Abstract
This Classic article is a reprint of the original work by William S. Baer, MD, The Treatment of Chronic Osteomyelitis With the Maggot (Larva of the Blow Fly). An accompanying biographical sketch on William Baer, is available at DOI 10.1007/s11999-010-1415-4. The Classic Article is ©1931 by the Journal of Bone and Joint Surgery, Inc. and is reprinted with permission from Baer WS. The treatment of chronic osteomyelitis with the maggot (larva of the blow fly). J Bone Joint Surg Am. 1931;13:438–475.
Ever since the days of Pasteur and Lord Lister surgery has been basing its antiseptic and aseptic treatment upon their discoveries, and we have almost come to the belief that the antiseptic and aseptic techniques were purely on a chemical basis. During the late World War an Observation which I made among the wounded soldiers led me to believe that the prevention of an infection, and the curing of an infection, could be brought about by means other than chemical. At a certain battle during 1917, two soldiers with Compound fractures of the femur and large flesh wounds of the abdomen and scrotum were brought into the hospital. These men had been wounded during an engagement and in such a part of the country, hidden by brush, that when the wounded of that battle were picked up they were overlooked. For seven days they lay on the battlefield without water, without food, and exposed to the weather and all the insects which were about that region. On their arrival at the hospital I found that they had no fever and that there was no evidence of septicaemia or blood poisoning. Indeed, their condition was remarkably good, and if it had not been for their starvation and thirst, we would have said they were in excellent condition. When I noticed the extent of the wounds, of the thigh particularly, I could not but marvel at the good constitutional condition of the patients. At that time the mortality of Compound fractures of the femur was about seventy-five to eighty per cent.—even when the wounded had the best of medical and surgical care that the Army and Navy could provide. Later, of course, the mortality was reduced as the splinting improved in the advance area, and when finally the splinting was made to a Compound fracture of this nature where the men feel, the mortality was cut down to about twenty-five per cent.
Here, however, were two men in the earlier part of our engagement in the War, when the mortality of Compound fractures of the femur was high, who, to all intents and purposes, were constitutionally well. This unusual fact quickly attracted my attention. I could not understand how a man who had lain on the ground for seven days with a Compound fracture of the femur, without food and water, should be free of fever and of evidences of sepsis. On removing the clothing from the wounded part, much was my surprise to see the wound filled with thousands and thousands of maggots, apparently those of the blow fly. These maggots simply swarmed and filled the entire wounded area. The sight was very disgusting and measures were taken hurriedly to wash out these abominable looking creatures. Then the wounds were irrigated with normal salt Solution and the most remarkable picture was presented in the character of the wound which was exposed. Instead of having a wound filled with pus, as one would have expected, due to the degeneration of devitalized tissue and to the presence of the numerous types of bacteria, these wounds were filled with the most beautiful pink granulation tissue that one could imagine. There was practically no bare bone to be seen and the internal structure of the wounded bone, as well as the surrounding parts, was entirely covered with the pink, rosy granulation tissue which filled the wound. Bacterial cultures were made and, while one found a few staphylococci and Streptococci still remaining, they were very few in number and not sufficient at that time to cause a pus formation. These patients went on to healing, notwithstanding the fact that we removed their friends which had been doing such noble work.
The character of these wounds made such an impression upon me that I could not help but revolve the question in my mind for the next ten years, until I finally decided to put the observations made on the battlefield into practical use in civil surgery. There have been notes made by other military surgeons, notably, Larrey, Millingen, Keen, Zacharias, of the apparently harmless and perhaps beneficial effect of blow-fly infestation of war wounds, but no mention is to be found of any experimental or voluntary use of these scavengers in wound treatment in times of peace.1 All of us have had many cases of osteomyelitis, both in children and in adults, which have failed to heal under the antiseptic treatments which have been used from time to time. Whether treated by irrigation with mild bichlorid solution, or the packing with iodoform gauze, or the use of Dakin solution, or the use of secondary closure after a complete toilet of the wound, or immediate closure as recommended by Halsted, these cases have formerly recurred and recurred.
In September 1928 there were four cases of children that came into the hospital, each one of whom had been operated upon three or four times and treatment had covered a period of from one to five years. Being baffled in their cure by the means usually employed, I thought it was time to put into active use the Observation that I had made on the battlefield. We, therefore, obtained the maggots from the blow fly from our immediate neighborhood and, without sterilization of the fly or maggot, we loaded the wound up with these maggots and proceeded to watch the results. It must be remembered that at this time no attempt was made to sterilize the maggot or the egg. We were copying, as we thought, the same conditions under which the maggots had performed their work on the battlefield. First a thorough operation was performed, with removal of all dead tissue, sequestra, devitalized muscle, fascial tags,—a complete macroscopic houser cleaning of the region performed surgically. Nothing was used upon the skin of the patient except some slight scrubbing with normal salt Solution, and no antiseptic whatsoever was brought in the neighborhood of the wound. The operation was done with the bare hands, washed only in water, and while no gloves were used, no iodin or any other chemical preparation was applied to the wound itself before operation. The idea was that, if the wounds healed up by means of the introduction of the maggot, the maggot alone would be responsible for the cure and no chemical agent could be said to have had anything to do with the result; and, if the wound healed, the maggot would not be injured or its activity decreased by bringing it in contact with any chemical substance. I realized the days on the battlefields and in the war hospitals could not be entirely reproduced, in that while on the battlefield the action of the maggot would be to prevent infection, in the cases now under treatment the action would be to cure infection. Nevertheless, at the end of about six weeks the wounds had entirely healed, not only in the deeper structures but even as to the skin.
As all the flies leave Baltimore about the fifteenth of October of each year for Florida and points south, we were unable to continue the treatment during that fall in many other cases, and so had to await the return of spring in order to carry on our experiments. In April and May 1929 further cases were submitted to this method of treatment and with the same results. Today we have eighty-nine cases which have been treated here in the City of Baltimore. An abstract of each will be given later in this paper.
It has been noted that in these early cases no sterilization was done. Owing to this fact we very unfortunately ran into certain secondary infections, particularly by anaerobes, such as tetanus bacillus and the gas bacillus of Welch. In the early experiments the Board of Trustees of the Children’s Hospital School had the lawn in front of the hospital upturned so as to make a better sod. What was my surprise, therefore, when in three of the cases I found gas bacilli in the wound This was rather a disconcerting observation, yet one also noticed that the gas bacillus was doing no actual harm even though found in the wound. All experiments were discontinued until that difficulty had been overcome. Six guinea-pigs were given gas bacilli by traumatizing the part and having gas bacilli injected into the wound. All these specimens quickly succumbed to gas bacillus infection, and the gas bacilli were recovered from the organs of these pigs. Then six other guinea-pigs were inoculated with gas bacilli after bone and periosteal tissue had been markedly traumatized. The wounds through which the gas bacillus had been implanted were then sewed so as to make the conditions as anaerobic as possible. Twelve hours, sixteen hours, and twenty hours after the injections had been given these wounds were opened and the maggots introduced into the wound. Each and every one of these guinea pigs made a rapid recovery with complete function in the infected parts. The Observation was therefore drawn that in order to overcome the gas bacillus one would only have to increase the number of maggots, provided the maggots were free of all gas bacillus when they were inserted into the wound. Treatment of patients was started again and a little later tetanus (lock-jaw) bacilli were found in about eight cases. All wounds were immediately washed out and all the patients thus infected were given doses of antitoxin. Four of the cases had no clinical Symptoms but two of the cases went on to marked Symptoms of lock-jaw. Both of these cases were of the severest type of osteomyelitis. One case of a colored man, who had tuberculosis of the ankle, tuberculosis of the lung, and tuberculosis of the spine, with a large abscess in the psoas region, died on the third day, notwithstanding the large amount of antitoxin he received. The other case had an extensive osteomyelitis of the right and left humerus and of the entire left femur. Large amounts of antitoxin were given through the small veins of the hand. Avertin was used to control the marked rigidity and spasm of the muscles and the patient finally made a complete recovery from the tetanus infection. This brought home to us the fact that in civil practice it would be necessary to have sterile maggots, and it would also be necessary to cultivate these maggots so that we might have a constant production of the larvae throughout both winter and summer time. They would have to be grown under our Observation and under perfectly sterile technique. We also realized that, before sending these maggots out for use, the bacteriology of each group would have to be thoroughly studied and the maggots guaranteed free from the presence of all bacteria, both anaerobic and aerobic. This, therefore, took us into the field of the entomology of the blow fly. The flies selected for our use were the blue bottle fly and two varieties of the green bottle fly.
One had, therefore, to learn the life history of the fly,—its cycle of life; conditions under which it bred and laid its eggs; the conditions under which the eggs were laid as to temperature, as to moisture, as to the numerical proportion between the sexes. On what does the fly feed? Where and on what character of material does it lay its eggs? On what does the maggot feed? What is essential for its growth? How is it affected by sunlight? How is it affected by the lack of moisture? How is it affected in various temperatures, and so, on the whole, the problem of the growth of the fly, of the sterilization of the maggot, was a new and extensive under-taking and much experimentation had to be done. It was found that the best method of sterilization was to sterilize the egg. At first we tried to sterilize the maggot, and the maggot on its outside can be sterilized, but the maggot has an intestinal tract and the bacteria which were in the intestinal tract were contaminating the results even though the maggot on its outside had been made perfectly sterile. So finally it was determined that we could absolutely sterilize the surface of the eggs, that we could grow these sterilized eggs on sterilized food, and hence we could produce a sterilized maggot. Following is an account of the experiments and results obtained in the growing and sterilization of the maggot from the blow fly by Mrs. Elgin and myself. It must be remembered that now no batch of maggots leaves the laboratory until those maggots have been tested and found to be absolutely free of any bacteria.
Historical Review
Animal Life Under Aseptic Conditions
Pasteur (1885) was the first to ask the question as to whether animal life is possible without the aid of micro-organisms. He believed that the question would be answered in the negative.
Nencki (1886) brought up theoretical considerations supporting the opposite view, reasoning that various digestive enzymes of the pancreas, stomach, and intestines split up the food into nourishing end products without the aid of bacteria, and that many or most of the end products of bacterial decomposition in the intestine,—such as indol, shatol, phenol, carbon dioxid, and carbon monoxid, are not foods and are actually harmful to the organism. He predicted that chickens, dogs, rabbits, or guinea-pigs might be successfully grown aseptically but he did not make any experimental tests.
In (1901) Mme. O. Metchnikoff raised sterile tadpoles of the frog, Rana temporaria.
Gelcourt and Guyenot in 1910 were the first to devise a method for rearing sterile larvae on sterile media.
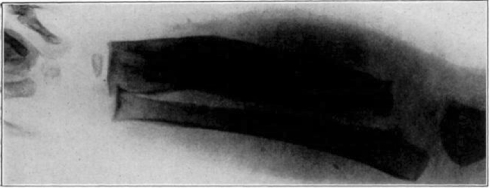
Fig. 1.
Showing male and female fly.
Fig. 2.

Full-grown maggots.
Fig. 3.

Container for sterile maggots.
Fig. 4.
