Abstract
PAPA syndrome (Pyogenic Arthritis, Pyoderma gangrenosum, and Acne) is an autosomal dominant, hereditary auto-inflammatory disease arising from mutations in the PSTPIP1/CD2BP1 gene on chromosome 15q. These mutations produce a hyper-phosphorylated PSTPIP1 protein and alter its participation in activation of the “inflammasome” involved in interleukin-1 (IL-1β) production. Overproduction of IL-1β is a clear molecular feature of PAPA syndrome. Ongoing research is implicating other biochemical pathways that may be relevant to the distinct pyogenic inflammation of the skin and joints characteristic of this disease. This review summarizes the recent and rapidly accumulating knowledge on these molecular aspects of PAPA syndrome and related disorders.
Keywords: Auto-inflammatory disease, PAPA syndrome, PSTPIP1, CD2BP1, PTP-PEST, pyrin, neutrophils, microarray transcript profiling, anakinra, IL-1β.
INTRODUCTION
The auto-inflammatory disorders bear resemblance to classic autoimmune diseases such as systemic lupus in that they present with seemingly unprovoked inflammation but, unlike most autoimmune diseases, high-titer auto-antibodies or antigenic-specific T lymphocytes are not found [1-3]. Considered broadly, this disease class is characterized by abnormalities in the innate immune system. Familial Mediterranean Fever (FMF), a recessive disorder caused by mutations in the gene encoding the pyrin protein, is the founding member of this disease class [3] that is marked by episodic inflammation of serosal or synovial tissue, fever, and occasional lesions of the skin. Many disorders are now recognized as auto-inflammatory. Some, like FMF, display simple Mendelian inheritance and are caused by mutations in single genes [2-4]. Examples include TNF receptor-associated periodic syndrome (TRAPS) [2], hyperimmunoglobulinemia D with periodic fever syndrome (HIDS) [5, 6], familial cold auto-inflammatory syndrome (FCAS)/Muckle-Wells syndrome (MWS), neonatal onset multisystem inflammatory disease (NOMID) [7], deficiency of the interleukin-1-receptor antagonist (DIRA) [8, 9]; the granulomatous diseases such as Blau syndrome [10], early onset sarcoidosis [11], and chronic granulomatous disease (CGD) [12]; and the pyogenic disorders Majeed syndrome [13] and PAPA syndrome [14]. Auto-inflammatory underpinnings are now also recognized in common, less heritable conditions including Crohn’s disease and systemic-onset juvenile idiopathic arthritis (SoJIA) [15-17]. Over the past decade our understanding of both the clinical characteristics and molecular pathogenesis of the auto-inflammatory diseases has expanded dramatically (for an extensive review of this disease class please see reference [3]). Accordingly, a new classification system was recently proposed that defines auto-inflammatory disease by one of six categories according to its underlying molecular pathology [3]. The chronicle of PAPA syndrome, a relatively recent addition and one of the more clinically dramatic members of the auto-inflammatory disease family, is a relevant illustration of diagnostic, molecular, and clinical advances in the field. In this review we summarize the ongoing elucidation of PAPA syndrome pathogenesis, with particular regard to the impact of molecular discovery on the diagnosis and management of affected patients and families.
CLINICAL CHARACTERISTICS OF PAPA SYNDROME
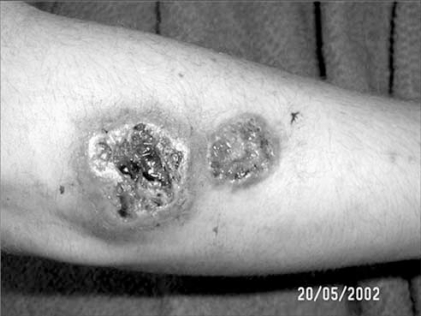
PAPA syndrome (OMIM #604416) was described as a heritable disease in an extended family in 1997 [14]. We now appreciate that PAPA syndrome was in fact first reported in a single male patient described with “streaking leukocyte factor”, arthritis, and pyoderma gangrenosum in 1975 [18]. “Streaking leukocyte factor” referred to a partially purified, ~160 kd component of this patient’s serum that enhanced the random migration of normal mononuclear cells and neutrophils in laboratory studies. (The identity of this “factor” is unresolved.) Both reports described joint inflammation as generally responsive to high dose corticosteroids. A second extended family was described in 2000 as “familial recurrent arthritis” [19]. Additional families and cases are now reported (described later in this review). Distinguishing features of PAPA syndrome include early onset, florid, and painful flares of recurrent sterile arthritis involving a prominent neutrophilic infiltrate, and autosomal dominant inheritance. Skin involvement is more variable and may present as ulcerations, frank pyoderma gangrenosum, or severe cystic acne (Fig. 1). Standard laboratory findings typically reflect systemic inflammation but are otherwise non-diagnostic; however elevated production of interleukin-1 beta (IL-1β) and tumor necrosis factor (TNFα) in peripheral blood leukocytes has been reported in more recent literature [20-22]. Symptoms persist into adulthood, with a natural history of significant joint destruction. Anecdotally, some individuals report significant psycho-social impairment due to physical disability, steroid-induced Cushingoid appearance, and permanent, wide-spread cutaneous scarring.
Fig. (1).
Pyoderma gangrenosum in a child with PAPA syndrome.
