A novel TSH receptor inverse agonist was developed and shown to inhibit stimulation of TSH receptor by all 30 sera from patients tested who had Graves' disease.
Abstract
Context:
Graves' disease (GD) is caused by persistent, unregulated stimulation of thyrocytes by thyroid-stimulating antibodies (TSAbs) that activate the TSH receptor (TSHR). We previously reported the first small-molecule antagonist of human TSHR and showed that it inhibited receptor signaling stimulated by sera from four patients with GD.
Objective:
Our objective was to develop a better TSHR antagonist and use it to determine whether inhibition of TSAb activation of TSHR is a general phenomenon.
Design:
We aimed to chemically modify a previously reported small-molecule TSHR ligand to develop a better antagonist and determine whether it inhibits TSHR signaling by 30 GD sera. TSHR signaling was measured in two in vitro systems: model HEK-EM293 cells stably overexpressing human TSHRs and primary cultures of human thyrocytes. TSHR signaling was measured as cAMP production and by effects on thyroid peroxidase mRNA.
Results:
We tested analogs of a previously reported small-molecule TSHR inverse agonist and selected the best NCGC00229600 for further study. In the model system, NCGC00229600 inhibited basal and TSH-stimulated cAMP production. NCGC00229600 inhibition of TSH signaling was competitive even though it did not compete for TSH binding; that is, NCGC00229600 is an allosteric inverse agonist. NCGC00229600 inhibited cAMP production by 39 ± 2.6% by all 30 GD sera tested. In primary cultures of human thyrocytes, NCGC00229600 inhibited TSHR-mediated basal and GD sera up-regulation of thyroperoxidase mRNA levels by 65 ± 2.0%.
Conclusion:
NCGC00229600, a small-molecule allosteric inverse agonist of TSHR, is a general antagonist of TSH receptor activation by TSAbs in GD patient sera.
Graves' disease (GD) is caused by persistent, unregulated stimulation of thyroid cells by thyroid-stimulating antibodies (TSAbs) that activate the TSH receptor (TSHR) (1–3). TSAbs, like TSH, bind primarily to the large amino-terminal ectodomain of TSHR. For the majority of antibodies tested, TSAbs and TSH compete for binding to TSHR. We previously reported the first small-molecule TSHR antagonist (NIDDK/CEB-52) (4), which inhibited TSH-stimulated signaling, and the first TSHR inverse agonist (NCGC00161856) (5), which is an antagonist that inhibits basal (or constitutive or agonist-independent) TSHR signaling in addition to TSH-stimulated signaling. TSHR is one of a minority of G protein-coupled receptors that exhibit easily measurable basal signaling activity in vitro (6). In our previous reports, we provided compelling evidence that these drug-like compounds bind to TSHR in its serpentine region at what is termed an allosteric site and do not compete for binding with TSH. We also reported that one of these antagonists inhibited activation of TSHR by sera from four patients with GD (4). Because we measured the effect of the antagonist on a small number of GD sera, it was possible that allosteric ligands would not inhibit TSHR activation by some TSAbs.
We have continued in our attempts to optimize small-molecule TSHR ligands and have developed a better inverse agonist (NCGC00229600) (1), which is an analog of NCGC00161856. Herein we show that 1 is a TSHR inverse agonist in a model system overexpressing TSHRs and in human thyrocytes in primary culture and show that 1 inhibits TSHR activation by all 30 sera from patients with GD tested.
Subjects and Methods
The clinical features of the patients are summarized in Table 1.
Table 1.
