TGF-β3 mediates leiomyoma-induced uterine bleeding and infertility by a direct effect on endometrium.
Abstract
Context:
Uterine leiomyomas occur in 30–70% of reproductive-age women. Leiomyoma reduce implantation, increase miscarriage risk, and increase menstrual bleeding. We hypothesized that endometrial defects induced by leiomyoma result in menorrhagia and reproductive dysfunction.
Objectives:
We evaluated the effect of leiomyoma on endometrial gene expression essential for implantation and hemostasis both in vivo and in primary endometrial stromal cells (ESC).
Design and Setting:
We conducted a case control and in vitro study at a university medical center.
Patients:
The study included 24 subjects with or without leiomyoma.
Intervention/Main Outcome Measured:
Endometrium, myometrium, leiomyoma, and ESC were obtained. Immunohistochemistry was used to evaluate TGF-β3, bone morphogenetic protein (BMP) receptors (BMPRs), plasminogen activator inhibitor 1 (PAI-1), and thrombomodulin in vivo. BMP-2 secretion was assessed by ELISA. ESC were treated with recombinant human (rh) BMP-2 or rhTGF-β3. Expression of HOXA10, LIF, BMPRs, antithrombin III (ATIII), thrombomodulin, and PAI-1 was assessed by quantitative RT-PCR.
Results:
ESC from controls secreted more BMP-2 than those from women with leiomyoma. HOXA10 and LIF expression increased after rhBMP-2 treatment of normal but not leiomyoma-associated ESC. In vivo leiomyoma-associated endometrium expressed lower levels of BMPR 1A, 1B, and 2 than controls. Leiomyoma expressed high levels of TGF-β3; TGF-β3 treatment of ESC reduced expression of BMPRs. Similarly, leiomyoma-associated endometrium expressed less PAI-1 and thrombomodulin in vivo. In ESC, TGF-β3 reduced expression of PAI-1, ATIII, and thrombomodulin.
Conclusions:
Leiomyoma-secreted TGF-β3 induces BMP-2 resistance in endometrium by down-regulation of BMPR-2, likely causing defective endometrial decidualization. TGF-β3 also reduces expression of PAI-1, ATIII, and thrombomodulin in endometrium, likely contributing to menorrhagia. A single molecular signal targeting endometrium may mediate both leiomyoma-induced infertility and bleeding.
Uterine leiomyoma (fibroids) are benign myometrial neoplasms and the most common gynecological tumor in women of reproductive age. They occur in 30–70% of women (1). Of these, approximately 25% are symptomatic (2). They have significant effects on the female reproductive tract by causing impaired implantation, an increased risk of spontaneous abortion, and menorrhagia (1, 3–6).
Most reproductive dysfunction suspected to be due to defective endometrial development shows no apparent histological abnormalities; it is likely that subcellular and molecular defects are responsible for most instances of diminished endometrial receptivity. Similarly, endometrial defects in animal models are largely molecular, with no histological evidence to indicate impaired fertility. For example, Hoxa10-deficient mice have a uterine defect that does not allow embryo implantation, yet the endometrium is histologically normal. Hoxa10 (−/−) mice produce viable embryos that readily implant in a wild-type surrogate; however, even wild-type embryos do not implant in the these mice. Multiple molecular defects in pathways associated with normal implantation as well as ultrastructural morphological changes have been demonstrated in Hoxa10-deficient mice (7). In the adult human uterus, the levels of HOXA10 gene expression are significantly elevated at the time of peak functional differentiation of the endometrial epithelium and stroma, which coincides with the time of embryo implantation (8). HOXA10 appears to be essential for human endometrial receptivity, and altered HOXA10 expression is common in women with implantation defects (9–11).
In women with submucous myomas, we have previously demonstrated that there is a global decrease in endometrial HOXA10 gene expression in the endometrium, rather than a focal defect isolated to the site of the myoma (12). The deficient expression of this gene was not localized to the area over or adjacent to the myoma, suggesting that a diffusible factor may lead to endometrial dysfunction remote from the lesion. These findings may explain, in part, the reproductive dysfunction observed in women with myoma. However, to date the molecular mechanism underlying altered gene expression in the endometrium of women with leiomyoma has not been identified.
Decidualization is critical for establishment of blastocyst attachment, implantation, and proliferation. Although many steroid receptors, cytokines [including leukemia inhibitory factor (LIF)], and growth factors are implicated in this process, bone morphogenetic protein 2 (BMP-2) has been established as a critical factor in the process of embryo implantation (13–17).
BMP-2 is a growth factor that belongs to the TGF-β superfamily. It regulates cell proliferation and differentiation. Conditional ablation of BMP-2 in murine endometrium results in complete infertility because BMP-2d/d mice are unable to form implantation sites and demonstrate a complete lack of decidual response (13). Here we implicate BMP-2 in the induction of HOXA10 expression and demonstrate impairment in this pathway in the endometrial cells of women with leiomyoma.
Menorrhagia is also known to be associated with uterine leiomyoma. In the normal uterus, the endometrium undergoes extensive cyclic variations each month throughout the reproductive life of women. It multiplies and expands in response to estradiol in the proliferative stage and differentiates in response to progesterone in the secretory phase. Hormone withdrawal results in menstruation. The endometrial release of plasminogen activator (PA) activity in uterine fluid increases during the proliferative phase to a maximum at midcycle, is low in the secretory phase, and then increases again premenstrually (18–21). PA inhibitor 1 (PAI-1), a fibrinolytic modulator that binds to urokinase PA (u-PA), increases from the late proliferative phase, through the secretory phase, to a maximum in the menstrual phase (22–26). This pattern of gene expression contributes to the hemostatic mechanisms that allow normal menstrual blood flow in the normal uterus. However, PAI-1 expression is increased 4-fold in the presence of leiomyoma, compared with normal myometrium (24). The elevated levels of PAI-1 expression in leiomyomatous tissue suggests that leiomyoma may be unable to initiate the fibrinolytic process, thus making thrombolysis difficult or even impossible in leiomyoma-associated vessels (27, 28). We may hypothesize that elevated endometrial PAI-1 expression may contribute to the impaired hemostatic process in women with leiomyoma, resulting in menorrhagia.
