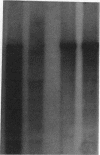
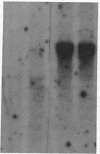
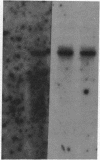
Abstract
The hereditary goitre of Afrikander cattle is an autosomal recessive disease characterized in homozygotes by the production of abnormal thyroglobulin (Tg) and the coexistence in the thyroid of normal-sized 8.4-kilobase (kb) Tg mRNA with a misspliced 7.3-kb message having lost exon 9. We have cloned and sequenced the cDNA segment corresponding to the abnormal exon 8-exon 10 junction and the relevant genomic DNA region. The mutation responsible for the disease is a cytosine to thymine transition creating a stop codon at position 697 in exon 9. The original reading frame is maintained in the 7.3-kb mRNA, which, as it lacks the mutated exon, is translatable into a potentially functional protein. This puzzling phenotype in which a mutated exon is apparently removed selectively from transcripts by alternative splicing leads us to suggest that the 7.3-kb transcript could be present in normal animals. Using a sensitive oligonucleotide hybridization assay, we have demonstrated that a 7.3-kb mRNA lacking exon 9 does exist in normal thyroids as a minor mRNA species. As it is fully translatable, the 7.3-kb mRNA is expected to be more stable than the normal-sized 8.4-kb message. This probably accounts for the higher proportion of 7.3-kb transcript found in the goitre.
Full text
PDF

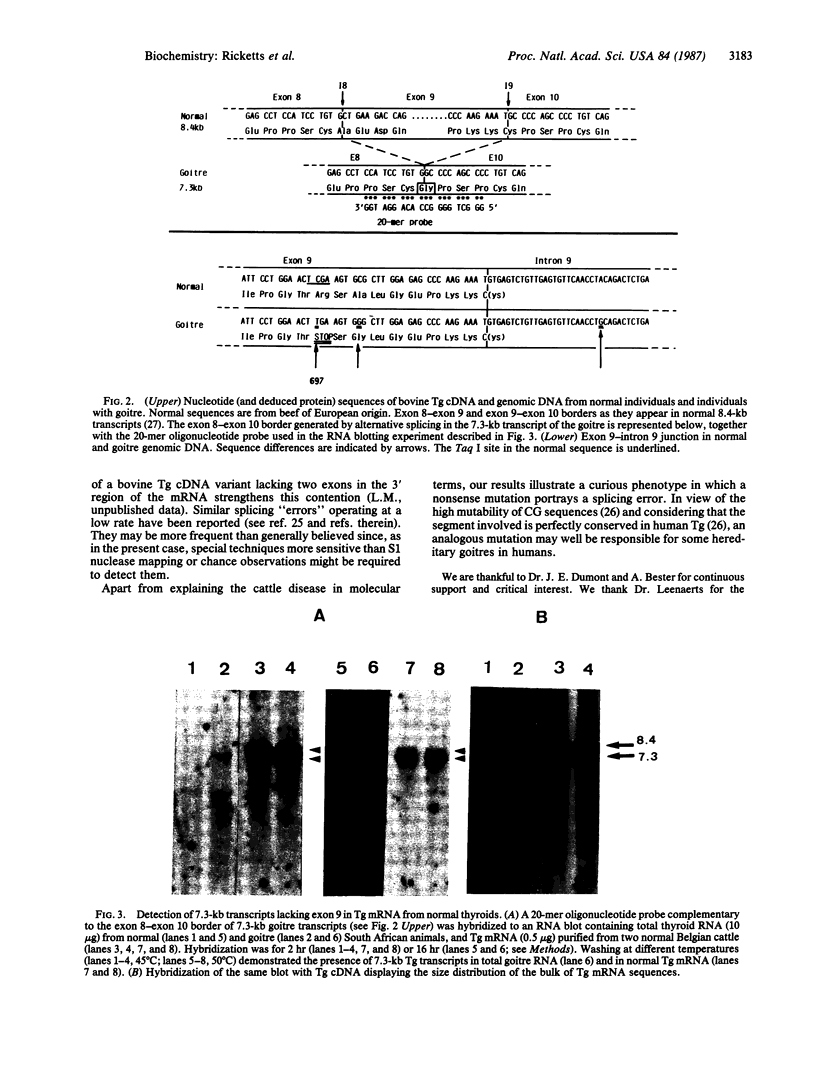

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G. Peptide diversity from exon choice: RNA processing regulation in the neuroendocrine system. Mol Cell Endocrinol. 1985 Oct;42(3):191–205. doi: 10.1016/0303-7207(85)90049-8. [DOI] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. beta 0 thalassemia, a nonsense mutation in man. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2886–2889. doi: 10.1073/pnas.76.6.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe D., Mercken L., Brocas H., Pohl V., Vassart G. Molecular cloning of the 8000-base thyroglobulin structural gene. Eur J Biochem. 1982 Mar 1;122(3):461–469. doi: 10.1111/j.1432-1033.1982.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Malthiéry Y., Lissitzky S. Sequence of the 5'-end quarter of the human-thyroglobulin messenger ribonucleic acid and of its deduced amino-acid sequence. Eur J Biochem. 1985 Feb 15;147(1):53–58. doi: 10.1111/j.1432-1033.1985.tb08717.x. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken L., Simons M. J., Swillens S., Massaer M., Vassart G. Primary structure of bovine thyroglobulin deduced from the sequence of its 8,431-base complementary DNA. Nature. 1985 Aug 15;316(6029):647–651. doi: 10.1038/316647a0. [DOI] [PubMed] [Google Scholar]
- Moschonas N., de Boer E., Grosveld F. G., Dahl H. H., Wright S., Shewmaker C. K., Flavell R. A. Structure and expression of a cloned beta o thalassaemic globin gene. Nucleic Acids Res. 1981 Sep 11;9(17):4391–4401. doi: 10.1093/nar/9.17.4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pammenter M., Albrecht C., Liebenberg W., van Jaarsveld P. Afrikander cattle congenital goiter: characteristics of its morphology and iodoprotein pattern. Endocrinology. 1978 Mar;102(3):954–965. doi: 10.1210/endo-102-3-954. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. A role for exon sequences and splice-site proximity in splice-site selection. Cell. 1986 Aug 29;46(5):681–690. doi: 10.1016/0092-8674(86)90343-0. [DOI] [PubMed] [Google Scholar]
