Abstract
Background
Arthroscopically assisted anterior cruciate ligament reconstruction using a bioabsorbable tibial fixation screw is occasionally complicated by pretibial cyst formation. The few case reports describing pretibial cyst formation noted several graft types and fixation techniques, making it difficult to establish one etiology. Some literature suggests cysts form from communication between the joint and pretibial area leading to extravasation of joint fluid, maturing into a cyst. We propose the development of cysts after PLLA screw use may be related to a foreign body reaction.
Questions/purposes
We propose this foreign body reaction (1) relates to the biochemical breakdown of bioabsorbable materials; and (2) differs from cystic formations resulting from joint communication.
Methods
We retrospectively reviewed seven patients who developed pretibial cysts at least 2 years after original primary ACL reconstruction surgery. MRI was used to visualize the extent of cystic formation. Cysts were treated by débridement with specimens sent for histologic analysis. Cyst appearance had a 3-year incidence of 5%.
Results
No cyst had an infectious etiology. In all cases, the tibial screw outline was present on MRI, although intraoperatively, the screw was substantially decomposed. Grafts were well incorporated and none of the knees demonstrated anterior laxity. Histologically, cyst material contained fragments of PLLA surrounded by foamy histiocytes, suggesting a foreign body reaction. No cysts recurred.
Conclusions
Tibial cysts occur in a subset of patients undergoing ACL reconstruction using a bioabsorbable PLLA interference screw. We suspect they arise from a foreign body response to the screw breakdown. Removal is well tolerated.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Over the last few decades, arthroscopically assisted ACL reconstruction has become a widespread operation resulting in pain relief and improved knee stability and ROM. The procedure is, nevertheless, fraught with potential complications, including, rarely, pretibial cyst formation. Case reports have mentioned cyst formation in the tibial tunnel after ACL reconstruction [4–6, 8, 9, 13–15]. These reports suggest several different graft types and fixation techniques preceding cyst appearance, making it difficult to establish one specific etiology for the emergence of the cyst. Several studies [14–16] propose that cysts form as a result of communication between the joint and pretibial area that leads to extravasation of joint fluid, which then matures into a cyst. Another study has suggested bioabsorbable screw breakdown incites an inflammatory reaction [15]. We posit that the development of cysts may be related to a foreign body reaction based on clinical presentation, radiographic and intraoperative evaluation, and histologic analysis of specimens.
Specifically, we propose that this foreign body reaction (1) relates to the biochemical breakdown of bioabsorbable materials; and (2) differs from cystic formations as a result of joint communication.
Patients and Methods
We retrospectively reviewed seven patients (mean age, 39 years; range, 22–57 years) (Table 1) diagnosed with pretibial cyst formation after arthroscopically assisted ACL reconstruction with a hamstring autograft or allograft. During the time period between the initial reconstruction surgeries (arbitrarily set between January 1, 2003, and December 31, 2006) and the appearance of all the cysts, one author (WNL) identified the development of seven cysts (5.0%) out of 140 primary arthroscopically assisted ACL reconstructions. Of the seven patients who developed cysts, four reconstructions had been completed with allograft tissue and three were completed with hamstring autografts (Table 1). Of the 140 patients, 21 (15%) had received allograft and 119 (85%) had received autografts with an incidence of cyst development in each group of 19.0% and 2.5%, respectively. Allografts were subjected to low-dose gamma irradiation and antibiotic solution soaking according to the manufacturers’ sterilization techniques (Osteotech, Eatontown, NJ; Musculoskeletal Transplant Foundation, Edison, NJ). All grafts had been secured in the tibia with an Arthrex Cannulated Delta Tapered Biointerference Screw (Arthrex Inc, Naples, FL) composed of 100% noncrystalline poly-L-lactic acid (PLLA) with no intra-articular penetration as documented during followup arthroscopy. The minimum followup after cyst débridement was 5 months (mean, 6 months; range, 5−6 months) (Table 2). No patients were lost to followup.
Table 1.