PSTPIP1 / CD2BP1
Positional cloning methods subsequently identified co-segregating missense mutations in the proline-serine-threonine phosphatase-interacting protein 1 gene (PSTPIP1, also known as CD2 binding protein 1 (CD2BP1)) in the original family that defined the syndrome and a second extended family (originally described with “familial recurrent arthritis”) [23-24]. Other PAPA syndrome patients with either of these PSTPIP1 mutations have been reported more recently [25, 26]. Specifically, missense mutations (c.904G>A) or (c.964G>C) occur in exons 10 and 11, creating A230T or E250Q variants, respectively, in affected individuals. The observation of the same mutation in seemingly unrelated families suggest either a founder effect, particularly as these were each observed in cases within the same ethnic group, i.e. European descent, or a mutational hotspot. Otherwise, two additional missense mutations are reported, both in exon 11 and creating E250K or D260N amino acid changes. This genotype and phenotype information is documented and continuously updated for PAPA syndrome and the other auto-inflammatory diseases within the INFEVERS database, a web-based resource (please see http://fmf.igh.cnrs.fr/ISSAID/infevers/) [17]. It is interesting that other putative cases of PAPA syndrome have proven negative for PSTPIP1 coding or splicing mutations (Table 1) [our unpublished data and reference 27]. This suggests the possibility that alterations in other gene(s) could evoke features of PAPA syndrome. Notably, also, cases with isolated pyoderma gangrenosum or Crohn’s disease-associated pyoderma gangrenosum, have thus far proven negative for PSTPIP1 mutations. However, recently a CCTG repeat in the PSTPIP1 promoter of patients with Crohn’s disease or aseptic abscesses syndrome was identified [28] that may play a pathogenic role in these diseases. CD2BP1 (PSTPIP1) and CARD15 mutations are not associated with pyoderma gangrenosum in patients with inflammatory bowel disease (Table 1) [29]. Further study is required to explain PSTPIP1 mutation-negative cases of putative PAPA syndrome. In the meantime, the low complexity of disease-associated mutations in PSTPIP1 simplifies diagnostic screening for patients with a tentative diagnosis of PAPA syndrome, and a DNA sequence-based test for PSTPIP1 coding mutations is now commercially available. It is worth noting that the PAPA-associated mutations presently identified are highly predictive, as reflected by the essentially complete disease penetrance they confer, and thus provide useful information in the context of genetic counseling for families.
Table 1.
Summary of PSTPIP1 Mutation Screening for Cases of Putative PAPA Syndrome. Cases were screened in the order given. The last three cases were accepted for screening based on the presence of three or more features of PAPA syndrome. *Exons 10 and 11 only were screened.
| Subject | Country of Origin | Sex | Family History | Onset | Clinical Characteristics | PSTPIP1 Mutation |
|---|---|---|---|---|---|---|
| 1 | Spain | M | affected brother, mother | Child | arthritis; psoriasis; pyoderma gangrenosum | A230T (confirmed in affected brother) |
| 2 | Italy | M | mother mildly affected | Child | “consistent with PAPA syndrome” | Negative |
| 3 | USA | M | negative | Adult | inflammations around surgical incisions-pyoderma gangrenosum? Subsequent severe bleeding. Responsive to cortico-steroids | Negative |
| 4 | USA | F | multiple individuals with reported arthritis, fibromyalgia, diabetes mellitus | 7 | severe pauci-articular corticosteroid responsive arthritis; recurrent destructive pyoderma gangrenosumat age 11; recent dep venous thrombosis, pulmonary hypertension and congestive heart failure | None detected in screens of exon 10 or 11 |
| 5 | Canada | M | undetermined; father’s history incomplete | 1 | Recurrent sterile pyogenic arthritis in knee; fever, synovial expansion into muscle above and below knee | A230T |
| 6 | New Zealand | M | positive with apparent dominant inheritance | 1.5 | Recurrent pyogenic arthritis | E250Q (confirmed in affected parent) |
| 7 | USA | M | mother with positive ANA and acne | 14 | arthritis in fingers, toes, and large joints; severe acne; ulcerated lesions bilaterally on hands, forearms, elbows described as pyoderma gangrenosum; aphthous ulcers; negative laboratory findings | None detected in screens of exons 10 and 11 |
| 8 | France (Toulouse) | M | negative | ? | recurrent arthritis of knees; painful involvement of chest, lower back, and sacrum regions; acute episodic abdominal pain and fever; severe cystic acne; recurrent pyoderma of axillae, groin, and face | Negative |
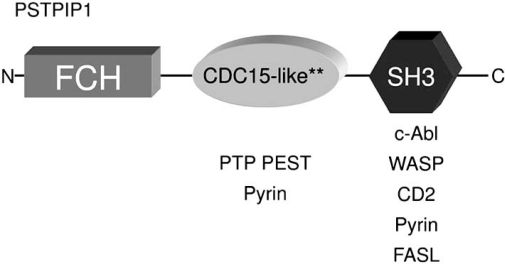
Interestingly, there is a homolog of PSTPIP1, named proline-serine-threonine-phosphatase-interacting protein 2 (PSTPIP2), encoded on chromosome 18 in both humans and the mouse. PSTPIP1 and PSTPIP2 predicted proteins share approximately 41% amino acid identity, but PSTPIP2 is lacking the terminal src-homology 3 (SH3) domain found in PSTPIP1 (Fig. 2) [30]. At present it is unclear if PSTPIP1 and PSTPIP2 are completely distinct, or if they participate in overlapping biochemical pathways. Both proteins possess Fer-CIP4 and coiled-coil domains involved in binding with PEST-type phosphatases. Fer-CIP4 domains also bind phosphatidyl inositol (4, 5) bisphosphonate (PI(4,5)P2) on artificial liposomes with high affinity, inducing membrane tubulation and suggesting that both could participate in coupling membrane deformation to cytoskeletal reorganization (described in more detail below) [31]. Several lines of evidence link PSTPIP2 with yet another auto-inflammatory condition. In the mouse, spontaneous recessive mutations in PSTPIP2 produce a phenotype most closely resembling human chronic multifocal osteomyelitis (CRMO), another auto-inflammatory disease of bone and skin. Human PSTPIP1 is encoded on chromosome 18q21.3-22 and overlaps a susceptibility locus for CRMO [32]. Based on these observations, PSTPIP2 could be an attractive candidate gene for cases with PAPA-like features that are negative for PSTPIP1 mutations, however, no mutations have been identified. Further functional studies, particularly utilizing PSTPIP2 mouse mutants, will provide insight into the biochemical, and possibly clinical relationship of these two proteins.
