Abstract
Background
Articulating spacers used in two-stage revision surgery of infected prostheses have the potential to abrade and subsequently induce third-body wear of the new prosthesis.
Questions/purposes
We asked whether particulate material abraded from spacers could be detected in the synovial membrane 6 weeks after implantation when the spacers were removed for the second stage of the revision.
Patients and Methods
Sixteen hip spacers (cemented prosthesis stem articulating with a cement cup) and four knee spacers (customized mobile cement spacers) were explanted 6 weeks after implantation and the synovial membranes were removed at the same time. The membranes were examined by xray fluorescence spectroscopy, xray diffraction for the presence of abraded particles originating from the spacer material, and analyzed in a semiquantitative manner by inductively coupled plasma mass spectrometry. Histologic analyses also were performed.
Results
We found zirconium dioxide in substantial amounts in all samples, and in the specimens of the hip synovial lining, we detected particles that originated from the metal heads of the spacers. Histologically, zirconium oxide particles were seen in the synovial membrane of every spacer and bone cement particles in one knee and two hip spacers.
Conclusions
The observations suggest cement spacers do abrade within 6 weeks. Given the presence of abrasion debris, we recommend total synovectomy and extensive lavage during the second-stage reimplantation surgery to minimize the number of abraded particles and any retained bacteria.
Introduction
Periprosthetic infections occur in less than 1% of patients but nevertheless are a serious complication of hip and knee arthroplasties [12, 15, 26, 36, 37]. When infections occur within 4 weeks of implantation, the implant can be left in place with a high probability of implant survival of between 80% and 100%, whereas late infections generally require prosthesis revision to control the infection [7, 22, 44, 45].
Many orthopaedic surgeons remove the infected prosthesis, débride the area, and then perform a two-stage revision, which involves implantation of an antibiotic-loaded cement spacer for 6 to 12 weeks [4, 9, 11, 16, 24, 34, 38]. Survival rates between 90% and 100% are reported for infected hip arthroplasties [11, 14, 15, 33], and between 91% and 96% for infected knee arthroplasties [18, 24, 35]. The function of the spacer is to release the antibiotic into the infected bed of the prosthesis, minimize soft tissue contractures, and maintain reasonable functionality until a prosthesis can be reimplanted [4]. There are several different types of spacers: monoblock and two-part spacers, commercially available and customized spacers made in the operating theater, and for the knee, static and mobile spacers. The advantage of the mobile, two-part spacer is related to the maintenance of function, and in comparison to static spacers, they reportedly result in a lower degree of scarring and facilitate reimplantation of the new prosthesis [10, 13, 17, 19, 24, 35]. In addition, the loss of bone is less with mobile spacers than with static spacers [5, 10]. A potential disadvantage of a mobile spacer used for hip and knee arthroplasties is the development of abraded particles. Cement particles in the joint can embed in bearing surfaces and create scratches with the risk of wear and decreased survival of the implant [6, 28, 32]. Of these cement particles, the radiopaque agent zirconium dioxide seems to be the most important because of its high hardness according to the Mohs’ scale [27]. Villa and Carnelli [43] found material abraded from mobile knee spacers in vitro and Affatato et al. [1] found the same for hip spacers. Although no in vivo studies exist in the literature verifying these laboratory findings, we presume abrasion of spacers would occur in vivo. Abrasion particles of spacers in the joint could lead to third-body-wear of the new prosthesis influencing survival although we are unaware of evidence suggesting prostheses after septic two-stage revision have lower survival rates than those after aseptic loosening. We assume evidence of abraded particles in the synovial membrane (where they are histologically detectable) is a sign of release of particles into the joint space.
We asked whether abraded material from mobile spacers is present in the synovial membrane after a 6-week spacer implantation period as part of two-stage revision surgery of infected knee and hip endoprostheses.
Patients and Methods
Between January and December 2008, 20 patients underwent two-stage revision surgery of infected endoprostheses, and during the reimplantation procedure, the synovial membrane was removed and examined for the presence of abraded particles. The cohort included nine women and seven men (16 hip arthroplasties) with a mean (± SD) age of 69.7 ± 11.7 years (range, 47–89 years) and one woman and three men (four knee arthroplasties) with a mean age of 65.0 ± 19.6 years (range 26–79 years). As a control, we analyzed the synovial membrane of three patients (two women, one man with a mean age of 73.0 ± 3.0 years; two hips, one knee) removed during primary joint arthroplasty. All patients had osteoarthritis as the indication for the primary joint replacement. Our Institutional Review Board approved the study protocol, ensuring all investigations were conducted in conformity with ethical principles of research and informed consent had been obtained.
