Abstract
Introduction
Peripheral sudomotor dysfunction is present in many peripheral neuropathies, but structural assessments of sudomotor fibers rarely occur.
Methods
We evaluated 36 diabetic and 72 healthy control subjects who underwent detailed neurologic examinations and punch skin biopsies. Physical exam findings were quantified by neuropathy impairment score in the lower limb. Skin biopsies quantified intra-epidermal nerve fiber density (IENFD) and sweat gland nerve fiber density (SGNFD) by a manual, automated and semi-quantitative method.
Results
The automated and manual SGNFD correlated with the IENFD at the same site (r=0.62, P<0.05 automated method, r=0.67, P<0.05 manual method). As neuropathy worsened the SGNFD at the distal leg declined (automated counting r=−0.81, P<0.001; manual counting r=−0.88, P<0.001). The semi-quantitative method displayed poor inter- and intra-reviewer reliability and correlated poorly with standard neuropathy evaluation scores.
Discussion
Our results suggest that sudomotor fibers can be rapidly and reproducibly quantified, and results correlate well with physical exam findings.
Keywords: Sweat glands, sudomotor, neuropathy, autonomic, skin biopsy
INTRODUCTION
Many length dependent neuropathies prominently target small nerve fibers and cause pain, impaired sensation and increased risks of ulceration, infection and amputation.1 The evaluation of sudomotor nerve fibers through functional testing is well established and has direct clinical relevance; quantitative sudomotor axon reflex testing, thermoregulatory sweat testing and other post-ganglionic sudomotor function tests may provide the earliest evidence of distal small fiber neuropathy.2–4
Skin biopsy evaluation of intra epidermal nerve fiber density (IENFD) is a useful tool in the investigation of neuropathies that affect unmyelinated nociceptive C-fibers.5, 6 Despite the clinical utility of functional sudomotor testing, there are few reports of structural quantitation of sudomotor nerve fibers.7–9
We recently described a method to quantitate the density of nerve fibers that innervate sweat glands using tissue obtained for standard punch biopsy IENFD analysis9. The technique requires manual quantification of sudomotor fibers by a trained observer. Although the technique is reproducible and correlates with the gold standard unbiased stereology, it is labor intensive. In this paper, we introduce an automated method to quantitate sudomotor nerve fibers and compare this to our previously published manual technique and a descriptive, semi-quantitative clinical approach in healthy control and diabetic subjects with and without neuropathy.9.
METHODS
Participants
Thirty-six patients with diabetes and seventy-two control subjects were recruited between March 2005 and December 2008. Diabetic subjects between the ages of 18–70 were recruited from the clinical practices of the investigators. Healthy control subjects between the ages of 18–70 were recruited by local advertisement and had no medical illnesses. Control subjects were taking no medications and had normal general and neurologic examinations. Control subjects had normal fasting blood glucose levels within the past year; subjects with impaired fasting glucose or elevated glycosylated hemoglobin levels were excluded. Exclusion criteria for subjects with diabetes included use of anti-coagulants and antiplatelet agents (e.g., warfarin, aspirin or clopidogrel), a history of lidocaine allergy, impaired wound healing, or other medical conditions that could pose a risk to the patient. Our study was approved by the institutional review board at Beth Israel Deaconess Medical Center. All patients gave informed consent at the time of the study.