Patient TSH, free T4, TPO-Ab, and TSAb levels and treatment at the time of serum acquisition
| Sample | TSH (IU/liter) | Free T4 (ng/dl) | TPO-Ab (IU/ml) | TSAb (AU) | TR-Ab (U/liter) | Thyroid volume (ml) | RX |
|---|---|---|---|---|---|---|---|
| GD10 | <0.01 | 4.2 | 225 | 23.5 | 4.7 | 22.2 | At diagnosis |
| GD13 | 2.6 | 0.7 | <15 | 24.1 | 5.7 | 20.2 | MMI for 3 months |
| GD15 | 0.01 | 2.8 | NA | 23.6 | NA | 21.4 | MMI for 30 d |
| GD16 | 5.2 | 0.6 | 166 | 19.7 | 49.8 | 46.8 | MMI for 21 months |
| GD19 | <0.01 | 2.5 | NA | 55.9 | 3.2 | 36.3 | At diagnosis |
| GD20 | 0.015 | 1.3 | <15 | 53.5 | 4.5 | 15.2 | At diagnosis |
| GD23 | 0.01 | 1.5 | <15 | 63.1 | 1.6 | 15.5 | MMI for 2 months |
| GD24 | 0.9 | 1.3 | NA | 276 | NA | 0.3 | Post-TX/RAI on l-T4 for 10 yr; ophthalmopathy |
| GD25 | 0.01 | 2.3 | <15 | 56.2 | 7.4 | 27 | MMI for 9 months |
| GD26 | 0.01 | 1.4 | >2000 | 96.6 | 2.9 | 50.9 | MMI for 4 months |
| GD27 | 5.9 | 0.7 | <15 | 34.7 | 1.4 | 11.3 | MMI for 3 months |
| GD28 | 0.66 | 1.1 | 849 | 26.4 | 2.1 | 18.4 | MMI for 4 months |
| GD31 | 0.02 | 1.6 | 101 | 9.5 | 3.5 | 12.5 | MMI for 30 d |
| GD32 | 0.01 | 1.7 | >2000 | 7.6 | 5.1 | 11.7 | At diagnosis |
| GD33 | 0.06 | 1.2 | >2000 | 10.6 | 1.3 | 12.5 | At diagnosis |
| GD38 | 0.09 | 0.8 | 438 | 33 | 7.8 | 10.9 | MMI for 20 d |
| GD42 | 0.04 | 2.2 | NA | 133 | 2.7 | 17 | At diagnosis |
| GD43 | 0.06 | 1.4 | NA | 28.6 | NA | 18.7 | MMI for 2 months |
| GD44 | 0.01 | 1.9 | 1058 | 5.9 | 3.4 | 18.1 | MMI for 15 d |
| GD45 | 24.8 | 0.5 | >2000 | 5.39 | 5.6 (at diagnosis) | 12.6 | MMI for 4 months |
| GD54 | 18.9 | 0.7 | >2000 | 387 | 11.4 | 32.5 | MMI for 4 months |
| GD55 | 13.6 | 0.6 | 101 | 86 | 6.2 | 13.3 | MMI for 24 months |
| GD56 | 0.7 | 1.2 | 790 | 263 | 4.1 | 2.4 | RAI on l-T4 for 3 months |
| GD57 | 1.2 | 1.1 | <15 | 1.1 | 0.57 | 10.6 | Remission of GD |
| GD66 | 0.01 | 4.4 | >2000 | 45.9 | 4.4 | 16.8 | At diagnosis |
| GD67 | 13.2 | 0.6 | 99 | 1.34 | 3.4 | 16.1 | PTU for 6 months |
| GD68 | 0.01 | 3.6 | 931 | 5.18 | 9.7 | 10.8 | At diagnosis |
| GD69 | 3.8 | 0.6 | <15 | 11.2 | 0.8 | 30.1 | MMI for 7 months |
| GD71 | 2.3 | 1 | 287 | 6.68 | 1.64 | 16.2 | MMI for 5 months |
| GD80 | 0.01 | 1.2 | 226 | 5.25 | 3.1 | 12.2 | MMI for 30 d |
TSH normal range = 0.4–4.2 IU/liter; free T4 normal range = 0.76–1.42 ng/dl; TPO-Ab normal value is less than 15 IU/ml; TSAb was measured using a biological assay (7). Cutoff of normal values was determined by the mean of at least five samples from normal subjects. The sd between these normal samples was determined, and this value was multiplied by two and added to the calculated mean. The cutoff value obtained in this way was arbitrarily considered equal to 1 arbitrary unit (AU). Normal range was therefore 0–1 AU. TR-Ab (RIA rTSHAb CT assay normal value <1 U/liter, values between 1.1–1.5 U/liter were considered uncertain or gray zone; Kronus Ltd., Boise, ID). Thyroid volume was calculated by using the volumetric ellipsoid method (height × width × depth × correction factor 0.524) for each lobe and then adding the obtained values. At diagnosis indicates untreated patients at first visit; remission of GD indicates patient untreated in the last 36 months after 18 months treatment with methimazole (MMI). NA, Not applicable; PTU, propylthiouracil; RAI, radioactive iodine; RX, treatment; TX, thyroidectomy.