Periprosthetic infection was diagnosed by aspiration or biopsy of the joint and subsequent incubation of the sample for 14 days [11]. An antibiogram was used to assess the sensitivity of the detected microorganism and the appropriate antibiotics added at a maximal concentration of 10% to the commercially available Palacos® R+G (five hips, two knees) or Copal® (11 hips, two knees) spacer cement (Heraeus Medical GmbH, Wehrheim, Germany). As described previously [11], a cup-shaped acetabulum spacer was hand-formed from cement for hip revision surgery; for the stem component, antibiotic-loaded cement was placed around a prosthesis stem and coated with the patient’s blood to facilitate removal at the second stage of revision. The two spacer modules were connected to each other by a metal head (Fig. 1). In nine cases, this was a Protasul™-20 and in seven cases, a Protasul™-S30 (Zimmer GmbH, Winterthur, Switzerland). The Protasul™ heads used to prepare the spacers consisted of the alloys Co28Cr6Mo (Protasul™-20) and FeCr22Ni10Mn4Mo2NNb (Protasul™-S30). Compared with pure-cement spacers, the two-part mobile spacer has the advantage of minimizing the risk of spacer fractures [11]. For the knee we made hand-formed femoral and tibial components from antibiotic-loaded bone cement (Heraeus Medical) (Fig. 2).
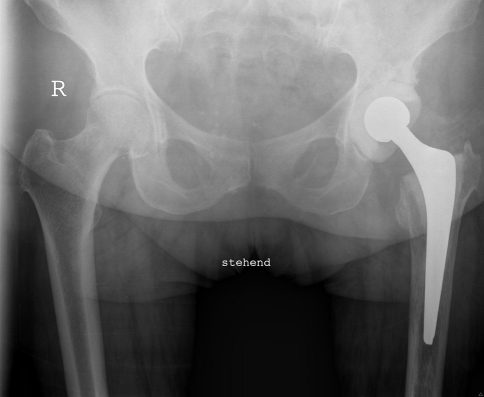
Fig. 1.
A radiograph shows the pelvis of a 60-year-old woman with a spacer implanted in the left hip.
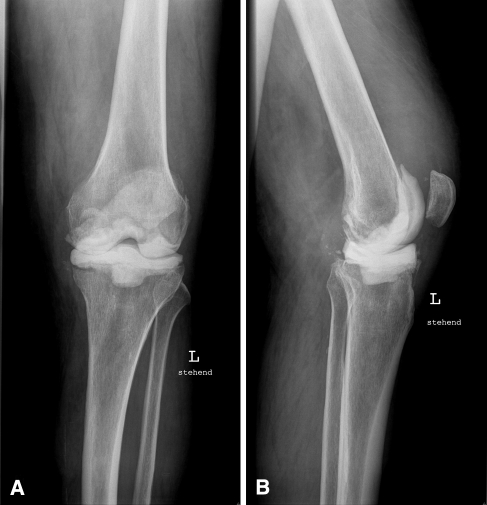
Fig. 2A–B.
(A) AP and (B) lateral radiographs show the left knee of a 71-year-old man with an implanted mobile spacer.
While these spacers were in place, the patient was mobilized with crutches and partial weightbearing of 20 kg on the surgically treated leg. Antibiotics were administered for 6 weeks: parenterally for 2 weeks and orally for 4 weeks (according to recommendations of the consulting microbiologist). At the end of the 6 weeks, a new hip or knee prosthesis was reimplanted. During the reimplantation procedure, a complete synovectomy with débridement of the whole capsule was performed and the whole synovial membrane was examined for the presence of abraded material. For each analyzing technique one sample approximately 1 × 1 × 0.3 cm was taken from the inner surface of each synovial membrane. We determined the presence of zirconium dioxide from the cement and metal particles abraded from the spacers and the elements present in the alloys: cobalt, chromium, molybdenum, manganese, and niobium. Human tissues contain minute amounts of chromium, copper, and manganese as essential trace elements [3, 39]. In contrast, zirconium dioxide is not normally present in human tissue and is used in Palacos® R+G and Copal® as opaquing agents for purposes of radiographic detection [29].
We used xray fluorescence spectroscopy (XRF) [29–31] to determine whether zirconium was present in the spacer membrane and xray diffraction (XRD) [25] to obtain structural evidence for the presence of zirconium dioxide in the spacer membrane. XRF functions by excitation of electrons in the innermost shells of elements with atomic numbers greater than 9. If a primary xray from an xray tube or a radioactive source is absorbed by an atom by transferring all of its energy to an innermost electron, electrons are ejected from the inner shells, creating vacancies. These vacancies present an unstable state for the atom. As the atom returns to its stable condition, electrons from the outer shells are transferred to the inner shells, and in the process, give off a characteristic xray whose energy is the difference between the two binding energies of the corresponding shells. Each element has a unique set of energy levels so each element produces a unique set of xrays according to Moseley’s law that then can be detected and analyzed [25, 29–31]. Thus, XRF can be used to detect the presence of elements with an atomic number greater than 9. XRD is based on the fact that crystal lattices diffract xrays. This means that every crystalline substance can be characterized structurally and identified by XRD according to the lattice parameters of the substance [25]. Samples of spacer membrane with a mass of approximately 0.5 g were lyophilized and investigated with XRD and XRF. XRF was performed using a Nanomaster (Röntgenanalytik Apparatebau GmbH, Berlin, Germany) and XRD using the D8 Advance xray diffractometer (Bruker AXS GmbH, Karlsruhe, Germany).