Study protocol
All subjects had a complete medical history, diabetic history, anthropomorphic measurements and general examination. Subjects were assessed using the Neuropathy Impairment Score in the Lower Limb (NIS-LL), a quantifiable examination of the peripheral nervous system.10, 11 The NIS-LL system ranges from 0 (no neuropathy) to 88 (the most severe neuropathy). Muscle strength in the lower extremities (measured by extension and flexion at the hip, knee, ankle and toe) is graded for a maximal score of 64 if the subject is paraplegic. Reflexes are graded at the knee and ankle for a maximum of 8 points if the subject is areflexic in the lower extremities. Sensation is assessed at the terminal phalanx of the great toe (touch pressure, pinprick, vibration and joint position) for a maximum of 16 points if the subject is insensate in both great toes. In our study, diabetic subjects with NIS-LL scores >8 were excluded to ensure a homogenous group of patients with mild or no neuropathy (an NIS-LL score of 8 might equate to absent ankle reflexes and absent vibration at the great toes). All healthy control subjects had NIS-LL scores of 0. All participants had 3mm punch skin biopsies from the distal leg, distal thigh, and proximal thigh at standard sites.12, 13
Immunohistochemistry
Skin biopsy specimens were fixed with PLP (paraformaldehyde-lysine-periodate) and cryoprotected.14 Tissue blocks were cut by freezing microtome into 50 μm sections.15 Four randomly selected sections from each biopsy were evaluated. The sections were selected from the middle 30 tissue sections by a random number generator (a typical biopsy has 50–60 sections; the first 10 sections were excluded as were any remaining sections beyond the 40th). These sections on the extreme ends of the biopsy are prone to artifacts and are routinely excluded.16 Tissues were stained with antibodies to PGP 9.5 (ubiquitin hydrolase, Chemicon) using standard techniques.5, 13, 17 We studied 3 additional biopsies from the proximal thighs of 3 healthy control subjects. Tissue sections were fixed with PLP and cut into 50 μm sections. The biopsies were sectioned exhaustively and co-stained with PGP 9.5 and tyrosine hydroxylase (TH - a marker of sweat gland neuroendocrine cells).9 Briefly, the sections were treated with 0.25% potassium permanganate (Sigma, St. Louis, MO) for 15 minutes and 5% oxalic acid (Sigma, St. Louis, MO) for 2 minutes. The sections were placed in block solution for one hour, incubated with rabbit polyclonal PGP 9.5 (Chemicon) and mouse monoclonal TH (Chemicon) antibodies for one hour and moved to 4°C overnight. Samples were incubated with mixtures of donkey anti-rabbit IgG conjugated to the fluorophores cyanine 3 (1:500) and biotinylated donkey anti-mouse IgG (Jackson ImmunoResearch Inc. 1:700) for 5 hours, followed by streptavidin labeled fluorophores cyanine 2 (Jackson ImmunoResearch Inc. 1:400).18 Sections were washed with TBS containing 0.5% Triton x100 3×20 minutes then mounted on coverslips for viewing. Z-stack images of sweat glands were taken in 2–5 μm optical sections acquired at successive levels through the 50 μm thick tissue sections (Zeiss LSM Pascal Exciter).
Intra-epidermal nerve fiber density
Biopsies underwent blinded IENFD counting using standard light microscopy techniques.19 Briefly, epidermal nerve fibers that pierced the basement membrane were counted, while those that did not were excluded.16 The total number of nerve fibers counted was averaged by length of each biopsy and the number of sections with results reported as nerve fibers per millimeter.16
Sweat gland imaging
Every sweat gland was digitally captured during light microscopy using a 6 megapixel camera (PixeLINK PL-686, Ottawa, ON) mounted on an Olympus BH-2 trinocular head microscope, with images taken at 20x magnification. In addition, an out of focus image of each sweat gland was taken and color-subtracted from the in-focus image to form a composite image.20, 21 This digital image processing technique, the ‘unsharp mask filter’, reduces non-specific background staining and visually accentuates nerve fibers (Image Pro Plus, Media Cybernetics, Bethesda, MD). It has been used to augment pathologic studies in a variety of disciplines.9, 20 This technique is more reproducible than visual thresholding using computer assistance because of the variance in nonspecific polyclonal anti-PGP antibody binding to sweat glands. The composite image is used for both the automated and manual quantitative methods.
The variability in sweat gland size requires that nerve density be normalized by sweat gland area. The area of interest (AOI) of the sweat gland was selected using an out of focus image as previously described.9 This was automated (Image Pro Plus, Media Cybernetics, Bethesda, MD) using the freeform selection tool in order to remove individual reviewer variability entirely.
Semi-quantitative evaluation of sweat gland innervation
The composite images of sweat glands were reviewed and scored semi-quantitatively on a 5 point scale. Scores of 0 (no identifiable nerve fibers), 1 (severely reduced nerve fiber density), 2 (moderately reduced nerve fiber density), 3 (mildly reduced nerve fiber density) and 4 (normal nerve fiber density) were provided for each image. The two reviewers with extensive experience in this technique jointly assessed 50 biopsies prior to the study to establish agreement and consistency on the 5 point scoring system. All study images were reviewed in a blinded fashion twice at two different time points by the two reviewers to assess reliability and reproducibility. Reviewers were blinded to each others assessment.