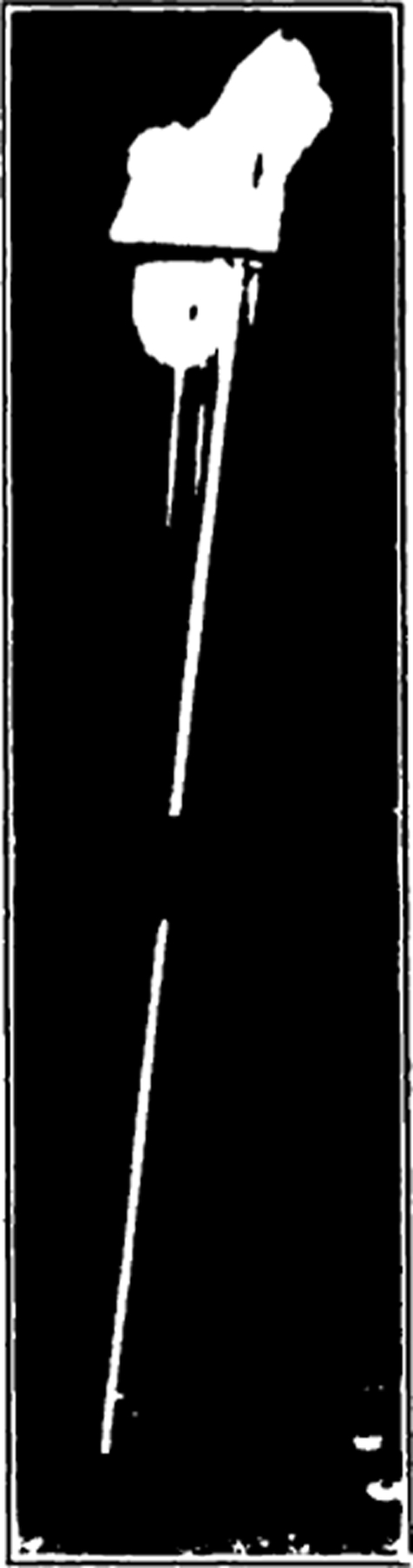
Test tube with applicator stick. Used as container when sterilizing the eggs.
In (1912) Cohendy experimented on chickens obtained from sterilized eggs. He compared the general condition and weight of (a) sterile chickens supplied with sterile air and food, (b) chickens from sterilized eggs, infected, and then fed with sterilized food and kept in conditions similar to those of a, and (c) chickens treated normally. The chickens of both a and b grew much less and were weaker than the normal chickens, but there was no great difference between the chickens of a and b, and he concluded that a complete absence of micro-organisms does not in itself entail any deterioration in higher vertebrates.
Studies on Blow Fly Larvae (Not Under Aseptic Conditions)
These larvae were secured during the summer by exposing raw beef outdoors, and when the flies had deposited a large mass of eggs the meat was transferred to a glass jar. The eggs hatched in eight to twenty-four hours, and the larvae were allowed to feed on the raw beef for two days. Water was then poured into the jar and the larvae would leave the meat. The water containing the larvae was then poured through a wire strainer and the larvae were collected with a small spoon and placed in bottles. The bottles were stopped with cotton wrapped in a fine grade of cheesecloth. The larvae were now ready to place in the patient’s wound.
Cultures were taken from the wounds several days after the introduction of the maggots, and when studied were found to contain great numbers of gram negative bacilli, including bacillus coli, bacillus pyocyaneus, bacillus proteus vulgaris, and many spore-forming aerobes. A number of the cultures were found to contain anaerobic bacilli, such as bacillus tetani, bacillus Welchii, and bacillus sporogenes. Cultures were then taken from maggots and found to contain the same organisms.
We attempted to free the larvae or maggots from the pathogenic anaerobes by chemical sterilization. The larvae were immersed in full strength hydrogen peroxid and allowed to remain for two hours; following this they were immersed in bichlorid of mercury, strength one part in 1,000, for two hours. They were then washed in sterile distilled water. Cultures were made by allowing the larvae to crawl over a plate of sterile nutrient agar; and by streaking plates with the water that had been used in washing the larvae. Anaerobic cultures were also made from this water. These cultures were all negative. Some of the larvae treated in this manner were crushed and cultures made. These cultures were all positive for the gram negative bacilli as well as the pathogenic anaerobes; therefore, we concluded that the organisms were carried in the intestinal tract of the larvae and we were unable to reach them with a sterilizing solution.
Studies on Blow Fly Larvae (Under Aseptic Conditions)
These flies were secured by exposing raw beef outdoors and when the flies had deposited a mass of eggs, the meat was transferred to a glass jar and the jar stopped with cotton wrapped in a fine grade of cheesecloth. The eggs hatched in eight to twenty-four hours, and the larvae were given fresh beef each day for seven days. The larvae were now full-grown and the jar containing them was placed in a larger jar which contained about two inches of sand in the bottom. This sand had been baked in a hot-air oven one hour at 160 degrees centigrade. The stopper was removed from the jar containing the larvae and they immediately left their food and dropped into the jar containing the sand. In twenty-four to thirty-six hours all these larvae had pupated. The jar in which they had fed was removed and the jar containing the sand and pupae was covered with a piece of cheesecloth and an elastic band slipped over it. The pupal period of these larvae lasted from five to seven days when the adult flies emerged. The jar was now placed in a cage, twenty-four inches Square, made of wire Screening. Food was supplied at once and consisted of granulated sugar (sucrose) dissolved in water. The cage was kept in the laboratory. In two days all the flies were dead.
Fig. 5.

Sterile food used in growing maggots for the patient’s wound.
Fig. 6.

Gooch crucible. Complete for washing the eggs.
Fig. 7.
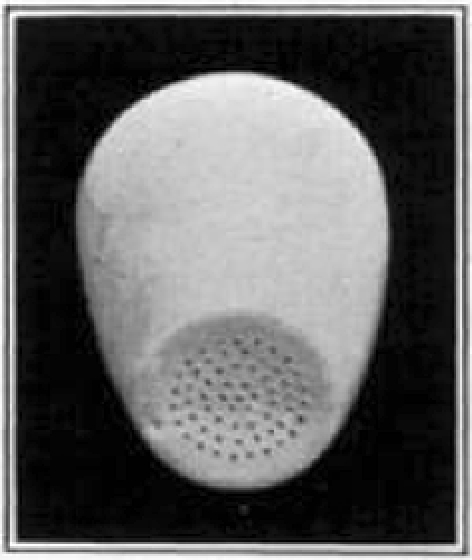
Gooch crucible showing perforations in the bottom.
Fig. 8.
Strainer used in washing maggots.
Several generations of flies were secured in the same manner but lived only a few days after hatching. We, therefore, concluded that the laboratory air did not contain enough moisture, the temperature was not constant, the food was, no doubt, insufficient, and there was a possibility of the flies being poisoned from the wire screening.
A large glass incubator was then constructed in which the temperature was controlled by a thermostat, the humidity controlled, artificial light supplied, and circulation of the air carried on by a small motor. For housing the flies, small cages were built to fit inside the incubator; these cages consisted of a circular bottom made of light wood and a wire frame. A bag made of a fine grade of cheesecloth, fitted with a sleeve on one side was slipped over this, the sleeve being used when feeding or capturing the flies. Another generation of flies was secured as before and, when hatching began, the flies were placed in the gauze cages. Food for these flies consisted of strained honey, water, and yeast. Small pieces of raw beef were given to them to receive their eggs.
These flies lived, were very healthy, and when they were seven days old began laying eggs.
An attempt was made to free the outside of the egg from bacteria by chemical sterilization, believing the inside of the egg was free from bacteria.
A great many experiments were carried out, using various sterilizing Solutions, such as bichlorid of mercury, strength one part in 1,000, phenol, alcohol, argyrol, mercurochrome, gentian violet, hexylresorcinol and silver nitrate. The experiments were carried out under various lengths of time and temperatures. Most of these experiments were very unsuccessful, due to the fact that either the egg embryo was killed or the bacteria survived.
With the bichlorid Solution used at room temperature for one hour, we were able to destroy the bacterial flora, but most of the eggs were destroyed as well.
Some sterile larvae were obtained, however, and when hatched on sterile agar culture media no evidence of contamination could be found, but the agar culture media was not a satisfactory food for the larvae, and after crawling over and burrowing down into the agar they did not increase in size and lived only a few days. Various sterile synthetic foods were tried, such as dextrose agar, meat extract agar, sterilized cheese, liver extract media, and sterilized ground beef. All of these foods were unsatisfactory. A food was then made which consisted of nutrient agar, peptone, Fleischmann’s yeast and liver, sterilized by autoclaving. This proved to be very successful and has been used throughout the entire work.
We carried out many experiments and used the larvae in the treatment of the wounds by sterilizing the eggs in bichlorid, strength one part in 1,000, plus one-half per cent. of hydrochloric acid, for one hour.
The sterile eggs were allowed to hatch and the larvae feed on the agar and liver food mentioned before. On this food the larvae reached full growth, pupated normally, and emerged as well developed sterile flies.
Present Technique
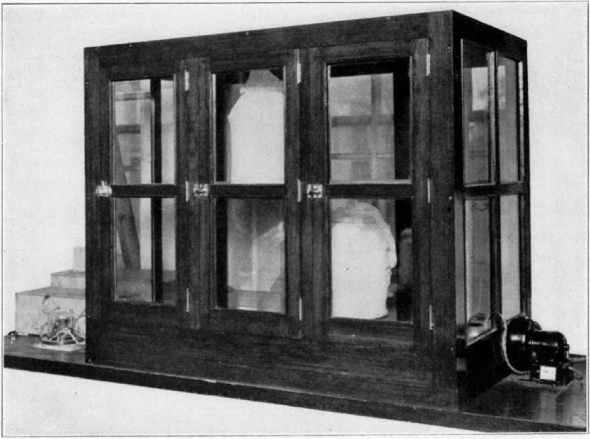
An incubator should be constructed in which the temperature is controlled by a thermostat and operated continuously between seventy-five and eighty degrees Fahrenheit, humidity controlled to fifty per cent., artificial illumination given with electric-light bulbs, and circulation of air supplied by a small motor.
Fig. 9.

Cage for housing the flies. The side sleeve is used when feeding or catching the flies. When transferring the flies from one cage to another the top opening is untied and a clean cage is inverted over the old one. A light is placed over the top cage and the flies will at once fly into the clean cage. When they have all been captured the gauze can be drawn together and tied.
Fig. 10.
Incubator used for housing the flies. On the right the blower can be seen and on the left two boxes. The large box contains water to supply moisture and the smaller one contains the heating coils. On the left also is the thermostat control. The thermostat can be seen on the inside of the incubator on the right-hand side.
For housing the flies, small cages should be built to fit inside the large incubator. A satisfactory cage consists of a circular bottom made of wood and a wire frame. A cheesecloth bag is slipped over this, one side being fitted with a sleeve which may be slipped over the arm when feeding or capturing the flies. Sunshine is very necessary for the flies, and the cages should be removed from the incubator and placed in the direct sun rays (not Coming through window glass) at least once or twice each week.
To Culture Flies
A culture of flies may be started during the summer months by exposing raw beef outdoors, and the generation of flies carried through as already explained.
The desired species of flies can be identified and kept for egg-laying and breeding purposes. We have found the following species satisfactory for this work:—Phormia Regina, Sucilia Sericata, Lucilla Ceaesar.
Life History of Maggots
The fly, after being hatched, produces eggs in four to seven days. Eggs hatch in eight to twenty-four hours. Larvae turn into pupae in five to seven days. Adult flies emerge in seven to ten days.
In growing the larvae to carry on the generations of flies we use the following food:—
Ground raw beef, 1 part
Ground raw liver, 1 part
1 cake of Fleischmann’s yeast to every 250 grams of the combined raw meat.
This mixture is placed in bottles of six inches in height by two inches in diameter to a depth of two inches. Plugs for the bottles are made of cotton wrapped with cheesecloth. All bottles and plugs before they are used are sterilized in hot air at 160 degrees, centigrade, for one hour.
This food is autoclaved for thirty-five minutes under fifteen pounds’ pressure; allowed to cool and then incubated at thirty-seven degrees, centigrade, for thirty-six hours; autoclaved again for thirty-five minutes under fifteen pounds’ pressure and stored on ice.
Larvae from sterilized eggs are placed on this food and the bottles kept in the fly incubator; the bottles should be wrapped in dark paper to protect the larvae from the light. If too many larvae are placed in the bottles they will leave their food before they are full grown, so care must be used at this point.