Fig. (2).
PSTPIP1 protein schematic. FCH, coiled-coil, and SH3 domains are shown. Asterisks denote the relative positions of the A230T and E250Q mutations. Interacting proteins are shown below single domains for simplicity. It should be noted that some in vitro studies indicate more complex binding patterns than depicted here.
PSTPIP1 BINDING PROTEINS
PSTPIP1 is a cytoskeletal adaptor protein that was originally identified in the mouse through its interaction with PEST (rich in proline (P), glutamic acid (E), serine (S), and threonine (T) residues)-type protein tyrosine phosphatase (PTP-PEST, also known as PTPN12) [33]. The human homolog, called CD2BP1, was identified near the same time by interaction with the T cell surface protein CD2 [34]. The gene and protein are now generally cited as “PSTPIP1”. The fact that PAPA syndrome mutations clustered in the coiled-coil region of the protein immediately cast suspicion that the PTP-PEST interaction mediated through this domain was central to pathology. Indeed, the E250Q and A230T variants of PSTPIP1 were shown to severely abrogate binding to PTP-PEST in yeast two hybrid and co-immunoprecipitation experiments [21, 24]. At least one consequence of this is hyperphosphorylation of PSTPIP1 itself. Tryptic peptide mapping identified tyrosine 344 as a primary phosphorylation site of PSTPIP1. Experiments using a combination of co-transfection, immunoprecipitation and anti-phosphotyrosine antibody western blots have shown that binding of PTP-PEST to PSTPIP1 is essential for its dephosphorylation [21, 35, 36]. Interestingly, PTP-PEST−/− fibroblasts contain hyperphosphorylated PSTPIP1 and a defect in cytokinesis [35].
PSTPIP1 is highly expressed in hematopoietic cells, and the protein interacts with other immune-related proteins in addition to CD2 and PTP-PEST. These include Wiskott Aldrich Syndrome protein (WASP), c-Abl kinase, and Fas ligand (FasL) [36-38]. Cumulative evidence suggests that each of these proteins acts as a “substrate”, binding primarily via the PSTPIP1 SH3 domain (Fig. 2) to be delivered to PTP-PEST (or homolog) for de-phosphorylation [39-41]. It is not obvious whether A230T or E250Q mutations directly alter these interactions, or may mediate effects due to hyperphosphorylation of the “substrate” or of PSTPIP1 itself. For example, the PSTPIP1/WASP interaction is phosphorylation-dependent, and disease mutations consequently predict reduced interaction in vivo. Thus WASP-mediated cytoskeletal reorganization events are implicated in PAPA pathogenesis via post-translational mechanisms [24, 36]. In the case of FasL, it is clear that this protein forms a ternary complex with PSTPIP1/PTP-PEST, and that PSTPIP1 mediates its sequestration away from the cell surface and into intracellular secretory lysosomes [38, 39]. This suggests a general inhibitory effect on cytotoxic T cell functioning. Further study will elucidate the effects of PAPA causative mutations on these interactions and their relationship to manifestations of the disease (see more discussion later in this review). Taken altogether, current knowledge suggests multiple functions for a PSTPIP1/PTP-PEST complex in hematopoietic cells that may have direct consequences for immune cell adhesion, invasion, and migration [36-46]. It is worth noting that PSTPIP1 also interacts with other PTP-PEST homologs such as PTP hematopoietic stem cell factor (HSCF) [33], suggesting possible roles in functions such as lymphocyte activation, antigen processing, granule exocytosis, phagocytosis and apoptosis, as the PEST family phosphatases have been implicated in these processes [47].
PSTPIP1 also binds itself. Western blots of patient and control monocyte lysates run on native gels clearly revealed a major band twice the size of PSTPIP1 monomer when probed with an anti-PSTPIP1 polyclonal antibody. Weaker bands possibly corresponding to PSTPIP1 trimers or tetramers were also observed [48]. In silico modeling predicted that the dimerized protein assumes a gently curved structure that is not altered by disease-causing mutations. Other work has suggested the PSTPIP1 exists as a homotrimer [49]. Either way, evidence suggests that disease-causing mutations in PSTPIP1 do not alter its self-binding capacity.
PSTPIP1 AND PYRIN
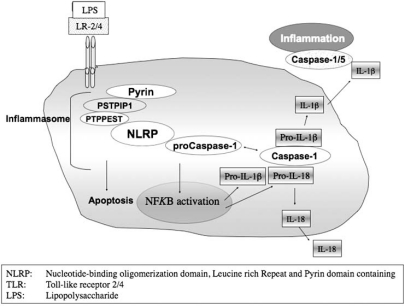
As noted earlier, mutations in the pyrin protein are responsible for FMF, a disease that shows some clinical similarities to PAPA syndrome, including a neutrophil-rich sterile infiltrate of the joints, neutrophilic dermatoses, and elevated production of IL-1 by peripheral blood leukocytes [50-52]. The relationship of these two diseases was clarified by the discovery of molecular overlap, when a pyrin bait identified PSTPIP1 in yeast two hybrid screens of a monocyte library. Subsequent work confirmed the direct interaction of pyrin and PSTPIP1. Furthermore, hyperphosphorylated variants of PSTPIP1 (e.g. containing disease-causing mutations) displayed a higher affinity for pyrin than wild type forms [21]. This discovery was a watershed moment, as it linked the two diseases within the same previously described pathway and suggested a perturbation that could be targeted therapeutically. Specifically, pyrin has been proposed to activate IL-1β production via a complex known as the “inflammasome”. Although the composition of inflammasomes may vary, for the purposes of this discussion we focus on a canonical complex of three proteins: NLRP3/ASC/Caspase-1. NLRP3, also called cryopyrin, contains a pyrin-like domain through which it interacts with apoptosis-associated speck-like protein with a caspase recruitment domain (ASC). ASC in turn interacts with caspase 1 that catalytically cleaves pro-IL-1β into its active form. Upon activation, the inflammasome complex cleaves pro-IL-1β to produce mature IL-1β that is subsequently secreted, perhaps through a pathway of lysosome exocytosis [53, 54]. Mutations in NLRP3 itself cause the auto-inflammatory syndromes FCAS/MWS/NOMID described earlier. How pyrin participates in this or other inflamma-somes is the subject of ongoing investigations. However, the experiments of Shoham et al. clearly demonstrated overproduction of IL-1β in the presence of PAPA-associated mutations relative to wild type in a heterologous, reconstituted system, as well as elaboration of IL-1β in lipopolysaccharide (LPS)-stimulated monocytes of a PAPA syndrome patient [21]. IL-1β precursor transcripts are also highly over-expressed in circulating neutrophils from PAPA syndrome patients as compared to healthy control individuals by transcript profiling (our unpublished observations).
