Number of microorganisms | 0.52 | ||
| Polybacterial | 2 (7) | 4 (11) | |
| Monobacterial | 27 (93) | 31 (89) | |
| PIIP | 0.02* | ||
| Retention | 10 (35) | 22 (63) | |
| Removal | 19 (65) | 13 (37) | |
Percentages are shown in parentheses. PIIP = postoperative infection index procedure.
* Statistically significant predictor.
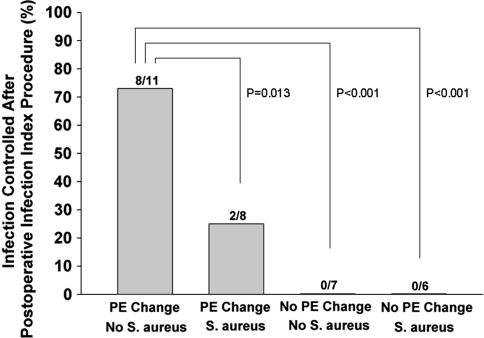
With the subgroup analysis for the retention group, we identified two independent predictors of failure for infection control: S. aureus infection (p = 0.03) and lack of PE exchange (p < 0.001) (Table 5). Infection control rates were higher when PE was exchanged in non-S. aureus infection than PE exchange with S. aureus and no PE exchange with or without S. aureus. Regardless of the causative organisms, lack of PE exchange resulted in poor outcome (Fig. 2). We found no risk factor related to our latest followup results of infection control in both overall and retention subgroups.
Table 5.
Subgroup analysis: factors associated with infection control after postoperative infection index procedure: retention subgroup (N = 32 knees)
| Variable | Infection controlled (N = 10) | Infection not controlled (N = 22) | Multivariable p value (logistic regression) |
|---|---|---|---|
| Age at PIIP (years) | 0.9 | ||
| ≤ 65 | 5 (50) | 11 (50) | |
| > 65 | 5 (50) | 11 (50) | |
| Gender | 0.36 | ||
| Female | 5 (50) | 10 (46) | |
| Male | 5 (50) | 12 (54) | |
| Host factor | 0.15 | ||
| Uncompromised | 5 (50) | 8 (36) | |
| Compromised | 5 (50) | 14 (64) | |
| Diabetes mellitus | 0.71 | ||
| Yes | 4 (40) | 5 (23) | |
| No | 6 (60) | 17 (77) | |
| Pre-PIIP number of operations | 0.1 | ||
| One | 6 (60) | 12 (55) | |
| Two or more | 4 (40) | 10 (45) | |
| Pre-PIIP treatment for infection | 0.99 | ||
| Yes | 0 (0) | 2 (9) | |
| No | 10 (100) | 20 (91) | |
| Pre-PIIP implant | 0.07 | ||
| Primary | 9 (90) | 16 (73) | |
| Revision | 1 (10) | 6 (27) | |
| Type of infection | 0.66 | ||
| Early postoperative | 2 (20) | 4 (18) | |
| Acute hematogenous | 7 (70) | 16 (73) | |
| Chronic | 1 (10) | 2 (9) | |
| Microorganism | 0.03* | ||
| Staphylococcus aureus | 2 (20) | 12 (54) | |
| Other | 8 (80) | 10 (46) | |
| Number of microorganisms | 0.35 | ||
| Polybacterial | 1 (10) | 2 (9) | |
| Monobacterial | 9 (90) | 20 (91) | |
| PE Change | < 0.001* | ||
| Yes | 10 (100) | 9 (41) | |
| No | 0 (0) | 13 (59) | |
Percentages are shown in parentheses. PIIP = postoperative infection index procedure.
PE = polyethylene.
* Statistically significant factor influencing infection control after retention treatment.
Fig. 2.
Logistic regression indicated patients without Staphylococcus aureus infection and who have polyethylene (PE) exchange are more likely to control infection after a postoperative infection index procedure compared with the other three subgroups (odds ratio: 25.3, 95% confidence interval: 3.5–18.2, p < 0.001).
Discussion
Although treatment results of I/D with component retention, in general, are considered less successful than two-stage exchange arthroplasty for treatment of infected TKA [10, 18, 29, 34, 36], available data for comparison of different therapeutic approaches are limited [17, 29, 30]. Therefore, we compared the infection control rates with the number of additional procedures, total length of hospital stay, and total treatment duration between these two treatment groups. We also tried to identify contributing factors for infection control and performed retention subgroup analysis to assess the influence of various factors on the result of infection control of retention treatment.