When the larvae have reached their full growth (which requires from five to seven days) place the jar in a larger jar containing about two inches of baked sand in the bottom. Remove the cotton plug and the larvae will pupate in the sand. Remove the feeding jar and place cheesecloth over the jar containing the sand and pupae. In seven to ten days the flies will emerge. The jar is now placed in the gauze cage. (For perfect breeding twice the number of males to females should be kept in each cage.)
Food for Flies
Food should be supplied at once, and consists of
Strained honey—30 cubic centimeters
Fleischmann’s yeast—3 grams
Water—70 cubic centimeters
Stir the mixture well and pour into small Petri dishes containing absorbent cotton in the bottom. Fresh dishes of food should be supplied each day. The flies must be given abundant food at all times.
After hatching, the flies require from five to seven days to develop before they will begin to lay eggs. When egg-laying begins, the flies are supplied with a small cube of fresh beef from which the outside surfaces have been cut off. We place the beef in the cages at nine o’clock in the morning. After about seven hours’ exposure to the flies, the meat is taken out and the eggs are removed by gently washing them with water and freeing them from the meat with a small probe or knife. Then the eggs are placed in a small bottle containing sterile water. This is done to protect the eggs from drying out. The eggs are now placed in the ice-box kept at a temperature of forty degrees Fahrenheit; at this temperature the eggs will not hatch and they may be kept for twenty-four hours without injury.
Sterilizing the Eggs
Test tubes, six inches in height by three-fourths inches in diameter, fitted with small wooden applicators, and plugged with cotton and sterilized in hot air at 160 degrees, centigrade, for one hour, are used as Containers for sterilizing the eggs. The clumps of eggs are separated by vigorously stirring the water they are now in. After this is accomplished they are divided and placed in test tubes to a depth of one-half of an inch. The eggs will settle to the bottom of the tube and any supernatant water present can be poured off. The sterilizing solution is then poured on to the eggs. This solution is made as follows:
Stock Solution of bichlorid of mercury, strength one in 1,000 parts.
Stock Solution of fifty per cent. ethyl alcohol.
Take equal volume of each Solution, mix, and add one-half of one per cent. chemically pure hydrochloric acid.
The final Solution will contain bichlorid of mercury, strength one in 2,000 parts, twenty-five per cent. alcohol, and one-half of one per cent. hydrochloric acid.
The eggs should remain immersed in this Solution for thirty minutes; they should be gently agitated several times during this period by stirring with the wooden applicator. The neck of the test tube is passed through a flame and allowed to cool. They are now ready to be washed.
A satisfactory Container for use in washing the eggs consists of a Gooch crucible with a cover; a piece of gauze is placed over the perforated plate and the crucible is fitted into a bottle. This is sterilized in hot air at 160 degrees, centigrade, for one hour. The eggs are strained through the gauze and washed by pouring sterile distilled water over them. After washing, the cloth with the eggs is transferred, using aseptic technique, to a bottle containing sterile food and the bottle placed in an incubator at seventy degrees Fahrenheit, moisture being supplied by keeping open pans of water in the incubator.
Preparing Sterile Food
Two-ounce, wide-mouth bottles, stopped with cotton wrapped in muslin, are used as Containers for food; these are sterilized in hot air at 160 degrees, centigrade, for one hour. The food consists of agar culture media liquified, one liter; one cake of Fleischmann’s yeast; one-half pound of pig liver. The liver is cut into small cubes, placed in a pan, and covered with water and boiled over the flame for twenty minutes. The yeast cake is added to liquified agar and stirred well. One cube of liver is placed in each bottle and allowed to rest on the bottom of the bottle and slant to the side. Now the agar and yeast mixture is poured into the bottle until the liver is covered, except for about one-quarter of an inch. The bottles containing the food are autoclaved thirty-five minutes at fifteen pounds’ pressure; then allowed to cool. They are incubated at thirty-seven degrees, centigrade, for thirty-six hours; then autoclaved again for thirty-five minutes at fifteen pounds’ pressure. This food may be stored in the ice-box and kept several weeks.
To culture the larvae:—The second day after the eggs have hatched, the cloth containing the unhatched eggs is removed with a sterile forcep and cultures are made by taking scrapings from the agar and one or two larvae. These are crushed and anaerobic and aerobic cultures made. On the third day, provided the cultures are negative, the sterile larvae are ready for use.
-
Example:—Eggs sterilized on Monday will be hatched Tuesday.
Cultures are taken Wednesday.
Larvae ready for use Thursday.
When needed, these larvae, still small, are suspended by pouring sterile water into the bottles and straining them through a sixty-mesh wire strainer. The larvae are collected with a sterile spoon and placed in sterile bottles stopped with cotton wrapped in muslin.
If desired, the larvae may be held for several days by placing the bottles of food containing them in the ice-box at a temperature of forty degrees, Fahrenheit. They will become very inactive at this temperature and cease to grow until again placed at a higher temperature.
The Method of Treatment of Wounds
The skin is washed off with sterilized water (normal salt Solution) simply to remove the grease and dirt from around the wound. No sterilization or chemical of any other nature is used on the part to be operated upon.
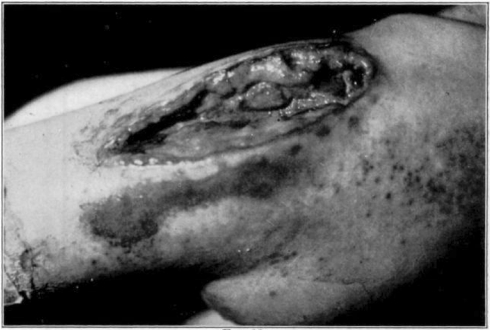
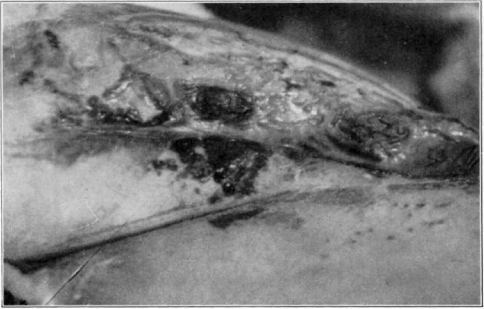
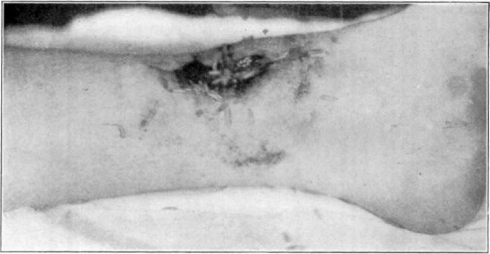
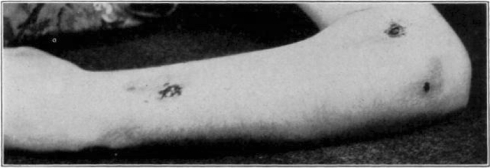
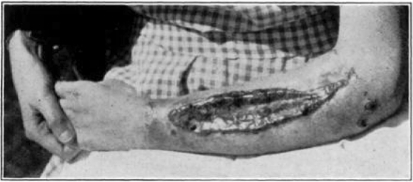
Fig. 11.
Showing cage turned back.
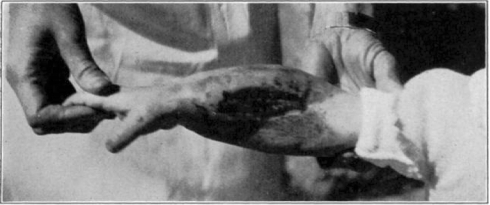
Fig. 12.
Showing maggots at work in wound.
Fig. 13.
Showing maggots at work in wound.
Fig. 14.

Showing maggots at work in wound.
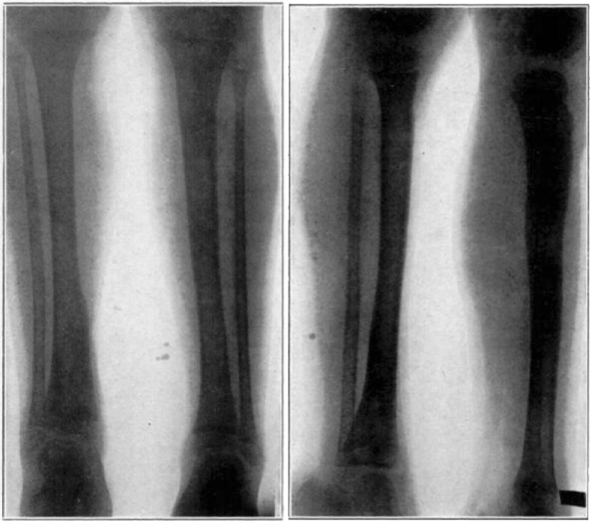
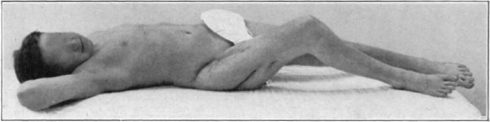
Fig. 15.
Cured cases of osteomyelitis following maggot treatment.
Fig. 16.
Another group of cases cured by the maggot treatment.
Fig. 17.
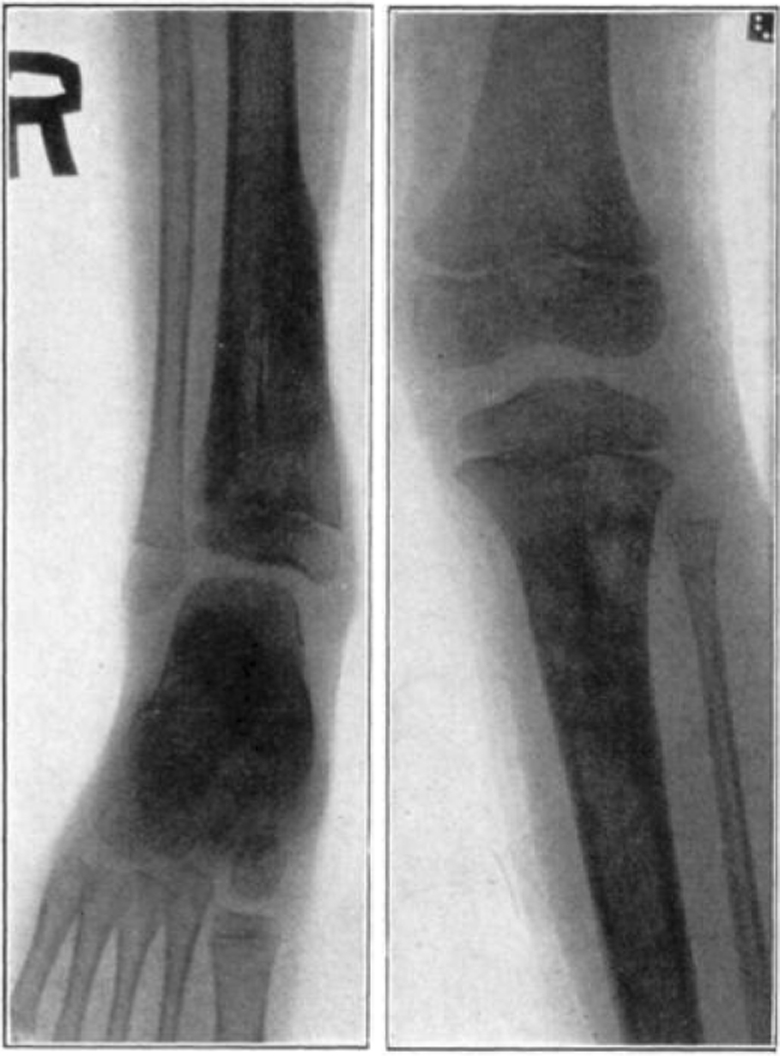
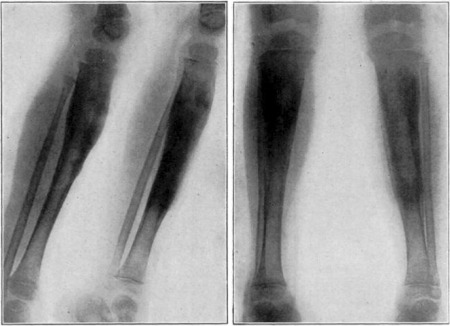
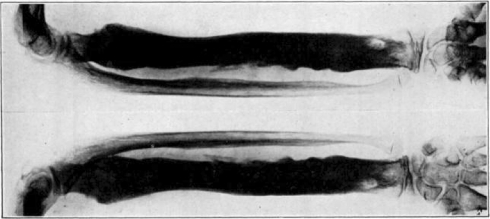
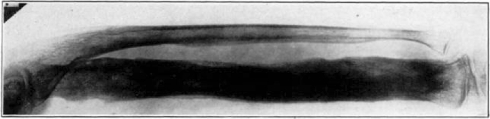
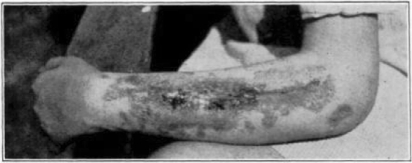
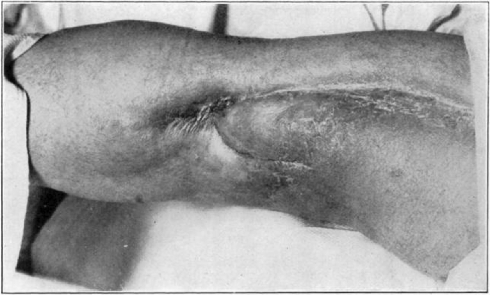
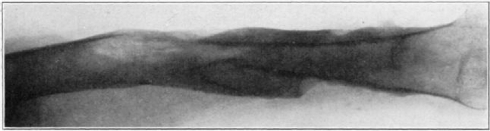
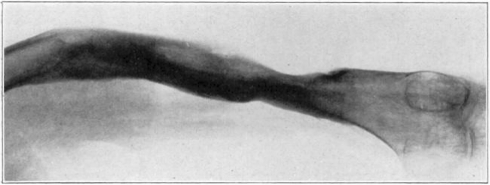
Case 1. B. M. Before operation.