Automated quantification of sweat gland innervation
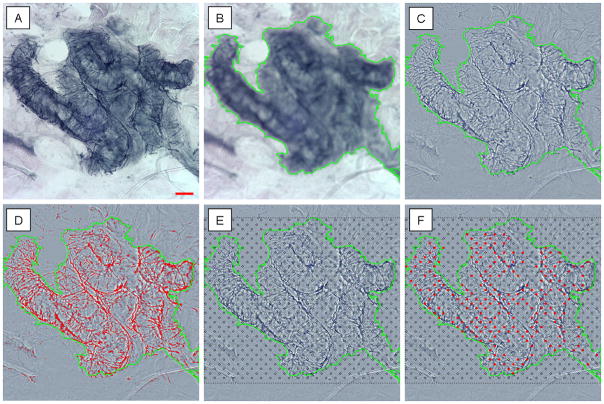
The nerve fibers in the area of interest were selected using computer assisted image analysis,21 to determine the percent surface area covered by nerve fibers. Briefly, the composite image (created from the base image and the blurred image) has no background staining leaving nerve fibers clearly identifiable. The nerve fibers are highlighted within the selected area of interest using a histogram based segmentation tool. In brief, the selection tool traces the dark nerve fibers and quantifies the number of highlighted pixels within the area of interest (Image Pro Plus, Media Cybernetics, Bethesda, MD). The results are expressed as the percent area of nerve fibers within the area of interest. This approach is demonstrated in detail in Figure 1A–D. Intra- and inter-reviewer reliability was measured by randomly selecting 48 images (30 control and 18 diabetic) to be re-measured by the primary reviewer and by two other observers.
Figure 1. The quantification of sudomotor nerve fibers.
The unadjusted image of a sweat gland is seen (A). The out of focus image of the same sweat gland can be seen in (B), the out of focus image is used to select the area of interest through the use of the ‘freeform magic wand’ tool (shown in green). The composite image (C) is created by subtracting the background color in the out of focus image (B) from the base image (A), a technique known as the ‘unsharp mask filter’. The composite image undergoes a histogram based segmentation selection to automatically highlight the nerve fibers within the area of interest (D), with the results expressed as a percent area. The manual counting method applies a grid over the composite image (E), and any intersections between nerve fibers and the circles are counted (F). The results are expressed as the percent of intersections (out of the total number possible). Scale bar = 50 μm. Because results of the manual and automated SGNFD are expressed in different units, direct comparison of values is not possible.
Manual quantification of sweat gland innervation
Every sweat gland was also analyzed through manual quantitation, as previously reported.9 Briefly, a standardized grid of circles 10 μm in diameter spaced 50 μm apart horizontally, and offset 25 μm vertically was placed over the sweat gland image area of interest. Nerve fibers that intersect the circles were counted. The results are expressed as the number of circles intersected out of the total number of circles present within the area of interest (percent intercept density) as shown in Figure 1E–F.9 Intra- and inter-reviewer reliability was measured by randomly selecting 48 images (30 control and 18 diabetic) that were re-measured by the primary reviewer and by two other observers.9
Unbiased stereologic quantification of sweat gland innervation
Skin biopsies from 3 healthy control subjects were analyzed by an unbiased stereologic technique using a cycloid test system to estimate total nerve fiber length surrounding sweat glands as previously described.22 Complete Z-stack images of the sweat glands were studied using a cycloid test system and compared against a 20 μm extended depth of field image for SGNFD analysis using both the automated and manual counting methods. This technique has been described in detail in our previous paper comparing the manual method to unbiased stereology.9 Briefly, a grid of curved cycloids is placed over the sweat gland image. The intersections between the cycloids and nerve fibers are counted manually using a 3 dimensional stepping pattern.9, 23 This labor intensive approach provides an estimate of the total length of nerve fibers within a given area of tissue (in this case, within the sweat gland structure). The stereologic results were compared to both the automated and manual quantitative approaches as a gold standard.
Statistical analysis
Statistical analysis was performed using SPSS v15 (Chicago, IL). Results are expressed as mean ± standard deviation. Subgroups of patients are reported for demographic purposes, but all data for analysis is grouped into control or diabetic subjects. Pearson correlation coefficients (r) were calculated to assess simple relationships between variables. Interclass correlation coefficients were calculated to assess reliability within and between examiners. Inter- and intra-reviewer reliability testing was measured for the entire process: from the recreation of the composite image, selection of the area of interest and counting of the SGNFD.
RESULTS
Demographics
Thirty-six diabetic (17 male and 19 female) and 72 control subjects (35 male and 37 female) completed the study. Demographic data for the diabetic subjects is shown in Table 1; control subjects had similar age, sex, height, weight and body mass index. Control subjects all had NIS-LL scores of 0. A total of 526 sweat glands were identified from 324 skin biopsies.
Table 1. Demographic Characteristics of Subjects with Diabetes and Controls.
