Parathyroid hormone-like hormone acts in an autocrine/paracrine manner to repress human decidualization.
Abstract
Context:
Parathyroid hormone-like hormone (PTHLH) is abundantly expressed by human endometrial stromal cells during decidualization. However, the role for PTHLH in the decidualization process is unknown.
Objective:
To examine the effects of PTHLH on the induction and maintenance of decidualization of human uterine fibroblast (HUF) cells in vitro.
Design:
HUF cells were treated with a PTHLH siRNA or a PTHLH receptor antagonist (bPTH7-34) before or after decidualization with medroxyprogesterone acetate (MPA), estradiol (E2), and prostaglandin E2 (PGE2). Decidualization was monitored by immunocytochemistry and the induction of decidualization-specific marker genes, including IGFBP-1, prolactin, lefty, and transcription factor FOXO1.
Results:
HUF cells decidualized after pretreatment with a PTHLH siRNA showed greater morphologic changes of decidualization, greater IGFBP-1 protein, and two- to threefold more IGFBP-1, prolactin, lefty, and FOXO1 mRNAs than cells pretreated with a nonsilencing RNA. The PTHLH siRNA pretreated cells also had 31% less DNA fragmentation (TUNEL assay) and 30–35% less caspase 3 levels during decidualization than cells pretreated treated with nonsilencing RNA. Treatment of HUF cells with PTHLH siRNA or bPTH7-34 at 9 d after the induction of decidualization also resulted in 2.1- to 3.2-fold greater IGFBP-1, prolactin, lefty, and FOXO1 mRNA levels than that noted in control cells treated with nonsilencing RNA.
Conclusions:
These finding strongly suggest that PTHLH represses the induction of human decidualization, stimulates stromal cell apoptosis, and limits the extent of uterine stromal cell differentiation. Because PTHLH and its receptor are expressed by HUF cells and placental cells, the inhibitory effect of PTHLH on decidualization appears to be due, at least in part, to an autocrine/paracrine mechanism.
Parathyroid hormone-like hormone (PTHLH; also known as parathyroid hormone-related protein, PTHrP) was originally identified in the 1980s in tumors associated with the syndrome of humoral hypercalcemia of malignancy (1, 2). The name derives from the fact that the first 13 amino acids of PTHLH are highly homologous with those in the same position in parathyroid hormone (PTH) and both peptides act via PTH/PTHLH receptors. In marked contrast to PTH, which is only expressed by the parathyroid glands, PTHLH is expressed in many tissues in both fetuses and adults, including endocrine, fetoplacental, mesenchymal, and epithelial tissues as well as the central nervous system.
Several studies in the rat strongly suggest a role for PTHLH in uterine physiology during pregnancy. Both immunocytochemical and molecular studies have demonstrated that PTHLH is expressed by rat decidual cells and deciduoma (3). PTHLH mRNA expression during pregnancy begins at d 5.5 in the antimesometrial crypts of the uterine epithelium that mark the site of implantation. PTHLH expression in decidual cells occurs by 48 h after the expression of the gene in uterine epithelium (3). The infusion of the PTHLH antagonist (Asn10, Leu11)PTHLH(7-34) amide into the uterine lumen of pregnant rats results in excessive decidualization (4). At d 13 of pregnancy, the number of apoptotic decidual cells in the antagonist-infused uterine horns is less than that in vehicle or noninfused horns, suggesting that the decrease in apoptosis may contribute to the “overdecidualization” (4). Taken together, these studies suggest that PTHLH is important for normal decidualization and blastocyst implantation during rat pregnancy.