More recent studies have suggested an alternative mechanism in which the balance of interaction between PSTPIP1, pyrin and ASC is altered by PSTPIP1 variants E250Q and A230T in monocytes, leading to activation of the so-called “pyroptosome” that leads to cell death and release of cytokines such as IL-1β [49, 55]. PSTPIP1 associates with microtubules in native and transfected cells and does not associate with the ASC compartment in the absence of pyrin [48, 49]. Pyrin modulates PSTPIP1 intracellular distribution and is apparently necessary for PSTPIP1-mediated regulation of the ASC pyroptosome. Importantly, either “inflammasome” or “pyroptosome” mechanisms predict overproduction of IL-1β in the presence of PAPA causal mutations (Fig. 3). It is worth noting that while such disease-causing mutations affect pyrin-mediated pathways, the reverse is apparently not the case, i.e. FMF causal mutations do not affect binding to PSTPIP1. This is generally explained by the fact that such mutations almost always occur in pyrin outside the PSTPIP1 binding site, a B box domain that mediates pyrin autoinhibition in the absence of PSTPIP1 [56]. Two B box variants, P369S and R408Q, may play a role in FMF susceptibility but do not appear to alter PSTPIP1 interaction [56].
Fig. (3).
Schematic depiction of the “inflammasome.” IL-1β and IL-18 are substrates for caspase.
PAPA SYNDROME AS AN “INFLAMMASOMOPATHY”
Studies described above firmly established PAPA syndrome as a member of the auto-inflammatory disease family, and more specifically, an “inflammasomopathy” according to the new classification system proposed by Masters et al. [3]. IL-1β hence emerged as a prime therapeutic target for PAPA syndrome. Fortunately, biological agents that target this cytokine cascade are available. Anakinra, a recombinant IL-1 receptor antagonist that is administered by daily parenteral injection, has proven to be effective in controlling flares for PAPA syndrome patients [57, 58]. Indeed, in our experience, this treatment is very effective in resolving joint inflammation altogether for some patients.
Pharmaceuticals that target downstream events in IL-1β signaling may also prove effective in some patients. For example, IL-1β is a potent inducer of TNFα, a major stimulant of apoptosis and inflammation, and an anti-TNFα monoclonal antibody, infliximab, has shown promising results in controlling symptoms of PAPA syndrome, including dramatic resolution of severe pyoderma gangrenosum in one patient [59-61]. In contrast, cystic acne, the second cutaneous symptom of PAPA syndrome, does not seem as responsive to IL1β and TNFα blockade (unpublished observations), raising interesting questions about the pathogenesis of each of the manifestations of the disease. More clinical experience with PAPA syndrome should help to clarify and prioritize best treatment options in the face of varying symptoms in specific target organs.
INSIGHTS INTO DISEASE PATHWAYS
Il-1β and TNF-α-targeted therapies (so-called “biologics”) have provided a generally superior alternative to high-dose corticosteroids in relieving inflammatory flares of PAPA syndrome. However, in collective experience these biologics have not been consistently effective in all cases and do not necessarily hasten remission of all the disease manifestations. This may not be surprising in view of the evidence that PSTPIP1 likely functions in multiple biochemical pathways in several immune-related cells (T cells, neutrophils, monocyte-derived cells, NK cells). IL-1β/TNF-α antagonists suppress innate immune mechanisms fairly broadly and convey significant risk of infection, a particularly troublesome problem when faced with managing simultaneous open skin lesions. Clearly, more detailed studies of PSTPIP1-mediated pathways are needed to better understand disease mechanisms in relevant tissues (i.e. joints and skin) and thereby inform the development of more specific therapies. Genetic studies in appropriate animal models will likely be invaluable to these efforts. Meanwhile studies in patient cells have provided some initial insights. A recent study of macrophages cultured from monocytes of four PAPA syndrome patients (with confirmed A230T or E250Q mutations) revealed deficiencies in chemotaxis and invasive migration. In contrast, impairment of these functions was not observed in similar tests of patient T cells. In addition, PAPA syndrome macrophages formed significantly fewer podosomes than controls, and podosome structures were altered in that they contained increased numbers of focal complexes [62]. This is consistent with expression data revealing increased transcript levels in PAPA individuals for proteins involved in focal adhesion complexes (e.g. gelsolin, integrin-beta3, talin, PTP-PEST) [our unpublished observations]. The authors also noted that the dismorphology of macrophages observed in PAPA syndrome is reminiscent of that seen in Wiskott-Aldrich Syndrome, suggesting a common PSTPIP1/WASP pathway is disrupted in both diseases, at least in monocytes/macrophages. It was further hypothesized that the dramatic recruitment of neutrophils during PAPA syndrome flares is a response to signaling from macrophages retained in affected sites [62]. Certainly prior studies of patient peripheral blood leukocytes and serum suggest a gain-of-function, pro-inflammatory state in these tissues, as evidenced by overproduction of IL-1β and TNF-α, and the original ~160 kd “streaking leukocyte factor” that stimulated random migration of normal neutrophils and mononuclear cells in vitro [18].