Semiquantitative analysis of the detected elements was performed by inductively coupled plasma-mass spectrometry (ICP-MS; SGS Fresenius, Taunusstein, Germany) using an ELAN DRC II machine (Perkin Elmer Inc, Waltham, MA, USA). ICP technology is built on the principles used in atomic emission spectrometry [40]. Samples are decomposed to neutral elements in a high-temperature argon plasma, which has been produced by the effect of high-frequency electromagnetic fields, and after accelerating the elements through an electrical field, they are analyzed on the basis of their mass-to-charge ratios. The slightest trace of elements can be identified and quantified in this way [2, 30, 40]. Samples of spacer membrane with a wet weight of approximately 0.7 g were ultrasonically nebulized in nitric acid before being introduced into the ELAN DRC II apparatus in the TotalQuant mode. The values obtained are semiquantitative because there is no available reference substance for zirconium for calibrating the ICP-MS method for biologic tissue.
We performed histologic examinations of the synovial membrane using previously described methods of fixation [20, 21]. After the specimens were dehydrated and fat removed in an ascending composition of several alcohol concentrations (70%, 80%, 96%, 100%) for 4 days, they were infiltrated by exposing them to an increasing ethanol-methylmethacrylate solution (Technovit 7200, Kulzer, Germany) (30%–70%, 50%–50%, 70%–30%). The Technovit with added benzylperoxide was used to continue the infiltration process for an additional 14 days under vacuum. This preparation sequence enabled complete preservation of the bone cement in the tissue. An additional 10 hours elapsed until the polymerization process (blue light) was completed. We used three specimens from each patient to prepare ultrathin ground specimens of 25 μm thickness using an EXAKT machine (Norderstedt, Germany) [8]. After the polishing process, the specimens were stained with toluidine blue. The stained grinding specimens were used for semiquantitative evaluation of the amount and dimensions of bone cement debris. The size of the particles were measured with an image analyzing system (ImageJ 1.34s, National Institutes of Health, Bethesda, MD, USA) using a magnification of ×400. The histologic analyses were performed by two observers (BF, MH) independently with intrarater intraclass correlation coefficients of 1.0 and 1.0, respectively.
Results
Zirconium was detected by XRF in all samples of the spacers but not in the synovial membrane of the control group (Fig. 3). XRD showed zirconium dioxide in all cases (Table 1). The ICP-MS analyses also showed all spacer membrane samples contained zirconium (Table 1). Traces of chromium and copper also were detected in all samples. Some hip spacer membrane samples contained very high concentrations of chromium whereas the knee membranes only exhibited traces of this element. In contrast, copper was present in all samples at more or less similar levels. Cobalt was detectable only in the hip spacer membrane samples, at times in high concentrations, but could not be identified in any of the knee spacer membrane samples or in the synovial membranes of the control group. We found nickel, manganese, and molybdenum in trace amounts in the hip spacer membrane samples but not in the knee membrane samples or the synovial membranes of the control group (Table 1). Niobium was not detected in any of the samples. Iron, phosphorus, sodium, potassium, calcium, and magnesium, which are elements typical for human tissues, all were detected, although these are not included in Table 1. In some spacer membranes, there were also minimal concentrations of zinc, strontium, and barium detected (data not shown).
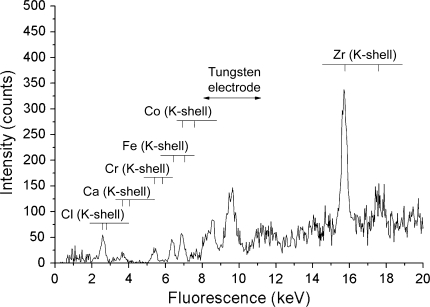
Fig. 3.
An XRF spectrum of a synovial membrane sample shows the characteristic peaks of zirconium, chlorine, calcium, chromium, iron, and cobalt.
Table 1.
