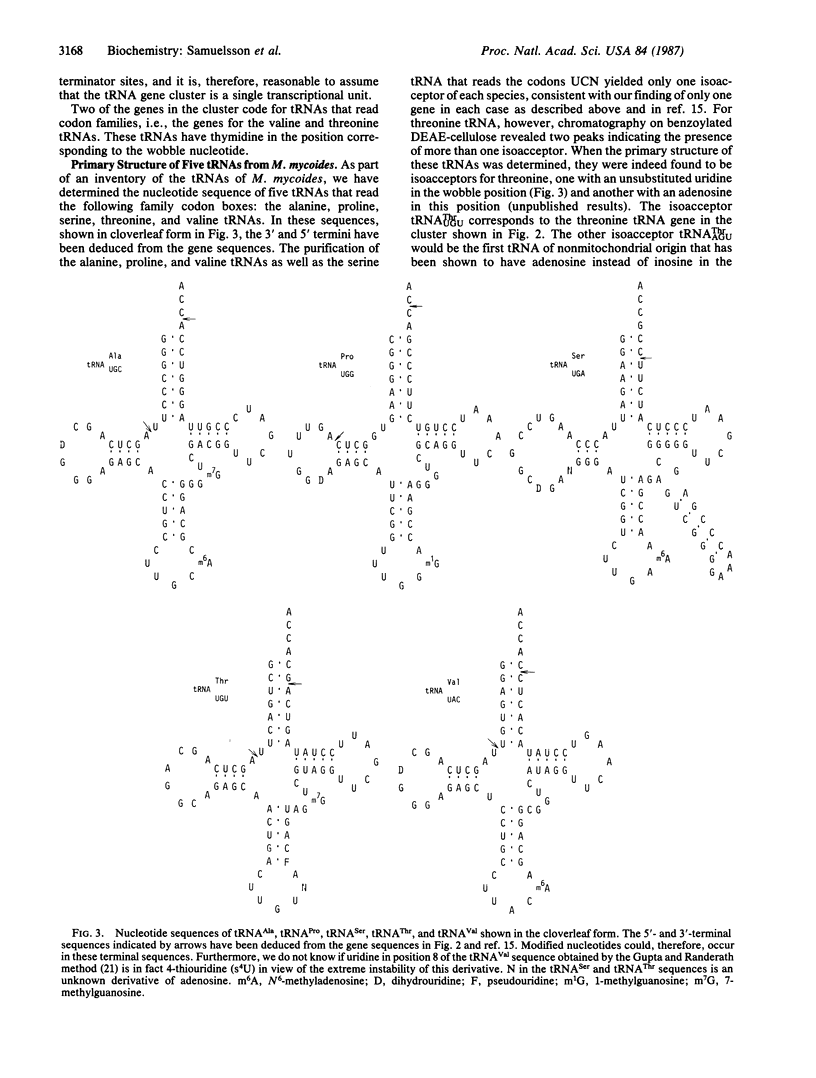
Abstract
We report a cluster of four tRNA genes from Mycoplasma mycoides as well as the sequence of the alanine, proline, and valine tRNAs and the serine tRNA reading the UCN codons (where N stands for G, A, C, or U). This brings the total number of tRNA genes that we have so far characterized in this organism to 14, 6 of which code for tRNAs that read the codons of family boxes. In each of these latter cases, we found only one gene per family box, and the gene sequence contains a thymidine in the position corresponding to the wobble nucleotide, with the exception of the arginine tRNA gene that has an adenosine in this position. Furthermore, all of the tRNA structures reported here have an unsubstituted uridine in the wobble position. These findings are similar to those reported for mitochondria, especially yeast mitochondria, that contain an arginine tRNA with the anticodon ACG. However, the resemblance is not complete since we have demonstrated the presence of two isoacceptor tRNAs for threonine having uridine and adenosine, respectively, in the wobble position. It is suggested that in the M. mycoides at least some of the family codon boxes are read by only one tRNA each, using an unconventional method without discrimination between the nucleotides in the third codon position.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonitz S. G., Berlani R., Coruzzi G., Li M., Macino G., Nobrega F. G., Nobrega M. P., Thalenfeld B. E., Tzagoloff A. Codon recognition rules in yeast mitochondria. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3167–3170. doi: 10.1073/pnas.77.6.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Garel J. P., Garber R. L., Siddiqui M. A. Transfer RNA in posterior silk gland of Bombyx mori: polyacrylamide gel mapping of mature transfer RNA, identification and partial structural characterization of major isoacceptor species. Biochemistry. 1977 Aug 9;16(16):3618–3624. doi: 10.1021/bi00635a018. [DOI] [PubMed] [Google Scholar]
- Gillam I., Blew D., Warrington R. C., von Tigerstrom M., Tener G. M. A general procedure for the isolation of specific transfer ribonucleic acids. Biochemistry. 1968 Oct;7(10):3459–3468. doi: 10.1021/bi00850a022. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Rapid print-readout technique for sequencing of RNA's containing modified nucleotides. Nucleic Acids Res. 1979 Aug 10;6(11):3443–3458. doi: 10.1093/nar/6.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick M. W., Walker R. T. The nucleotide sequence of glycine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1980 Jun 25;8(12):2783–2786. doi: 10.1093/nar/8.12.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerkvist U. "Two out of three": an alternative method for codon reading. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1759–1762. doi: 10.1073/pnas.75.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerkvist U. Unconventional methods in codon reading. Bioessays. 1986 May;4(5):223–226. doi: 10.1002/bies.950040509. [DOI] [PubMed] [Google Scholar]