Antithrombin III (ATIII) and thrombomodulin (TM) are also involved in the anticoagulant/thrombosis system. These molecules interrupt the coagulation cascade by preventing platelet activation and by the production of nitric oxide and prostacyclin. They also trigger and control fibrinolysis by the synthesis and release of u-PA, tissue PA, and its inhibitor PAI-1. Together, these molecules normally balance thrombosis and fibrinolysis in blood vessels (27–29).
We hypothesized that failure of blastocyst implantation in women with uterine leiomyomas is secondary to impaired BMP-2-mediated decidualization. We also hypothesized that menorrhagia in women with leiomyoma is due to a reduction of ATIII, PAI-1, and TM expression in endometrial stromal cells (ESC). Furthermore, we implicate TGF-β in the regulation of these processes. TGF-β, a dimeric polypeptide, has three isoforms, TGF-β1, TGF-β2, and TGF-β3. Each is a multifunctional cytokine whose primary role is to modulate cell development (32). They act as stimulators or inhibitors, depending on the type of tissue (31). In reproductive tissues, TGF-β3 is the most abundant isoform and is secreted in large amounts by leiomyoma (32–37). Here we demonstrate that the cytokine TGF-β3 may be a common signal resulting in both impaired fertility and excessive menstrual blood loss.
Subjects and Methods
Materials
Recombinant human (rh) BMP-2 and TGF-β3 were purchased from R&D Systems (Minneapolis, MN). Anti-BMPR1A (ab59945), BMPR1B (ab78417), and BMPR-2 (ab78422) antibodies were obtained from Abcam (Cambridge, MA). TGF-β3 (sc-80348), PAI-1 (sc-59636), and TM (sc-13164) antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The secondary antibodies, goat antirabbit IgG (sc-2040) and goat antimouse IgG (sc-2039), were also obtained from Santa Cruz Biotechnology.
Tissue sampling and cell culture
Approval from the Institutional Review Board of Yale University School of Medicine was obtained. Patients with symptomatic leiomyoma who were scheduled for hysterectomy or hysteroscopic myomectomy were recruited to this study. All recruits gave written informed consent for participation. Tissue samples were obtained from 12 subjects undergoing hysteroscopic myomectomy (n = 8) or hysterectomy (n = 4) for infertility or menorrhagia, respectively. The mean age was 32.2 yr (range, 27-47 yr). All subjects were free of preoperative hormonal therapy. Matched sets of specimens of normal myometrium, uterine leiomyoma tissue, and leiomyoma-associated endometrial tissue (1–3 g) were collected in the operating room. The control endometrial samples were obtained from 12 subjects undergoing laparoscopic (n = 7) or hysteroscopic tubal sterilization (n = 5) and without evidence of uterine pathology.
Primary cell culture
The tissues were dissected into minced 3- to 5-mm pieces. After washing with Hank's balanced salt solution, the endometrial tissues were placed in serum-free media containing 100 U/liter penicillin, 0.1 g/liter streptomycin, and 1.25 mg/liter fungizone. They were incubated at 37 C for 1 h. The tubes were then gently mixed, and the supernatant was discarded to remove the endometrial epithelial cell aggregates. The partially digested tissues were washed twice in PBS, then placed in serum-free media containing 0.5 g/liter collagenase. After incubation for 1 h at 37 C, the tubes were vortexed for 10–12 sec until the supernatant became turbid with dispersed ESC. The contents of the tube were then passed through a 70-mm gauze filter (Millipore, Billerica, MA). Cells were resuspended in DMEM-F12 (with 100 U/liter penicillin, 0.1 g/liter streptomycin, and 1.25 mg/liter fungizone) containing 2% heat-inactivated fetal calf serum. Then, 1 × 105 cells were seeded in six-well culture plates. The unattached cells were removed by washing with PBS, and the cell culture was continued after addition of fresh medium supplemented with progesterone (1 μm) and estrogen (10 nm). After 24 h, the primary cell cultures of both leiomyoma-associated ESC and nonleiomyoma ESC were treated with rhBMP-2 for 24 h. A dose-response curve was generated by treating primary stromal cell cultures with increasing amounts of rhBMP-2 (300, 500, and 1000 ng/ml) for 24 h.
RNA extraction and cDNA synthesis
Total RNA was extracted from primary ESC from three separate biopsies by using the QIAGEN RNeasy isolation kit (QIAGEN, Valencia, CA). All samples were treated with RNase-free DNase (Ambion, Austin, TX) to remove the possibility of genomic DNA contamination. The RNA samples were then analyzed by spectrophotometry to determine the RNA concentration, yield, and purity. mRNA (1 μg) was reverse-transcribed into cDNA using the iScript cDNA Synthesis Kit at 46 C for 40 min in a reaction mixture of 20 μl (Bio-Rad Laboratories, Hercules, CA). The resultant cDNA mixtures were stored at −20 C. All RNA samples were reverse-transcribed in triplicate.