Description of the demographic and analytic data
| Demographic data | Spacer position | Prosthetic head | Cement | XRF | XRD | Semiquantitative element analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | Gender | Age (years) | Zirconium detection | ZrO2 detection | Zirconium [mg/kg] | Chromium [mg/kg] | Cobalt [mg/kg] | Manganese [mg/kg] | Copper [mg/kg] | Nickel [mg/kg] | Molybdenum [mg/kg] | |||
| 1 | M | 59 | Hip | Protasul S30 | Copal | + | + | 0.6 | 0.9 | < 0.05 | 0.1 | 0.6 | 0.2 | < 0.05 |
| 2 | F | 76 | Hip | Protasul S30 | Palacos | + | + | 6.1 | 0.1 | < 0.05 | < 0.1 | 0.6 | < 0.1 | < 0.05 |
| 3 | F | 77 | Hip | Protasul 20 | Copal | + | + | 1.1 | 25.3 | 2.5 | 0.1 | 0.6 | < 0.1 | 0.7 |
| 4 | F | 71 | Hip | Protasul 20 | Copal | + | + | 1.3 | 0.1 | < 0.05 | < 0.1 | 0.7 | < 0.1 | < 0.05 |
| 5 | F | 76 | Hip | Protasul 20 | Copal | + | + | 0.2 | 0.1 | < 0.05 | < 0.1 | 0.4 | < 0.1 | < 0.05 |
| 6 | F | 48 | Hip | Protasul 20 | Copal | + | + | 2.1 | 13 | 11.2 | 0.2 | 1 | 0.1 | 0.6 |
| 7 | M | 61 | Hip | Protasul 20 | Copal | + | + | 1.7 | 30.4 | 8.9 | < 0.1 | 0.7 | < 0.1 | 1.1 |
| 8 | M | 64 | Hip | Protasul 20 | Copal | + | + | 23.8 | 98.5 | 18.7 | 0.3 | 1.6 | 0.7 | 4.7 |
| 9 | M | 73 | Hip | Protasul S30 | Palacos | + | + | 2 | 14 | 2 | < 0.1 | 1.2 | 0.1 | 0.6 |
| 10 | F | 72 | Hip | Protasul S30 | Palacos | + | + | 0.2 | 0.6 | < 0.05 | < 0.1 | 0.8 | 0.2 | < 0.05 |
| 11 | F | 66 | Hip | Protasul 20 | Copal | + | + | 2.5 | 42 | 17 | 0.5 | 0.8 | 0.5 | 1.1 |
| 12 | F | 72 | Hip | Protasul S30 | Palacos | + | + | 0.4 | 0.5 | 0.1 | < 0.1 | 0.9 | 0.2 | < 0.05 |
| 13 | M | 82 | Hip | Protasul 20 | Copal | + | + | 0.7 | 50 | 14 | 0.4 | 1.3 | < 0.1 | 1.7 |
| 14 | F | 83 | Hip | Protasul S30 | Copal | + | + | 1.2 | 3.1 | 0.3 | 0.3 | 1.4 | 1.6 | 0.2 |
| 15 | M | 89 | Hip | Protasul S30 | Palacos | + | + | 2.3 | 0.87 | 0.12 | 0.12 | 2.8 | 26 | < 0.05 |
| 16 | M | 47 | Hip | Protasul 20 | Copal | + | + | 8.8 | 61 | 10.1 | 0.23 | 1.7 | 0.23 | 2.5 |
| 17 | M | 79 | Knee | Copal | + | + | 0.1 | 0.3 | < 0.05 | < 0.1 | 0.6 | < 0.1 | < 0.05 | |
| 18 | M | 75 | Knee | Palacos | + | + | 8.5 | 0.2 | < 0.05 | < 0.1 | 0.7 | < 0.1 | < 0.05 | |
| 19 | F | 36 | Knee | Palacos | + | + | 2.5 | 0.2 | < 0.05 | < 0.1 | 1.1 | < 0.1 | < 0.05 | |
| 20 | M | 70 | Knee | Copal | + | + | 4.8 | 0.34 | < 0.05 | < 0.1 | 1.3 | < 0.1 | < 0.05 | |
| 21 | F | 73 | Hip | Control | – | – | – | < 0.05 | 0.32 | < 0.05 | < 0.1 | 1.0 | < 0.1 | < 0.05 |
| 22 | M | 76 | Hip | Control | – | – | – | < 0.05 | 0.36 | < 0.05 | < 0.1 | 0.76 | < 0.1 | < 0.05 |
| 23 | F | 70 | Knee | Control | – | – | – | < 0.05 | 0.16 | < 0.05 | < 0.1 | 0.66 | < 0.1 | < 0.05 |
ZrO2 = zirconium dioxide.
In the histologic analyses, zirconium oxide particles as the opaquing compound of the bone cement typically were shaped like mulberries, had a diameter of 20 to 25 μm on average, and were seen in every synovial membrane (Fig. 4). Moreover, in each sample, smaller particles of zirconium less than 1.5 μm were found, as a sign of abrasion of the zirconium particles (Fig. 4). The distribution of the different particles was inhomogeneous. Complete bone cement parts with diameters of 100 to 1400 μm and smooth surfaces were observed in three cases (one knee and two hip spacers) (Fig. 5).