- Lustig F., Elias P., Axberg T., Samuelsson T., Tittawella I., Lagerkvist U. Codon reading and translational error. Reading of the glutamine and lysine codons during protein synthesis in vitro. J Biol Chem. 1981 Mar 25;256(6):2635–2643. [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Axberg T., Elias P., Lagerkvist U. Relative efficiency of anticodons in reading the valine codons during protein synthesis in vitro. J Biol Chem. 1979 Jul 25;254(14):6397–6401. [PubMed] [Google Scholar]
- Mitra S. K., Lustig F., Akesson B., Lagerkvist U. Codon-acticodon recognition in the valine codon family. J Biol Chem. 1977 Jan 25;252(2):471–478. [PubMed] [Google Scholar]
- Samuelsson T., Axberg T., Borén T., Lagerkvist U. Unconventional reading of the glycine codons. J Biol Chem. 1983 Nov 10;258(21):13178–13184. [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Axberg T., Fölsch G., Akesson B., Lagerkvist U. Aberrations of the classic codon reading scheme during protein synthesis in vitro. J Biol Chem. 1980 May 25;255(10):4583–4588. [PubMed] [Google Scholar]
- Samuelsson T., Elias P., Lustig F., Guindy Y. S. Cloning and nucleotide sequence analysis of transfer RNA genes from Mycoplasma mycoides. Biochem J. 1985 Nov 15;232(1):223–228. doi: 10.1042/bj2320223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibler A. P., Dirheimer G., Martin R. P. Codon reading patterns in Saccharomyces cerevisiae mitochondria based on sequences of mitochondrial tRNAs. FEBS Lett. 1986 Jan 1;194(1):131–138. doi: 10.1016/0014-5793(86)80064-3. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Prochiantz A., Haenni A. L., Rajbhandary U. L. Studies on the sequence of the 3'-terminal region of turnip-yellow-mosaic-virus RNA. Eur J Biochem. 1977 Feb;72(3):465–478. doi: 10.1111/j.1432-1033.1977.tb11270.x. [DOI] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., McMillan C., 3rd, Cline S., Bradley D., Snyder M. Construction of a composite tRNA gene by anticodon loop transplant. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5092–5096. doi: 10.1073/pnas.77.9.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M. Translational efficiency of transfer RNA's: uses of an extended anticodon. Science. 1982 Nov 12;218(4573):646–652. doi: 10.1126/science.6753149. [DOI] [PubMed] [Google Scholar]