Fig. 18.
Case 1. B. M. After operation.
Fig. 19.
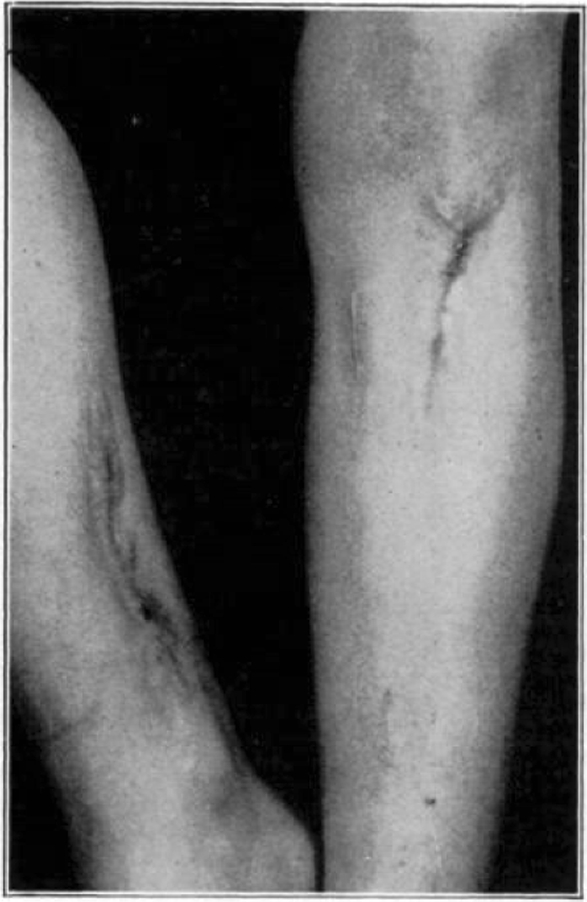
Case 1. B. M. Almost healed.
Fig. 20.
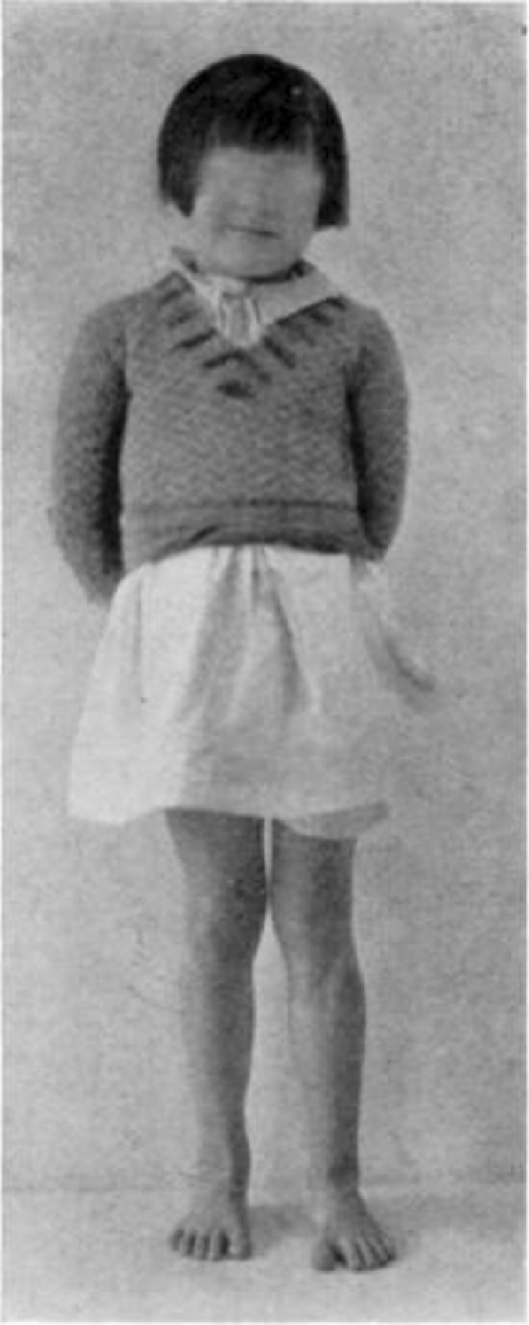
Case 1. B. M. Completely healed.
Iodin in any form, iodoform, bichlorid, mercurochrome, Scott’s Solution, or any of the chemical materials usually used in the preparation of the field of operation are absolutely tabooed. No gloves are used, so as to prove the point that it is the maggots, and the maggots alone, and not disinfection that does the work. One, therefore, lays bare the infected bone and cuts through the cortex and opens wide the medullary cavity. All granulation tissue and sequestra are completely removed. It is not always necessary to remove all cloaca for the maggots themselves seem to get around and through the cloaca. Therefore, a great deal of the structure of the bone may be saved rather than doing a complete saucerization of the wound. After the wound has been thoroughly cleaned in this manner, simply by débridement and without antiseptics, the wound is packed with pain sterile gauze. This is in order to check whatever hemorrhage may occur, and these packs are left in twenty-four to forty-eight hours, according to the amount of hemorrhage started up. At that time the pack is removed and the sterilized maggots are turned into the wound. The wound should be entirely filled with the maggots so that every part may be attacked by the maggots at the same time. It very often happens that a certain part of a wound offers more delicious food than any other part of the wound, and hence with only a few maggots they all seek the green pastures. Thus it is better to have so many maggots that the cleaning-up process which they carry on is universal throughout the entire wound. Before the maggots are turned in, however, the wound is bounded on its edges by adhesive plaster. This is done for two reasons. In the first place, it offers a firm and secure basis on which to place the cages which are put on to prohibit the maggots from getting out of the wound. In the second place, it protects the skin from the Sensation of tickling which is very often caused by the maggots when they walk on the normal skin. This tickling may be, and generally is, the only disagreeable feature of the treatment, because it may irritate the patient constantly. Hence, if all skin areas within the cage are thoroughly covered by adhesive plaster, firmly covered with collodion, these elements of irritation are abolished. After the maggots have been introduced into the wound in a sterilized State, they are sluggish because of their recent bath. Maggots are generally sent out in sterilized bottles. In order to get the maggots out of the bottles a little sterilized water is poured into the bottle. The solution, with the maggots, is then poured into a sterile sieve. The water runs off and the sieve is turned upside down and the maggots, though wet, are precipitated into the wound. This sluggishness is quite acceptable as it allows a little more time for the placement of the cage over the wound. The cages, as seen in the pictures, are made of various sizes and shapes. The edges of the cage are made of soft sponge rubber, and over the top of this soft rubber is placed a very fine wire mesh which is sewn on to the rubber. These cages are cut to suit any size and contour of wound to be treated. Before being placed over the wound the bottom edge of the cage is covered with adhesive plaster, and the gum side exposed so that it attaches itself to the adhesive already placed around the periphery of the wound. This is strapped to the leg, or part involved, and by this means the maggots are retained within the wound itself. The top of the cage should be thoroughly exposed to the sunlight and air, and should not be covered up by gauze or other dressings which keep out sunlight and air. Both air and sunlight are necessary for the well-being and the activity of the maggots themselves. Maggots are like dogs—they seek the shade. The more sunlight, therefore, the deeper they penetrate into the cavernous aspects of the bone, and if one desires to send them more quickly into the lower depths one may use artificial light directly over the cage. By this means, also, the temperature of the maggot is kept more constant and it works better on account of the warmth and on account of the air. At the end of the fifth day the cage is removed, the adhesive plaster is removed, and the wound is thoroughly washed out with normal salt Solution. All maggots that are in the wound are, therefore, washed away. One may use even little sterilized pledgets to take the membrane which is formed in the early stages of the process away from the depths of the wound.
Fig. 21.
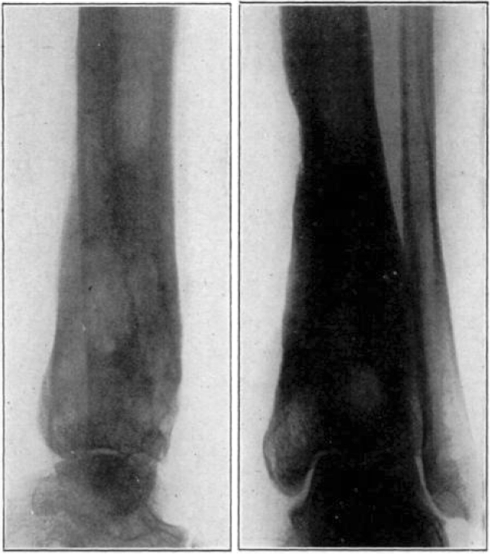
Case 3. A. W. Before operation.
Fig. 22.
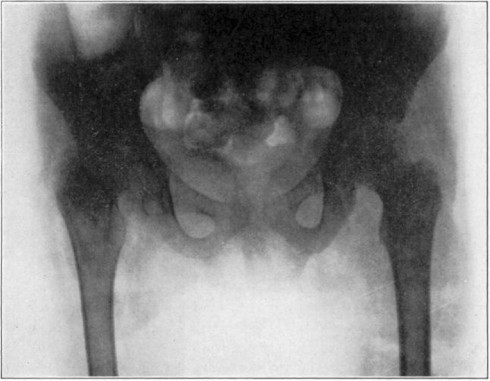
Case 3. A. W. After operation. Tuberculous hip.
Fig. 23.
Case 3. A. W. During treatment. Tuberculous hip.
Fig. 24.
Case 3. A. W. Almost healed. Tuberculous hip.
Fig. 25.