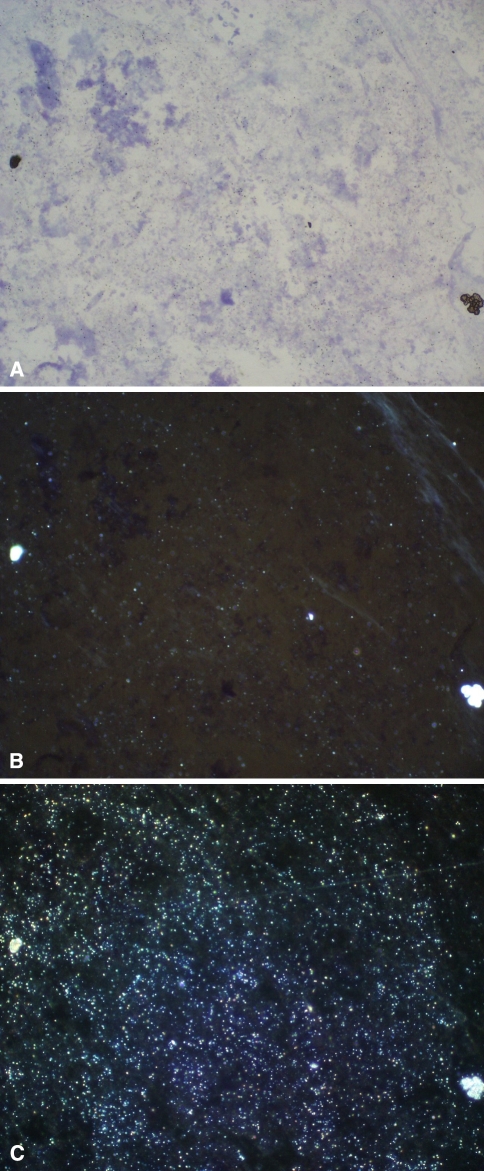
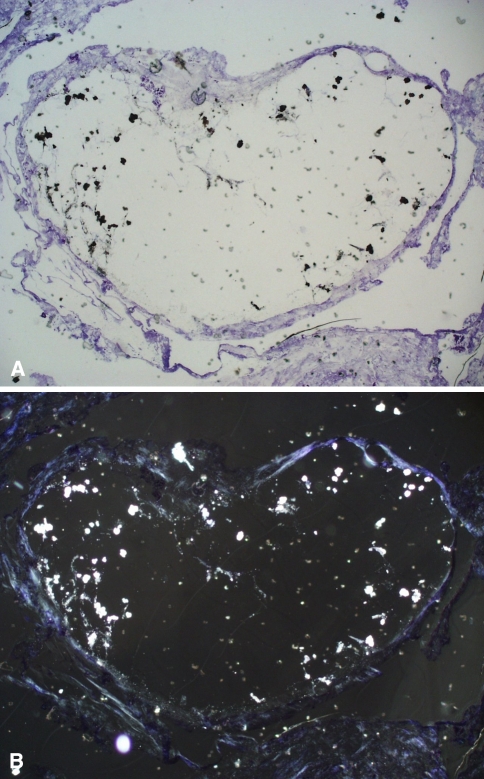
Fig. 4A–C.
Histologic analysis of the synovial lining of a hip spacer from a 60-year-old man shows abrasion particles of zirconium dioxide (Stain, toluidine blue; original magnification, ×200). (A) Light microscopy shows the mulberry-shaped zirconium dioxide particles and some smaller particles (black). (B) The smaller abrasion particles (< 1.5 μm, light) from the same sample are seen better with polarized light microscopy (C) The total amount of small abrasion particles of zirconium dioxide is seen in the dark-field illumination.
Fig. 5A–B.
Histologic analysis of the synovial lining of a hip spacer from a 71-year-old man shows a greater piece of cement (1.2 mm) with smooth, rounded surfaces as a sign of abrasion (Stain, toluidine blue; original magnification, ×50). (A) With light microscopy, zirconium particles of the cement are dark and (B) can be seen better as light particles with polarized light microscopy.
Discussion
Spacers in two-stage procedures for treatment of periprosthetic infections are the most frequently used concept. A potential disadvantage is abrasion of the spacers leading to the risk of third-body wear in the new prosthesis. Therefore we determined whether abraded material from mobile spacers is present in the synovial membrane after 6 weeks of spacer implantation as part of two-stage revision surgery for infected knee and hip endoprostheses.
Our study has some limitations. First, the zirconium and metal particles detected could have originated from the original, infected prostheses, especially because Keen et al. [29] reported detection of zirconium particles in the synovial membrane after many years of endoprosthesis implantation. Although this cannot be totally excluded, we assumed most of the abraded material we detected came from the spacers because part of the treatment of a periprosthetic infection usually involves radical débridement and synovectomy before the spacer is implanted [11, 14, 15, 19, 24, 33, 35]. Analyses of synovial controls obtained at the time of prosthesis removal could have addressed this question but we had no such controls. Second, we assumed that the evidence of abraded particles in the synovial membrane is a sign of release of spacer particles into the joint space. Although we could not prove this hypothesis we believe it is the most obvious explanation. Moreover, the analysis of abrasion particles (especially histologically) can technically be realized better in tissue. Third, the question of whether the amount and size of the zirconium particles is different for hip and knee spacers cannot be answered in this initial descriptive study because there is no available reference substance for zirconium for calibration of the ICP-MS method for biologic tissue. This may explain the high variability of the results of the ICP-MS analysis. Although the semiquantitative data indicate there are similar concentrations of abraded material for both sets of spacers, statistical analyses could not be performed. The histologic analyses showed no difference in particle sizes of the hip and knee spacers. To be able to perform quantitative analyses in future studies, development of a quantitative method to measure zirconium dioxide and metal particles in biologic membranes is needed because such a method has not yet been established. Fourth, we examined only one sample of each synovial membrane by each analyzing method. Therefore, the amount of abraded material could be different in another sample of the synovial membrane. Because the goal of this study was to verify the presence of abrasion and not a quantitative analysis of abraded material, this method is sufficient for the aim of this study. Fifth, our observations are not necessarily relevant for other types of cement spacers. However, it can be assumed all articulating cement surfaces, whether handmade or commercially produced (eg, the PROSTALAC® spacer used by Haddad et al. [18] and Meek et al. [35]) will generate abraded material.