Real-time PCR amplification
Gene transcripts were quantified using real-time PCR using the Bio-Rad iCycler iQ system (Bio-Rad Laboratories). Primers and probes were obtained from W. M. Keck Oligonucleotide Synthesis Facility, Yale University. Real-time PCR was performed using the iQ SYBR Green Supermix Kit (Bio-Rad Laboratories). Reaction conditions included cDNA template, primer, RNase-free water, and the iQ SYBR Green Supermix, for a final reaction volume of 25 μl. The thermal cycling conditions were initiated by uracil-N-glycosylase activation at 50 C for 2 min and an initial denaturation at 95 C for 10 min, then 40 cycles at 95 C for 15 sec, annealing at 60 C for 20 sec. Melting analysis was performed by heating the reaction mixture from 74 to 99 C at a rate of 0.2 C/sec. Threshold cycle (Ct) and melting curves were acquired by using the quantitation and melting curve program of the Bio-Rad iCycler iQ system (Bio-Rad Laboratories). Only genes with clear and single melting peaks were taken for further data analysis. Samples with irregular melting peaks were excluded from the calculation. The threshold was set manually, using identical threshold levels for one gene in all analyzed samples. Reaction efficiency was established for each set of primers after quantification of four different dilutions of a reference cDNA. Each reaction was performed in triplicate, and each assay was repeated three times using independent samples. Omission of reverse transcriptase was used as a negative control. The mRNA level of each sample was normalized to that of the 18S mRNA level, which was used as the internal control. Relative mRNA level was presented using the formula 2−∧Ct, and results are expressed as relative fold values.
Immunohistochemistry
Archival specimens fixed in neutral buffered formalin were sectioned at a thickness of 4 μm, deparaffinized, and dehydrated through a series of xylene and ethanol washes followed by permeabilization in 95% cold ethanol. After a 5-min rinse in distilled water, an antigen-presenting step was performed by steaming the slides in 0.01 mol/liter sodium citrate buffer for 20 min, followed by cooling for 20 min. Slides were rinsed for 5 min in PBS with 0.1% Tween 20 (PBST), and sections were circumscribed with a hydrophobic pen. Endogenous peroxidase was inactivated by incubation in 3% hydrogen peroxide for 5 min, followed by a 5-min PBST wash. After a preincubation with 2% normal goat serum to block nonspecific sites, the sections were incubated with primary antibodies in a humidified chamber for 18 h at 4 C. Anti-BMPR-1A, anti-BMPR-1B, anti-BMPR-2, anti-TGF-β3, anti-PAI-1, and anti-TM antibodies were used at a concentration of 4 μg/ml. Antigenic binding sites were visualized with a serial incubation with biotinylated secondary antibody, followed by avitin-biotin-horseradish peroxidase complex and diaminobenzidine tetrahydrochloride before counterstaining with Gill's hematoxylin (ABC kit; Vector Laboratories, Burlingame, CA). Negative control sections were processed in an identical manner by substitution of primary antibody with a normal rabbit IgG fraction. All negative control sections showed no color reaction. Multiple high-power fields were reviewed for each case. Immunohistochemistry was performed in triplicate on each patient sample.
BMP-2 ELISA
The ELISA was used to quantify the total amount of BMP-2 in leiomyoma-associated and control ESC. A monoclonal anti-BMP-2 was precoated onto a microplate (R&D Systems, catalog no. DBP200). Color intensity was measured by spectrophotometry at 450 nm. A standard curve was generated, and the BMP-2 concentration of each sample was calculated by regression analysis.
Statistics
Statistical analyses were performed using two-sample, two-tailed, equal variance Student's t test. Mann-Whitney U test was used to determine the difference in protein expression between leiomyoma-associated ESC and healthy ESC. P values of less than 0.05 were considered significant.
Results
BMP-2 secretion and response was decreased in ESC from leiomyomatous uteri
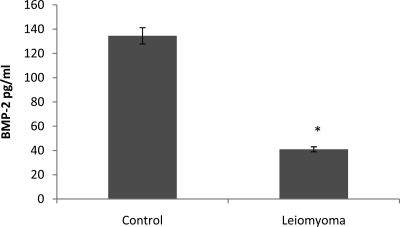
BMP-2 expression and secretion by ESC is essential for decidualization. To determine whether the presence of leiomyomata impairs BMP-2 production, we assayed BMP-2 secretion in the culture media of ESC obtained from women with and without leiomyoma. ESC from controls produced nearly 3-fold more BMP-2 than ESC from leiomyomatous uteri. BMP-2 levels in ESC cultures obtained from women with leiomyoma were 42.83 ± 1.76 pg/ml compared with 111.23 ± 21.35 pg/ml in control ESC (Fig. 1).
Fig. 1.
ELISA was performed to quantify the concentration of BMP-2 secreted by ESC in the myomatous and normal uterus. BMP-2 secretion by leiomyoma-associated ESC into the culture media was significantly lower than in normal (control) ESC. *, P = 0.0009.
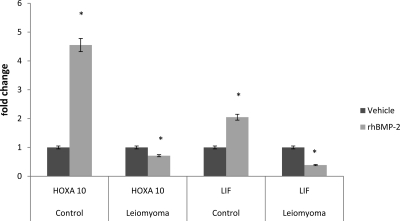
BMP-2 acts in a paracrine fashion on surrounding ESC. To determine whether these cells had a normal response to BMP-2 treatment, we assessed markers of decidualization after administration of rhBMP-2. HOXA10 gene expression increased 4.6-fold (P < 0.05) in normal ESC treated with rhBMP-2 compared with those treated with vehicle control. Similarly, LIF gene expression increased 2.1-fold (P < 0.05) when treated with rhBMP-2 (Fig. 2).
Fig. 2.
HOXA10 and LIF gene expression were measured in control ESC and compared with expression in ESC from the leiomyomatous uterus when treated with rhBMP-2. The expression of HOXA10 (*, P = 0.04) and LIF (*, P = 0.04) was significantly increased in control ESC when treated with rhBMP-2. In contrast, the expression of both implantation markers, HOXA10 (*, P < 0.003) and LIF (*, P < 0.05), was paradoxically decreased after treatment with rhBMP-2, suggesting BMP-2 resistance in the leiomyoma-associated ESC.