Pretibial cyst case series
| Patient number | Age (years) | Previous knee surgery? | Type of reconstruction | Femoral fixation | Graft | Graft size (mm) | Bioabsorbable (PLLA) screw size (mm) | Tibial tunnel size (mm) | Years before cyst emergence | Symptoms? | Size of cyst |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | N | BioTransFix (Arthrex Inc, Naples, FL) | TransFix 5-mm PLLA cross pin | Allograft | 8 × 110 | 10 × 35 | 8 | 2 | Mass | 5 cm |
| 2 | 48 | N | BioTransFix (Arthrex Inc) | TransFix 5-mm PLLA cross pin | Allograft | 10 × 130 | 12 × 35 | 10 | 2 | Mass tenderness | 4 cm |
| 3 | 57 | N | BioTransFix (Arthrex Inc) | TransFix 5-mm PLLA cross pin | Allograft | 9 × 130 | 11 × 35 | 9 | 2.5 | Mass tenderness | 5 cm |
| 4 | 22 | N | BioTransFix (Arthrex Inc) | TransFix 5-mm PLLA cross pin | Hamstring autograft | 7 × 180 | 10 × 35 | 7 | 3 | Mass | 5 cm |
| 5 | 26 | N | BioTransFix (Arthrex Inc) | TransFix 5-mm PLLA cross pin | Hamstring autograft | 9 × 130 | 10 × 35 | 9 | 3 | Drainage, no erythema | Draining cyst |
| 6 | 51 | N | BioTransFix (Arthrex Inc) | TransFix 5-mm PLLA cross pin | Allograft | 9 × 140 | 11 × 35 | 9 | > 2 | Mass | 2 cm |
| 7 | 23 | N | BioTransFix (Arthrex Inc) | TransFix 5-mm PLLA cross pin | Hamstring autograft | 8 × 140 | 10 × 35 | 8 | 2.5 | Mass, no erythema, nontender | 5 cm |
PLLA = L-poly-L-lactide; N = no.
Table 2.
Pretibial cyst case series
| Patient number | Age (years) | Concurrent pathology at the time of initial surgery and management | Prior treatment before excision | Concurrent pathology at time of cyst removal | ACL intact in notch and tibial tunnel? | MRI | Pathology | Cultures | Followup |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 48 | None | Observation | None | Y | Multiloculated cyst tracking from the tibial screw into the pretibial soft tissues | Multiloculated cyst set in aponeurotic tissue and adipose tissue with strongly birefringent PLLA material; cystic cavities borne out of degenerated, lyzed collagen in the midst of particles of PLLA; many foci of collagen degeneration attended by foamy histiocytes | Y | 5 months |
| 2 | 48 | Lateral meniscus tear—débrided; medial meniscus tear—repaired | Observation | Lateral meniscus tear; LFC/patellar chondromalacia | Y | Osteolysis surrounding the tibial screw; edema surrounding screw | Cystic cavities of degenerated, lyzed collage with particles of PLLA; foci of collagen degeneration attended by foamy histiocytes | Y | 5 months |
| 3 | 57 | None | Observation | None | Y | Fluid tracking down the tibial tunnel | Fibrous and fibroadipose tissue; lymphocytic palisading; inflammation around cystic space; foreign body giant cell reaction around refractile, nonbirefringent and rare birefringent material and focal acute inflammation within the fibrotic wall | Y | 5 months |
| 4 | 22 | None | Aspiration and antibiotics | None | Y | Large joint effusion with fluid tracking down the tibial tunnel and extending into the soft tissues anterior to the tibia | Foreign body reaction around birefringent material; focal acute inflammation within the fibrotic wall | Y | 6 months |
| 5 | 26 | None | Antibiotics and observation | None | Y | Fluid tracking down the tibial tunnel | Birefringent foreign material in bone; granulation tissue, necrosis, acute and chronic inflammatory infiltration and fibrosis | Y | 6 months |
| 6 | 51 | Lateral meniscus tear—débrided | Observation | Lateral meniscus tear; Baker’s cyst | Y | Bone marrow edema; transcortical tibial cystic lesion | Focal perivascular small lymphoid aggregates; fibrohistiocytic palisading pseudogranulomatous inflammation around necrotic tissue and debris; small birefringent particles; no acute inflammation (ie, infection) | Y | 5 months |
| 7 | 23 | None | Observation | None | Y | Anteromedial cyst communicating with joint | Foreign body reaction around birefringent material and focal acute inflammation | Y | 5 months |
LFC = lateral femoral condyle; Y = yes; PLLA = L-poly-L-lactide.