Clinical tests
TSH was measured by an immunofluorometric assay (TSH ultra; normal range, 0.4–4.2 IU/liter; Perkin-Elmer Italia spa, Monza, Italy). Free T4 was measured by an immunofluorometric assay (normal range, 0.76–1.42 ng/dl; Perkin-Elmer Italia). Thyroid peroxidase antibody (TPO-Ab) was measured by TPO-Ab RIA (normal value, <15 IU/ml; Becton Dickinson Co., Franklin Lakes, NJ). TSAb was measured using a biological assay (7). Cutoff of normal values was determined by the mean of at least five samples from normal subjects. The sd between these normal samples was determined, and this value was multiplied by two and added to the calculated mean. The cutoff value obtained in this way was arbitrarily considered equal to 1 arbitrary unit.
Synthesis of 1
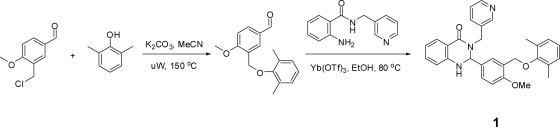
To a solution of 3-(chloromethyl)-4-methoxybenzaldehyde (300 mg, 1.625 mmol, 1.0 equivalent) and 2,6-dimethylphenol (218 mg, 1.787 mmol, 1.1 equivalent) in 10 ml acetonitrile was added potassium carbonate (1.1 g, 8.12 mmol, 5.0 equivalents). The mixture was heated to 150 C in a microwave for 30 min. Upon completion, the mixture was filtered and dried down to give 3-[(2,6-dimethylphenoxy)methyl]-4-methoxybenzaldehyde (400 mg, 91% yield) as a yellow solid. A portion of which (100 mg, 0.370 mmol, 1.0 equivalent) was taken up in ethanol (4 ml), and to it was added 2-amino-N-(pyridin-3-ylmethyl)benzamide (92 mg, 0.407 mmol, 1.1 equivalent) followed by ytterbium(III) trifluoromethanesulfonate (45.9 mg, 0.074 mmol, 0.2 equivalent). The mixture was heated to 80 C for 2 h. Upon completion, the mixture was dried down and chromatographed on silica gel with 0–30% EtOAc (ethyl acetate)/hexanes gradient elution to give the desired 2-{3-[(2,6-dimethylphenoxy)methyl]-4-methoxyphenyl}-3-(pyridin-3-ylmethyl)-2,3-dihydroquinazolin-4(1H)-one (110 mg, 62.0% yield) as a tan solid. 1H nuclear magnetic resonance (400 MHz, DMSO-d6) δ ppm 8.39–8.45 (m, 2 H), 7.60–7.67 (m, 2 H), 7.48 (d, J = 2.35 Hz, 1 H), 7.15–7.35 (m, 4 H), 6.94–7.02 (m, 3 H), 6.84–6.93 (m, 1 H), 6.60–6.69 (m, 2 H), 5.82 (d, J = 2.35 Hz, 1 H), 5.07 (d, J = 15.65 Hz, 1 H), 4.64 (d, J = 2.74 Hz, 2 H), 3.99 (d, J = 15.45 Hz, 1 H), 3.73 (s, 3 H), 2.12 (s, 6 H); liquid chromatography mass spectrometry: (electrospray +ve), m/z 480.2 (MH)+ (mass of molecular weight plus 1); HPLC: tR = 5.05 min,UV254 = 100%. High-resolution mass spectroscopy (electrospray ionization): m/z calculated for C30H30N3O3 [M+H]+ 480.2282, found 480.2291.
Cell culture
The generation of a stable HEK-EM 293 cell line expressing TSHRs (HEKTSHR cells) was described previously (4). HEKTSHR cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 10 μg/ml streptomycin (Life Technologies Inc., Carlsbad, CA) at 37 C in a humidified 5% CO2 incubator.