In an earlier DNA microarray study, our laboratory demonstrated that PTHLH mRNA levels increase markedly during the in vitro decidualization of human stromal fibroblast cells to a decidual cell phenotype (5). To determine whether PTHLH regulates decidualization, we examined in the present study whether silencing of PTHLH expression by a PTHLH siRNA or blocking PTHLH action by a PTHLH receptor antagonist (bPTH7-34) affects decidualization. The studies were performed using cultured human uterine fibroblast (HUF) cells that differentiate to a phenotype that expresses abundant amounts of IGFBP1, prolactin, lefty, and other decidualization-specific genes and closely mimics the phenotype of human endometrial stromal cells (6). Decidualization was monitored by changes in cellular morphology and the expression of several of the decidualization-specific genes, including the transcription factor FOXO1. FOXO1 was studied because earlier investigations using HUF cells (7) and endometrial stromal cells (8) demonstrated that FOXO1 is induced during the initial stages of decidualization and is a critical component of the transcriptional network that regulates the decidualization process. The results of the present study strongly suggest that PTHLH inhibits human decidualization by an autocrine/paracrine mechanism.
Methods and Materials
Cell culture
Approval to use term human placentas was obtained by the Institutional Review Boards at the University of Cincinnati, College of Medicine and the Children's Hospital Medical Center, Cincinnati, OH; informed consent was given in each instance. Decidual tissue was dissected from the chorionic layer, and fibroblast cells were isolated and purified to >95% purity by differential plating on plastic as previously described (5, 6). After reaching 90% confluence, the cells were decidualized by the addition of medroxyprogesterone acetate (MPA) (1 μm), estradiol (E2) (10 nm), and prostaglandin E2 (PGE2) (1 μm) in RPMI medium that contained 2% fetal bovine serum and antibiotics. The medium in each well was changed at 3-d intervals unless indicated otherwise.
Three sets of experiments were performed. In the first set, HUF cells were exposed for 3 d to a PTHLH siRNA or a nonsilencing RNA in RPMI medium containing 2% fetal calf serum. The medium was then changed to fresh medium containing MPA, E2, and PGE2. Three days later, the medium was removed from each culture well, and RNA was extracted from the cells as indicated below. In the second set of experiments, the cells were exposed to MPA, E2, and PGE2 for 9 d. The medium in each well was then removed and replaced by medium containing the hormones and the PTHLH siRNA or the nonsilencing RNA. The cultures were stopped 4 d later, and RNA was extracted from the cells. The PTHLH siRNA and nonsilencing RNA were purchased from Qiagen (Valencia, CA). The sequence of the PTHLH siRNA was as follows: sense strand (5′-3′) CTCTTTGTACGTCTCCACCdTdT and antisense strand (3′-5′) GAGAAACATGCAGAGGTGGdTdT. The cells were transfected with 100 nm of the PTHLH siRNA or the control RNA using 4 μl Dharmafect #4 reagent (Fisher Scientific, Pittsburgh, PA) according to the manufacturer's protocol. The third set of experiments was identical to the second set of experiments, except that the HUF cells were exposed to the PTHLH antagonist bovine PTH7-34 (11) (Bachem, Torrance, CA, 0.5 μm) in place of the PTHLH siRNA.
Immunocytochemistry studies
In immunocytochemistry studies, HUF cells were exposed to the PTHLH siRNA or the nonsilencing RNA and decidualized as above except that the cells were cultured on glass two-well chamber slides (Lab Tek, Thermo Scientific, Rochester, NY) rather than six-well plates. After the fourth day of treatment with MPA, E2, and PGE2, the cells were fixed in methanol and immunostained for IGFBP-1 using a highly specific rabbit anti–IGFBP-1 polyclonal antiserum (Santa Cruz Biotechnology, Santa Cruz, CA). Control slides were stained using an equivalent amount of nonimmune rabbit serum in place of the antiserum.