Results
Patients presented after the emergence of an enlarging, anteromedial, often painless (five patients without pain, two patients with tenderness) mass near the pretibial tunnel entrance 2 to 3 years after reconstruction (Table 1). The size of the mass varied from 2 to 5 cm. Patients denied symptoms of infection such as fevers, chills, or local erythema. Physical examination was notable for soft tissue fullness over the pretibial area, no erythema, and tenderness in two patients. Laboratory tests, including complete blood cell count, erythrocyte sedimentation rate, and C-reactive protein, were normal in all patients.
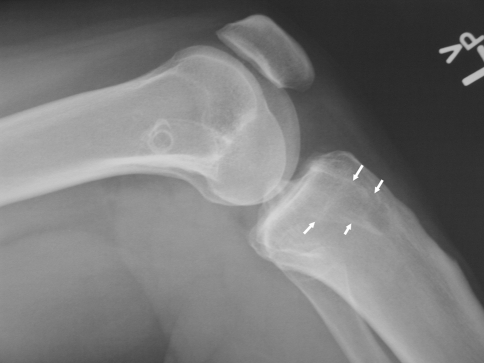
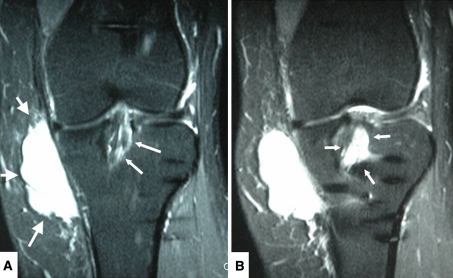
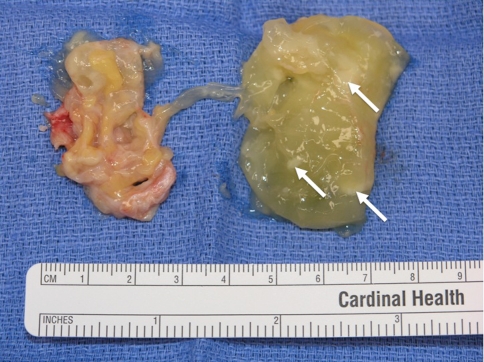
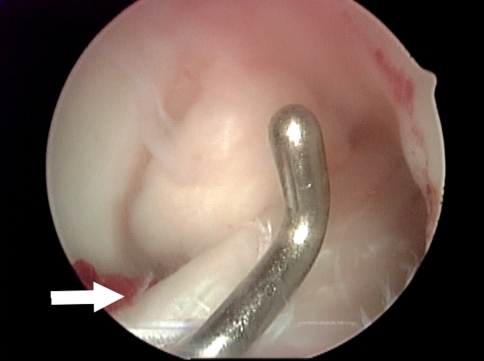
Radiographs demonstrated tibial tunnel widening (Fig. 1), whereas MRI showed multiloculated cyst tracking from the tibial screw into the soft tissues and fluid within the tunnel (Fig. 2A−B). Incisions were made over the tibial tunnel, yellowish gelatinous matter was expressed and, macroscopically, the absorbable screw was in its resorptive phase interlaced with cyst material (Fig. 3). Diagnostic knee arthroscopy revealed intact ACL grafts (Fig. 4). The femoral tunnel did not look excessively widened.
Fig. 1.
Lateral radiograph of a left knee 3 years after arthroscopically assisted ACL reconstruction with an allograft using a delta taper tibial interference screw composed of L-poly-L-lactide demonstrates tibial tunnel widening (white arrows).
Fig. 2A−B.
MRI coronal T2-weighted images of a left knee 3 years after arthroscopically assisted ACL reconstruction with allograft using a delta taper tibial interference screw composed of L-poly-L-lactide demonstrates a growing pretibial nontender mass initially not fluctuant but becoming fluctuant as it became larger. (A) Large white arrows point to the pretibial cyst. Small white arrow points to edema in the tibial tunnel surrounding the ACL graft. (B) Small white arrows point to fluid within the tibial tunnel.
Fig. 3.
Intraoperative picture demonstrating gelatinous material removed from a pretibial cyst and from a tibial tunnel. Pieces of screw debris are visible (arrows) interlaced with the cyst material.
Fig. 4.
Intraoperative arthroscopic image demonstrating an intact, well-fixed ACL allograft despite the presence of fluid in tibial tunnel and pretibial cyst. White arrow points to tibial insertion of the ACL graft.