General characteristics of patients are outlined by sex and diabetes type (with corresponding control group). There was no significant difference in Neuropathy Impairment Score in the Lower Limb (NIS-LL) among those with diabetes.
| Patient Type | Number | Age | Duration Diabetes (years) | Body Mass Index | NIS-LL |
|---|---|---|---|---|---|
| Diabetes | |||||
| Type 1 Female | 10 | 34±11 | 13±7.1 | 18.3±2.2 | 4.1±3.2 |
| Type 1 Male | 8 | 34±12 | 12±7.2 | 22.3±2.3 | 4.2±3.4 |
| Type 2 Female | 9 | 47±11 | 4.6±4.6 | 32.4±4.5 | 4.1±3.2 |
| Type 2 Male | 9 | 49±11 | 5.1±4.2 | 34.2±5.8 | 4.8±3.3 |
| Controls | |||||
| Type 1 Female | 18 | 34±9 | 0 | 18.5±2.6 | 0 |
| Type 1 Male | 17 | 35±10 | 0 | 23.0±2.6 | 0 |
| Type 2 Female | 19 | 47±13 | 0 | 32.3±5.4 | 0 |
| Type 2 Male | 18 | 49±11 | 0 | 34.7±6.2 | 0 |
Intra-epidermal nerve fiber density
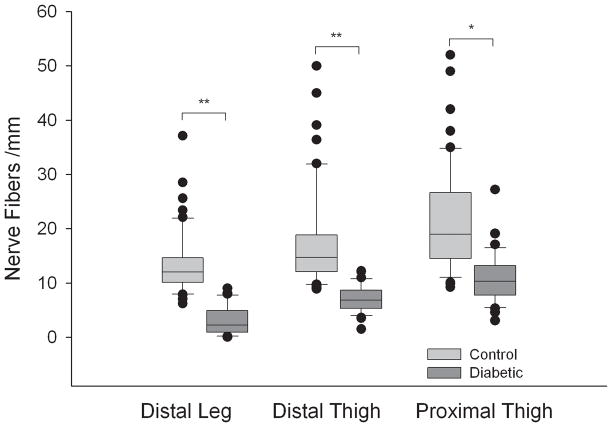
Intra-epidermal nerve fiber density was lower at all sites in the group of patients with diabetes compared to the healthy control subjects (P<0.05); the greatest difference was seen at the distal leg (P<0.01). The results are shown in Figure 2. The IENFD was also lower at the distal leg in patients with diabetes that had evidence of neuropathy on examination compared to those with diabetes that had no evidence of neuropathy (NIS-LL 2–8, IENFD 1.5±1.9 nerve fibers/mm; NIS-LL=0–1, IENFD7.4±5.1 nerve fibers/mm; P<0.001). The difference was less at the distal thigh (IENFD 6.9±2.7 vs. 10.4±3.1 nerve fibers/mm; P<0.05) and proximal thigh (IENFD 9.7±5.4 vs. 12.5±4.8 nerve fibers/mm; P=NS).
Figure 2. Intra-epidermal nerve fiber density by location in control and diabetic subjects.
The intra epidermal nerve fiber density at the distal leg, distal thigh and proximal thigh are shown for diabetic and control subjects. Diabetic subjects are denoted by the dark box plots, healthy controls by the clear box plots. The box plots demonstrate the median value, with first and third quartiles outlined by the box, tenth and ninetieth percentiles by the whisker lines and individual outliers shown as solid dots. ** P<0.001, * P<0.01.
Unbiased Stereology
As previously described, a total of 20 sweat glands had complete Z-stack images (with 213 individual images) within the 3 sample biopsies.9 Using the 20 μm extended depth of field view from the confocal images, the 3 sample biopsies showed strong correlations between our gold standard unbiased stereologic estimate of sweat gland nerve fiber length, the manual counting technique (r=0.93, p<0.01) and the automated counting method (r=0.76, p<0.05).