Isolation of total RNA and PCR analysis
Decidualization was also monitored by determination of the mRNA levels of decidualization-specific marker genes by real-time quantitative PCR using the GAPDH mRNA for normalization. Total RNA was extracted from the cells using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's specifications. Two micrograms of total RNA was reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). PCR reactions were performed using the Stratagene Mx30000P instrument. Quantitative PCR amplifications were performed using the Eppendorf HotMasterMix supplemented with SYPR Green and ROX according to the manufacturers' instructions. The PCR reaction was performed with a 2-min incubation at 95 C, followed by 40 cycles of 95 C for 30 sec, 55 C for 1 min, and 72 C for 30 sec. Dissociation curves were determined after the 40th cycle. A single dissociation curve was noted for each primer. The sequences of the primers for IGFBP-1, prolactin, lefty, FOXO1, and GAPDH have been published elsewhere (7, 9). The sequences for the PTHLH (accession number M17183) primers, which amplify both PTHLH mRNA isoforms, were as follows: sense (5′-3′) CGGTGGAGGGTCTCAGC and antisense (3′-5′) ACACAACGTCGCACCTTTC.
TUNEL assay
HUF cells were seeded on glass two-well chamber slides (Lab-Tek, Thermo Scientific, Rochester, NY) and cultured as described above. The cells were exposed for 72 h to either nonsilencing or target-silencing siRNA (Qiagen, Valencia, CA) at 150 nm with Dharmafect #4 transfection reagent (Fisher Scientific, Pittsburgh, CA) and then treated with MPA, E2, and PGE2 for four days. The cells were fixed for 20 min at −20 C with ice-cold methanol; and the TUNEL assay was performed using the Dead End Colorimetric TUNEL System (Promega, Madison, WI) per the manufacturer's directions except that the permeabilization time was increased to 20 min and the development time with diaminobenzidine (DAB) solution was increased to 60 min. By this method, biotinylated nucleotide is incorporated at the 3′-OH DNA ends using recombinant terminal deoxynucleotidyl transferase (rTdT) enzyme. Horseradish peroxidase-labeled streptavidin (Streptavidin HRP) was then bound to these biotinylated nucleotides, which are detected using the peroxidase substrate, hydrogen peroxide, and DAB. For analysis, photographs were taken of ten representative 10× sections of slides from control and PTHLH siRNA-treated cells. The total numbers of labeled and unlabeled HUF cells were determined for each photograph, and the percentage of positive cells (mean ± sem) for the ten fields in each group was calculated.
Caspase 3 measurement
Cellular extracts were used for detection of caspase 3 protein. The extracts were prepared as previously described (7, 10), and 25 μg of total protein (Bradford) was separated by electrophoresis on 12% stacking/5% resolving PAGE gels and transferred to Protran nitrocellulose membranes. The membranes were then blocked and incubated overnight at room temperature with caspase 3 IgG antibodies (Cell Signaling Technology, Danvers, MA) at a final dilution 1:500. After additional washing and blocking, the blots were incubated with donkey antirabbit secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at a 1:1000 dilution at room temperature for 1 h. Chemiluminescence was measured using SuperSignal West Femto (Pierce, Thermo Fisher Scientific, Rockford, IL) followed by autoradiography on Hyperfilm. The autoradiographic signals were quantitated using Kodak Digital Science ID Image Analysis software.
Results
PTHLH mRNA levels increased markedly in HUF cells during in vitro decidualization. As shown in Fig. 1, PTHLH mRNA levels, which were undetectable in undecidualized cells, increased during the first 6 d of exposure to MPA, E2, and PGE2, reaching a peak on d 6 of 4.7 ± 0.2 (relative PTHLH mRNA/GAPDH mRNA level). The PTHLH levels remained relatively constant over the next 6 d. At the end of d 12, the relative level was 3.7 ± 0.1.
Fig. 1.
PTHLH mRNA levels during the decidualization of HUF cells. HUF cells were decidualized in vitro in response to MPA, E2, and PGE2 as described in Materials and Methods. Each point represents the mean of triplicate wells, and the bars enclose ± 1 sem. Similar patterns of PTHLH mRNA expression were observed during the decidualization of two other preparations of HUF cells from different pregnancies.