In contrast, HOXA10 gene expression was reduced by approximately 20% (P < 0.003), and LIF gene expression was reduced by 65% (P = 0.03), compared with vehicle control, in leiomyoma-associated ESC treated with rhBMP-2. Both findings demonstrate impaired decidualization in these cells (Fig. 2).
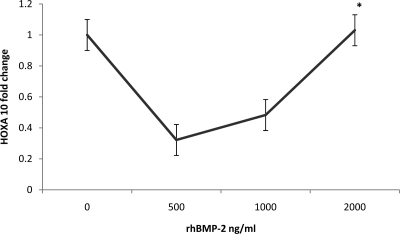
A dose-response curve was then generated to determine whether expression of decidualization markers could be restored with increasing doses of BMP-2. These data further demonstrate the diminished responsiveness of leiomyoma-associated ESC to rhBMP-2 because even high concentrations of rhBMP-2 failed to elicit an increased expression of HOXA10 above control values (Fig. 3). Low doses (500 ng/ml) paradoxically resulted in a 7-fold reduction in HOXA10 gene expression, whereas doses of 2000 ng/ml still failed to enhance expression of decidualization markers above that of the control group (0-fold). These data confirm that ESC in women with leiomyomas are BMP-2 resistant.
Fig. 3.
Dose-response curve in leiomyoma-associated ESC treated with rhBMP-2. Low doses (500 ng/ml) of rhBMP-2 resulted in a 7-fold reduction in HOXA10 gene expression, whereas doses of 2000 ng/ml still failed to enhance expression of decidualization markers above that of the control group (0-fold, P < 0.05). These data confirm that ESC in women with leiomyomas are BMP-2 resistant.
TGF-β3 induces BMP-2 resistance
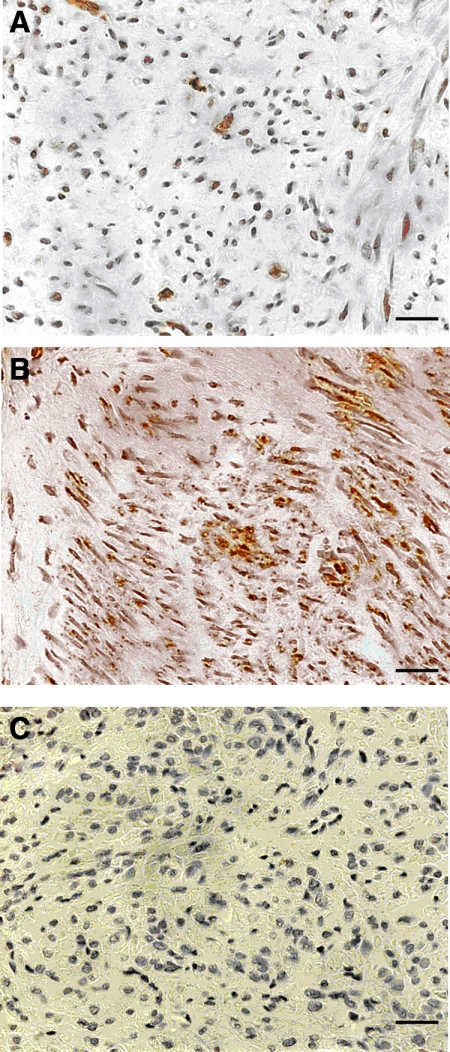
To confirm previous reports of increased TGF-β3 secretion by leiomyoma compared with normal myometrium, three archival leiomyoma and three control myometrium samples were examined for TGF-β3 expression using immunohistochemistry. Figure 4 shows representative immunohistochemical staining for TGF-β3 in normal myometrium and leiomyoma. There was consistently increased staining demonstrated in leiomyoma, compared with normal myometrium. These data were consistent with previous evidence of a 3- to 5-fold increased expression of TGF-β3 in leiomyoma compared with normal myometrium (27).
Fig. 4.
TGF-β3 expression in leiomyoma and normal myometrium. A, Normal myometrium; B, leiomyoma; C, negative control. There was significantly increased TGF-β3 staining in leiomyoma compared with normal myometrium. Scale bar, 100 μm; original magnification, ×40.
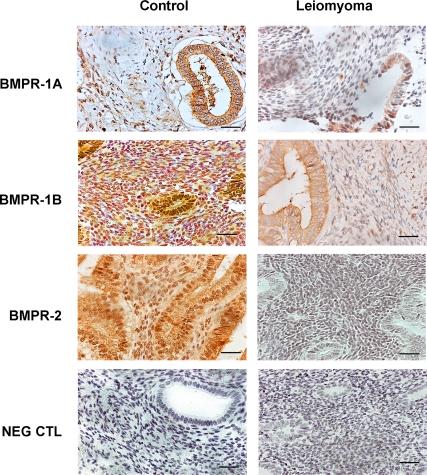
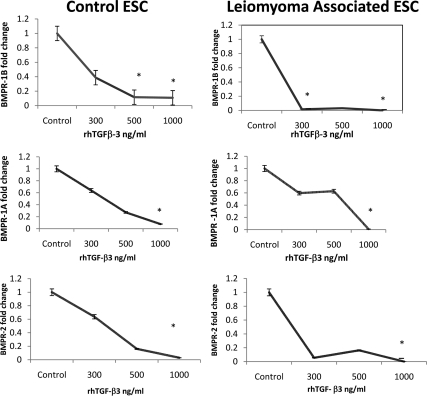
We hypothesized that TGF-β3 induces BMP-2 resistance in ESC by down-regulation of BMP-2 receptors (BMPR-2). Leiomyoma-associated endometrium demonstrated reduced expression of BMPR-1A, BMPR-1B, and BMPR-2 compared with endometrium from normal controls as assessed by immunohistochemistry (Fig. 5). To further demonstrate that TGF-β3 reduces BMPR gene expression, we performed real-time PCR to measure receptor expression in ESC after treatment with TGF-β3. Untreated control ESC expressed readily detectable levels of all BMPRs (Fig. 6). Although there was a 9-fold reduction of BMPR-1B gene expression in normal ESC treated with TGF-β3 (500 ng/ml, P < 0.005; and 1000 ng/ml, P < 0.0005; Fig. 6), we observed more than 9-fold reduction of BMPR-1B gene expression in leiomyoma-associated ESC treated even with low doses of TGF-β3 (300 ng/ml, P = 0.002; Fig. 6). Similarly, there was a greater than 9-fold reduction of BMPR-1B gene expression in leiomyoma-associated ESC when treated with higher doses of TGF-β3 (1000 ng/ml, P = 0.0005; Fig. 6). Similar profound reductions in BMPR-1A and BMPR-2 are also seen after TGF-β3 treatment. Both BMPR-1A and BMPR-2 repression occurred at lower doses of TGF-β3 in leiomyoma-associated ESC compared with control ESC.