Although cutaneous involvement is variable in PAPA syndrome it is a particularly troubling feature of the disease, not only in terms of clinical management but also cosmesis. Regarding the latter, some PAPA syndrome patients become quite reclusive because of concerns about their appearance. Pyoderma gangrenosum (and cystic acne) are characterized by neutrophil infiltration; given the strong PSTPIP1 expression in neutrophils we hypothesize that this is at least in part secondary to an intrinsic mechanism in these cells. Recent cell transfection studies by Cooper et al. found that PSTPIP1 localizes to the neutrophil uropod and alters motility and endocytosis. However, A230T and E250Q mutants did not alter these functions, and thus, the role of disease mutations in neutrophil behavior is unresolved [42]. As noted, PSTPIP1 pathways in non-myeloid cells such as T cells may also factor in PAPA syndrome.
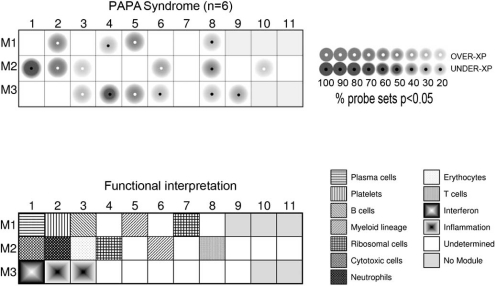
Global gene expression analysis has proved to be a useful tool for dissecting underlying pathways in blood of patients with various inflammatory diseases [63-67]. This approach has been particularly powerful as a molecular diagnostic tool for certain complex diseases with relatively non-specific features, such as SoJIA. Like PAPA syndrome, SoJIA is an auto-inflammatory disease marked by overproduction of IL-1β. Gene expression in blood of SoJIA and PAPA shows extensive overlaps (our unpublished observations), yet So-JIA predictive genes can distinguish it from PAPA [66]. Our preliminary observations of gene expression in peripheral blood mononuclear cells of PAPA patients using modular analyses have revealed strongest up-regulation of genes expressed in low-density neutrophils as well as in platelets and myeloid cells (Modules 2.1, 1.2, and 1.5, respectively, Fig. 4). As examples, over-expressed neutrophil-related genes included defensin A4 (DEFA4), peptidyl arginine deiminase 14 (PAD14), lipocalin 2 (LCN2), and forkhead box O3A (FOXO3A) among others. In addition, genes involved in cytotoxic cell functions were strongly under-expressed relative to controls (Fig. 4). Over-expression of neutrophil-related genes in PBMC fractions has been observed in other inflammatory diseases such as SLE and SoJIA and may indicate over-representation of neutrophil precursors in PAPA blood, although further study is required to determine this.
Fig. (4).
Modular analyses of PAPA blood gene expression reveals over-expression of neutrophil and platelet-related genes. mRNA expression level differences in PBMCs between PAPA patients and age- and sex-matched healthy controls were obtained by statistical group comparison. We further performed modular analyses as described in [67, 68]. Briefly, genes are assigned a priori to particular functional units (i. e. modules) as derived from extensive analyses of expression in PBMCs. Statistical comparisons are then made between disease groups and healthy groups on a module-by-module basis, where the proportion of under- or over-expressed genes in the module is depicted in the cartoon by differences in color and intensity. This approach simplifies analysis and produces a distinct “fingerprint” of disease. A complete list of differentially expressed gene transcripts in PAPA syndrome individuals versus controls is available upon request.
SUMMARY
The convergence of biochemical, cell biological, molecular genetic, and clinical studies in various experimental systems has brought PAPA syndrome (and afflicted patients) out of obscurity, clearly revealing causation and pathways to therapy. It has also raised intriguing new questions. The classification of PAPA syndrome as an inflammasomopathy implies a pro-inflammatory state with heightened response to environmental triggers. If so, are patients protected from generalized infections? This is difficult to determine from limited patient studies but an interesting concept to explore, perhaps in animal models. The disease pathogenesis of PAPA syndrome may be explained by the mutations in PSTPIP1 that alter its interaction with pyrin and the inflammasome. Alternatively, is pyroptosome formation and cell death relevant in vivo, for patients with PAPA syndrome? Either way, an apparent consequence of this is an overproduction of active IL-1β. In this scenario, are there specific microbial pathogens, other stimuli, or simply trauma that provoke an exuberant response and consequent flares? It will be particularly important to investigate these mechanisms not only for sterile compartments (i.e. joints) but also in the skin, where interactions with comensal and pathogenic microbes abound [3]. Given the localization of PSTPIP1 along microtubules and its association with pyrin, why is colchicine ineffective in treating for PAPA syndrome patients? As PSTPIP1 is a cytoskeletal protein, could it regulate IL1β secretion rather than activation? Therapies targeting IL1β also lack efficacy in some individuals. Could this be explained by upregulation of interleukin 18, which is also activated by the inflammasome, in PAPA patients? Finally, as with virtually all Mendelian disorders the variability in disease severity even within single families is baffling. The emerging era of whole genome sequencing may provide the opportunity to identify genetic modifiers that will truly enlighten interventions for episodic diseases such as PAPA syndrome.
ACKNOWLEDGEMENTS
We thank J. Brandon and M. Walford for help with figures. We particularly thank the patients and families for their inspiration and participation in these studies. This work was funded by the TSRHC Research Grant #05-02-592.