Thyroid tissue samples were collected from normal thyroid tissue from patients undergoing total thyroidectomy for thyroid cancer at the National Institutes of Health Clinical Center. Patients provided informed consent on an Institutional Review Board-approved protocol, and materials were received anonymously via approval of research activity through the Office of Human Subjects Research. Thyrocytes monodispersed with collagenase IV (Life Technologies) were plated in 10 ml DMEM with 10% FBS in 10-cm tissue culture dishes and incubated at 37 C in a humidified 5% CO2 incubator, and after 24 h, the primary cultures of adherent thyroid cells were obtained as described previously (8). For determination of TPO mRNA expression, thyrocytes were seeded into 24-well plates at a density of 6 × 104 cells per well. The cells were incubated in DMEM with 2% FBS for 48 h in the presence or absence of 1 and without or with the designated dilutions of GD sera.
Determination of cAMP production
HEKTSHR cells were seeded into 48-well plates at a density of 11 × 104 cells per well in DMEM containing 10% FBS. Cells were cultured for 24 h before incubation for 30 min in Hanks' balanced salt solution (HBSS)/10 mm HEPES (pH 7.4). To determine basal cAMP production, cells were incubated for 1 h at 37 C in a humidified incubator in HBSS/HEPES containing 1 mm 3-isobutyl-1-methylxanthine (IBMX) (Sigma, St. Louis, MO) in the presence or absence of 1. To determine the effect of 1 on human TSH (hTSH) (Thermo Fisher Scientific, Fremont, CA) or GD sera activation of TSHRs, cells were incubated for 30 min with 1 in HBSS/HEPES without IBMX and subsequently for 45 min with hTSH or GD sera and 1 in HBSS/HEPES supplemented with 1 mm IBMX at 37 C. The levels of cAMP in cells incubated in HBSS/HEPES without IBMX were subtracted in all experiments. After aspiration of the incubation medium, cells were lysed using lysis buffer of the cAMP-Screen Direct System (Applied Biosystems, Carlsbad, CA) and total cAMP content determined as described by the manufacturer. The chemiluminescence signal was measured in a VICTOR3 V 1420 Multilabel Counter (PerkinElmer, Shelton, CT). Data analysis was performed with GraphPad Prism 4 for Windows.
Effect of 1 on [125I]TSH binding
HEKTSHR cells were seeded into 24-well plates at a density of 2.2 × 105 cells per well. Cell surface binding was measured after 18 h by incubation in 0.25 ml binding buffer (HBSS containing 2.5% milk powder and 0.2% BSA) containing 60,000 cpm bovine [125I]TSH (Brahms Aktiengesellschaft, Hennigsdorf, Germany) without or with 30 μm 1 for 2 h at room temperature; nonspecific binding was measured in the presence of 1.8 μm unlabeled bovine TSH (Sigma, St. Louis, MO) (9). Cells were washed three times with 0.5 ml ice-cold HBSS and lysed with 0.5 ml 0.4 n NaOH, and the cell-associated radioactivity was counted in a 1470 Wallac Wizard γ-counter (PerkinElmer).
Effect of 1 on antibody binding
HEKTSHR cells were seeded into 12-well plates at a density of 4.4 × 105 cells per well. After 24 h, cells were incubated with 30 μm 1 for 1 h in PBS and then harvested with 1 mm EDTA/1 mm EGTA in PBS, washed with PBS containing 0.1% BSA and 0.1% sodium azide and incubated in 1:200 dilutions of one of two antibodies that bind to different epitopes within the amino-terminal ectodomain of TSHR, 2C11 (epitope between amino acid residues 354 and 359) (Serotec, Raleigh, NC), or 4C1 (epitope between amino acid residues 381 and 384) (Santa Cruz Biotechnology, Santa Cruz, CA) in the presence of 30 μm 1 for 90 min. Cells were then washed and incubated with a 1:200 dilution of an Alexa-Fluor 633-conjugated F(ab)2 fragment of goat antimouse IgG (Molecular Probes, Carlsbad, CA) for 30 min. After a final wash, cells were fixed with 1% paraformaldehyde and analyzed by fluorescence-activated cell analyzer (FACSCalibur; BD Biosciences, San Jose, CA). Antibody binding was determined as the number of singlet cells and the level of fluorescence present in a gate that contained no cells in an analysis of cells incubated without primary antibody.