There were several limitations related to our study. First, as a retrospective analysis, we could not clarify the rationale for choosing attempted retention versus staged exchange arthroplasty and exchanging PE or not in retention treatment. A large portion of the decision-making was surgeon-dependent, which might have led to an inconsistent treatment strategy. Second, we had a short followup period. As infection can recur after followup, it is possible a few patients with short-term followup may have presented with infection at a later date leading us to understate the failure rates. Third, because our hospital was a tertiary referral center, many patients presented with complex histories related to infection or surgeries. These histories could reflect an uncontrolled negative selection bias of a single-center study and might not be generalizable to other centers. Finally, we focused our evaluation only on infection control because there was not enough information regarding patients’ functionality. Without evaluation of clinical function, we could not make a clear conclusion of the effect of multiple additional operative procedures required for infection control on the final outcome.
In many reports, I/D with retention of implant has resulted in an approximately 20% to 40% of infection control or successful retention of the implant [4–6, 14, 19, 22, 26, 29, 30], whereas two-stage exchange arthroplasty has resulted in control of infection in approximately 90% of patients [7, 12, 18, 36]. Many of these reports demonstrated treatment outcome at latest followup evaluation based on recurrence rates, which was acquired by additional surgical procedures. Schoifet and Morrey [26] reported five knees were débrided twice and three knees needed débridement three times to get their 23% success free of infection in their 31 knees. Mont et al. [22] reported that the implant was successfully retained in 83% of their patients after one to three repeated débridements, which would have been 41% if they had stopped treatment after one failed attempt (Table 6). In these reports, repeated débridements were considered a unit of retention treatment, which might have overestimated the effect of retention treatment. We evaluated the results of initial surgical treatment and any type of additional surgeries was considered as failure of infection control. The infection control rate was 31% in the retention group and 59% in the removal group after this initial surgical treatment (PIIP). These control rates improved to 81% in the retention group and 91% in the removal group by average of 1.7 additional operative procedures.
Table 6.
Comparison of retention treatment of infected TKA
| Study | Treatment option | Number of cases (TKA) | Definition of success/failure | Success rates after initial treatment (%) | Number of additional procedures (mean or median) | Success rates at latest followup (%) | Mean followup (months) | Risk factors/comments |
|---|---|---|---|---|---|---|---|---|
| Brandt et al. [4] | Retention | 33 (26 TKA and 7 THA) | Recurrence | 36% | 4 (1–9) | – | 78 | Symptom duration (> 2 days) |
| Burger et al. [5] | Retention | 39 | Salvage of prosthesis | 18% | 3.4 (1–7) | – | 49 | Symptom duration (2 weeks), susceptibility of organism, wound drainage, radiographic loosening |
| Deirmengian et al. [6] | Retention | 31 | Recurrence | – | 4 knees (2x) 1 knee (3x) | 35%* | 48 | Staphylococcus aureus infection |
| Hartman et al. [14] | Retention | 33 | Recurrence | 39% | Mixed additional procedures | – | 53 | Within 4 weeks of index surgery |
| Laffer et al. [17] | Retention and removal | 40 | Recurrence | – | – | 95% (retention) 85% (removal) | 28 | Similar outcome of retention and removal treatment |
| Marculescu et al. [19] | Retention | 99 (THA and TKA) | Relapse or reinfection | – | 1 (1–4) | 46% | 23 | Presence of sinus tract, symptom duration (≥ 8 days) |
| Mont et al. [22] | Retention | 24 | Positive culture after operation | 41% | 1–3† | 83% | 48 | Symptom duration |
| Schoifet and Morrey [26] | Retention | 31 | Eradication of infection | – | 5 knees (2x) 3 knees (3x) | 23% | 96 | Duration of infection, virulence of organism, age of patient, type of prosthesis |
| Tattevin et al. [29] | Retention and removal | 69 (53 THA and 17 TKA) | Relapse of infection | 38% (retention) 82% (removal) | ‡ | 80% (overall) | 19 | Symptom duration |
| Teeny et al. [30] | Retention | 21 | Recurrence | 29% | – | – | 48 | Time from initial arthroplasty (2 weeks) |
| Choi et al. [current study] | Retention and removal | 64 | Recurrence/chronic suppression | 31% (retention) 59% (removal) | 1.7 (0–11) | 81% (retention) 91% (removal) | 36 | Retention treatment, Staphylococcus aureus infection, polyethylene nonexchange |
* Includes five patients of indefinite course of oral antibiotics; †routine postoperative multiple attempts based on their criteria; ‡62% of initial retention treatment required additional surgeries.
We found no difference in number of additional procedures, total length of hospital stay between two treatment groups, and the treatment duration was shorter in the retention group than the removal group. These results might suggest that although early infection control rate is lower in retention treatment, the ultimate control rate could be comparable to that of removal treatment without any disadvantage in these three parameters. However, further investigation is also required to clarify the meaning of final infection control rates related to additional procedures since there were various unknowns such as change of host condition and complexity of additional procedures after failed PIIP.