- Ricketts M. H., Pohl V., de Martynoff G., Boyd C. D., Bester A. J., Van Jaarsveld P. P., Vassart G. Defective splicing of thyroglobulin gene transcripts in the congenital goitre of the Afrikander cattle. EMBO J. 1985 Mar;4(3):731–737. doi: 10.1002/j.1460-2075.1985.tb03690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts M. H., Schulz K., van Zyl A., Bester A. J., Boyd C. D., Meinhold H., van Jaarsveld P. P. Autosomal recessive inheritance of congenital goiter in Afrikander cattle. J Hered. 1985 Jan-Feb;76(1):12–16. doi: 10.1093/oxfordjournals.jhered.a110008. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasekhar M. B., Mertz J. E. Exon mutations that affect the choice of splice sites used in processing the SV40 late transcripts. Nucleic Acids Res. 1985 Aug 12;13(15):5591–5609. doi: 10.1093/nar/13.15.5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi V. P., Di Lauro R., Van Jaarsveld P., Alvino C. G. Two abnormal thyroglobulin-like polypeptides are produced from Afrikander cattle congenital goiter mRNA. J Biol Chem. 1984 Aug 25;259(16):10507–10510. [PubMed] [Google Scholar]
- VANZYL A., SCHULZ K., WILSON B., PANSEGROUW D. THYROIDAL IODINE AND ENZYMATIC DEFECTS IN CATTLE WITH CONGENITAL GOITER. Endocrinology. 1965 Mar;76:353–361. doi: 10.1210/endo-76-3-353. [DOI] [PubMed] [Google Scholar]
- Van Herle A. J., Vassart G., Dumont J. E. Control of thyroglobulin synthesis and secretion (second of two parts). N Engl J Med. 1979 Aug 9;301(6):307–314. doi: 10.1056/NEJM197908093010605. [DOI] [PubMed] [Google Scholar]
- Van Heuverswyn B., Streydio C., Brocas H., Refetoff S., Dumont J., Vassart G. Thyrotropin controls transcription of the thyroglobulin gene. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5941–5945. doi: 10.1073/pnas.81.19.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassart G., Brocas H., Lecocq R., Dumont J. E. Thyroglobulin messenger RNA: translation of a 33-S mRNA into a peptide immunologically related to thyroglobulin. Eur J Biochem. 1975 Jun 16;55(1):15–22. doi: 10.1111/j.1432-1033.1975.tb02133.x. [DOI] [PubMed] [Google Scholar]
- Vassart G., Verstreken L., Dinsart C. Molecular weight of thyroglobulin 33 S messenger RNA as determined by polyacrylamide gel electrophoresis in the presence of formamide. FEBS Lett. 1977 Jul 1;79(1):15–18. doi: 10.1016/0014-5793(77)80340-2. [DOI] [PubMed] [Google Scholar]
- de Martynoff G., Pays E., Vassart G. Synthesis of a full length DNA complementary to thyroglobulin 33 S messenger RNA. Biochem Biophys Res Commun. 1980 Apr 14;93(3):645–653. doi: 10.1016/0006-291x(80)91127-4. [DOI] [PubMed] [Google Scholar]
- van Voorthuizen W. F., de Vijlder J. J., van Dijk J. E., Tegelaers W. H. Euthyroidism via iodide supplementation in hereditary congenital goiter with thyroglobulin deficiency. Endocrinology. 1978 Dec;103(6):2105–2111. doi: 10.1210/endo-103-6-2105. [DOI] [PubMed] [Google Scholar]