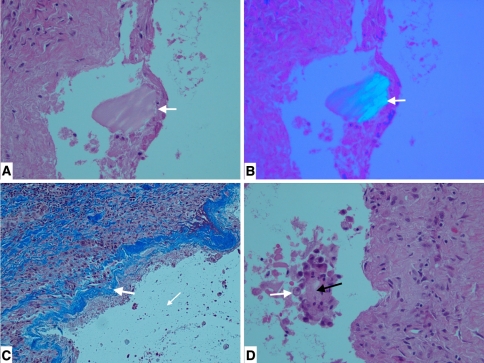
Histology (Table 2) showed multiloculated cysts in aponeurotic tissue and adipose tissue with histiocytic infiltrate around the PLLA debris with surrounding acellular cystic material. The debris appeared embedded in fragmented collagen surrounded by macrophages and was birefringent under polarized light, signifying the presence of fragments of the PLLA screw (Fig. 5A−B). Residual layers of collagen still attached to the lining with foci of collagen degeneration surrounded by foamy histiocytes (Fig. 5C−D) suggested the cysts were borne of fragmented collagen in the midst of particles of PLLA. On average, cysts have not recurred for at least 5 months after débridement.
Fig. 5A−D.
(A) Histologic section of cyst material with white arrow pointing to PLLA particle embedded in necrotic collagen and surrounded by macrophages, suggestive of foreign body reaction (Stain, hematoxylin and eosin, original magnification ×200). (B) PLLA particle (arrow) demonstrates strong birefringence under polarized light (Stain, hematoxylin and eosin, original magnification ×200). (C) Histologic section showing histiocytic infiltrate around debris with residual layers of collagen (blue, arrow) in the lining (Stain, trichrome stain, original magnification ×100). (D) White arrow points to foamy histiocytes adjacent to necrotic fibrous tissue (black arrow) (Stain, hematoxylin and eosin, original magnification ×200).
Discussion
We describe seven cases of pretibial cyst formation after use of a bioabsorbable interference screw composed of PLLA and propose how this foreign body reaction (1) relates to the biochemical breakdown of bioabsorbable materials; and (2) differs from cystic formations resulting from joint communication.
There are limitations to this study. First, the graft type used was not uniform and we solely used hamstring grafts. The use of allograft or autograft or other graft sources such as bone-patellar tendon-bone may influence cyst development. The number of cysts we saw was too small to draw definitive conclusions about these relationships. Second, we did not obtain 2- to 3-year followup on all 140 patients nor after the initial cyst removal. Some cysts may have occurred in patients who were lost to followup. Our results therefore represent the minimum possible cyst incidence in our cohort. As of January 2010, no patients have returned with development or redevelopment of cysts. Third, although we believe these cysts are caused by a foreign body reaction incited by bioabsorbable screw fragmented particles, one cannot infer a causal relationship given the small number of patients, use of only light microscopy, and lack of biochemical analysis.
Pretibial cysts are a known complication of arthroscopically assisted ACL reconstruction, reported to occur 3 to 4 years postoperatively [14], but their mechanism for development remains unclear. Proposed etiologies often invoke extra-articular leakage of joint fluid through the tibial tunnel [16, 17] caused by graft-tunnel diameter mismatch [14], eccentric placement of graft in the bone tunnel [17], intraosseous graft necrosis [16, 18], breakdown of bioabsorbable interference screw [15], and micromotion of graft leading to tunnel expansion [14–16]. One study proposed that bioabsorbable screw breakdown incites a foreign body reaction [15]. Our study, at least histiologically, supports this notion.
Several bioabsorbable screw materials exist, including polyglyconate (PGA) [1], poly-D-L-lactide (PDLLA), poly-lactic-co-glycolic-acid (PLGA), and composites of PLLA. These bioabsorbable materials pass through five stages of degradation: hydration, depolymerization, loss of mass integrity, absorption, and elimination [12]. During degradation, once the screw has been hydrolyzed, it fragments. These particles then undergo phagocytosis by macrophages. Notably, hydrolysis itself may release acid products harmful to surrounding tissue. Material composition of the screw therefore likely has an effect on degradation rate and absorption and possibly cyst development [17]. Indeed, factors such as polymer crystallinity, isomeric composition, and degradation rate reportedly affect cyst formation [18].