Quantitative RT-PCR
Total RNA was purified using RNeasy Micro kits (QIAGEN, Valencia, CA). cDNA was prepared using a High Capacity cDNA Archive Kit (Applied Biosystems). RT-PCR was performed in 25-μl reactions using cDNA prepared from 100 ng or less of total RNA and Universal PCR Master Mix (Applied Biosystems). mRNA levels were measured using primers and probes from Applied Biosystems. Quantitative RT-PCR results were normalized to GAPDH to correct for differences in RNA input as described (5).
Statistical analysis
Data are expressed as mean ± se. The data were analyzed by Student's t test or one-way ANOVA; P < 0.05 was considered significant.
Results
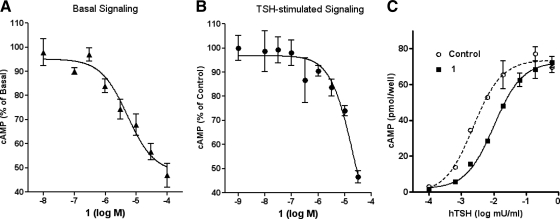
We synthesized and tested six analogs of NCGC00161856 (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org) and found that 1 was as potent and effective as NCGC00161856 as a TSHR antagonist using bovine TSH but was a better drug because of its increased solubility (Fig. 1). In this study, we characterize 1 as an antagonist of basal and hTSH stimulation of hTSHR in human cells. Figure 2A illustrates that in HEKTSHR cells, 1 inhibited basal cAMP production by TSHRs by 53% at 30 μm. Figure 2B shows that 1 inhibited cAMP production stimulated by an EC50 concentration of hTSH by 53% at 30 μm. Figure 2C shows that the inhibition of cAMP production by 30 μm 1 was overcome at high doses of hTSH and was, therefore, competitive. Because 1 is a competitive antagonist, the extent of inhibition is dependent on the concentration of TSH. Competitive inhibition may have been caused by competition of hTSH and 1 for binding; however, 1 had no effect on [125I]TSH binding to HEKTSHR cells (Fig. 3). The finding that 1 did not affect TSH binding was expected because we have previously shown that these small-molecule ligands do not affect TSH binding and appear to bind in the serpentine region of TSHR (4, 5, 8, 10, 11). Allosteric antagonists that inhibit signaling by G protein-coupled receptors are well known (12).
Fig. 1.
Synthetic scheme and structure of 1. See Subjects and Methods for details of the synthesis.
Fig. 2.
1 is an inverse agonist at TSHR and a competitive antagonist of TSH-stimulated signaling. A, HEKTSHR cells were exposed to the noted concentrations of 1 for 60 min in HBSS with 1 mm IBMX (Basal Signaling). Total cAMP levels were measured by ELISA. The data are from three experiments with duplicate samples and are presented as mean ± se. B, HEKTSHR cells were exposed to the indicated concentrations of 1 for 20 min in HBSS and then were incubated in HBSS with 1 mm IBMX, 1, and 1 mU/ml hTSH. After 40 min, the incubation was stopped, and total cAMP levels were measured by ELISA. The data are from two experiments with duplicate samples and are presented as mean ± se. C, HEKTSHR cells were incubated in HBSS (Control or) exposed to 30 μm 1 for 30 min in HBSS and then 1 mm IBMX, and various concentrations of hTSH were added. After an additional 45 min, the incubation was stopped, and total cAMP levels were measured by ELISA. The data are from two experiments with duplicate samples.