Case 3. A. W. After treatment. Tuberculous hip.
Fig. 26.
Case 3. A. W. Healed tuberculous hip.
Fig. 27.
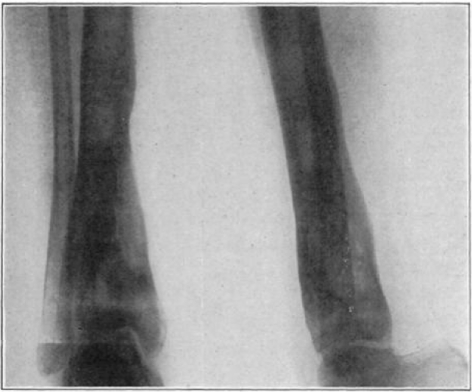
Case 4. B. C. Before operation.
Fig. 28.
Case 4. B. C. After operation.
Fig. 29.
Case 4. B. C. During treatment.
Fig. 30.
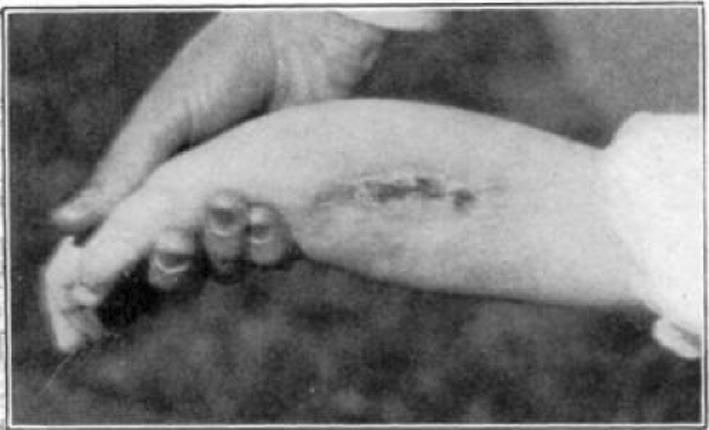
Case 4. B. C. Almost healed.
Fig. 31.
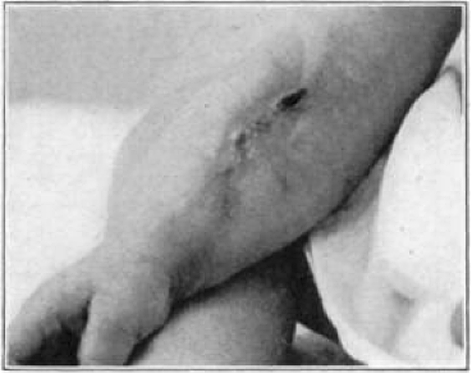
Case 4. B. C. Healed.
After one application of maggots, indeed after the second or third day of the first application of the maggots, all wound’s become alkaline. Most wounds have an acid reaction— either weak or quite strong—but it takes hardly twenty-four hours before this reaction becomes alkaline after the introduction of the maggots. I mention this fact because I believe that the alkaline reaction has a great deal to do with the sterilization of the wound and the killing of the bacteria. This period of changing every fifth day is done for the following reasons: The life history of the maggot under normal conditions is seven days. The maggot is about forty-eight hours old when he is inserted into the wound. The first day is occupied with his hatching and in sterilization, and the second is utilized in studying the bacteria and to show that the cultures which are made after the sterilization are perfectly negative. The following four days, therefore, he works in the wound. He is washed out then on about his seventh day, at which time, if left, he would change into a pupa and hence be of no service in the process of healing.
Fig. 32.
Case 15. F. R. Before operation.
Fig. 33.
Case 15. F. R. During treatment.
Fig. 34.
Case 15. F. R. During treatment.
Fig. 35.
Case 15. F. R. Almost healed.
Fig. 36.
Case 15. F. R. Healed osteomyelitis.
Even at the early stage of the first dressing one notices the change in the character of the wound. There may have been a slight amount of odor during the first two days after the insertion of the maggots, when the bacteria have been stirred up by their activity and when they are eating and destroying the dead tissue which is there. But, at the end of the first dressing, whatever odor there may have been practically ceases, and now one notices the beautiful reddish granulation that is springing up throughout the wound. At the insertion of the maggots, therefore, for the second time, at the end of five days, this process of cleaning up and of the growth of new granulation tissue is seen to be going on. The wound still remains alkaline and is becoming more and more filled with granulation tissue. There may be a scum over the granulation tissue at this time but this scum is due mostly to the proteus bacillus, which seems to be the most persistent organism to remain in the wound. The wound now fills up from the bottom with the continuous application of maggots every five days, and in about six to seven weeks the wounds in children are usually healed. In adults a little longer time is taken, particularly where the osteomyelitis has been of very long standing and where there has been a great eburnation of bone and dense scar-tissue formation. Generally, the wound fills up with granulation tissue which becomes flat with the end surface, and the epithelial cells gradually grow over this granulation tissue and the resulting wounds are usually found entirely flat so that no deep sulci are left on the affected part, as in our former treatment by drainage, and the contour of the wound more nearly resembles the original shape of the part.
Fig. 37.

Case 20. L. P. Before operation.
Fig. 38.
Case 20. L. P. After operation.
Fig. 39.
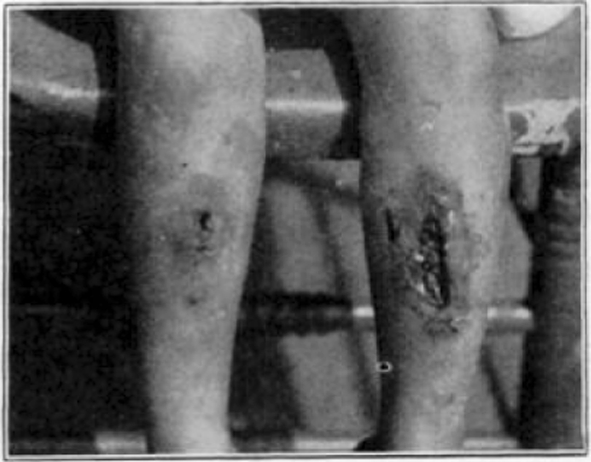
Case 20. L. P. During treatment.
Fig. 40.
Case 20. L. P. Completely healed.
Fig. 41.
Case 23. E. W. Before operation.
Fig. 42.
Case 23. E. W. After operation.
Fig. 43.

Case 23. E. W. Completely healed.
Fig. 44.
Case 23. E. W. Showing method of treatment.
As soon as the skin of the patient has entirely healed, one may allow the use of the part. Before that time the patient is, as a rule, at rest so as to prevent any traumatism or strain on account of use.
Action of the Maggot Within the Wound
At first the maggot seems to act as a scavenger. He busily sucks up the bacteria and consumes the dead tissue. He passes these through his intestinal tract. Some bacteria are thus killed, while others pass through the intestinal tract in a perfectly live State. These live bacteria may be the source of further contamination. The maggots of this type destroy only dead tissue or tissue which is pathological. They will work around and separate a small sequestrum or dead particles of bone which are still attached to normal bone, and seem to gnaw down through the bone until the bleeding area is arrived at. Until the bleeding area is reached the granulation tissue will not grow over and amalgamate or attach itself to the bone. One can see the process of scarification by the maggots by closely observing them at work in the wound. So voracious are they in their struggle for food that they will stand upright on their heads with their tails in the air, as puppies do to crowd around a basin of food where the basin is too small for the number of puppies. They apparently continue this process of sucking day and night and seem never to tire. Apparently the time for inactivity is only when they go into the pupal stage. They do not like too much secretion, although they make some secretion by their own activity in the wound, and in certain cases it is rather wise to draw off the supernatant juice which is formed in the wound by their activity, lest they drown. Personally, I believe that the scavenger reaction is not the only method by which they heal the wound. I am inclined to think that there is some biological reaction which is formed between the activity of the maggot and the fluids of the human body which plays an important part in the healing of the wound. Just what this biochemical substance is, has not yet been absolutely determined by investigations. When the wound reaches the state of almost healing, when the bacteria become less from day to day, there comes a time when maggots placed in the wound will be killed after a stay of a few hours. This, too, is another problem and one which necessitates more biochemical work.
Fig. 45.
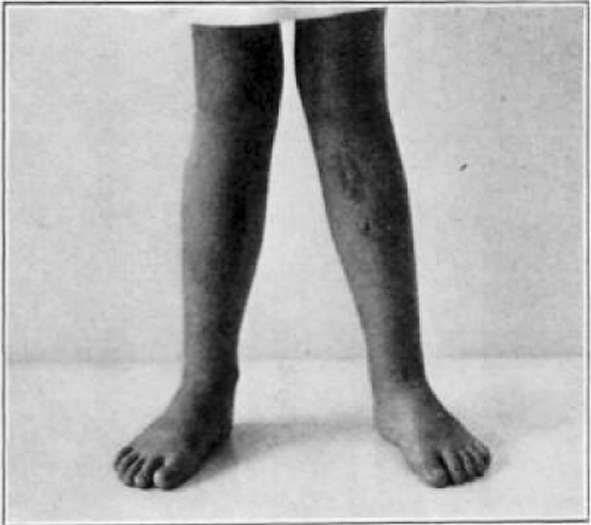
Case 25. R. Y. Healed osteomyelitis.
Fig. 46.
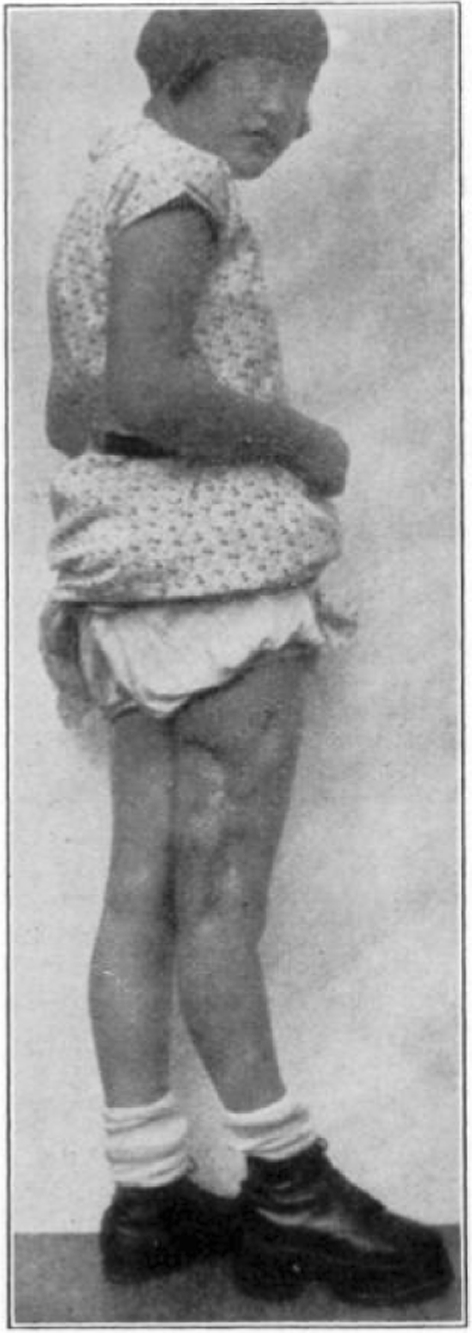
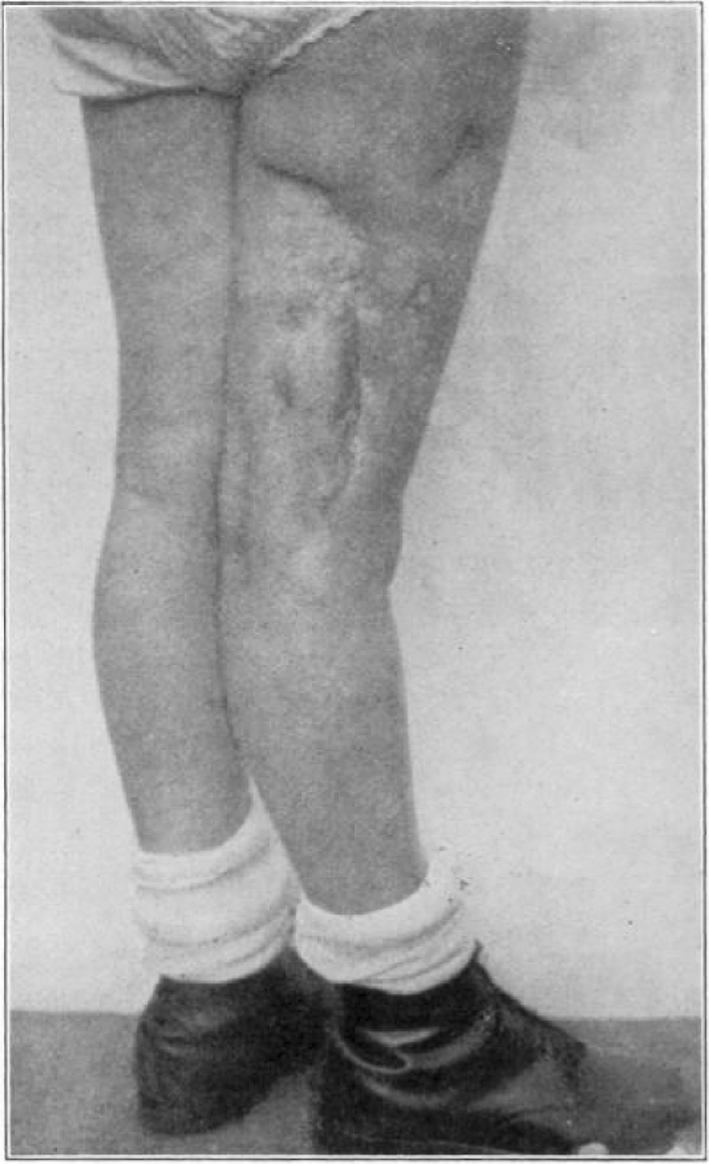
Case 25. R. Y. Healed osteomyelitis.