Of the many factors that should be considered in treating periprosthetic joint infection, duration of symptoms has been suggested as the most important, affecting results of retention treatment [4, 5, 19, 29]. Many investigators suggested a wide range of cutoffs for successful acute phase treatment from 2 days to 6 weeks [4, 5, 11, 14, 17, 19, 22, 29, 30, 33]. In the present study, we arbitrarily defined acute phase treatment as the treatment performed within 4 weeks of symptoms because most algorithms suggested 3 to 4 weeks as an acute onset [20, 21, 33, 34, 36]. Based on this time criteria, we classified infection into three types. For most infection of acute phase (early postoperative and acute hematogenous infection), retention treatment was performed and for most of chronic phase infection (chronic infection), removal treatment was applied. There was no association between type of infection and infection control rates.
In the retention subgroup analysis, we identified two predictors for failure of infection control: S. aureus infection and PE nonexchange. Considering its impact as a principal pathogen with frequent antibiotic resistance, identification of the causative organism remains critically important [6, 29, 33]. However, the dilemma regarding the identification of the organism is that delay in treatment is not feasible until the aspiration culture results return in the acute phase. Furthermore, in seven of 64 patients (11%), organisms could not be identified, which was similar to the incidence of 5% to 11% in the literature [2, 17, 19, 25, 36]. Although the identification of the causative organism(s) is an important factor, it may not always be able to guide surgical strategy. PE exchange is usually recommended as a part of thorough débridement [6, 18]. It can be a factor related to treatment results [6], but it is not always performed [19]. In our study, PE was not changed in 13 of 32 cases resulting in a poor infection control rate.
In summary, half of infections after TKA were managed by initial retention treatment. Although infection control rate of initial retention treatment was substantially lower than that of removal treatment, the final infection control rate was comparable at the latest followup by additional operative procedures. Retention treatment should be restrictively considered in non-S. aureus infection, and when it is applied in selected patients, a PE insert exchange should be performed. Further investigation for relation between additional procedures after initial failure and final infection control rate is necessary.
Acknowledgments
We thank Dr Andrew Freiberg, Dr Dennis Burke, Dr Joseph McCarthy, and Dr Harry Rubash for their help in preparing this article.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Harris Orthopedic Laboratory, Massachusetts General Hospital, Boston, MA
References
- 1.Altman DG. Practical Statistics for Medical Research. Boca Raton, FL: Chapman & Hall/CRC; 1991. pp. 194–214. [Google Scholar]
- 2.Berbari EF, Marculescu C, Sia I, Lahr BD, Hanssen AD, Steckelberg JM, Gullerud R, Osmon DR. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45:1113–1119. doi: 10.1086/522184. [DOI] [PubMed] [Google Scholar]
- 3.Blom AW, Brown J, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total knee arthroplasty. J Bone Joint Surg Br. 2004;86:688–691. doi: 10.1302/0301-620X.86B5.14887. [DOI] [PubMed] [Google Scholar]
- 4.Brandt CM, Sistrunk WW, Duffy MC, Hanssen AD, Steckelberg JM, Ilstrup DM, Osmon DR. Staphylococcus aureus prosthetic joint infection treated with debridement and prosthesis retention. Clin Infect Dis. 1997;24:914–919. doi: 10.1093/clinids/24.5.914. [DOI] [PubMed] [Google Scholar]
- 5.Burger RR, Basch T, Hopson CN. Implant salvage in infected total knee arthroplasty. Clin Orthop Relat Res. 1991;273:105–112. [PubMed] [Google Scholar]
- 6.Deirmengian C, Greenbaum J, Lotke PA, Booth RE, Jr, Lonner JH. Limited success with open debridement and retention of components in the treatment of acute Staphylococcus aureus infections after total knee arthroplasty. J Arthroplasty. 2003;18:22–26. doi: 10.1016/S0883-5403(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 7.Durbhakula SM, Czajka J, Fuchs MD, Uhl RL. Antibiotic-loaded articulating cement spacer in the 2-stage exchange of infected total knee arthroplasty. J Arthroplasty. 2004;19:768–774. doi: 10.1016/j.arth.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Ellingsen DE, Rand JA. Intramedullary arthrodesis of the knee after failed total knee arthroplasty. J Bone Joint Surg Am. 1994;76:870–877. doi: 10.2106/00004623-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Falahee MH, Matthews LS, Kaufer H. Resection arthroplasty as a salvage procedure for a knee with infection after a total arthroplasty. J Bone Joint Surg Am. 1987;69:1013–1021. [PubMed] [Google Scholar]