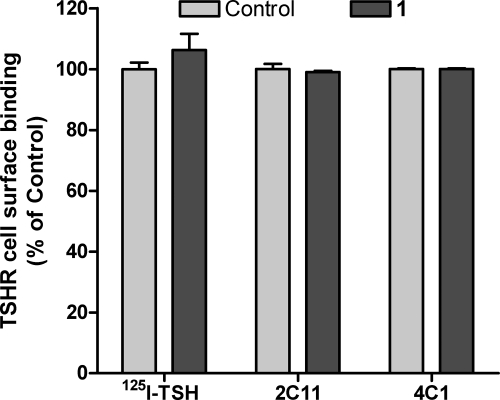
Fig. 3.
1 does not inhibit TSH or TSAb binding to TSHR. Specific binding of [125I]TSH to HEKTSHR cells was performed in the absence or presence of 30 μm 1 as described in Subjects and Methods. The data are from two experiments with triplicate samples. There was no effect of 1 on TSH binding (P > 0.1). Binding of 2C11 or 4C1 antibodies to HEKTSHR cells was performed in the absence or presence of 30 μm 1 and analyzed by FACS as described in Subjects and Methods. The data are from two experiments with triplicate samples. There was no effect of 1 on antibody binding (P > 0.1).
Before testing whether 1 inhibited signaling by GD sera, we showed that 1 had no effect on the binding of two monoclonal anti-TSHR antibodies, 2C11 and 4C1 (Fig. 3). Although we could not test whether 1 had any effect on the binding of TSAbs in GD sera, we assume that because it did not inhibit binding of 2C11 or 4C1 that it does not inhibit TSAb binding also. We tested whether 1 would inhibit cAMP production in HEKTSHR cells stimulated by three GD sera that increased cAMP by more than 10-fold at 1:30 or 1:100 dilution (Fig. 4A), and 1 inhibited the increase in cAMP production stimulated by these three sera by 30–75%. In experiments in which 30 GD sera that increased cAMP production at a 1:30 dilution in HEKTSHR cells were tested, 1 inhibited cAMP production by 39 ± 2.6% (mean ± sem) in all sera tested (Fig. 4B). Thus, 1 is a general antagonist of TSHR activation of cAMP by TSAbs in GD patient sera in the model cell system.
Fig. 4.
1 is an antagonist of GD sera stimulation of cAMP production. A, HEKTSHR cells were incubated without (control) or with 30 μm 1 for 20 min, and then 1 mm IBMX and 1:30 or 1:100 dilutions of each of three GD sera were added. After an additional 40 min, the incubation was stopped, and total cAMP levels were measured by ELISA. The data are from one experiment with triplicate samples and are representative of three experiments. The effects of 1 were significant by paired t test (P < 0.01). B, HEKTSHR cells were incubated without (control) or with 30 μm 1 for 20 min, and then 1 mm IBMX and 1:30 dilutions of 30 GD sera were added. After an additional 40 min, the incubation was stopped, and total cAMP levels were measured by ELISA. The data are presented as percent inhibition = 100 − (100 × samples exposed to 1/control). The data are representative of two experiments performed in duplicate.
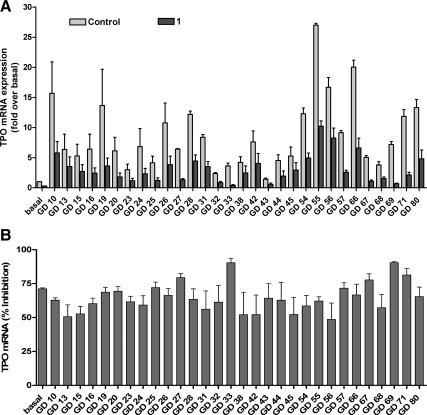
To test the effect of 1 in a more physiologically relevant cell system in which an important thyroid-specific biological response can be measured, we next determined whether 1 inhibited TSHR-mediated up-regulation of TPO mRNA in human thyrocytes in primary culture, and 1 inhibited TSH-induced increases in TPO mRNA (data not shown). Figure 5 illustrates that for all 30 GD sera tested, 1 inhibited GD sera up-regulation of TPO mRNA by 65 ± 2.0% (mean ± sem). This effect of 1 was not because 1 is toxic to human thyrocytes because although 1 decreased the control (or basal) levels of TPO mRNA (Fig. 5) and the mRNAs of thyroglobulin and TSHR (Supplemental Fig. 2), it had no effect on the level of deiodinase type 2 mRNA (Supplemental Fig. 2). Thus, 1 is a nontoxic antagonist of TSHR activation by TSAbs in GD patient sera in human thyrocytes.