Keen et al. [29] reported the presence of zirconium dioxide particles in the synovial membrane, and Schuh et al. [42] reported the presence of these particles in granulomas of loosened hip arthroplasties after long-term implantation of cemented endoprostheses. Our data suggest articulating materials also can be abraded during a short, 6-week period of implantation of a cement spacer. The actual type of particles detected is variable because we studied various hip and knee spacers. The hip membrane, for example, exhibited not only zirconium dioxide and polymethylmethacrylate abrasion products but also metals originating from the heads used to join the spacer components (such as chromium, cobalt, molybdenum, and nickel). The detection of chromium and copper in the case of knee spacers can be explained by the fact that normal human tissues naturally contain chromium, copper, and manganese as essential trace elements [39]. It also is possible the abraded material originates from the original, infected prostheses. However, the fact that extensive débridement and radical synovectomy were performed at the time the infected prosthesis was removed suggests the majority of the detected abrasion products came from the spacer components. Despite this, it is possible traces of the alloy used for the original prosthesis do persist and thus are detectable. This also could explain the presence of barium in some of the tissue samples as barium sulfate is used as an opaquing agent in some bone cements and such particles reportedly are detectable in the synovial membrane after many years of prosthesis implantation [29]. However, we have no information regarding the brands of cement used in the original implantations.
Another possible option for treatment would be using static spacers. However, these are used only for the knee and not for the hip, and mobile spacers are preferred for the interim phase of the procedure because they permit a certain degree of mobility, they are easier to replace than static spacers at the time of reimplantation, and they are associated with a lower degree of bone loss than that observed with static spacers [5, 10, 13, 17, 19, 24, 35]. Moreover, abrasion also can be expected for static spacers because some movement between the spacer and the bone can be assumed. Hofmann et al. [23, 24] attempted to counter the problem of abrasion products by combining a resterilized metal femoral component with a polyethylene tibial component or a polyethylene cup. However, we presume this method does not provide as effective a release of antibiotic into the joint space as does the surface of the cement spacer.
Cement spacers usually generate abrasion products even during a short implantation period of a few weeks. Although we are unaware of evidence suggesting prostheses after septic two-stage revision have lower survival rates than those after aseptic loosening, we believe abrasion should be taken into account when designing treatments for periprosthetic infections. The presence of such material underlines the importance of total synovectomy and extensive lavage performed at the time of reimplantation not only to ensure radical débridement of residual organisms but also to reduce the amount of abraded material. Another step in addressing the problem of abrasion products would be to stop using zirconium as a contrast medium in spacer cement, especially as zirconium oxide particles reportedly cause third-body wear of new prostheses, and zirconium dioxide, owing to its hardness, induces more scratches and damage to metal heads than barium sulfate or cement without radiopaque additives [6, 26, 29, 32]. However, abrasion products of another opaquing compound, barium sulfate, also are disadvantageous in that they induce bone resorption more severe than zirconium dioxide or polymethylmethacrylate without radiopaque additives [41]. Thus, further development work on spacer cement appears necessary.
Acknowledgments
We thank Matthias Schnabelrauch PhD and Christian Schrader PhD (Innovent e.V. Technologieentwicklung) for performing the XRF and XRD measurements.
Footnotes
One or more of the authors (AR) received funding from Heraeus Medical GmbH for the XRF and XRD analyses and the ICP-MS analyses.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Orthopaedic Clinic Markgröningen gGmbH.
References
- 1.Affatato S, Mattarozzi A, Taddei P, Robotti P, Soffiatti R, Sudanese A, Toni A. Investigations on the wear behaviour of the temporary PMMA-based hip Spacer-G. Proc Inst Mech Eng H. 2003;217:1–8. doi: 10.1243/095441103762597665. [DOI] [PubMed] [Google Scholar]
- 2.Ammann AA. Inductively coupled plasma mass spectrometry (ICP MS): a versatile tool. J Mass Spectrom. 2007;42:419–427. doi: 10.1002/jms.1206. [DOI] [PubMed] [Google Scholar]
- 3.Burch RE, Hahn HK. Trace elements in human nutrition. Med Clin North Am. 1979;63:1057–1068. doi: 10.1016/s0025-7125(16)31659-5. [DOI] [PubMed] [Google Scholar]