Fig. 5.
Immunohistochemistry was used to demonstrate reduced expression of BMPR-1A, BMPR-1B, and BMPR-2 in leiomyoma-associated endometrium compared with endometrium from normal controls. Scale bar, 100 μm; original magnification, ×40.
Fig. 6.
Cultured primary ESC from controls (left) and leiomyomatous uteri (right) were treated with TGF-β3. TGF-β3 treatment reduced BMPR gene expression. Untreated control ESC expressed readily detectable levels of all BMPRs. Reductions in BMPR-1A and BMPR-2 are also seen after TGF-β3 treatment. Both BMPR-1B and BMPR-2 repression occurred at lower doses of TGF-β3 in leiomyoma-associated ESC compared with control ESC. *, P < 0.0005.
TGF-β3 reduces hemostatic factors in ESC
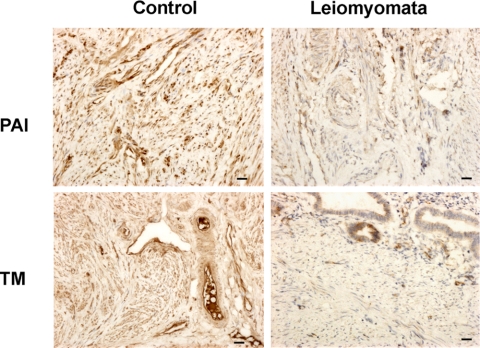
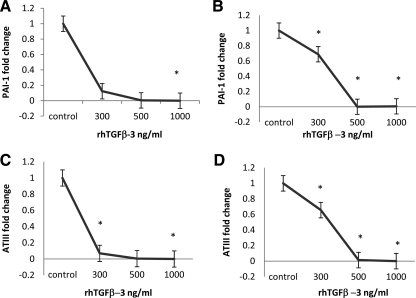
PAI-1 normally increases in the secretory and premenstrual phase of the menstrual cycle, reducing the release of u-PA from normal ESC. Real-time PCR demonstrates that the gene expression of PAI-1 was reduced more than 9-fold in ESC obtained from either the leiomyomatous (P = 0.006; Fig. 7) or normal uterus (P = 0.0008; Fig. 7), when treated with TGF-β3 (1000 ng/ml). A similar pattern was observed in vivo in leiomyoma-associated endometrial stromal tissue, in which low levels of staining for PAI-1 protein were observed in both glands and stroma (Fig. 8). In contrast, there was intense staining throughout the stroma in normal endometrium (Fig. 8).
Fig. 7.
Protein expression of hemostatic factors in control ESC and leiomyoma-associated ESC. PAI-1 and TM protein expression is significantly reduced in ESC from the leiomyomatous uterus (P < 0.005), compared with controls. Scale bar, 100 μm; original magnification, ×40.
Fig. 8.
Reduced expression of hemostatic factors in ESC treated with TGF-β3. PAI-1 gene expression is significantly reduced in control ESC (A) (*, P = 0.0008) and leiomyoma-associated ESC (B) (*, P = 0.006), when treated with TGF-β3 (1000 ng/ml). ATIII gene expression is significantly reduced in control ESC (C) (*, P =0.002), and leiomyoma-associated ESC (D) (*, P = 0.0002) when treated with TGF-β3 (1000 ng/ml).
ATIII demonstrated a similar pattern of expression and regulation by TGF-β3, with a significant reduction in gene expression in both leiomyoma-associated (P = 0.0002) and normal ESC (P = 0.002) treated with 1000 ng/ml of TGF-β3 (Fig. 7).
Likewise, TM mRNA levels were significantly reduced in leiomyoma-associated ESC by TGF-β3 (data not shown). Immunohistochemistry confirmed these findings with weak staining throughout the stroma in endometrial tissue obtained from the leiomyomatous uterus (Fig. 8). Again, this contrasted significantly with the intense immunostaining demonstrated in normal endometrial stroma (Fig. 8).
Discussion
Uterine leiomyomas are associated with menorrhagia and poor reproductive outcomes (1–6). We have previously reported that endometrial defects occur globally rather than being focally confined to the endometrium overlying the myoma (8). However, histological examination of endometrium from myomatous uteri shows no evidence of abnormality (13). Attempts to identify a molecular etiology of defective endometrial receptivity have identified alterations in HOXA10, HOXA11, and LIF gene expression in leiomyoma-associated ESC (8). Hoxa10, Hoxa11, and Lif have been shown to be necessary for embryo implantation in mice. The targeted disruption of any of these markers of implantation results in infertility due to failed uterine receptivity (7, 37–42). All are decreased in endometrium obtained from women with leiomyoma.