Fig. 5.
Inhibition of basal and TSAb-induced up-regulation of the expression of TPO mRNA by 1 in primary cultures of human thyrocytes. Thyrocytes were incubated in DMEM containing 2% FBS without (control) or with 30 μm 1 without (basal) or with 1:10 dilutions of GD sera at 37 C. After 48 h, the buffers were aspirated, the cells were lysed, and the levels of TPO mRNA were measured and normalized to GAPDH mRNA. The mRNA levels are presented as fold of basal levels (control) (A) and as percent inhibition = 100 − (100 × samples exposed to 1/control) (B). The data from two independent experiments with duplicate samples are shown.
Discussion
Graves' hyperthyroidism may be treated with antithyroid drugs, radioactive iodine, or surgery (13). Each of these therapies has been shown to be effective in many clinical trials and is well tolerated by patients, but there is a significant rate of relapse with each, especially antithyroid drugs and radioiodine. For example, in a randomized trial of 179 patients, these three options were equally effective as initial therapy, each was well accepted by over 90% of patients, but the relapse rate was approximately 40% for patients treated with antithyroid drugs, 21% for those treated with radioactive iodine, and 5% after thyroidectomy (14). None of these therapies is pathogenesis based; that is, none attempts to inhibit the production of TSAbs or TSAb activation of TSHR signaling. This type of therapy might be more easily titrated to achieve a euthyroid state and might be used to treat recurrences associated with antithyroid drugs and radioactive iodine. Inhibition of TSAb production would perhaps be the most desirable approach, but no benign treatment to accomplish this has been developed. Herein we report the further development of a TSHR antagonist that inhibits TSAb activation of TSHR. An orally effective TSHR antagonist, if shown to be without significant side effects, might be used as initial chronic therapy for GD or for recurrent disease, and its dosing could be varied to maintain a euthyroid state. To be used as therapy for GD, it would be important that an antagonist inhibit TSHR activation by the majority of TSAbs. It is noteworthy, therefore, that we found that 1 inhibited activation of TSHR by the sera from all 30 GD patients tested.
The observation that 1 is an allosteric antagonist at TSHR may explain its efficacy to antagonize stimulation by all GD sera tested. It has been shown that TSAbs, like TSH, bind primarily to the amino-terminal ectodomain of TSHR at multiple epitopes. It was unlikely that a small molecule like 1 would inhibit binding of TSAbs (or TSH) because it has been found that it is very difficult to inhibit protein-protein interactions with small molecules (15). Indeed, we showed that 1 does not inhibit TSAb (or TSH) binding to TSHR. Therefore, the mechanism of its inhibition most likely involves a restraint on the conformational changes needed for TSHR activation when 1 is bound at an allosteric binding site, and that may account for its ability to inhibit TSHR signaling by all GD sera.
In conclusion, 1 is an antagonist of activation of the TSHR by TSAbs and is a lead drug for the development of a therapeutic agent to treat the hyperthyroidism of GD.
Supplementary Material
Acknowledgments
We thank Francesco S. Celi and Anna Teresa Alberobello for providing human thyroid tissue and William Leister for analytical support.
This work was supported by the Intramural Research Programs of the National Institute of Diabetes and Digestive and Kidney Diseases (Z01-DK047044 Z01-DK011006), the National Human Genome Research Institute, and the Molecular Libraries Initiative of the Roadmap for Medical Research, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 1
- NCGC00229600
- FBS
- fetal bovine serum
- GD
- Graves' disease
- HBSS
- Hanks' balanced salt solution
- hTSH
- human TSH
- IBMX
- 3-isobutyl-1-methylxanthine
- TPO-Ab
- thyroid peroxidase antibody
- TSAb
- thyroid-stimulating antibody
- TSHR
- TSH receptor.