REFERENCES
- 1.Kastner DL, O'Shea JJ. A fever gene comes in from the cold. Nat. Genet. 2001;29:241–242. doi: 10.1038/ng1101-241. [DOI] [PubMed] [Google Scholar]
- 2.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, McCarthy J, Frucht DM, Aringer M, Torosyan Y, Teppo AM, Wilson M, Karaarslan HM, Wan Y, Todd I, Wood G, Schlimgen R, Kumarajeewa TR, Cooper SM, Vella JP, Amos CI, Mulley J, Quane KA, Molloy MG, Ranki A, Powell RJ, Hitman GA, O'Shea JJ, Kastner DL. Germline mutations in the extracellular domains of the 55kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell. 1999;97:133–144. doi: 10.1016/s0092-8674(00)80721-7. [DOI] [PubMed] [Google Scholar]
- 3.Masters SL, Simon A, Aksentijevish I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu. Rev. Immunol. 2009;27:621–668. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aksentijevich I, Deng Z, Sood R, Balow Jnr JE, Wood G, Zaks N, Mansfield E, Tiqva P, Chen X, Eisenberg S, Vedula A, Shafran N, Raben N, Pras E, Pras M, Richards N, Shelton DA, Kastner DL, Gumucio D, Yokoyama CY, Mangelsdorf M, Orsborn A, Richards RI, Blake T, Baxevanis AD, Robbins C, Krizman D, Collins FS, Liu P, Ricke DO, Buckingham JM, Moyzis RK, Deavan LL, Doggett NA, Chen X, Shohat M, Hamon M, Kahan T The International FMF Consortium . Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 5.Drenth JP, Cuisset L, Grateau G, Vasseur C, van de Velde-Visser SD, de Jong JG, Beckmann JS, van der Meer JW, Delpech M. Mutations in the gene encoding mevalonate kinase cause hyper-IgD and periodic fever syndrome. International Hyper-IgD Study Group. Nat. Genet. 1999;22:178–181. doi: 10.1038/9696. [DOI] [PubMed] [Google Scholar]
- 6.Houten SM, Kuis W, Duran M, de Koning TJ, van Royen-Kerkhof A, Romeijn GJ, Frenkel J, Dorland L, de Barse MM, Huijbers WA, Rijkers GT, Waterham HR, Wanders RJ, Poll-The BT. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat. Genet. 1999;22:175–177. doi: 10.1038/9691. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001;29:301–305. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, Laxer R, Tedgård U, Cowen EW, Pham TH, Booty M, Estes JD, Sandler NG, Plass N, Stone DL, Turner ML, Hill S, Butman JA, Schneider R, Babyn P, El-Shanti HI, Pope E, Barron K, Bing X, Laurence A, Lee CC, Chapelle D, Clarke GI, Ohson K, Nicholson M, Gadina M, Yang B, Korman BD, Gregersen PK, van Hagen PM, Hak AE, Huizing M, Rahman P, Douek DC, Remmers EF, Kastner DL, Goldbach-Mansky R. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N. Engl. J. Med. 2009;360:2426–2437. doi: 10.1056/NEJMoa0807865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy S, Jia S, Geoffrey R, Lorier R, Suchi M, Broeckel U, Hessner MJ, Verbsky J. An autoinflammatory disease due to homozygous deletion of the IL1RN locus. N. Engl. J. Med. 2009;360:2438–2444. doi: 10.1056/NEJMoa0809568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miceli-Richard C, Zouali H, Lesage S, Thomas G, Hugot JP, Said-Nahal R, Breban M. CARD15 mutations in Blau syndrome. Nat. Genet. 2001;29:19–20. doi: 10.1038/ng720. [DOI] [PubMed] [Google Scholar]
- 11.Kanazawa N. Early-onset sarcoidosis and CARD15 mutations with constitutive nuclear factor-kappaB activation: common genetic etiology with Blau syndrome. Blood. 2005;105:1195–1197. doi: 10.1182/blood-2004-07-2972. [DOI] [PubMed] [Google Scholar]
- 12.Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Reichenbach, J.; Zychlinsky, A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. 2010 doi: 10.1182/blood-2010-01-264218. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson PJ, Chen S, Tayeh MK, Ochoa L, Leal SM, Pelet A, Munnich A, Lyonnet S, Majeed HA, El-Shanti H. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J. Med. Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo. Clin. Proc. 1997;72:611–615. doi: 10.1016/S0025-6196(11)63565-9. [DOI] [PubMed] [Google Scholar]
- 15.Brydges S, Kastner DL. The systemic autoinflammatory diseases: inborn errors of the innate immune system. Curr. Top. Microbiol. Immunol. 2006;305:127–160. doi: 10.1007/3-540-29714-6_7. [DOI] [PubMed] [Google Scholar]
- 16.Ozen S, Hoffman HM, Frenkel J, Kastner D. Familial Mediterranean fever (FMF) and beyond: a new horizon. Fourth International Congress on the Systemic Autoinflammatory Diseases held in Bethesda, USA. Ann. Rheum. Dis. 2006;65:961–964. doi: 10.1136/ard.2006.052688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Touitou I, Lesage S, McDermott M, Cuisset L, Hoffman H, Dode C, Shoham N, Aganna E, Hugot JP, Wise C, Waterham H, Pugnere D, Demaille J, Sarrauste de Menthiere C. Infevers: an evolving mutation database for auto-inflammatory syndromes. Hum. Mutat. 2004;24:194–198. doi: 10.1002/humu.20080. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JC, Goetzl EJ. "Streaking leukocyte factor," arthritis, and pyoderma gangrenosum. Pediatrics. 1975;56:570–578. [PubMed] [Google Scholar]
- 19.Wise CA, Bennett LB, Pascual V, Gillum JD, Bowcock AM. Localization of a gene for familial recurrent arthritis. Arthritis Rheum. 2000;43:2041–2045. doi: 10.1002/1529-0131(200009)43:9<2041::AID-ANR15>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 20.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D’Urbano LE, Ugazio AG. Abnormal production of the tumour necrosis factor alpha and clinical efficacy of the TNF Inhibitor Etanercept in a patient with PAPA syndrome. J. Pediatr. 2004;145:851–855. doi: 10.1016/j.jpeds.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, Kastner DL. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. USA. 2003;100:13501–13506. doi: 10.1073/pnas.2135380100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edrees AF, Kaplan DL, Abdou NI. Pyogenic arthritis pyoderma gangrenosum, and acne syndrome (PAPA syndrome) associated with hypogammaglobulinemia and elevated serum tumor necrosis factor-alpha levels. J. Clin. Rheumatol. 2002;8:273–275. doi: 10.1097/00124743-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Yeon HB, Lindor HM, Seidman JG, Seidman CE. Pyogenic Arthritis, Pyoderma Gangrenosum, and Acne Syndrome Maps to Chromosome 15q. Am. J. Hum. Genet. 2000;66:1443–1448. doi: 10.1086/302866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, Lovett M. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 2002;11:961–969. doi: 10.1093/hmg/11.8.961. [DOI] [PubMed] [Google Scholar]