Bacteria in the Wound
Whatever the bacteria of the original wound, be they aerobes or anaerobes, and generally they are the former, the amount of bacteria begins to diminish from the first application of maggots. As I stated before, the wound becomes alkaline after twenty-four hours’ activity on the part of the maggot, which can be easily tested out by litmus paper inserted into the wound. Daily the number of bacteria diminishes,—the Streptococci and staphylococci seeming to be destroyed first and the proteus remaining until the end. There may still be, at the end, some few bacteria living on the surface but they are very few in number.
Fig. 47.
Case 28. R. L. Before operation.
Fig. 48.
Case 28. R. L. After operation.
Fig. 49.
Case 28. R. L. Before treatment.
Fig. 50.
Case 28. R. L. During treatment.
Fig. 51.
Case 28. R. L. Nearly healed.
Fig. 52.
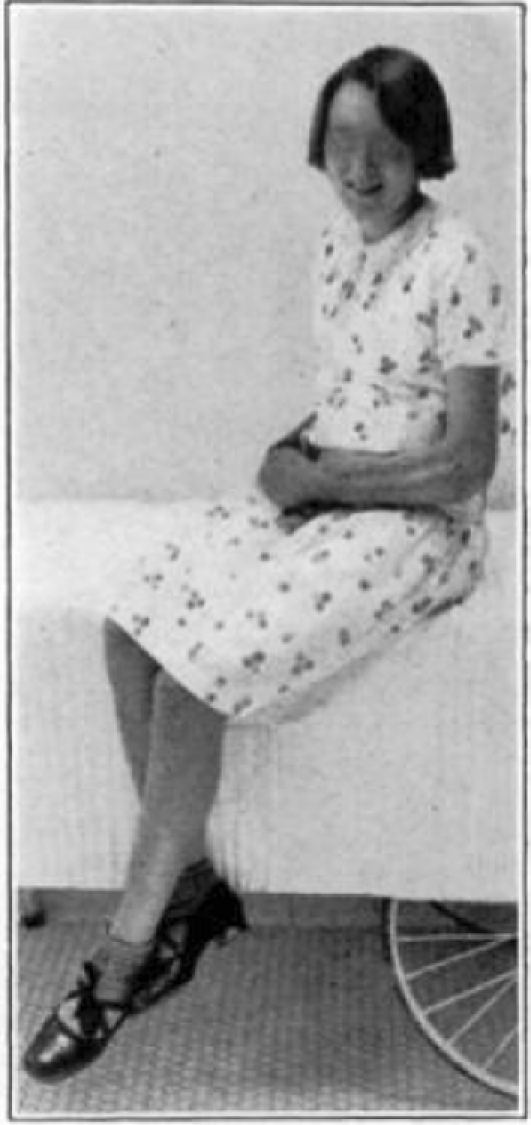
Case 28. R. L. Healed.
Ages
It is very much easier to heal up the wounds in children than in adults. Growing bone naturally aids the process of repair. The duration of time in children is about six weeks in the ordinary long bone. Of course, I am speaking again of chronic osteomyelitis. It is known that osteomyelitis in children generally occurs at the diaphyseal portion of the epiphysis, but the more I have studied osteomyelitis, the more I have been impressed by the fact that the point of rupture of the pus in the long bones—as the femur—is generally in the posterior part of the shaft near the epiphyseal line. And hence, often, I fear, the operation for acute osteomyelitis does not go down far enough to reach the point of infection. Later, then, one is confronted with a rupture of the pus on the deep surface of the bone, and I have seen such cases go directly into the Joint by breaking through the periosteum on the posterior surface of the bone. Before doing a debriding operation on children one should be sure that the involucrum is perfectly well formed. In adults the question of healing is a more difficult one. The disease has often been present for years and years and the bone is extremely eburnated. Here also, the occurrence of the osteomyelitis in the shaft of the bone is more frequent than is the case in children, and yet the occurrence of infection of the diaphysis, next to the epiphyseal structures, is more apt to be the case than true diaphyseal osteomyelitis except in gun-shot wounds. Owing to a lack of growing cells, new bone formation is less active than in children and there is a greater quantity of old eburnated bone present to deal with, and the progress of healing is slower than in children. If the average child heals in approximately six weeks, the adult healing is at least a third longer; but, if the technique be carried out and the maggots allowed to remain until they are killed off by the nature of the secretion in the wound, healing will take place.
Fig. 53.
Case 57. B. G. Nine years’ duration. Seven previous operations. Seven operations. Healed.
Fig. 54.
Case 57. B. G. Three months after operation.
Fig. 55.
Case 57. B. G. Three months after operation.
Fig. 56.
Case 57. B. G. Ten months after operation. Nine years’ duration. Seven previous operations. Seven operations. Healed.
Recurrence
There is a certain recurrence in about five per cent. of the children and five per cent. of the adult cases. Be they children or adults, this recurrence consists generally of small spicules of bone which work up to the surface and are extruded through a small sinus. Almost invariably they close up by themselves in a few days and need no further operative or maggot treatment. It is simply an effort on the part of nature to throw off some fragments of foreign material which in our stupidity we have overlooked. There have been comparatively few cases where a complete operation has had to be done over again where we have felt that we had done a complete operation the first time. In acute osteomyelitis, by treatment with the same method, we have shortened the time tremendously in the cure of the patient. In acute osteomyelitis, treated within the first few days from the beginning of the infection, one drills down to the spot where the osteomyelitis exists. The tension being relieved, the focus walls itself off or becomes circumscribed at that area. A slight drain is put into the drill hole so as to keep the skin from closing over. About the fifth day, after there has been an attempted walling off, the maggots are placed in the wound, which may have to be slightly enlarged to allow for their admission. These rapidly clean out the pus and separate whatever sequestra are going to be formed. The maggot has an intuition as to just where the line of demarcation is going to appear, and eats down to that line and thus removes all potential sequestra. The wound fills up rapidly by granulation tissue and is soon healed.
Table 1.
Summary of case reports
| Case Number | Diagnosis* | Age | Onset before admission | Admission | Previous operations | Operations | First insertion of maggots | Number of insertions of maggots | Healed | Duration | Infection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 1, B. M. | Right tibia | 4 years | 3 months | Aug. 25, 1928 | 2 | Sept. 18, 1928 | Sept. 18, 1928 | 8? | |||
| Oct. 9, 1928 | Oct. 11, 1928 | Bacillus Welchii | Dec. 17, 1928 | 13 months | Staphylococcus | ||||||
| Left tibia | May 14, 1929 | May 16, 1929 | 6? | Aug. 27, 1929 | |||||||
| Right tibia | Aug. 6, 1930 | Aug. 9, 1930 | 7 | ||||||||
| Case 2, T. M. | Left tibia Right tibia |
8 years | 3 years | Nov. 29, 1929 | 2 | Dec. 18, 1929 July 23, 1930 |
Dec. 24, 1929 July 25, 1930 |
6 24 |
June 1, 1930 Nov. 18, 1930 |
6 months 4 months |
Streptococcus hemolyticus |
| Case 3, A. W. | Left femur | 6 years | 6 months | Mar. 14, 1929 | 0 | Dec. 11, 1929 | Jan. 9, 1930 | 5 | June 1, 1930 | 6½ months | Tuberculosis and staphylococcus |
| Case 4, W. C. | Right radius | 15 months | 2½ months | Jan. 25, 1930 | 0 | Feb. 12, 1930 | Mar. 29, 1930 | 9 | |||
| Feb. 26, 1930 | |||||||||||
| Aug. 26, 1930 | Aug. 28, 1930 | 4 | Oct. 30, 1930 | 7 months | Staphylococcus | ||||||
| Oct. 10, 1930 | |||||||||||
| Case 5, S. K. | Left humerus | 11 years | 2 years | July 11, 1930 | 2 | July 16, 1930 | July 18, 1930 | 16 | Oct. 30, 1930 | 3 months, 11 days | Streptococcus pyocyaneus |
| Case 6, A. V. | Left femur | 4 years | 10 months | May 22, 1930 | 2 | June 4, 1930 | June 9, 1930 | 10 | Dec. 20, 1930 | Staphylococcus | |
| Nov. 12, 1930 | Practically healed | ||||||||||
| Case 7, M. S. | Left femur | 10 years | 4 years | July 7, 1930 | 3 | July 9, 1930 | July 12, 1930 | 19 | Healed | Staphylococcus | |
| Case 8, E. R. | Left femur | 18 years | 6 years | June 30, 1930 | 2 | July 2, 1930 | July 5, 1930 | 23 | May 18, 1931 | Staphylococcus | |
| Feb. 22, 1931 | 4 | Oct. 22, 1930 | Oct. 25, 1930 | Not healed | |||||||
| Feb. 25, 1931 | Feb. 28, 1931 | 5 | |||||||||
| Case 9, M. D. | With multiple abscesses | 11 years | 1 year | Jan. 31, 1930 | 3 | April 23, 1930 Feb. 18, 1931 |
May 8, 1930 Feb. 21, 1931 |
36 3 |
Improved, still under treatment | Staphylococcus | |
| Case 10, N. T. | Right tibia | 9 years | 2 years | Oct. 23, 1930 | 1 | Oct. 31, 1930 | Nov. 4, 1930 | 3 | May 18, 1931 Almost healed |
||
| Case 11, P. B. | Right femur | 14 years | 1½ years | July 2, 1929 | 1 | July 16, 1929 Jan. 8, 1930 June 4, 1930 |
Nov. 21, 1929 Jan. 11, 1930 June 16, 1930 |
5 3 23 |
Still under treatment | Staphylococcus | |
| Case 12, J. I. | Right hip | 11 years | 1 year | Sept. 29, 1930 | 4 | Oct. 15, 1930 | Oct. 21, 1930 | 6 | Improved, still under treatment | Negative Blood agar and broth |
|
| Case 13, S. Z. | Right tibia | 13 years | 5 months | May 22, 1930 | 1 | May 28, 1930 | May 31, 1930 | 31 | May 18, 1931 | Staphylococcus | |
| Feb. 18, 1931 | Feb. 21, 1931 | 3 | AImost healed | ||||||||
| Case 14, M. S. | Right femur | 8 years | 1 month | June 30, 1930 | 0 | July 2, 1930 | July 5, 1930 | 6 | May 18, 1931 | Staphylococcus | |
| Aug. 13, 1930 | Aug. 16, 1930 | 1 | Almost healed | Streptococcus | |||||||
| Aug. 26, 1930 | |||||||||||
| Case 15, F. R. | With multiple abscesses | 14 years | 6 years | Nov. 4, 1929 | 2 | Nov. 6, 1929 | Nov. 8, 1930 | 2 | May 18, 1931 | Staphylococcus | |
| Mar. 27, 1930 | Mar. 31, 1930 | 12 | Much improved | ||||||||
| Sept. 24, 1930 | Sept. 29, 1930 | 10 | |||||||||
| Case 16, E. MacM. | Right femur | 4 years | 4 years | Sept. 15, 1930 | 2 | Sept. 17, 1930 | Sept. 20, 1930 | 18 | May 15, 1931 Healed |
Staphylococcus | |
| Case 17, E. S. | Right os calcis | 8 years | 5 months | Nov. 4, 1929 | 5 | Nov. 6, 1929 | Nov. 8, 1929 | 6 | Dec. 31, 1929 | Staphylococcus | |
| Left humerus | 4 months | 4 | Mar. 21, 1930 | Mar. 31, 1930 | 2 | May 23, 1930 | Staphylococcus | ||||
| Case 18, A. D. | Right humerus | 3 years | 7 months | July 2, 1929 | 7 | July 9, 1929 | July 13, 1929 | 11 | Nov. 20, 1929 | Staphylococcus | |