Sweat gland nerve fiber density
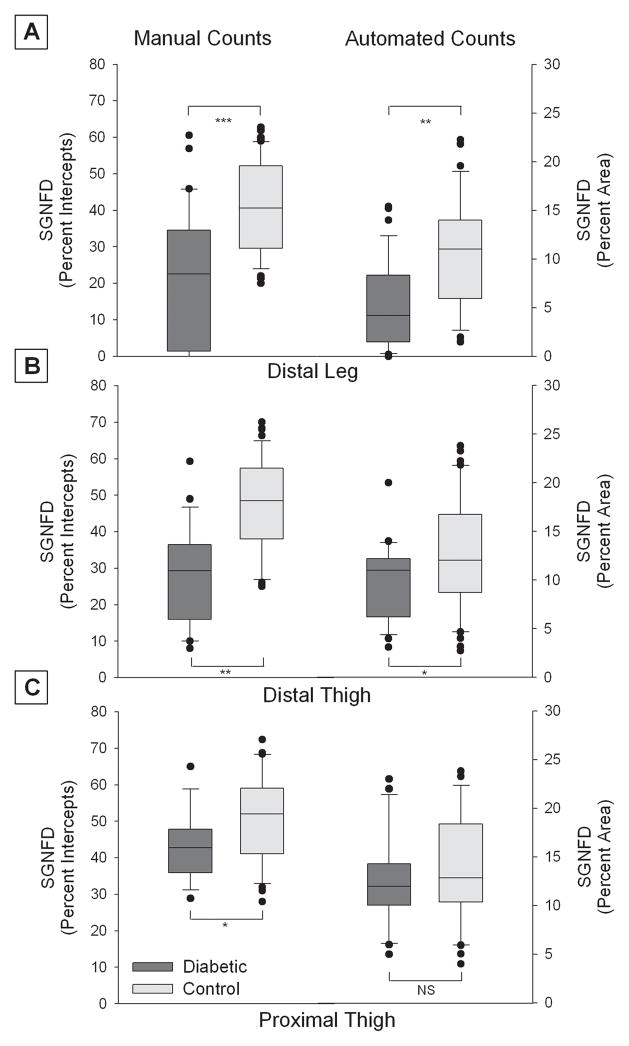
Sweat gland nerve fiber density was lower in the diabetic subjects than the healthy control subjects at all biopsy sites using both the manual and automated methods. The ranges of values for SGNFD by location for both methods are shown in figure 3. There was a greater overlap of confidence intervals between healthy controls and diabetic subjects using the automated method, with greater separation in confidence intervals using the manual method.
Figure 3. Sweat gland nerve fiber density (SGNFD) by location in control and diabetic subjects.
The results of the automated counting method and the manual counting methods are shown at the distal leg (A), distal thigh (B) and proximal thigh (C). The manual count values are denoted on the left Y axis (expressed as intercept density) and the automated count values by the right Y axis (expressed as percent area). Diabetic subjects are denoted by the dark box plots and healthy controls by the clear box plots. The box plots demonstrate the median value, with first and third quartiles outlined by the box, tenth and ninetieth percentiles by the whisker lines and individual outliers shown as solid dots. *** P<0.001, ** P<0.01, * P<0.05, NS = non significant.
The SGNFD was also lower at all sites in patients with diabetes who had evidence of neuropathy on examination (NIS-LL>1) compared to those who had no evidence of neuropathy. The automated SGNFD at all sites for NIS-LL=0–1 is 10.2±3.0, for NIS-LL 2–8 is 7.0±5.5 (P<0.05). The manual SGNFD at all sites for NIS-LL 0–1 is 38.4±12.5, for NIS-LL 2–8 is 23.9±18.5 (P<0.01).
The manual and automated sweat gland nerve fiber density correlated with the intra-epidermal nerve fiber density at the same site (r=0.62, P<0.05 automated method, r=0.67, P<0.05 manual method). The automated and manual sweat gland nerve fiber densities correlated with each other at the distal leg (r=0.81, P<0.01), distal thigh (r=0.69, P<0.05) and proximal thigh (r=0.61, P<0.05). There was limited correlation between semi-quantitative evaluation of sweat gland innervation and NIS-LL scores (r=−0.19, P=NS) and IENFD (r=−0.22, P=NS). If extreme examples were compared (i.e. healthy control vs. diabetic subjects with NIS-LL scores >6), then visual differences could be easily identified. However, at lower NIS-LL scores semi-quantitative differentiation quickly became less reliable.
Relationships between nerve densities and examination scores
The SGNFD was also lower at all sites in patients with diabetes who had evidence of neuropathy on examination (NIS-LL>1) compared to those who had no evidence of neuropathy. The automated SGNFD at all sites for NIS-LL=0–1 is 10.2±3.0, for NIS-LL 2–8 is 7.0±5.5 (P<0.05). The manual SGNFD at all sites for NIS-LL 0–1 is 38.4±12.5, for NIS-LL 2–8 is 23.9±18.5 (P<0.01).
As neuropathy worsened (denoted by increasing NIS-LL score) the sweat gland nerve fiber density at the distal leg decreased (automated counting r=−0.81, P<0.001; manual counting r=−0.88, P<0.001). At more proximal sites, the relationship between the sweat gland nerve fiber density and the NIS-LL score was less (automated counting r=−0.36 distal thigh, r=−0.06 proximal thigh; manual counting r=−0.52 distal thigh, r=−0.43 proximal thigh).
