The clinical, biochemical, and molecular features of macronodular adrenocortical hyperplasia of the zona reticularis in a man are described and characterized.
Abstract
Context:
Macronodular adrenocortical hyperplasia classically presents with progressive hypercortisolemia and Cushing syndrome. We describe a 29-yr-old man with massive macronodular adrenocortical hyperplasia without hypercortisolemia but rather markedly elevated and nonsuppressible production of dehydroepiandrosterone (DHEA) and its sulfate (DHEAS).
Objective:
To characterize the clinical and molecular features of this case and to determine whether the tissue biochemically resembles the zona reticularis or fetal adrenal.
Setting:
University clinic, hospital, and laboratories.
Design:
Static and dynamic blood and urine testing were performed preoperatively. Tissue was studied by light microscopy, immunoblot, RNA microarray, and enzyme assay.
Participant:
A 29-yr-old man with incidentally discovered bilateral adrenal enlargement.
Intervention:
Bilateral adrenalectomy.
Main Outcome Measures:
Molecular studies compared with control samples.
Results:
Hypercortisolism and 21-hydroxylase deficiency were excluded. DHEA, DHEAS, and 17-hydroxypregnenolone were markedly elevated and did not suppress with dexamethasone 2 mg/d for 4 d. Homogenates of the adrenals demonstrated high 17-hydroxylase, good 17,20-lyase, and low or absent 21-hydroxylase and 3β-hydroxysteroid dehydrogenase activities. Immunoblots confirmed robust expression of cytochrome P450c17 and AKR1C3 but not P450c21. Microarray analysis demonstrated high CYP11A1 and CYP17A1 expression but low or absent HSD3B1, HSD3B2, and CYP21A2 expression. Expression of mRNA for cytochrome b5 (CYB5A) and AKR1C3, markers of the zona reticularis, were markedly elevated.
Conclusion:
This is the first case of macronodular hyperplasia of the adrenal zona reticularis confirmed with studies of enzyme activity, mRNA expression, and protein identification. We speculate that this condition can be clinically silent in men but might cause severe hyperandrogenemia in women.
Dehydroepiandrosterone sulfate (DHEAS) circulates at much higher concentrations than any other steroid during most of human life (1, 2). The source of DHEAS is the innermost zone of the adrenal cortex, the zona reticularis, which is present in a limited number of species (3). The zona reticularis grows exponentially during childhood (4), and the excretion of 19-carbon steroids in urine rises in parallel (5). DHEAS production peaks in the third decade of life and then declines gradually, in part due to the involution of the zona reticularis, while the zona glomerulosa and zona fasciulata change little throughout life. The rise in DHEAS production and its metabolism to active androgens leads to the phenomenon of adrenarche (6), which is clinically manifested by the growth of axillary and pubic hair late in the first decade of life in boys and girls.
The zona reticularis resembles the fetal adrenal, which also produces massive quantities of DHEAS, the precursor to estrogens during pregnancy. Detailed molecular studies reveal many similarities yet also slight differences in the biochemical features of these two adrenal cell types. For example, both cell types express abundant cytochrome P450scc and P450c17 (CYP11A1, CYP17A1) as well as cytochrome b5 (b5) and DHEA sulfotransferase (SULT2A1) but low 3β-hydroxysteroid dehydrogenase/isomerase type 2 (3βHSD2) (7). This biochemical signature restricts the flux of cholesterol to only Δ5-steroids and assures efficient DHEAS production. In contrast, the zona reticularis expresses much more AKR1C3, also known as 17β-hydroxysteroid dehydrogenase type 5 (17βHSD5), than the fetal adrenal, which probably accounts for the small amount of testosterone directly secreted by the postnatal adrenal glands (8).
Tumors of the adrenal gland are common, and adenomas of the zona glomerulosa and zona fasciculata cause Conn (9) and Cushing (10) syndromes, respectively. Tumors of the zona reticularis are rare, although adrenocortical carcinomas often produce 19-carbon steroids. Another form of autonomous adrenal hyperfunction is nodular hyperplasia, which can be micronodular, often in the context of the Carney complex (11), or macronodular. Macronodular hyperplasia is found in McCune-Albright syndrome, familial adenomatous polyposis, and hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome, but the majority of cases are sporadic (12). Ectopic expression of G protein–coupled transmembrane receptors, which inappropriately drives second messenger production and augments steroidogenesis, is a hallmark of this disorder, explaining the unusual patterns of steroid production in many cases of macronodular adrenal hyperplasia with hypercortisolism (13, 14) and possibly in adrenal adenomas as well (15, 16). To our knowledge, macronodular hyperplasia of the zona reticularis has never been described and confirmed using molecular and biochemical studies. Herein, we describe the clinical, biochemical, and molecular features of such a case.