- 10.Garvin KL, Cordero GX. Infected total knee arthroplasty: diagnosis and treatment. Instr Course Lect. 2008;57:305–315. [PubMed] [Google Scholar]
- 11.Haddad FS, Adejuwon A. The management of infected total knee arthroplasty. Orthopedics. 2007;30:779–780. doi: 10.3928/01477447-20070901-29. [DOI] [PubMed] [Google Scholar]
- 12.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements. Prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 13.Hanssen AD, Rand JA. Evaluation and treatment of infection at the site of a total hip or knee arthroplasty. Instr Course Lect. 1999;48:111–122. [PubMed] [Google Scholar]
- 14.Hartman MB, Fehring TK, Jordan L, Norton HJ. Periprosthetic knee sepsis. The role of irrigation and debridement. Clin Orthop Relat Res. 1991;273:113–118. [PubMed] [Google Scholar]
- 15.Isiklar ZU, Landon GC, Tullos HS. Amputation after failed total knee arthroplasty. Clin Orthop Relat Res. 1994;299:173–178. [PubMed] [Google Scholar]
- 16.Katz MH. Multivariable Analysis: A Practical Guide for Clinicians. 2. New York, NY: Cambridge University Press; 2006. pp. 117–136. [Google Scholar]
- 17.Laffer RR, Graber P, Ochsner PE, Zimmerli W. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin Microbiol Infect. 2006;12:433–439. doi: 10.1111/j.1469-0691.2006.01378.x. [DOI] [PubMed] [Google Scholar]
- 18.Leone JM, Hanssen AD. Management of infection at the site of a total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2335–2348. doi: 10.2106/00004623-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components. Clin Infect Dis. 2006;42:471–478. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- 20.McPherson EJ, Tontz W, Jr, Patzakis M, Woodsome C, Holtom P, Norris L, Shufelt C. Outcome of infected total knee utilizing a staging system for prosthetic joint infection. Am J Orthop (Belle Mead NJ) 1999;28:161–165. [PubMed] [Google Scholar]
- 21.McPherson EJ, Woodsome C, Holtom P, Roidis N, Shufelt C, Patzakis M. Periprosthetic total hip infection: outcomes using a staging system. Clin Orthop Relat Res. 2002;403:8–15. doi: 10.1097/00003086-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Mont MA, Waldman B, Banerjee C, Pacheco IH, Hungerford DS. Multiple irrigation, debridement, and retention of components in infected total knee arthroplasty. J Arthroplasty. 1997;12:426–433. doi: 10.1016/S0883-5403(97)90199-6. [DOI] [PubMed] [Google Scholar]
- 23.Moyad TF, Thornhill T, Estok D. Evaluation and management of the infected total hip and knee. Orthopedics. 2008;31:581–588. doi: 10.3928/01477447-20080601-22. [DOI] [PubMed] [Google Scholar]
- 24.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–308. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoifet SD, Morrey BF. Treatment of infection after total knee arthroplasty by debridement with retention of the components. J Bone Joint Surg Am. 1990;72:1383–1390. [PubMed] [Google Scholar]
- 27.Segawa H, Tsukayama DT, Kyle RF, Becker DA, Gustilo RB. Infection after total knee arthroplasty. A retrospective study of the treatment of eighty-one infections. J Bone Joint Surg Am. 1999;81:1434–1445. doi: 10.2106/00004623-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Silva M, Tharani R, Schmalzried TP. Results of direct exchange or debridement of the infected total knee arthroplasty. Clin Orthop Relat Res. 2002;404:125–131. doi: 10.1097/00003086-200211000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Tattevin P, Cremieux AC, Pottier P, Huten D, Carbon C. Prosthetic joint infection: when can prosthesis salvage be considered? Clin Infect Dis. 1999;29:292–295. doi: 10.1086/520202. [DOI] [PubMed] [Google Scholar]
- 30.Teeny SM, Dorr L, Murata G, Conaty P. Treatment of infected total knee arthroplasty. Irrigation and debridement versus two-stage reimplantation. J Arthroplasty. 1990;5:35–39. doi: 10.1016/S0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 31.Trampuz A, Hanssen AD, Osmon DR, Mandrekar J, Steckelberg JM, Patel R. Synovial fluid leukocyte count and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117:556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Trebse R, Pisot V, Trampuz A. Treatment of infected retained implants. J Bone Joint Surg Br. 2005;87:249–256. doi: 10.1302/0301-620X.87B2.15618. [DOI] [PubMed] [Google Scholar]
- 33.Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78:512–523. doi: 10.2106/00004623-199604000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85-A Suppl 1:S75–S80. doi: 10.2106/00004623-200300001-00014. [DOI] [PubMed] [Google Scholar]
- 35.Wiedel JD. Salvage of infected total knee fusion: the last option. Clin Orthop Relat Res. 2002;404:139–142. doi: 10.1097/00003086-200211000-00024. [DOI] [PubMed] [Google Scholar]
- 36.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351:1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]