Reliability
The intraclass correlation coefficient (ICC) for intra-reviewer reliability for the automated method was ICC>0.91, P<0.001 and for the manual method ICC>0.88, P<0.001. The intraclass correlation coefficient for inter-reviewer reliability for the automated method was ICC>0.93, P<0.001 and for the manual method ICC>0.89, P<0.001. The intraclass correlation coefficient (ICC) for intra-reviewer reliability for the semi-quantitative method was ICC=0.46, P=NS and for the inter-reviewer reliability was ICC=0.39, P=NS despite pre-study training.
DISCUSSION
We have successfully quantified the density of nerve fibers surrounding sweat glands using a rapid automated method. The results show that the automated method has a high degree of inter- and intra-reviewer reliability. It is easily reproducible, and it correlates with IENFD, standardized neuropathy examination scores, unbiased stereology and our previously reported manual method. In contrast, the semi-quantitative method displays poor inter- and intra-reviewer reliability and correlates poorly with standard neuropathy evaluation scores.
Autonomic testing is widely used in the assessment of distal small fiber neuropathy.24 Similarly, there is a growing body of evidence that supports the clinical use of skin biopsy analysis of intra-epidermal nerve fiber density in the evaluation of distal sensory polyneuropathy.25 To date, there are few reports that quantify the density of autonomic nerve fibers in the skin.7, 8, 26 The clinical utility of skin biopsies could be expanded if a quantitative structural assessment of cutaneous autonomic nerves was combined with quantitation of small sensory nerve fibers.
We have previously reported a method to quantify the density of autonomic nerve fibers surrounding sweat glands.9 This technique correlated well with intra-epidermal nerve fiber density, clinical neuropathy exam scores and an unbiased confocal microscopy stereologic analysis of sweat gland nerve fiber density. Although not as labor intensive as the confocal imaging technique, which takes many hours per sample, the manual counting technique takes approximately 15 minutes per sweat gland for analysis. The automated technique to quantify sweat gland nerve fiber density reported in this study is substantially faster than the manual quantification technique. It requires only 1 minute per image for analysis while still showing a strong correlation with the unbiased stereologic technique. The correlations between the SGNFD and the NIS-LL were strongest in the distal leg and distal thigh; the lower correlations in the proximal thigh are due to the relatively mild degree of neuropathy studied.
Although both techniques clearly differentiated a group of diabetic patients from healthy control subjects, the automated area analysis approach has a larger range of values for both diabetic and control subjects and greater overlap of confidence intervals (Figure 3). There may be several explanations for this variability. First, although the use of a fully automated analysis system reduces the time for image analysis, this method reduces the capacity to adjust to changes in image quality. Second, the use of polyclonal anti-PGP 9.5 antibodies creates non-specific tissue uptake that is often counted using an automated system despite optimal image thresholding and the ‘unsharp mask filter’. There are also some methodological differences between the two approaches to sweat gland nerve fiber density quantification. The two approaches appear to have higher levels of agreement at the distal leg site, but lower agreement in patients with intermediate levels of neuropathy (NIS-LL scores of 1–5) at more proximal biopsy sites. The differences between the two techniques are in part due to the presence of nerve fiber thickening that occurs together with a reduction in nerve fiber number in some patients with diabetes.
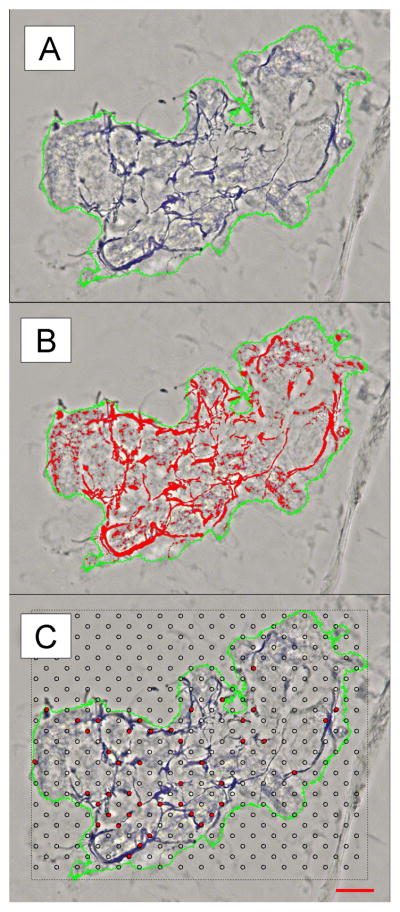
We have previously suggested that these thickened nerve fibers bear some similarities to reports of neuroaxonal dystrophy seen in the autonomic ganglia in animal models of diabetic neuropathy.9, 27, 28 The dystrophic nerve fibers result in an increase in SGNFD using the automated method (thickened fibers increase the surface area) while simultaneously decreasing the SGNFD using the manual method (due to a reduction in the number of fibers). This problem is highlighted in Figure 4, where the automated count is high, and the manual count is low in the sweat gland from the distal thigh of a diabetic subject with an NIS-LL score of 4. These dystrophic fibers appear to cause broader confidence intervals with the automated method and reduce the differences between patients and control subjects. More sophisticated counting algorithms that consider fiber thickness may improve this discrepancy.