TGF-β3 impairs BMP-2 responsiveness in endometrial decidualization
BMP-2, a member of the TGF superfamily, is a multifunctional growth factor that is critical to endometrial implantation (14). Conditional ablation of BMP-2 in the murine endometrium results in reproductive loss due to failure of decidualization, and therefore, the inability to support embryo implantation (14).
In this study, we identified defective BMP-2 signaling in ESC from women with leiomyoma; the defect persisted in vitro, when the cells were no longer in proximity to the leiomyoma. ESC from the leiomyomatous uterus were shown to secrete only small quantities of BMP-2 and have a diminished paracrine response to BMP-2 compared with ESC obtained from healthy women. Attempts to overcome this deficiency, and therefore improve decidualization, by treatment with increasing doses of rhBMP-2 revealed a distinct cellular resistance as manifested by failure to induce HOXA10 and LIF gene expression in the ESC from leiomyomatous uteri. We then sought to identify the cause of the BMP-2 resistance.
Expression of TGF-βs and their receptors in myometrium and leiomyoma is particularly pronounced in the secretory phase of the menstrual cycle and is most pronounced in midsecretory phase samples. There are three isoforms of TGF-β. TGF-β3 has been shown to be the dominant isoform produced by uterine leiomyomas, with a 3- to 5-fold higher level of expression than in normal myometrium (35). TGF-β3 is a cytokine that regulates cell proliferation, angiogenesis, apoptosis, and migration.
We confirmed that TGF-β3 is secreted by leiomyoma. We tested the hypothesis that TGF-β3 may cause impaired decidualization in ESC by inducing BMP-2 resistance. The significant reduction in HOXA10 and LIF gene expression in leiomyoma-associated ESC treated with rhTGF-β3 supports this hypothesis. Further exploration of the BMP-2 signaling pathway demonstrated that TGF-β3 down-regulated BMP receptors. All three isoforms of the BMP membrane receptors (BMPRs)—type IA, type IB, and type II—were analyzed by real-time PCR and immunohistochemistry in leiomyoma-associated ESC. The significant reduction in BMPR gene expression in response to treatment with rhTGF-β3 suggests that TGF-β3 induces BMP-2 resistance by down-regulation of the BMPRs. Persistently decreased receptor expression explains the continued lack of BMP-2 response of ESC in culture when no longer in proximity to the myoma.
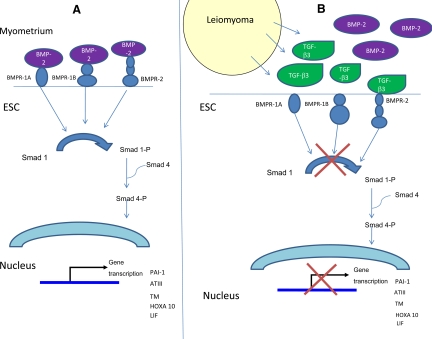
Based on these data, we propose a model that may explain the mechanism by which endometrial cells from the leiomyomatous uterus become BMP-2 resistant. BMP-2 normally binds to BMPR in ESC promoting decidualization (Fig. 9A). BMP is a member of the TGF-β superfamily, and TGF-β3 has weak affinity for the BMP receptor. In the presence of leiomyoma, excess TGF-β3 binds to BMP receptors resulting in their down-regulation and BMP-2 resistance (Fig. 9B).
Fig. 9.
Schematic representation demonstrating the effects of TGF-β3 on decidualization and hemostasis. A, In the non-leiomyomatous uterus, ESC respond to BMP-2 stimulation resulting in decidualization. To allow normal menstrual blood flow, ESC also make factors that contribute to efficient interruption of the coagulation cascade. These include ATIII, an inhibitor that neutralizes thrombin. ESC also express TM, a cellular receptor that catalyzes the activation of protein C by thrombin. u-PA is a fibrinolytic agent that is expressed by endometrial tissue and has been shown to increase in normal premenstrual and menstrual endometrial tissue. PAI-1 modulates the activity of u-PA, and higher levels are normally expressed in menstrual phase endometrium. B, In the presence of leiomyoma, excess TGF-β3 binds to BMPRs, resulting in their down-regulation, BMP-2 resistance, and thus, impaired decidualization. Excess TGF-β3 also impairs the production of PAI-1, ATIII, and TM in leiomyoma-associated ESC, resulting in defective hemostasis.
TGF-β3 also regulates hemostatic factors in ESC
Leiomyoma-associated menorrhagia has long been thought to be an anatomical issue, where the increased and abnormal surface area of endometrium resulting from these enlarged leiomyomatous tumors contributed to increased menstrual loss. Here we demonstrate that the mechanism may be directly related to impairment of the hemostatic factor production throughout the endometrium rather than a focal anatomic anomaly.
The coagulation pathway involves a cascade of reactions that culminate in the formation of thrombin and subsequently fibrin. Thrombin can further extend the coagulation cascade by activating the intrinsic coagulation pathway. To allow normal menstrual blood flow, the endothelium makes factors that contribute to efficient interruption of the coagulation cascade. These include ATIII, an inhibitor that neutralizes thrombin. It also expresses TM, a cellular receptor that catalyzes the activation of protein C by thrombin. Activated protein C counteracts the coagulation cascade by inactivation of factors Va and VIIIa. u-PA is a fibrinolytic agent that is expressed by endometrial tissue and has been shown to increase in normal premenstrual and menstrual endometrial tissue (25, 26). PAI-1 modulates the activity of u-PA, and higher levels are normally expressed in menstrual phase endometrium (21–23).
Here we show that the expression of PAI-1, TM, and ATIII in ESC is significantly altered in response to treatment with TGF-β3. This suggests that excessive levels of TGF-β3, secreted by leiomyomas, may suppress gene expression of these fibrinolytic and anticoagulant factors, thus resulting in menorrhagia.