References
- 1. Schott M, Scherbaum WA, Morgenthaler NG. 2005. Thyrotropin receptor autoantibodies in Graves' disease. Trends Endocrinol Metab 16:243–248 [DOI] [PubMed] [Google Scholar]
- 2. Latif R, Morshed SA, Zaidi M, Davies TF. 2009. The thyroid-stimulating hormone receptor: impact of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on multimerization, cleavage, and signaling. Endocrinol Metab Clin North Am 38:319–341 [DOI] [PubMed] [Google Scholar]
- 3. Rapoport B, McLachlan SM. 2007. The thyrotropin receptor in Graves' disease. Thyroid 17:911–922 [DOI] [PubMed] [Google Scholar]
- 4. Neumann S, Kleinau G, Costanzi S, Moore S, Jiang JK, Raaka BM, Thomas CJ, Krause G, Gershengorn MC. 2008. A low-molecular-weight antagonist for the human thyrotropin receptor with therapeutic potential for hyperthyroidism. Endocrinology 149:5945–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Neumann S, Huang W, Eliseeva E, Titus S, Thomas CJ, Gershengorn MC. 2010. A small molecule inverse agonist for the human TSH receptor. Endocrinology 151:3454–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cetani F, Tonacchera M, Vassart G. 1996. Differential effects of NaCl concentration on the constitutive activity of the thyrotropin and the luteinizing hormone/chorionic gonadotropin receptors. FEBS Lett 378:27–31 [DOI] [PubMed] [Google Scholar]
- 7. Tahara K, Ban T, Minegishi T, Kohn LD. 1991. Immunoglobulins from Graves' disease patients interact with different sites on TSH receptor/LH-CG receptor chimeras than either TSH or immunoglobulins from idiopathic myxedema patients. Biochem Biophys Res Commun 179:70–77 [DOI] [PubMed] [Google Scholar]
- 8. Neumann S, Huang W, Titus S, Krause G, Kleinau G, Alberobello AT, Zheng W, Southall NT, Inglese J, Austin CP, Celi FS, Gavrilova O, Thomas CJ, Raaka BM, Gershengorn MC. 2009. Small molecule agonists for the thyrotropin receptor stimulate thyroid function in human thyrocytes and mice. Proc Natl Acad Sci USA 106:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neumann S, Krause G, Chey S, Paschke R. 2001. A free carboxylate oxygen in the side chain of position 674 in transmembrane domain 7 is necessary for TSH receptor activation. Mol Endocrinol 15:1294–1305 [DOI] [PubMed] [Google Scholar]
- 10. Jäschke H, Neumann S, Moore S, Thomas CJ, Colson AO, Costanzi S, Kleinau G, Jiang JK, Paschke R, Raaka BM, Krause G, Gershengorn MC. 2006. A low molecular weight agonist signals by binding to the transmembrane domain of thyroid-stimulating hormone receptor (TSHR) and luteinizing hormone/chorionic gonadotropin receptor (LHCGR). J Biol Chem 281:9841–9844 [DOI] [PubMed] [Google Scholar]
- 11. Moore S, Jaeschke H, Kleinau G, Neumann S, Costanzi S, Jiang JK, Childress J, Raaka BM, Colson A, Paschke R, Krause G, Thomas CJ, Gershengorn MC. 2006. Evaluation of small-molecule modulators of the luteinizing hormone/choriogonadotropin and thyroid stimulating hormone receptors: structure-activity relationships and selective binding patterns. J Med Chem 49:3888–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kenakin T. 2004. Allosteric modulators: the new generation of receptor antagonist. Mol Interv 4:222–229 [DOI] [PubMed] [Google Scholar]
- 13. Brent GA. 2008. Clinical practice. Graves' disease. N Engl J Med 358:2594–2605 [DOI] [PubMed] [Google Scholar]
- 14. Törring O, Tallstedt L, Wallin G, Lundell G, Ljunggren JG, Taube A, Sääf M, Hamberger B. 1996. Graves' hyperthyroidism: treatment with antithyroid drugs, surgery, or radioiodine: a prospective, randomized study. Thyroid Study Group. J Clin Endocrinol Metab 81:2986–2993 [DOI] [PubMed] [Google Scholar]
- 15. Wilson AJ. 2009. Inhibition of protein-protein interactions using designed molecules. Chem Soc Rev 38:3289–3300 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.