Undecidualized HUF cells have a fibroblastoid appearance and do not express IGFBP-1, prolactin, lefty, or FOXO1 (5, 6). Following 1–2 d of exposure to MPA, E2, and PGE2, the cells acquire a cuboidal appearance and express abundant amounts of IGFBP-1, prolactin, lefty and FOXO1 (5). In initial studies to examine a possible role for PTHLH in the regulation of decidualization, we noted that silencing PTHLH in HUF cells for 3 d before decidualization using a PTHLH siRNA enhanced the induction of decidualization in response to MPA, E2, and PGE2. As shown in Fig. 2, the cells that had been pretreated with the PTHLH siRNA were more cuboidal in appearance after 3 d of decidualization than the cells pretreated with the control RNA, and the amount of IGFBP-1 staining of the PTHLH siRNA-exposed cells was greater than that of the controls. Furthermore, after 3 d of exposure to MPA, E2, and PGE2, the amounts of IGFBP-1, prolactin, and lefty mRNAs expressed by the HUF cells pretreated with the PTHLH siRNA were 3.4, 4.7, and 2.3-fold greater than those of cells pretreated with the control RNA (P < 0.01–0.001; Fig. 3). In addition, the mRNA for FOXO1, which is critical for the induction of decidualization (7, 8), was increased by 2.5-fold (P < 0.0001).
Fig. 2.
Silencing of PTHLH expression enhances the induction of decidualization. HUF cells were pretreated for 3 d with either a PTHLH siRNA (right) or a control non-silencing RNA (left) for 3 d and then exposed for 3 d to MPA, E2, and PGE2. At the end of this period, the cells were fixed and immunostained with a specific polyclonal antiserum to human IGFBP-1 and counterstained with hemotoxylin. No staining was observed in control slides in which the HUF cells were incubated with a nonimmune rabbit serum in place of the specific antiserum.
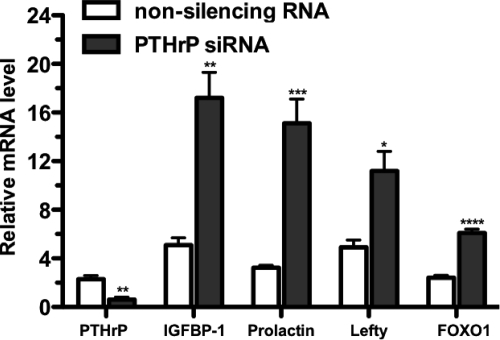
Fig. 3.
Silencing of PTHLH expression by a PTHLH siRNA enhances the induction of decidualization marker genes. HUF cells were exposed for 3 d to a specific PTHLH siRNA or a nonsilencing RNA. The medium in both groups of cells was then exposed to MPA, E2, and PGE2 for 3 d as in Fig. 2. The relative amounts of PTHLH, IGFBP-1, prolactin, and lefty mRNA levels, normalized to the amount of GAPDH mRNA in the same RNA sample, were determined by real-time PCR. Each bar represents the mean of triplicate wells, and the bracket above each bar represents + 1 sem. *, P < 0.05; **, P < 0.01; ****, P < 0.005; ****, P < 0.0001.
Silencing of PTHLH not only enhanced the induction of decidualization but also enhanced the expression of the decidualization-specific marker genes in HUF cells that had undergone decidualization. In the experiment depicted in Fig. 4, HUF cells were treated for 13 d with MPA, E2, and PGE2. On d 9, one group of cells was exposed to the PTHLH siRNA, and a second group was exposed to the control RNA. After the 4th day of exposure to the PTHLH siRNA (d 13), the cells contained 2.3- to 3.5-fold more of the decidualization marker genes than the cells exposed to the nonsilencing RNA (P < 0.05–0.001). Treatment of the HUF cells on d 9 with the PTHLH antagonist bPTH7-34 resulted in nearly identical results (Fig. 5). The amounts of the decidualization markers after 4 d of treatment with bPTH7--34 (d 9–13) were 2.7- to 3.6-fold greater than that of control cells (P < 0.05–0.005).