PAI-1 mRNA is normally localized in the walls of small vessels; however, the protein is also expressed in the stroma near to small blood vessels (26, 27). In the late secretory and menstrual phases, PAI-1 mRNA and protein increase in stromal cells around the vessels and beneath the luminal epithelium (20, 21). In contrast, our data show a significant reduction in PAI-1 gene expression in leiomyoma-associated endometrial stroma, both in vivo and in vitro.
ATIII and TM have not been previously investigated as possible mediators of coagulation in ESC. Here we demonstrate that they may be involved in the mechanism of increased cyclic menstrual blood loss associated with leiomyoma. The expression of ATIII was significantly altered in ESC treated with TGF-β3. Likewise, TM was significantly decreased in the setting of leiomyoma; higher levels of the anticoagulant cellular receptor in normal endometrial stroma may also help prevent the excessive menstrual loss associated with leiomyoma.
We conclude that TGF-β3 secreted by leiomyomas regulates BMP-2 responsiveness, PAI-1, ATIII, and TM gene expression in ESC. It induces the down-regulation of BMP receptors, which results in BMP-2 resistance. Impaired fertility in women with uterine leiomyomas may be a result of TGF-β3-induced BMP-2 resistance causing defective decidualization of ESC. TGF-β3 may also induce defective hemostasis by reducing the expression of PAI-1, ATIII, and TM in leiomyoma-associated ESC (Fig. 9). This mechanism may account for the clinical manifestation of menorrhagia, a common feature in women with uterine leiomyomas.
It is likely that a common signaling pathway exists by which TGF-β3 affects the decidualization of stromal cells and the anticoagulant mechanisms that regulate menstrual blood loss. TGF-β3 may be a potential target for therapeutic intervention to prevent the complications of leiomyoma.
Acknowledgments
The authors thank Kaitlin Haines for assistance with data analysis, Amos Brooks for preparation of histological slides, and Yuping Zhou for assistance with laboratory procedures.
This work was supported by Grant U54 HD052668 from the National Institutes of Health.
Disclosure Summary: No conflict of interest is declared.
Footnotes
- ATIII
- Antithrombin III
- BMP-2
- bone morphogenetic protein 2
- BMPR-2
- BMP-2 receptor
- ESC
- endometrial stromal cells
- LIF
- leukemia inhibitory factor
- PA
- plasminogen activator
- PAI-1
- PA inhibitor 1
- rh
- recombinant human
- TM
- thrombomodulin
- u-PA
- urokinase PA.
References
- 1. Ezzati M, Norian JM, Segars JH. 2009. Management of uterine fibroids in the patient pursuing assisted reproductive technologies. Womens Health (Lond Engl) 5:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahma PK, Martel KM, Christman GM. 2006. Future directions in myoma research. Obstet Gynecol Clin North Am 33:199–224, xiii [DOI] [PubMed] [Google Scholar]
- 3. Buttram VC, Jr, Reiter RC. 1981. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril 36:433–445 [DOI] [PubMed] [Google Scholar]
- 4. Vercellini P, Zàina B, Yaylayan L, Pisacreta A, De Giorgi O, Crosignani PG. 1999. Hysteroscopic myomectomy: long-term effects on menstrual pattern and fertility. Obstet Gynecol 94:341–347 [DOI] [PubMed] [Google Scholar]
- 5. Garcia CR, Tureck RW. 1984. Submucosal leiomyomas and infertility. Fertil Steril 42:16–19 [DOI] [PubMed] [Google Scholar]
- 6. Verkauf BS. 1992. Myomectomy for fertility enhancement and preservation. Fertil Steril 58:1–15 [DOI] [PubMed] [Google Scholar]
- 7. Daftary GS, Taylor HS. 2006. Endocrine regulation of HOX genes. Endocr Rev 27:331–355 [DOI] [PubMed] [Google Scholar]
- 8. Taylor HS, Arici A, Olive D, Igarashi P. 1998. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 101:1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Daftary GS, Taylor HS. 2002. Hydrosalpinx fluid diminishes endometrial cell HOXA10 expression. Fertil Steril 78:577–580 [DOI] [PubMed] [Google Scholar]
- 10. Cermik D, Selam B, Taylor HS. 2003. Regulation of HOXA10 expression by testosterone in vitro and in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab 88:238–243 [DOI] [PubMed] [Google Scholar]
- 11. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. 1999. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod 14:1328–1331 [DOI] [PubMed] [Google Scholar]
- 12. Rackow BW, Taylor HS. 2010. Submucosal uterine leiomyomas have a global effect on molecular determinants of endometrial receptivity. Fertil Steril 93:2027–2034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee KY, Jeong JW, Wang J, Ma L, Martin JF, Tsai SY, Lydon JP, DeMayo FJ. 2007. BMP-2 is critical for the murine uterine decidual response. Mol Cell Biol 27:5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Paria BC, Ma W, Tan J, Raja S, Das SK, Dey SK, Hogan BL. 2001. Cellular and molecular responses of the uterus to embryo implantation can be elicited by locally applied growth factors. Proc Natl Acad Sci USA 98:1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang H, Bradley A. 1996. Mice deficient for BMP-2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977–2986 [DOI] [PubMed] [Google Scholar]
- 16. Ying Y, Zhao GQ. 2000. Detection of multiple bone morphogenetic protein messenger ribonucleic acids and their signal transducer, Smad 1 during mouse decidualization. Biol Reprod 63:1781–1786 [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Kannan A, Wang W, Demayo FJ, Taylor RN, Bagchi MK, Bagchi IC. 2007. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem 282:31725–31732 [DOI] [PubMed] [Google Scholar]