| 3 | Sept. 24, 1929 | Sept. 30, 1929 | 8 | ||||||||
| Case 19, W. C. | Right fibula | 10 years | 6 weeks | Aug. 29, 1929 | 2 | Oct. 15, 1929 | Nov. 19, 1929 | 6 | Feb. 1, 1930 | 8 months | Staphylococcus |
| Case 20, L. P. | Both tibiae | 3 years | 8 month. | July 12, 1929 | 2 on each leg | July 16, 1929 | July 19, 1929 | 13 | Left Nov. 20, 1929 | 10 months | Staphylococcus |
| Right Jan. 17, 1930 | 8 months | ||||||||||
| Case 21, A. L. | Right fibula | 11 years | 6½ months | Apr. 29, 1929 | 1 | May 3, 1929 | May 4, 1929 | 11 | Sept. 29, 1929 | 1 year | Staphylococcus |
| Case 22, O. M. O. | Left tibia | 8 years | 1 month | Aug. 22, 1929 | 1 | Sept. 13, 1929 | Sept. 15, 1929 | 12 | Nov. 8, 1929 | 10 months | Staphylococcus |
| Case 23, E. W. | Right tibia | 14 years | 4 months | Sept. 1, 1927 | 4 | May 14, 1929 | May 16, 1929 | 7 | Aug. 27, 1929 | 9 months | Staphylococcus |
| Jan. 20, 1930 | |||||||||||
| Case 24, H. S. | Left femur | 10 years | 6 years | May 10, 1930 | 5 | May 21, 1930 | May 26, 1930 | 7 | July 22, 1930 | 2 months | Staphylococcus |
| Case 25, R. Y. | Right femur | 11 years | 3 years | June 26, 1928 | 3 | Nov. 6, 1928 | Nov. 11, 1928 | 1 | Apr. 18, 1929 | 18 months | Staphylococcus |
| Case 26, R. P. | Right femur | 6 years | 6 month, | Jan. 4, 1927 | 4 | Sept. 18, 1928 | Sept. 23, 1928 | 7 | Nov. 23, 1928 | 19 months | Staphylococcus |
| Feb. 20, 1929 | |||||||||||
| Case 27, M. M. | Right tibia | 9 years | 10 months | May 21, 1929 | 1 | May 22, 1929 | June 18, 1929 | 15 | Improved | Staphylococcus | |
| May 19, 1930 | May 21, 1930 | May 23, 1930 | 2? | ||||||||
| Case 28, R. L. | Left radius | 11 years | 6 months | Feb. 24, 1930 | 1 | Feb. 26, 1930 | Mar. 4, 1930 | 9 | May 23, 1930 | 4 months | Staphylococcus |
| Case 29, R. H. | Left tibia | 4 years | 1 year | Sept. 4, 1929 | 1 | Sept. 6, 1929 | Sept. 8, 1929 | 9 | Dec. 15, 1929 | 9 months | Negative, Hemolyticus Streptococcus, Staphylococcus aureus |
| Case 30, J. S. | Left tibia | 14 years | 1 year | July 8, 1929 Apr. 28, 1930 |
2 5 |
July 9, 1929 May 7, 1930 |
July 13, 1929 May 12, 1930 |
16 11 |
Sept. 24, 1929 July 12, 1930 |
2 months | Staphylococcus |
| Case 31, F. K. | Right tibia | 11 years | 3 years | Oct. 22, 1929 | 0 | Nov. 6, 1929 | Nov. 9, 1929 | 4 | Mar. 1, 1930 | 6½ months | Non-hemolyticius streptococcus, Staphylococcus |
| Case 32, R. N. | Left ankle, tuberculosis | 45 years | 2 years | July 1, 1930 | 1 | July 2, 1930 | July 4, 1930 | 12 | Sept. 1, 1930 | 2 weeks | Negative |
| Case 33, M. B. | With multiple abscesses | 13 years | 5 months | Nov. 3, 1927 | 4 | Nov. 29, 1927 Jan. 16, 1928 Nov. 20, 1928 Nov. 13, 1929 Apr. 2, 1930 July 16, 1930 |
Nov. 17, 1930 Apr. 7, 1930 July 18, 1930 |
15 20 16 |
May 18, 1931 Left humerus healed Tibia healed Two sinuses, left femur |
Staphylococcus | |
| Case 34, J. F. | Tuberculosis, right elbow | 3 years | 1 year | Feb. 20, 1929 | 0 | Apr. 3, 1929 Dec. 18, 1929 Jan. 9, 1930 May 28, 1930 |
Jan. 12, 1930 May 31, 1930 |
3 3 |
Sept. 2, 1930 | 1 year, 8 months | Tuberculosis |
| Case 35, D. Z. | Left femur | 10 years | 2 years | July 9, 1930 | 7 | July 9, 1930 | July 12, 1930 | 9 | Aug. 28, 1930 | 2 months | Negative Blood agar and broth |
| Case 36, F. L. | Tuberculosis, left hip | 8 years | 5 years | Aug. 10, 1926 | 0 | Aug. 12, 1926 | May 7, 1930 | 11 | Healed | Staphylococcus | |
| Feb. 16, 1927 | |||||||||||
| Apr. 2, 1930 | |||||||||||
| Case 37, R. M. | Left ankle | 15 years | 3 weeks | Aug. 22, 1930 | 1 | Aug. 26, 1930 | Aug. 29, 1930 | 6 | Healed | Staphylococcus | |
| Case 38, H. Y. | Lower end right tibia | 14 years | 8 months | Nov. 14, 1930 | 0 | Nov. 15, 1930 | Nov. 19, 1930 | 12 | Feb. 1, 1931 | ||
| Case 39, J. H. | Left tibia | 12 years | 2 years | Nov. 18, 1930 | 2 | Nov. 26, 1930 | Dec. 16, 1930 | 21 | Jan. 15, 1931 | ||
| Case 40, C. B. | Right tibia | 5 years | 8 months | Dec. 5, 1930 | 1 | Dec. 10, 1930 | Dec. 16, 1930 | 7 | May 18, 1931 Healed |
||
| Case 41, J. N. | Left leg | 11 years | 2 years | Feb. 17, 1931 | 2 | Feb. 20, 1931 | Feb. 21, 1931 | 3 | |||
| Case 42, E. W. | Left leg | 14 years | 11 months | Dec. 2, 1930 | 2 | Dec. 3, 1930 | Dec. 17, 1930 | 20 | May 18, 1931 Practically healed |
||
| Case 43, F. N. | Right femur | 7 years | 5 months | Jan. 12, 1931 | 0 | Jan. 21, 1931 | Jan. 26, 1931 | 4 | Healed | ||
| Case 44, M. T. | Left tibia | 18 months | 1 year | Feb. 27, 1931 | 1 | Mar. 4, 1931 | Mar. 7, 1931 | ||||
| Case 45, R. S. | Comminuted fracture, right femur | 53 years | 4 years | Mar. 19, 1929 | 1 | May 20, 1929 | July 20, 1929 | 11 | Oct. 6, 1929 Healed |
Staphylococcus | |
| Case 46. L. H. | Right and left humerus Left femur |
48 years | 3 years | Dec. 26, 1929 | 13 | Jan. 2, 1930 | Jan. 6, 1930 | 17 | Improved | ||
| Case 47, L. R. | Tuberculosis, right ankle | 29 years | 1 year | Jan. 6, 1930 | 0 | Jan. 13, 1930 | Jan. 16, 1930 | 2 | Jan. 25, 1930 Died, tetanus |
||
| Case 48, B. B. | Compound fracture, left radius, ulna and left humerus | 43 years | 4 days | Feb. 22, 1930 | 0 | Feb. 27, 1930 | Mar. 4, 1930 | 6 | Healed | Staphylococcus | |
| Case 49, F. T. | Right tibia | 9 years | 6 months | Mar. 4, 1930 | 1 | Mar. 6, 1930 | Mar. 8, 1930 | 7 | Sept. 29, 1930 Wound still slightly draining |
Staphylococcus | |
| Case 50, R. E. | Right tibia Amputation |
38 years | 1 year | Dec. 3, 1929 | 3 | Dec. 5, 1929 | Dec. 13, 1930 | 8 | Jan. 25, 1930 Healed |
||
| Case 51, A. M. | Old compound fracture, left tibia and fibula | 53 years | 2 years | Jan. 5, 1930 | Jan. 6, 1930 | Jan. 8, 1930 | Feb. 24, 1930 Completely healed |
||||
| Case 52, P. H. | Right humerus | 19 years | 1 year | Jan. 7, 1930 | 1 | Jan. 9, 1930 | Jan. 11, 1930 | 3 | Feb. 8, 1930 Improved |
Staphylococcus | |
| Case 53, B. M. | Right tibia | 10 years | 6 years | May 10, 1930 | 1 | May 15, 1930 | May 16, 1930 | 3 | July 3, 1930 Improved |
||
| Case 54, C. T. | With fracture of femur | 40 years | 23 years | Oct. 26, 1930 | 5 | Nov. 6, 1930 | Nov. 10, 1930 | May 18, 1931 Much improved |
|||
| Case 55, K. T. | Left femur | 20 years | 9 years | Sept. 24, 1930 | 4 | Oct. 4, 1930 | Oct. 6, 1930 | 11 | Apr. 15, 1931 Healed |
Staphylococcus | |
| Case 56, W. W. | Fracture of right tibia and fibula | 33 years | 5 months | Oct. 29, 1930 | 1 | Nov. 3, 1930 | Nov. 7, 1930 | Nov. 14, 1930 Improved |
|||
| Case 57, B. G. | Left femur | 39 years | 9 years | Apr. 15, 1930 | 7 | Apr. 18, 1930 | 29 | Feb. 15, 1931 | |||
| Case 58, C. S. | Left humerus | 24 years | 7 years | Aug. 26, 1930 | 5 | Aug. 28, 1930 | 15 | Unimproved | |||
| Case 59, A. N. | Right femur | 22 years | 16 years | May 29, 1930 | Several | May 31, 1930 | 38 | Oct. 27, 1930 Improved |
|||
| Case 60, F. R. | Left tibia | 26 years | 13 years | Feb. 17, 1930 | 4 | Feb. 18, 1930 | Unimproved | ||||
| Sept. 22, 1930 | Sept. 23, 1930 | Amputation | |||||||||
| Case 61, P. P. | Right femur Right tibia |
24 years | 12 years | May 7, 1930 | 6 | May 9, 1930 | 26 | Oct. 25, 1930 Tibia improved Femur unimproved |
|||
| Case 62, J. R. | Right tibia | 16 years | 7 years | May 12, 1930 | 3 | May 13, 1930 | May 17, 1930 | 9 | July 18, 1930 Practically healed |
||
| Case 63, J. U. | Right tibia | 15 years | 2 years | June 11, 1930 | 2 | June 13, 1930 | 12 | Sept. 3, 1930 Practically healed |
|||
| Case 64, E. G. | Left sacroiliac articulation | 27 years | 11 years | July 2, 1930 | July 10, 1930 | 9 | Sept. 24, 1930 Healed |
||||
| Case 65, A. S. | Right tibia | 30 years | 6 years | July 24, 1930 | 5 | July 26, 1930 | Aug. 4, 1930 | 7 | Improved | ||
| Case 66, P. M. | Right femur | 68 years | 10 years | July 1, 1930 | 4 | July 10, 1930 | 18 | Unimproved | |||
| Case 67, L. P. | Right second finger | 33 years | 5 months | Mar. 27, 1930 | 3 | Mar. 28, 1930 | 3 | Apr. 10, 1930 Unimproved |
|||
| Case 68, W. K. | Right femur | 18 years | 6 years | July 11, 1929 | 2 | July 13, 1929 | July 16, 1929 | 14 | Improved | ||
| Case 69, E. B. | Right femur | 21 years | 4 years | May 8, 1930 | 1 | May 13, 1930 | 28 | Unimproved | |||
| Right ulna | Aug. 21, 1930 | ||||||||||
| Oct. 28, 1930 | |||||||||||
| Case 70, R. R. | Left femur | 35 years | 10 years | Oct. 27, 1930 | 3 | Oct. 28, 1930 | 12 | Jan. 10, 1931 Improved |
|||
| Case 71, J. L. | Left femur | 18 years | 4 years | Sept. 23, 1930 | 4 | Sept. 26, 1930 | 18 | Mar. 10, 1931 Improved |
|||
| Case 72, J. O. | Left tibia | 15 years | 4 years | Oct. 1, 1930 | 9 | Oct. 3, 1930 | 9 | Improved | |||
| Case 73, C. B. | Right tibia | 17 years | 2 months | Aug. 22, 1929 | 1 | Aug. 23, 1929 | Aug. 26, 1929 | 7 | Aug. 1, 1930 Healed |
||
| Case 74, H. S. | Acute Left tibia |
16 years | Not known | Nov. 10, 1929 | 0 | Oct. 12, 1929 | 2 | Improved | |||
| Case 75, J. P. | Tuberculosis, left foot | 24 years | 7 years | Oct. 2, 1929 | 0 | Oct. 3, 1929 | 9 | Unimproved | |||
| Case 76, D. D. | Left tibia | 35 years | 11 years | Oct. 28, 1929 | 24 | Oct. 29, 1929 | 16 | Jan. 10, 1930 Improved |
|||