Figure 4. Discrepancies between automated and manual SGNFD in a subject with diabetes.
The sweat gland from the distal thigh of a subject with diabetes is shown in (A). Note the thickened nerve fibers that are highlighted in red by the automated counting method in (B) that give an overall high SGNFD. The manual counting method has fewer intercept points, shown in red in (C), because the thickened nerve fibers are not as evenly distributed throughout the sweat gland, reducing the SGNFD. Scale bar = 50 μm.
We also performed a semi-quantitative analysis of the sweat gland nerve fiber density as reported by other investigators. This technique is similar to the descriptive clinical analysis of intra-epidermal nerve fibers.7, 8, 29, 30 A simple descriptive method to quantify the innervation of sweat glands could significantly augment the utility of the skin biopsy for clinical diagnosis. The poor inter- and intra-reviewer reliability of the semi-quantitative method does not support the use in studies or clinical practice. A major factor that distinguishes our study from other published semi-quantitative methods is the relatively mild degree of neuropathy in our patient group. A study including more severely affected diabetic neuropathy subjects may have shown a stronger correlation because of the bimodal distribution. This observation highlights a limitation of semi-quantitative methodologies; robust results are seen in late stage progressive diseases but are of limited utility in detecting early nerve fiber damage.
We have successfully quantified the density of nerve fibers surrounding sweat glands using a novel automated technique. The technique distinguishes a group of patients with mild diabetic neuropathy (NIS-LL range of 0–8) from a group of healthy control subjects. While the diagnostic discrimination of the manual technique appears superior, the automation and speed of the analysis lends support for its use in screening or epidemiologic studies involving diabetic peripheral and autonomic neuropathy. Future studies are required to correlate these structural changes with assessments of sudomotor function, such as quantitative sudomotor axon reflex testing (QSART), in both control and diabetic subjects, and to provide normative data.
Acknowledgments
This research was supported by the Juvenile Diabetes Research Foundation 11-2007-143, NIH K23NS050209, the Langer Family Foundation and the Harriet Lewis Foundation.
Abbreviations
- AOI
Area of interest
- ICC
intraclass correlation coefficient
- IENFD
Intra-epidermal nerve fiber density
- NIS-LL
Neuropathy impairment score in the lower limb
- PLP
Paraformaldehyde lysine periodate
- PGP 9.5
Protein gene product 9.5
- SGNFD
Sweat gland nerve fiber density
- TH
Tyrosine hydroxylase
References
- 1.Abbott CA, Vileikyte L, Williamson S, Carrington AL, Boulton AJ. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21:1071–1075. doi: 10.2337/diacare.21.7.1071. [DOI] [PubMed] [Google Scholar]
- 2.Low VA, Sandroni P, Fealey RD, Low PA. Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve. 2006;34:57–61. doi: 10.1002/mus.20551. [DOI] [PubMed] [Google Scholar]
- 3.Low PA, Caskey PE, Tuck RR, Fealey RD, Dyck PJ. Quantitative sudomotor axon reflex test in normal and neuropathic subjects. Annals of Neurology. 1983;14:573–580. doi: 10.1002/ana.410140513. [DOI] [PubMed] [Google Scholar]
- 4.Fealey RD, Low PA, Thomas JE. Thermoregulatory sweating abnormalities in diabetes mellitus. Mayo Clinic Proceedings. 1989;64:617–628. doi: 10.1016/s0025-6196(12)65338-5. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann DN, Griffin JW, Hauer P, Cornblath DR, McArthur JC. Epidermal nerve fiber density and sural nerve morphometry in peripheral neuropathies. Neurology. 1999;53:1634–1640. doi: 10.1212/wnl.53.8.1634. [DOI] [PubMed] [Google Scholar]
- 6.Polydefkis M, Hauer P, Griffin JW, McArthur JC. Skin biopsy as a tool to assess distal small fiber innervation in diabetic neuropathy. Diabetes Technol Ther. 2001;3:23–28. doi: 10.1089/152091501750219994. [DOI] [PubMed] [Google Scholar]
- 7.Dabby R, Djaldetti R, Shahmurov M, et al. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm. 2006;113:1169–1176. doi: 10.1007/s00702-005-0431-0. [DOI] [PubMed] [Google Scholar]