- 18. Casslén B, Astedt B. 1983. Occurrence of both urokinase and tissue plasminogen activator in human endometrium. Contraception 28:553–564 [DOI] [PubMed] [Google Scholar]
- 19. Blasi F. 1999. Proteolysis, cell adhesion, chemotaxis, and invasiveness are regulated by the uPA-uPAR-PAI-1 system. Thromb Haemost 82:298–304 [PubMed] [Google Scholar]
- 20. Casslén B, Astedt B. 1981. Fibrinolytic activity of human uterine fluid. Acta Obstet Gynecol Scand 60:55–58 [DOI] [PubMed] [Google Scholar]
- 21. Casslen B, Gustavsson B. 1991. Expression of cell membrane receptors for urokinase plasminogen activator (u-PA) in the human endometrium increases during the ovarian cycle. Fibrinolysis 5:243–248 [Google Scholar]
- 22. Schatz F, Lockwood CJ. 1993. Progestin regulation of plasminogen activator inhibitor type-1 in primary cultures of endometrial stromal and decidual cells. J Clin Endocrinol Metab 77:621–625 [DOI] [PubMed] [Google Scholar]
- 23. Casslén B, Nordengren J, Gustavsson B, Nilbert M, Lund LR. 1995. Progesterone stimulates degradation of urokinase plasminogen activator (u-PA) in endometrial stromal cells by increasing its inhibitor (PAI-1) and surface expression of the u-PA receptor. J Clin Endocrinol Metab 80:2776–2784 [DOI] [PubMed] [Google Scholar]
- 24. Cubellis MV, Wun TC, Blasi F. 1990. Receptor-mediated internalization and degradation of urokinase is caused by its specific inhibitor PAI-1. EMBO J 9:1079–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gleeson NC. 1994. Cyclic changes in endometrial tissue plasminogen activator and plasminogen activator inhibitor type 1 in women with normal menstruation and essential menorrhagia. Am J Obstet Gynecol 171:178–183 [DOI] [PubMed] [Google Scholar]
- 26. Nordengren J, Casslen B, Lecander I. 1996. Plasminogen activator inhibitor 2 in menstrual endometrium and in primary cultures of endometrial cells. Fibrinolysis 10:295–302 [Google Scholar]
- 27. Danø K, Behrendt N, Høyer-Hansen G, Johnsen M, Lund LR, Ploug M, Rømer J. 2005. Plasminogen activation and cancer. Thromb Haemost 93:676–681 [DOI] [PubMed] [Google Scholar]
- 28. Lwaleed BA, Greenfield R, Stewart A, Birch B, Cooper AJ. 2004. Seminal clotting and fibrinolytic balance: a possible physiological role in the male reproductive system. Thromb Haemost 92:752–766 [DOI] [PubMed] [Google Scholar]
- 29. van Hinsbergh VW. 2001. The endothelium: vascular control of hemostasis. Eur J Obstet Gynecol Reprod Biol 95:198–201 [DOI] [PubMed] [Google Scholar]
- 30. van Zonneveld AJ, Curriden SA, Loskutoff DJ. 1988. Type 1 plasminogen activator inhibitor gene: functional analysis and glucocorticoid regulation of its promoter. Proc Natl Acad Sci USA 85:5525–5529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Massagué J. 1990. The transforming growth factor-β family. Annu Rev Cell Biol 6:597–641 [DOI] [PubMed] [Google Scholar]
- 32. Sozen I, Arici A. 2002. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril 78:1–12 [DOI] [PubMed] [Google Scholar]
- 33. Joseph DS, Malik M, Nurudeen S, Catherino WH. 2010. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor β-3. Fertil Steril 93:1500–1508 [DOI] [PubMed] [Google Scholar]
- 34. Chegini N, Zhao Y, Williams RS, Flanders KC. 1994. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor β1 (TGF-β1), TGF-β2, TGF-β-3, and TGF-β2 receptor messenger ribonucleic acid and protein contains [−125I]TGF-β1-binding sites. Endocrinology 135:439–449 [DOI] [PubMed] [Google Scholar]
- 35. Arici A, Sozen I, Olive D. 1994. Modulation of transforming growth factor-β3 (TGFβ-3) expression in myometrium and leiomyoma. Program of 50th Annual Meeting of American Fertility Society, San Antonio, TX, 1994; pp S31–S32 [Google Scholar]
- 36. Arici A, Sozen I. 2000. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 73:1006–1011 [DOI] [PubMed] [Google Scholar]
- 37. Kirkbride KC, Townsend TA, Bruinsma MW, Barnett JV, Blobe GC. 2008. Bone morphogenetic proteins signal through the transforming growth factor-β type III receptor. J Biol Chem 283:7628–7637 [DOI] [PubMed] [Google Scholar]
- 38. Kim IY, Lee DH, Ahn HJ, Tokunaga H, Song W, Devereaux LM, Jin D, Sampath TK, Morton RA. 2000. Expression of bone morphogenetic protein receptors type IA, -IB, and -II correlates with tumor grade in human prostate cancer tissues. Cancer Res 60:2840–2844 [PubMed] [Google Scholar]
- 39. Kimber SJ. 2005. Leukemia inhibitory factor in implantation and uterine biology. Reproduction 130:131–145 [DOI] [PubMed] [Google Scholar]
- 40. Taylor HS, Igarashi P, Olive DL, Arici A. 1999. Sex steroids mediate HOXA 11 expression in the human peri-implantation endometrium. J Clin Endocrinol Metab 84:1129–1135 [DOI] [PubMed] [Google Scholar]
- 41. Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. 1992. Blastocyst implantation depends on maternal expression of leukemia inhibitory factor. Nature 359:76–79 [DOI] [PubMed] [Google Scholar]
- 42. Charnock-Jones DS, Sharkey AM, Fenwick P, Smith SK. 1994. Leukemia inhibitory factor mRNA concentration peaks in human endometrium at the time of implantation and the blastocyst contains mRNA for the receptor at this time. J Reprod Fertil 101:421–426 [DOI] [PubMed] [Google Scholar]