Fig. 4.
Silencing of PTHLH expression in decidualized HUF cells by a PTHLH siRNA enhances the expression of decidualization-specific genes. HUF cells that had been decidualized with MPA, E2, and PGE2 (decidualizing medium) for 9 d were exposed to a PTHLH siRNA or a nonsilencing RNA and incubated for an additional 4 d in decidualizing medium. RNA was extracted from the cells at the end of this 4-d interval, and the relative amounts of PTHLH, IGFBP-1, prolactin, and lefty mRNA levels, normalized to the amount of GAPDH mRNA in the same RNA sample, were determined by real-time PCR. Each bar represents the mean of triplicate wells, and the bracket above each bar encloses + 1 sem. *, P < 0.05; **, P < 0.01; ****, P < 0.005.
Fig. 5.
Blocking PTHLH expression in decidualized HUF cells by the PTHLH antagonist bPTH7-34 enhances the expression of decidualization-specific genes. HUF cells that had been decidualized with MPA, E2, and PGE2 (decidualizing medium) for 9 d were exposed for an additional 4 d to bPTH7-34 in decidualizing medium or decidualizing medium alone. RNA was extracted from the cells at the end of the 4-d interval, and the relative amounts of PTHLH, IGFBP-1, prolactin, and lefty mRNA levels, normalized to the amount of GAPDH mRNA in the same RNA sample, were determined by real-time PCR. Each bar represents the mean of triplicate wells, and the bracket above each bar encloses + 1 sem. *, P < 0.05; **, P < 0.01; ****, P < 0.005.
To examine whether the action of PTHLH on decidualization is due, at least in part, to an effect on apoptosis, we examined the effects of the PTHLH siRNA on DNA fragmentation and caspase 3 expression, both of which increase during apoptosis. At the end of the 4th day of decidualization, HUF cells that had been pretreated with the PTHLH siRNA had 25.0 ± 2.4 apoptotic cells per ×10 field while HUF cell pretreated with the control RNA had 33.9 ± 1.1 apoptotic cells per ×10 field (P < 0.005). As shown in Fig. 6, the PTHLH siRNA pretreated cells contained 38% less caspase 3 protein compared with the cells transfected with the control RNA (P = 0.01).
Fig. 6.
Silencing of PTHLH expression in decidualized HUF cells by a PTHLH siRNA blocks caspase 3 expression. HUF cells were cultured as described in Fig. 3. Whole cell extracts were prepared 4-d after the cells had been transfected with the PTHLH siRNA or the nonsilencing RNA. Western blot analyses of the extracts for caspase 3 were performed as described in Materials and Methods. Relative signal sum density for each caspase 3 protein band was measured by autoradiography on Hyperfilm using Kodak Digital Science ID Image Analysis software. Each bar represents the density of triplicate wells, and the bracket encloses + 1 sem. **, P < 0.01.
Discussion
The demonstration that silencing of PTHLH expression with a PTHLH siRNA enhances the morphologic differentiation of HUF cells and the induction of the decidualization marker genes IGFBP-1, prolactin and lefty, as well as the FOXO1 gene, strongly suggests that PTHLH inhibits the induction of decidualization. Silencing of PTHLH expression by a PTHLH siRNA or by blocking PTHLH with the receptor antagonist bPTH7-34 also attenuated the expression of decidualization marker genes in decidualized HUF cells, suggesting that PTHLH also limits the extent of stromal cell differentiation. Because uterine stromal cells express PTHLH and the expression of the hormone increases markedly during uterine decidualization, the actions of PTHLH on decidualization appear to be attributable, at least in part, to an autocrine action. Because the placenta and amnion express abundant amounts of PTHLH (11, 12), the actions of PTHLH on decidualization are also likely to result in part from a paracrine mechanism.