- 8.Dabby R, Vaknine H, Gilad R, Djaldetti R, Sadeh M. Evaluation of cutaneous autonomic innervation in idiopathic sensory small-fiber neuropathy. J Peripher Nerv Syst. 2007;12:98–101. doi: 10.1111/j.1529-8027.2007.00128.x. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons CH, Illigens BM, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology. 2009;72:1479–1486. doi: 10.1212/WNL.0b013e3181a2e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyck PJ, Kratz KM, Lehman KA, et al. The Rochester Diabetic Neuropathy Study: design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology. 1991;41:799–807. doi: 10.1212/wnl.41.6.799. [DOI] [PubMed] [Google Scholar]
- 11.Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41 (Suppl 1):8–13. doi: 10.1159/000052074. [DOI] [PubMed] [Google Scholar]
- 12.McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Archives of Neurology. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- 13.Holland NR, Crawford TO, Hauer P, et al. Small-fiber sensory neuropathies: clinical course and neuropathology of idiopathic cases. Annals of Neurology. 1998;44:47–59. doi: 10.1002/ana.410440111. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 15.Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- 16.Lauria G, Cornblath DR, Johansson O, et al. EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12:747–758. doi: 10.1111/j.1468-1331.2005.01260.x. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons CH, Griffin JW, Polydefkis M, et al. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66:256–258. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy WR, Wendelschafer-Crabb G, Brelje TC. Innervation and vasculature of human sweat glands: an immunohistochemistry-laser scanning confocal fluorescence microscopy study. J Neurosci. 1994;14:6825–6833. doi: 10.1523/JNEUROSCI.14-11-06825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 20.Oberholzer M, Ostreicher M, Christen H, Bruhlmann M. Methods in quantitative image analysis. Histochem Cell Biol. 1996;105:333–355. doi: 10.1007/BF01463655. [DOI] [PubMed] [Google Scholar]
- 21.Underwood RA, Gibran NS, Muffley LA, Usui ML, Olerud JE. Color subtractive-computer-assisted image analysis for quantification of cutaneous nerves in a diabetic mouse model. J Histochem Cytochem. 2001;49:1285–1291. doi: 10.1177/002215540104901011. [DOI] [PubMed] [Google Scholar]
- 22.Gibbons CH, Illigens MW, Wang N, Freeman R. The quantification of sweat gland innervation: a clinical-pathological correlation. Neurology. 2009 doi: 10.1212/WNL.0b013e3181a2e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stocks EA, McArthur JC, Griffen JW, Mouton PR. An unbiased method for estimation of total epidermal nerve fibre length. J Neurocytol. 1996;25:637–644. doi: 10.1007/BF02284830. [DOI] [PubMed] [Google Scholar]
- 24.England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009;72:177–184. doi: 10.1212/01.wnl.0000336345.70511.0f. [DOI] [PubMed] [Google Scholar]
- 25.England JD, Gronseth GS, Franklin G, et al. Evaluation of distal symmetric polyneuropathy: The role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review) Muscle Nerve. 2009;39:106–115. doi: 10.1002/mus.21227. [DOI] [PubMed] [Google Scholar]
- 26.Donadio V, Nolano M, Provitera V, et al. Skin sympathetic adrenergic innervation: an immunofluorescence confocal study. Annals of Neurology. 2006;59:376–381. doi: 10.1002/ana.20769. [DOI] [PubMed] [Google Scholar]
- 27.Clark HB, Schmidt RE. Identification of dystrophic sympathetic axons in experimental diabetic autonomic neuropathy. Brain Research. 1984;293:390–395. doi: 10.1016/0006-8993(84)91250-2. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt RE, Dorsey DA, Beaudet LN, Parvin CA, Zhang W, Sima AA. Experimental rat models of types 1 and 2 diabetes differ in sympathetic neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:450–460. doi: 10.1093/jnen/63.5.450. [DOI] [PubMed] [Google Scholar]
- 29.Donadio V, Cortelli P, Falzone F, et al. Isolated generalised anhidrosis induced by postganglionic sympathetic skin nerve fibre degeneration: an incomplete Ross syndrome? J Neurol Neurosurg Psychiatry. 2008;79:959–961. doi: 10.1136/jnnp.2007.142802. [DOI] [PubMed] [Google Scholar]
- 30.Donadio V, Nolano M, Elam M, et al. Anhidrosis in multiple system atrophy: a preganglionic sudomotor dysfunction? Mov Disord. 2008;23:885–888. doi: 10.1002/mds.21972. [DOI] [PubMed] [Google Scholar]