Although PGA and PLLA have been used with good clinical results [10], they have different degradation characteristics. Earlier orthopaedic implants made of PGA placed in high mechanical stress areas rapidly degraded [18] and in some cases lead to the creation of sterile cysts in the implant-adjacent bone [18]. Polylactic acid implants with greater strength and slower degradation were developed to mitigate this clinical problem [18]. Similarly, device design has integrated varying levels of crystallinity, promoted d-isomer structures as well as incorporated phosphate buffers to ensure more gradual degradation and slower release of acid byproducts. More recently, calcium phosphate has been incorporated into screw design to improve biocompatibility and encourage new bone formation [7]. PLLA is purported to have slower rates of absorption and fewer complications than PGA. The theoretical advantage of slower absorption is diminished inflammation, which may curtail cyst formation [17]. Bostman reported a 5.3% incidence of aseptic foreign body reaction after PGA device implantation versus 0.2% rate with PLLA [3]. Studies in rabbit femurs failed to reveal widespread inflammatory reactions to PLLA implants [11]. Slowly degrading bioabsorbable composites, however, may decompose into smaller fractions that may incite delayed nonspecific foreign body reactions. Bostman described biopsy specimens from PGA screw implant inflammatory tissue showing a nonspecific foreign body reaction composed of neutrophilic polymorphonuclear leukocytes and giant cells phagocytizing polymer debris from decomposing implants [2]. Although the study looked at PGA screws, it is reasonable to infer that PLLA particles exert a similar effect. In our study, histologic analysis showed a foreign body reaction of foamy histiocytes surrounding PLLA debris and fragmented collagen. Our results support the findings of Tsuda et al. [15], whose histologic sections showed foreign body reaction to the degrading screw involving crystalline fractions of screw material with aggregated histiocytes.
Several authors advanced the idea that cysts may be related to pretibial communication with the joint before or after screw breakdown [15]. However, all fluid collections after screw implantation do not mature into cysts. Small fluid collections in the osseous tunnels commonly occur after ACL reconstruction. Sanders reported such collections resolved in seven of eight patients by 18 months postoperatively with no cyst formation in any patients [13]. Also, many screws are cannulated, so communication between the joint and pretibial area likely exists in all patients for at least several months to years afterward. Nevertheless, the majority of patients do not develop cysts.
In our cohort, the incidence of cyst formation was 5% occurring 2 to 3 years after reconstruction. The pattern of cyst appearance suggests it arose neither as a consequence of preexisting communication between the joint and pretibial area (leading to earlier cyst formation) nor from micromotion forming a larger tunnel (leading to more gradual, progressive cyst emergence). Furthermore, arthroscopically, we noted no cysts at the upper end of the tibial tunnel, and tendon probing revealed no cystic fluid. To our knowledge, no cysts recurred once the screw material was removed despite the existence of communication between the joint and pretibial area. The presence of screw material, therefore, had to play some part in cyst formation.
Given the number of ACL reconstructions performed with bioabsorbable screws and the infrequency of symptomatic cysts, there may be something particular about patients who develop them. Although PLLA appears well tolerated with little inflammatory response both experimentally and clinically, these patients may have greater sensitivity to it. Alternatively, they may have greater sensitivity to foreign body particles of a certain size. The factors that predispose to development of a sterile inflammatory reaction remain unclear. Future research should continue to delineate effects of biomaterials on screw degradation rates and cyst formation. Pretibial cysts should be considered a possible complication of ACL reconstruction surgery when tibial fixation is planned with bioabsorbable screws. Cyst and screw fragment removal is well tolerated and relieves symptoms.
Acknowledgment
We thank Ms Michele Roberts for her assistance in the handling of the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved or waived approval for the reporting of this case and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Center for Shoulder, Elbow and Sports Medicine, New York, NY, USA.