- 4.Burnett RS, Kelly MA, Hanssen AD, Barrack RL. Technique and timing of two-stage exchange for infection in TKA. Clin Orthop Relat Res. 2007;464:164–178. doi: 10.1097/BLO.0b013e318157eb1e. [DOI] [PubMed] [Google Scholar]
- 5.Calton TF, Fehring TK, Griffin WL. Bone loss associated with the use of spacer blocks in infected total knee arthroplasty. Clin Orthop Relat Res. 1997;345:148–154. doi: 10.1097/00003086-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Caravia L, Dowson D, Fisher J, Jobbins B. The influence of bone and bone cement debris on counterface roughness in sliding wear tests of ultra-high molecular weight polyethylene on stainless steel. Proc Inst Mech Eng H. 1990;204:65–70. doi: 10.1243/PIME_PROC_1990_204_230_02. [DOI] [PubMed] [Google Scholar]
- 7.Cui Q, Mihalko WM, Shields JS, Ries M, Saleh KJ. Antibiotic-impregnated cement spacers for the treatment of infection associated with total hip or knee arthroplasty. J Bone Joint Surg Am. 2007;89:871–882. doi: 10.2106/JBJS.E.01070. [DOI] [PubMed] [Google Scholar]
- 8.Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues: the Säge-Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–326. doi: 10.1111/j.1600-0714.1982.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 9.Evans RP. Successful treatment of total hip and knee infection with articulating antibiotic components: a modified treatment method. Clin Orthop Relat Res. 2004;427:37–46. doi: 10.1097/01.blo.0000143739.07632.7c. [DOI] [PubMed] [Google Scholar]
- 10.Fehring TK, Odum S, Calton TF, Mason JB. Articulating versus static spacers in revision total knee arthroplasty for sepsis: the Ranawat Award. Clin Orthop Relat Res. 2000;380:9–16. doi: 10.1097/00003086-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Fink B, Grossmann A, Fuerst M, Schäfer P, Frommelt L. Two-stage cementless revision of infected hip endoprostheses. Clin Orthop Relat Res. 2009;467:1848–1858. doi: 10.1007/s11999-008-0611-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fitzgerald RH., Jr Infected total hip arthroplasty: diagnosis and treatment. J Am Acad Orthop Surg. 1995;3:249–262. doi: 10.5435/00124635-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Freeman MG, Fehring TK, Odum SM, Fehring K, Griffin WL, Mason JB. Functional advantage of articulating versus static spacers in 2-stage revision for total knee arthroplasty infection. J Arthroplasty. 2007;22:1116–1121. doi: 10.1016/j.arth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 14.Garvin KL, Evans BG, Salvati EA, Brause BD. Palacos gentamicin for the treatment of deep periprosthetic hip infections. Clin Orthop Relat Res. 1994;298:97–105. [PubMed] [Google Scholar]
- 15.Garvin KL. Hanssen AD. Infection after total hip arthroplasty: past, present, and future. J Bone Joint Surg Am. 1995;77:1576–1588. doi: 10.2106/00004623-199510000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Goldman RT, Scuderi GR, Insall JN. 2-stage reimplantation for infected total knee replacement. Clin Orthop Relat Res. 1996;331:118–124. doi: 10.1097/00003086-199610000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein WM, Kopplin M, Wall R, Berland K. Temporary articulating methylmethacrylate antibiotic spacer (TAMMAS): a new method of intraoperative manufacturing custom articulating spacer. J Bone Joint Surg Am. 2001;83(suppl 2, pt 2):92–97. [PubMed]
- 18.Haddad FS, Masri BA, Campbell D, McGraw RW, Beauchamp CP, Duncan CP. The PROSTALAC functional spacer in two-stage revision for infected knee replacements: prosthesis of antibiotic-loaded acrylic cement. J Bone Joint Surg Br. 2000;82:807–812. doi: 10.1302/0301-620X.82B6.10486. [DOI] [PubMed] [Google Scholar]
- 19.Haddad FS, Muirhead-Allwood SK, Manktelow AR, Bacarese-Hamilton I. Two-stage uncemented revision hip arthroplasty for infection. J Bone Joint Surg Br. 2000;82:689–694. doi: 10.1302/0301-620X.82B5.9668. [DOI] [PubMed] [Google Scholar]
- 20.Hahn M. Quantitative analysis of the histological reactions at the interface and the surrounding bone tissue following the implantation of hip endoprostheses. In: Barbosa MA, Campilho A, eds. Imaging Techniques in Biomaterials. Amsterdam, The Netherlands: North-Holland/Elsevier Science Publishers; 1994:325–340.
- 21.Hahn M, Vogel M, Delling G. Undecalcified preparation of bone tissue: report of technical experience and development of new methods. Virchows Arch A Pathol Anat Histopathol. 1991;418:1–7. doi: 10.1007/BF01600238. [DOI] [PubMed] [Google Scholar]
- 22.Hanssen AD, Osmon DR. Evaluation of a staging system for infected hip arthroplasty. Clin Orthop Relat Res. 2002;403:16–22. doi: 10.1097/00003086-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann AA, Goldberg TD, Tanner AM, Cook TM. Ten-year experience using an articulating antibiotic cement hip spacer for the treatment of chronically infected total hip. J Arthroplasty. 2005;20:874–879. doi: 10.1016/j.arth.2004.12.055. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann AA, Kane KR, Tkach TK, Plaster RL, Camargo MP. Treatment of infected total knee arthroplasty using an articulating spacer. Clin Orthop Relat Res. 1995;321:45–54. [PubMed] [Google Scholar]