- 25.Renn CN, Helmer A, Megahed M. [Pyogenic arthritis, pyoderma gangrenosum and acne syndrome (PAPA syndrome)][Article in German] Hautarzt. 2007;58:383–384. doi: 10.1007/s00105-007-1331-z. [DOI] [PubMed] [Google Scholar]
- 26.Tallon B, Corkill M. Peculiarities of PAPA syndrome. Rheumatology (Oxford) 2006;45:1140–1143. doi: 10.1093/rheumatology/kei178. [DOI] [PubMed] [Google Scholar]
- 27.Hong JB, Su YN, Chiu HC. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome (PAPA syndrome): report of a sporadic case without an identifiable mutation in the CD2BP1 gene. J. Am. Acad. Dermatol. 2009;61:533–535. doi: 10.1016/j.jaad.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 28.André MF, Aumaître O, Grateau G, Chamaillard M, Costedoat-Chalumeau N, Cardoso MC, Henry-Berger J, Ramakrishna BS, Delpech M, Piette JC, Creveaux I. Longest Form of CCTG Microsatellite Repeat in the Promoter of the CD2BP1/PSTPIP1 Gene Is Associated with Aseptic Abscesses and with Crohn Disease in French Patients. Dig. Dis. Sci. 2010;55:1681–1688. doi: 10.1007/s10620-009-0929-7. [DOI] [PubMed] [Google Scholar]
- 29.Newman B, Cescon D, Domenchini A, Siminovitch KA. CD2BP1 and CARD15 mutations are not associated with pyoderma gangrenosum in patients with inflammatory bowel disease. J. Invest. Dermatol. 2004;122:1054–1056. doi: 10.1111/j.0022-202X.2004.22430.x. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y, Dowbenko D, Lasky LA. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. J. Biol. Chem. 1998;273:30487–30496. doi: 10.1074/jbc.273.46.30487. [DOI] [PubMed] [Google Scholar]
- 31.Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J. Cell Biol. 2006;172:269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golla A, Jansson A, Ramser J, Hellebrand H, Zahn R, Meitinger T, Belohradsky BH, Meindl A. Chronic recurrent multifocal osteomyelitis (CRMO): evidence for a susceptibility gene located on chromosome 18q21.3-18q22. Eur. J. Hum. Genet. 2002;10:217–221. doi: 10.1038/sj.ejhg.5200789. [DOI] [PubMed] [Google Scholar]
- 33.Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, Simanis V, Lasky LA. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J. Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Nishizawa K, An W, Hussey RE, Lialios FE, Salgia R, Sunder-Plassmann R, Reinherz EL. A cdc15-like adaptor protein (CD2BP1) interacts with the CD2 cytoplasmic domain and regulates CD2-triggered adhesion. EMBO J. 1998;17:7320–7336. doi: 10.1093/emboj/17.24.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angers-Loustau A, Côté JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Côté JF, Chung PL, Théberge JF, Hallé M, Spencer S, Lasky LA, Tremblay ML. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J. Biol. Chem. 2002;277:2973–2986. doi: 10.1074/jbc.M106428200. [DOI] [PubMed] [Google Scholar]
- 37.Cong F, Spencer S, Côté JF, Wu Y, Tremblay ML, Lasky LA, Goff SP. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol. Cell. 2000;6:1413–1423. doi: 10.1016/s1097-2765(00)00138-6. [DOI] [PubMed] [Google Scholar]
- 38.Baum W, Kirkin V, Fernández SB, Pick R, Lettau M, Janssen O, Zörnig M. Binding of the intracellular Fas ligand (FasL) domain to the adaptor protein PSTPIP results in a cytoplasmic localization of FasL. J. Biol. Chem. 2005;280:40012–40024. doi: 10.1074/jbc.M502222200. [DOI] [PubMed] [Google Scholar]
- 39.Qian J, Qian J, Chen W, Lettau M, Podda G, Zörnig M, Kabelitz D, Janssen O. Regulation of FasL expression: a SH3 domain containing protein family involved in the lysosomal association of FasL. Cell Signal. 2006;18:1327–1337. doi: 10.1016/j.cellsig.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 40.Badour K, Zhang J, Shi F, Leung Y, Collins M, Siminovitch K A. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J. Exp. Med. 2004;199:99–112. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Reinherz EL. CD2BP1 modulates CD2-dependent T cell activation via linkage to protein tyrosine phosphatase (PTP)-PEST. J. Immunol. 2006;176:5898–5907. doi: 10.4049/jimmunol.176.10.5898. [DOI] [PubMed] [Google Scholar]
- 42.Cooper KM, Bennin DA, Huttenlocher A. The PCH family member proline-serine-threonine phosphatase-interacting protein 1 targets to the leukocyte uropod and regulates directed cell migration. Mol. Biol. Cell. 2008;19:3180–3191. doi: 10.1091/mbc.E08-02-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiota M, Tanihiro T, Nakagawa Y, Aoki N, Ishida N, Miyazaki K, Ullrich A, Miyazaki H. Protein tyrosine phosphatase PTP20 induces actin cytoskeletal reorganization by dephosphorylation p190 RhoGAP in rat ovarian granulosa cells stimulated with follicle-stimulating hormone. Mol. Endocrinol. 2003;17:534–549. doi: 10.1210/me.2002-0187. [DOI] [PubMed] [Google Scholar]