The HUF cells pretreated with the PTHLH siRNA underwent less apoptosis after exposure to MPA, E2, and PGE2 than control cells as evidenced by a decrease in DNA fragmentation and caspase 3 expression. Modulation of apoptosis by PTHLH has also been demonstrated in other fetal and adult cells of human and non-human tissues. For example, in mesenchymal tissues such as cartilage, PTHLH functions in local feedback loops to control rates of proliferation and differentiation, thus allowing local tissue organization. Chondrocytes of PTHLH-knockout mice do not proliferate normally and undergo accelerated differentiation and early apoptosis (2). PTHLH has also been shown to inhibit major apoptosis signaling pathways in Saos human osteosarcoma cells by blocking signaling via p53, death receptors, and mitochondria and conferring chemoresistance of the cells to DNA damaging chemotherapeutic drugs (13). The level of expression of PTHLH in epidermis seems to control the size of the proliferative pool of basal keratinocytes, from which keratinocytes exit to undergo terminal differentiation, keratinization and apoptosis. In addition, PTHLH exerts similar effects on apoptosis in human embryonic kidney cells via the extrinsic and intrinsic apoptosis signaling pathways (14).
Earlier studies from our laboratory (7) and others (8) have shown that FOXO1 is a critical component of the pathway that regulates the induction of decidualization. Silencing of FOXO1 expression during decidualization attenuates both the morphologic changes normally observed during decidualization and the expression of IGFBP-1, prolactin, and other decidualization-specific marker genes (7, 8). The observation that HUF cells pretreated with a PTHLH siRNA express significantly more FOXO1 mRNA during decidualization than cells pretreated with a nonsilencing RNA therefore suggests that the effect of PTHLH on decidualization is mediated, at least in part, by a FOXO1-dependent mechanism.
Endocannabinoids (9) and prolactin (15), both of which are induced in uterine stromal cells, also attenuate decidualization and inhibit FOXO1 mRNA levels during decidualization. These findings and those of the current study therefore strongly suggest that the decidual cell synthesizes and secretes several factors that act by an autocrine mechanism to limit the extent of stromal cell differentiation. The findings also support further the hypothesis that FOXO1 plays a pivotal role in the regulation of decidualization. Factors that induce decidualization, such as progesterone, estradiol, and PGE2, induce FOXO1, while factors that attenuate decidualization, such as PTHLH, endocannabinoids, and prolactin, repress FOXO1.
In summary, the results of this study strongly suggest that PTHLH blocks the induction of decidualization and limits the extent of decidualization once the differentiation process is initiated. Because PTHLH expression is markedly induced in uterine stromal cells during decidualization and the placenta expresses abundant amounts of PTHLH, the action of PTHLH on decidualization is likely attributable to an autocrine/paracrine mechanism. PTHLH may act in part by the induction of caspase 3 via intrinsic and extrinsic apoptotic pathways. It is also possible that PTHLH has actions in uterine stromal cells in addition to the regulation of decidualization and apoptosis. Because PTHLH has dramatic effects on decidualization, PTHLH agonists and antagonists may be useful therapeutic agents to treat disorders of decidualization.
Acknowledgments
We thank Michael Hubert, Edith Markoff, and Sonjay Das for their suggestions.
This work was supported by National Institutes of Health Grant HD-15201.
Present address for R.S.-K.: Division of Endocrinology, Department of Pediatrics, Georgetown University Hospital, Washington DC 20007.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- DAB
- Diaminobenzidine
- E2
- estradiol
- HUF
- human uterine fibroblast
- MPA
- medroxyprogesterone acetate
- PGE2
- prostaglandin E2
- PTH
- parathyroid hormone
- PTHLH
- parathyroid hormone-like hormone.