- 25.Hook GR, Hosseini JM, Elin RJ. Analytical approaches for biomedical elemental analysis. J Am Coll Nutr. 1985;4:599–612. doi: 10.1080/07315724.1985.10720102. [DOI] [PubMed] [Google Scholar]
- 26.Huotari K. Lyytikäinen O; Hospital Infection Surveillance Team. Impact of postdischarge surveillance on the rate of surgical site infection after orthopedic surgery. Infect Control Hosp Epidemiol. 2006;27:1324–1329. doi: 10.1086/509840. [DOI] [PubMed] [Google Scholar]
- 27.Irifune T, Kurio A, Sakamoto S, Inoue T, Samiya H. Materials: ultrahard polycrystalline diamond from graphite. Nature. 2003;421:599–600. doi: 10.1038/421599b. [DOI] [PubMed] [Google Scholar]
- 28.Isaac GH, Atkinson JR, Dowson D, Kennedy PD, Smith MR. The causes of femoral head roughening in explanted Charnley hip prostheses. Eng Med. 1987;16:167–173. doi: 10.1243/EMED_JOUR_1987_016_035_02. [DOI] [PubMed] [Google Scholar]
- 29.Keen CE, Philip G, Brady K, Spencer JD, Levison DA. Histopathological and microanalytical study of zirconium dioxide and barium sulphate in bone cement. J Clin Pathol. 1992;45:984–989. doi: 10.1136/jcp.45.11.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kesteris U, Carlsson L, Haraldsson C, Lausmaa J, Lidgren L, Onnerfält R, Wingstrand H. Contamination of polyethylene cups with polymethyl methacrylate particles: an experimental study. J Arthroplasty. 2001;16:905–908. doi: 10.1054/arth.2001.25554. [DOI] [PubMed] [Google Scholar]
- 31.Lerouge S, Huk O, Yahia LH, Sedel L. Characterization of in vivo wear debris from ceramic-ceramic total hip arthroplasties. J Biomed Mater Res. 1996;32:627–633. doi: 10.1002/(SICI)1097-4636(199612)32:4<627::AID-JBM16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 32.Lewis G. Alternative acrylic bone cement formulations for cemented arthroplasties: present status, key issues, and future prospects. J Biomed Mater Res Part B: Appl Biomater. 2008;84:301–319. doi: 10.1002/jbm.b.30873. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman JR, Callaway GH, Salvati EA, Pellicci PM, Brause BD. Treatment of the infected total hip arthroplasty with a two stage reimplantation protocol. Clin Orthop Relat Res. 1994;301:205–212. [PubMed] [Google Scholar]
- 34.Masri BA, Panagiotopoulos KP, Greidanus NV, Garbuz DS, Duncan CP. Cementless two-stage exchange arthroplasty for infection after total hip arthroplasty. J Arthroplasty. 2007;22:72–78. doi: 10.1016/j.arth.2006.02.156. [DOI] [PubMed] [Google Scholar]
- 35.Meek RM, Masri BA, Dunlop D, Garbuz DS, Greidanus NV, McGraw R, Duncan CP. Patient satisfaction and functional status after treatment of infection at the site of a total knee arthroplasty with use of the PROSTALAC articulating spacer. J Bone Joint Surg Am. 2003;85:1888–1892. doi: 10.1302/0301-620X.85B8.14214. [DOI] [PubMed] [Google Scholar]
- 36.Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements. Clin Orthop Relat Res. 2001;392:15–23. doi: 10.1097/00003086-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. doi: 10.1302/0301-620X.88B7.17150. [DOI] [PubMed] [Google Scholar]
- 38.Pitto RP, Castelli CC, Ferrari R, Munro J. Pre-formed articulating knee spacer in two-stage revision for the infected total knee arthroplasty. Int Orthop. 2005;29:305–308. doi: 10.1007/s00264-005-0670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahil-Khazen R, Bolann BJ, Myking A, Ulvik RJ. Multi-element analysis of trace element levels in human autopsy tissues by using inductively coupled atomic emission spectrometry technique (ICP-AES) J Trace Elem Med Biol. 2002;16:15–25. doi: 10.1016/S0946-672X(02)80004-9. [DOI] [PubMed] [Google Scholar]
- 40.Riedel F, Hönle W, Göske J, Kachler W, Holzwarth U, Schuh A. [Examination of granuloma of revised cemented or cementless total hip arthroplasties using inductively coupled plasma atomic emission spectrometry (ICP-OES)] [in German] Biomed Tech (Berl) 2006;51:15–20. doi: 10.1515/BMT.2006.004. [DOI] [PubMed] [Google Scholar]
- 41.Sabokbar A, Fujikawa Y, Murray DW, Athanasou NA. Radio-opaque agents in bone cement increase bone resorption. J Bone Joint Surg Br. 1997;79:129–134. doi: 10.1302/0301-620X.79B1.6966. [DOI] [PubMed] [Google Scholar]
- 42.Schuh A, Thomas P, Kachler W, Göske J, Holzwarth U. [Characterisation of histologic sections of granulomas of revised total hip arthroplasties using a scanning electron microscope (SEM)] [in German] Biomed Technik. 2005;50(suppl):1577–1578. [Google Scholar]
- 43.Villa T, Carnelli D. Experimental evaluation of the biomechanical performances of the PMMA-based knee spacer. Knee. 2007;14:145–153. doi: 10.1016/j.knee.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Widmer AF, Gaechter A, Ochsner PE, Zimmerli W. Antimicrobial treatment of orthopedic implant-related infections with rifampin combinations. Clin Infect Dis. 1992;14:1251–1253. doi: 10.1093/clinids/14.6.1251. [DOI] [PubMed] [Google Scholar]
- 45.Zimmerli W, Widmer AF, Blatter M, Frei R, Ochsner PE. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. Foreign-Body Infection (FBI) Study Group. JAMA. 1998;279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]