| Case 77, B. O. | Right tibia | 24 years | 3 years | June 28, 1929 | 1 | June 30, 1929 | Aug. 9, 1929 | 10 | Aug. 11, 1929 Improved |
||
| Case 78, H. B. | With compound fracture of right femur | 17 years | 6 weeks | Feb. 1, 1929 | 1 | Feb. 4, 1929 | 16 | Dec. 6, 1929 Improved |
|||
| Case 79, J. E. | Right tibia | 5 years | Dec. 30, 1930 | Dec. 31, 1930 | Mar. 15, 1931 Almost healed |
||||||
| Case 80, M. N. | Left femur | 17 years | 11 years | Sept. 9, 1929 | 2 | Sept. 11, 1929 | 5 | Nov. 23, 1929 Almost healed |
|||
| Case 81, J. W. | Acute, with compound fracture of left tibia | 49 years | June 20, 1929 | June 29, 1929 | 7 | Feb. 20, 1930 Improved |
|||||
| Case 82, T. B. | Left tibia | 52 years | 3 years | Nov. 18, 1930 | 3 | Nov. 20, 1930 | 12 | Mar. 18, 1931 Healed |
|||
| Case 83, A. C. | Multiple tuberculous osteitis with amyloidosis | 14 years | 2 years | Aug. 27, 1930 | Aug. 29, 1930 | 12 | Apr. 17, 1931 Unimproved |
||||
| Case 84, L. B. | Left femur | 38 years | 12 years | Nov. 14, 1930 | Nov. 16, 1930 | 12 | Feb. 14, 1931 Improved |
||||
| Case 85. V. McC. | Left fibula | 22 years | 12 years | Nov. 14, 1930 | Nov. 16, 1930 | 12 | Jan. 10, 1931 Improved |
||||
| Case 86, M. G. | Left tibia | 15 years | 2 years | June 12, 1930 | 1 | June 14, 1930 | 10 | Aug. 8, 1930 Almost healed |
|||
| Case 87. J. D. | Left femur | 31 years | 2 years | June 25, 1930 | 2 | June 26, 1930 | 7 | Jan. 1, 1931 Almost healed |
|||
| Case 88. C. B. | Left femur | 48 years | 2 years | Nov. 19, 1929 | 5 | Nov. 20, 1929 | 6 | Dec. 12, 1929 Improved |
|||
| Case 89. G. L. | Left femur | 21 years | 2 years | May 28, 1930 | May 29, 1930 | 12 | Aug. 24, 1930 Improved |
* These are all cases of osteomyelitis unless otherwise stated.
While one recognizes, in the treatment of acute osteomyelitis, that an immediate operation on the infected part, with a secondary operation for removal of sequestra, does often result in the cure of the patient, the time taken for this procedure is much longer than it is when maggots are used.
The time has been too short yet to make any definite assertions in regard to tuberculous lesions of bone or joints, or tuberculous abscesses. In cases of tuberculosis of the spine and tuberculosis of the hip, where abscesses have occurred, and where the abscess is purely tuberculous with a lining of tuberculous membrane, those abscesses may be opened with impunity, the tuberculous material evacuated, and maggots inserted according to the lines which have been previously laid down. The tuberculous abscess will heal, and stay healed, if proper conservative treatment is used at the same time for the treatment of the Joint from which this abscess starts. We are in the habit of treating all mixed infections of the hip Joint, which have a tuberculous origin and which have become secondarily infected, by means of maggots, and as yet we have not failed to clear up the secondary infection when the seat of trouble can be directly attacked. In cases of tuberculosis of the hip which have become secondarily infected, owing to the formation of an abscess, the following method is used: An incision is made along the crest of the ilium and down between the tensor femoris muscle and the sartorius (Smith-Petersen), and the hip Joint laid widely open. Attempt is then made to remove the infected area, whether it be in the acetabular cavity or from the head of the femur itself. As much of the infected tissue as possible is removed by operative procedure. The maggots are then introduced into the hip Joint in the same manner as already described. These wounds, with a few exceptions, have healed so far as their secondary infection is concerned. It is too early to State, however, just what the ultimate results will be, because one knows the tenacity with which tuberculous material hangs on to the human System.
Conclusions
Maggots have been found to be a tremendously useful adjunct to thorough surgical treatment of chronic osteomyelitis, and, in our opinion, are far more successful in securing permanent healing of these extensive wounds than any other method tried by us.
Maggots, by their digestive action, clear away the minute fragments of bone and tissue sloughs caused by operative trauma in a way not accomplished by any other means. This is a tremendously valuable asset in the healing of a wound.
Maggots cause wounds to become alkaline and in this way diminish growth of pathogenic bacteria.
Maggots seem to have other more subtle biochemical effects within the wound itself and perhaps cause also a constitutional reaction inimical to bacterial growth. This is under investigation.
Maggots as raised and sterilized in the manner described may be used in any wound without risk to the patient.
The post-traumatic or postoperative general condition of the patient is better in maggot treatment than in the older forms of treatment where infection was combatted by chemicals or other types of dressing. There is less absorption and less toxic reaction.
In open tuberculous abscesses, with or without secondary infection, wide exposure followed by maggot treatment has proved surprisingly effective in a small number of cases and will be given further trial.
Note: It is evident that even in ancient times many surgeons had observed the effect of maggots on wounds, but it has remained for Dr. Baer to develop this idea in accordance with scientific medicine and surgery. The following article by Dr. Goldstein gives an interesting historical review of the subject of these observations referred to by Dr. Baer.—Editor.
Footnotes
D. J. Larrey, the famous military surgeon of Napoleon’s armies, observes as follows in his Memoirs of Military Surgery (Translation by R. W. Hall, I, 177, 1814): “During the progress of suppuration, the patients were only troubled by worms or larvae of the blue flies common in Syria. The hatching of the eggs, which these flies constantly deposit in the wounds or dressings, was assisted by the heat of the weather and by the quality of the dressings, which were of cotton, which alone could be procured in this country. The presence of these insects in the wounds, appeared to accelerate their suppuration; but they caused a disagreeable pruritis, and obliged us to dress them three or four times a day. They are produced in a few hours, and increase with such rapidity, that in the course of a night they grow to the size of the barrel of a small quill. It is necessary, at each dressing, to use lotions of a strong decoction of rue with a small portion of sage, which destroys them; but they were soon reproduced for want of proper means to prevent the approach of the flies and to destroy their eggs. Although these insects were troublesome, they expedited the healing of the wounds by shortening the work of nature, and causing the sloughs to fall off.”
Larrey again mentions maggots, commenting as follows:—“In spite of the importunities of these insects they have accelerated the cicatrization of the wounds by abbreviating the work of nature and in provoking the destruction of scar tissue, which they destroy”.
J. G. Millingen in his Curiosities of Medical Experience, American Edition, 1838, p. 171, says,—“During the retreat of our troops after the battle of Talavera (1809) I found the wounds of many of our men, that had not been dressed for three or four days, pullulating with maggots. This was not the case with the Spanish soldiers, who, to prevent this annoyance (which was more terrific than dangerous) had poured olive oil upon their dressings. I invariably resorted to the same practice when I subsequently had to remove the wounded in hot weather.”
W. W. Keen says, “During the Civil War maggots were very common in summer— the resulting maggots were certainly disgusting but so far as I ever observed they did no harm.”
Most interesting of all is a quotation from J. F. Zacharias of Cumberland, Md. who, as a surgeon in the Confederate Army, wrote as follows of his Civil War experiences:— “During my Service in the hospital at Danville, Virginia, I first used maggots to remove the decayed tissue in hospital gangrene and with eminent satisfaction. In a single day they would clean a wound much better than any agents we had at our command. I used them afterwards at various places. I am sure I saved many lives by their use, escaped septicaemia, and had rapid recoveries.”
Similar clinical observations were made by others in the World War, but no further investigation of the phenomenon was attempted by any one during the ten years following.
Read by Dr. Baer at the Annual Meeting of the American Orthopaedic Association, Chatham, Massachusetts, June 21, 1930. The final arrangement of this paper for publication has been completed since the death of Dr. Baer by his associate, Dr. George E. Bennett.
Richard A. Brand MD (✉) Clinical Orthopaedics and Related Research, 1600 Spruce Street, Philadelphia, PA 19103, USA e-mail: dick.brand@clinorthop.org