References
- 1. Strewler GJ, Nissenson RA. 1990. Hypercalcemia in malignancy. West J Med 153:635–640 [PMC free article] [PubMed] [Google Scholar]
- 2. Strewler GJ. 2000. The physiology of parathyroid hormone-related protein. N Engl J Med 342:177–185 [DOI] [PubMed] [Google Scholar]
- 3. Beck F, Tucci J, Senior PV. 1993. Expression of parathyroid hormone-related protein mRNA by uterine tissues and extraembryonic membranes during gestation in rats. J Reprod Fertil 99:343–352 [DOI] [PubMed] [Google Scholar]
- 4. Williams ED, Major BJ, Martin TJ, Moseley JM, Leaver DD. 1998. Effect of antagonism of the parathyroid hormone (PTH)/PTH-related protein receptor on decidualization in rat uterus. J Reprod Fertil 112:59–67 [DOI] [PubMed] [Google Scholar]
- 5. Brar AK, Handwerger S, Kessler CA, Aronow BJ. 2001. Gene induction and categorical reprogramming during in vitro human endometrial fibroblast decidualization. Physiol Genomics 7:135–148 [DOI] [PubMed] [Google Scholar]
- 6. Richards RG, Brar AK, Frank GR, Hartman SM, Jikihara H. 1995. Fibroblast cells from term human decidua closely resemble endometrial stromal cells: induction of prolactin and insulin- like growth factor binding protein-1 expression. Biol Reprod 52:609–615 [DOI] [PubMed] [Google Scholar]
- 7. Grinius L, Kessler C, Schroeder J, Handwerger S. 2006. Forkhead transcription factor FOXO1A is critical for induction of human decidualization. J Endocrinol 189:179–187 [DOI] [PubMed] [Google Scholar]
- 8. Buzzio OL, Lu Z, Miller CD, Unterman TG, Kim JJ. 2006. FOXO1A differentially regulates genes of decidualization. Endocrinology 147:3870–3876 [DOI] [PubMed] [Google Scholar]
- 9. Moghadam KK, Kessler CA, Schroeder JK, Buckley AR, Brar AK, Handwerger S. 2005. Cannabinoid receptor I activation markedly inhibits human decidualization. Mol Cell Endocrinol 229:65–74 [DOI] [PubMed] [Google Scholar]
- 10. Brar AK, Kessler CA, Handwerger S. 2002. An Ets motif in the proximal decidual prolactin promoter is essential for basal gene expression. J Mol Endocrinol 29:99–112 [DOI] [PubMed] [Google Scholar]
- 11. Bowden SJ, Emly JF, Hughes SV, Powell G, Ahmed A, Whittle MJ, Ratcliffe JG, Ratcliffe WA. 1994. Parathyroid hormone-related protein in human term placenta and membranes. J Endocrinol 142:217–224 [DOI] [PubMed] [Google Scholar]
- 12. Curtis NE, Ho PW, King RG, Farrugia W, Moses EK, Gillespie MT, Moseley JM, Rice GE, Wlodek ME. 1997. The expression of parathyroid hormone-related protein mRNA and immunoreactive protein in human amnion and choriodecidua is increased at term compared with preterm gestation. J Endocrinol 154:103–112 [DOI] [PubMed] [Google Scholar]
- 13. Gagiannis S, Müller M, Uhlemann S, Koch A, Melino G, Krammer PH, Nawroth PP, Brune M, Schilling T. 2009. Parathyroid hormone-related protein confers chemoresistance by blocking apoptosis signaling via death receptors and mitochondria. Int J Cancer 125: 1551–1557 [DOI] [PubMed] [Google Scholar]
- 14. Müller M, Gagiannis S, Nawroth PP, Brune M, Schilling T. 2009. Activation of the receptor for parathyroid hormone and parathyroid hormone related protein induces apoptosis via the extrinsic and intrinsic signaling pathway. Int J Mol Med 24:373–380 [DOI] [PubMed] [Google Scholar]
- 15. Eyal O, Jomain JB, Kessler C, Goffin V, Handwerger S. 2007. Autocrine prolactin inhibits human uterine decidualization: a novel role for prolactin. Biol Reprod 76:777–783 [DOI] [PubMed] [Google Scholar]