References
- 1.Benedetto KP, Fellinger M, Lim TE, Passler JM, Schoen JL, Willems WJ. A new bioabsorbable interference screw: preliminary results of a prospective, multicenter, randomized clinical trial. Arthroscopy. 2000;16:41–48. doi: 10.1016/S0749-8063(00)90126-9. [DOI] [PubMed] [Google Scholar]
- 2.Bostman OM. Intense granulomatous inflammatory lesions associated with absorbable internal fixation devices made of polyglycolide in ankle fractures. Clin Orthop Relat Res. 1992;278:193–199. [PubMed] [Google Scholar]
- 3.Bostman OM, Pihlajamaki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000;371:216–227. doi: 10.1097/00003086-200002000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Deie M, Sumen Y, Ochi M, Murakami Y, Fujimoto E, Ikuta Y. Pretibial cyst formation after anterior cruciate ligament reconstruction using auto hamstring grafts: two case reports in a prospective study of 89 cases. Magn Reson Imaging. 2000;18:973–977. doi: 10.1016/S0730-725X(00)00207-1. [DOI] [PubMed] [Google Scholar]
- 5.Feldmann DD, Fanelli GC. Development of a synovial cyst following anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:200–202. doi: 10.1053/jars.2001.8019. [DOI] [PubMed] [Google Scholar]
- 6.Ilahi OA, Younas SA, Sahni IK. Pretibial cyst formation after arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:E5. doi: 10.1053/jars.2003.50045. [DOI] [PubMed] [Google Scholar]
- 7.Lind M, Feller J, Webster KE. Tibial bone tunnel widening is reduced by polylactate/hydroxyapatite interference screws compared to metal screws after ACL reconstruction with hamstring grafts. Knee. 2009;16:447–451. doi: 10.1016/j.knee.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Malhan K, Kumar A, Rees D. Tibial cyst formation after anterior cruciate ligament reconstruction using a new bioabsorbable screw. Knee. 2002;9:73–75. doi: 10.1016/S0968-0160(01)00109-0. [DOI] [PubMed] [Google Scholar]
- 9.Martinek V, Friederich NF. Tibial and pretibial cyst formation after anterior cruciate ligament reconstruction with bioabsorbable interference screw fixation. Arthroscopy. 1999;15:317–320. doi: 10.1016/S0749-8063(99)70042-3. [DOI] [PubMed] [Google Scholar]
- 10.McGuire DA, Barber FA, Elrod BF, Paulos LE. Bioabsorbable interference screws for graft fixation in anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:463–473. doi: 10.1053/ar.1999.v15.015046001. [DOI] [PubMed] [Google Scholar]
- 11.Pihlajamaki H, Bostman O, Tynninen O, Laitinen O. Long-term tissue response to bioabsorbable poly-l-lactide and metallic screws: an experimental study. Bone. 2006;39:932–937. doi: 10.1016/j.bone.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Radford MJ, Noakes J, Read J, Wood DG. The natural history of a bioabsorbable interference screw used for anterior cruciate ligament reconstruction with a 4-strand hamstring technique. Arthroscopy. 2005;21:707–710. doi: 10.1016/j.arthro.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Sanders TG, Tall MA, Mulloy JP, Leis HT. Fluid collections in the osseous tunnel during the first year after anterior cruciate ligament repair using an autologous hamstring graft: natural history and clinical correlation. J Comput Assist Tomogr. 2002;26:617–621. doi: 10.1097/00004728-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Simonian PT, Wickiewicz TL, O’Brien SJ, Dines JS, Schatz JA, Warren RF. Pretibial cyst formation after anterior cruciate ligament surgery with soft tissue autografts. Arthroscopy. 1998;14:215–220. doi: 10.1016/S0749-8063(98)70044-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda E, Ishibashi Y, Tazawa K, Sato H, Kusumi T, Toh S. Pretibial cyst formation after anterior cruciate ligament reconstruction with a hamstring tendon autograft. Arthroscopy. 2006;22:691.e1−6. [DOI] [PubMed]
- 16.Victoroff BN, Paulos L, Beck C, Goodfellow DB. Subcutaneous pretibial cyst formation associated with anterior cruciate ligament allografts: a report of four cases and literature review. Arthroscopy. 1995;11:486–494. doi: 10.1016/0749-8063(95)90206-6. [DOI] [PubMed] [Google Scholar]
- 17.Weiler A, Hoffmann RF, Stahelin AC, Helling HJ, Sudkamp NP. Biodegradable implants in sports medicine: the biological base. Arthroscopy. 2000;16:305–321. doi: 10.1016/S0749-8063(00)90055-0. [DOI] [PubMed] [Google Scholar]
- 18.Williams RJ. Controversies in Knee Surgery. New York, NY: Oxford University Press, Inc; 2004. [Google Scholar]