- 44.Garton AJ, Tonks NK. PTP-PEST: a protein tyrosine phosphatase regulated by serine phosphorylation. EMBO J. 1994;13:3763–3771. doi: 10.1002/j.1460-2075.1994.tb06687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallé M, Liu YC, Hardy S, Théberge JF, Blanchetot C, Bourdeau A, Meng TC, Tremblay ML. Caspase-3 regulates catalytic activity and scaffolding functions of the protein tyrosine phosphatase PEST, a novel modulator of the apoptotic response. Mol. Cell Biol. 2007;27:1172–1190. doi: 10.1128/MCB.02462-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001;20:3414–3426. doi: 10.1093/emboj/20.13.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veillette A, Rhee I, Souza CM, Davidson D. PEST family phosphatases in immunity, autoimmunity, and autoinflammatory disorders. Immunol. Rev. 2009;228:312–324. doi: 10.1111/j.1600-065X.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- 48.Waite AL, Schaner P, Richards N, Balci-Peynircioglu B, Masters SL, Brydges SD, Fox M, Hong A, Yilmaz E, Kastner DL, Reinherz EL, Gumucio DL. Pyrin modulates the intracellular distribution of PSTPIP1. PLoS One. 2009;4:e6147. doi: 10.1371/journal.pone.0006147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, McCormick M, Zhang Z, Alnemri ES. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol. Cell. 2007;28:214–227. doi: 10.1016/j.molcel.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galon J, Aksentijevich I, McDermott MF, O'Shea JJ, Kastner DL. TNFRSFIA mutations and autoinflammatory syndromes. Curr. Opin. Immunol. 2000;12:479–486. doi: 10.1016/s0952-7915(00)00124-2. [DOI] [PubMed] [Google Scholar]
- 51.Callen JP. Neutrophilic dermatoses. Dermatol. Clin. 2002;20:409–419. doi: 10.1016/s0733-8635(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 52.Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL. Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell. 2003;11:591–604. doi: 10.1016/s1097-2765(03)00056-x. [DOI] [PubMed] [Google Scholar]
- 53.Pascual V, Chaussabel D, Banchereau J. A genomic approach to human autoimmune diseases. Annu. Rev. Immunol. 2010;28:535–571. doi: 10.1146/annurev-immunol-030409-101221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinon F, Burns K, Tschopp J. The Inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of pro-IL-1. Mol. Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 55.Fernandes-Alnemri T, Wu J, Yu JW, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007;14:1590–1604. doi: 10.1038/sj.cdd.4402194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryan JG, Masters SL, Booty MG, Habal N, Alexander JD, Barham BK, Remmers EF, Barron KS, Kastner DL, Aksentijevich I. Clinical features and functional significance of the P369S/R408Q variant in pyrin, the familial Mediterranean fever protein. Ann. Rheum. Dis. 2010;69:1383–1388. doi: 10.1136/ard.2009.113415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. British J. Dermatol. 2006;161:1199–1201. doi: 10.1111/j.1365-2133.2009.09404.x. [DOI] [PubMed] [Google Scholar]
- 58.Dierselhuis MP, Frenkel J, Wulffraat NM, Boelens JJ. Anakinra for flares of pyogenic arthritis in PAPA syndrome. Rheumatol. 2005;44:406–408. doi: 10.1093/rheumatology/keh479. [DOI] [PubMed] [Google Scholar]
- 59.Stichweh DS, Punaro M, Pascual V. Dramatic improvement of pyoderma ganrenosum with infliximab in a patient with PAPA syndrome. Ped. Derm. 2005;22:262–265. doi: 10.1111/j.1525-1470.2005.22320.x. [DOI] [PubMed] [Google Scholar]
- 60.Edrees AF, Kaplan DL, Abdou NI. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome (PAPA syndrome) associated with hypogammaglobulinemia and elevated serum tumor necrosis factor-alpha levels. J. Clin. Rheumatol. 2002;8:273–275. doi: 10.1097/00124743-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 61.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D'Urbano LE, Ugazio AG. Abnormal production of tumor necrosis factor (TNF) -- alpha and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome [corrected] J. Pediatr. 2004;145:851–855. doi: 10.1016/j.jpeds.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Cortesio CL, Cooper KM, Wernimont SA, Kastner DL, Huttenlocher A. Impaired podosome formation and invasive migration of macrophages from patients with a PSTPIP1 mutation and PAPA syndrome. Arthritis Rheum. 2010 doi: 10.1002/art.27521. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockage. J. Exp. Med. 2005;201:1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allantaz F, Chaussabel D, Stichweh D, Bennett L, Allman W, Mejias A, Ardura M, Chung W, Smith E, Wise C, Palucka K, Ramilo O, Punaro M, Banchereau J, Pascual V. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J. Exp. Med. 2007;204:2131–2144. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Allantaz F, Chaussabel D, Banchereau J, Pascual V. Microarray-based identification of novel biomarkers in IL-1-mediated diseases. Curr. Opin. Immunol. 2007;19:623–632. doi: 10.1016/j.coi.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and Granulopoiesis signatures in systemic lupus erythematosus. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chaussabel D, Allman W, Mejias A, Chung W, Bennett L, Ramilo O, Pascual V, Palucka AK, Banchereau J. Analysis of significance patterns identifies ubiquitous and disease-specific gene-expression signatures in patient peripheral blood leukocytes. Ann. N.Y. Acad. Sci. 2005;1062:146–154. doi: 10.1196/annals.1358.017. [DOI] [PubMed] [Google Scholar]
- 68.Chaussabel D, Quinn C, Shen J, Patel P, Glaser C, Baldwin N, Stichweh D, Blankenship D, Li L, Munagala I, Bennett L, Allantaz F, Mejias A, Ardura M, Kaizer E, Monnet L, Allman W, Randall H, Johnson D, Lanier A, Punaro M, Wittkowski KM, White P, Fay J, Klintmalm G, Ramilo O, Palucka AK, Banchereau J, Pascual V. A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity. 2008;29:150–164. doi: 10.1016/j.immuni.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]