Materials and Methods
Case description
A 29-yr-old white male was evaluated for a 6-yr history of progressive bilateral adrenal enlargement, which was incidentally discovered on a computed tomography (CT) scan for nephrolithiasis. He had fathered two children and had no family history of adrenal disorders, including adrenal hyperplasias, or children with genital ambiguity. Physical exam showed no features of Cushing syndrome or hyperandrogenism. Blood pressure, serum potassium, and serum gonadotropins were normal. Relevant laboratory data are shown in Table 1. CT scan of the abdomen showed slightly heterogenous bilateral adrenal masses of 7 cm each. The masses were 4 Hounsfield units before and 105 Hounsfield units after contrast. Two biopsies, performed at an outside hospital, showed “normal adrenal tissue” by report. Repeat CT scan 3 yr later showed enlargement to 9–10 cm each. After another 3 yr, progressive gastroesophageal reflux occurred, and another CT scan showed massively enlarged, heterogeneously enhancing adrenal glands (right 13.8 × 11.2 × 9.2 cm, left 10.5 × 9.5 × 11.4 cm, Figure 1A). The patient underwent bilateral adrenalectomy (Figure 1B) for intractable gastroesophageal reflux and progressive, worrisome adrenal enlargement. Histology showed macronodular cortical hyperplasia (Figure 1C). Control tissue was obtained from a patient with macronodular adrenocortical hyperplasia and Cushing syndrome (Figure 1D). The Institutional Review Board at the University of Texas Southwestern Medical Center approved the human experimentation protocol, and subjects gave written informed consent for use of the adrenal tissue in research studies.
Table 1.
Hormone data with dynamic testing
| Analyte | Basal (normal range) | After cosyntropin | After dexamethasone | SI factor |
|---|---|---|---|---|
| Cortisol | 8.9; 15.7 (3.1–22.4 μg/dl) | 26.8 | 0.8 | 27.59 |
| 11-Deoxysteorids | 0.9 (0–5 μg/dl) | 2.6 | 28.89 | |
| 17-Hydroxyprogesterone | 57; 201 (<220 ng/dl) | 181 | 30.26 | |
| DHEA | 17,477 (180–1,250 ng/dl) | 22,893 | 34.67 | |
| DHEAS | 1,645; 1,799 (89–457 μg/dl) | 2,036 | 27.21 | |
| 17-Hydroxypregnenolone | 1014 (20–450 ng/dl) | 1,536 | 30.08 | |
| Androstenedione | 2,177 (50–250 ng/dl) | 2,681 | 34.91 | |
| Testosterone | 327 (241–827 ng/dl) | 545 | 34.67 | |
| Urinary free cortisol | 10 (5–55 μg/24 h) | 2.79 | ||
| ACTH | 15; 32 (10–60 pg/ml) | 0.22 |
Conversion factors to SI units: multiply values by conversion factor to convert μg/dl to nmol/liter, μg/24 h to nmol/24 h, ng/dl to pmol/liter, and pg/ml to pmol/liter.
Fig. 1.
Macronodular adrenocortical hyperplasia of the zona reticularis. A, CT scan shows massively enlarged, heterogeneously enhancing adrenal glands. B, Gross specimen of one adrenal weighing 720 g and measures 20 × 15 × 8 cm; the other was 522 g and 17 × 10.5 × 4.5 cm. C, Hematoxylin and eosin (H&E) stained light micrograph (×10) showing benign adrenal cortex proliferation of varied cell types with scattered atypical cells (inset, ×40) and absence of zonation. D, H&E micrographs of macronodular hyperplasia with hypercortisolism, showing lipid-filled hyperplastic adrenal cells with clearer cytoplasm and less heterogeneity than C (×10, inset ×40).
Hormone and enzyme assays
Serum testosterone and cortisol were assayed in-house using an ADVIA Centaur immunoassay analyzer (Bayer Diagnostics, Tarrytown, NY). All other assays were performed by Mayo Medical Laboratories (Rochester, MN) or Quest Diagnostics (San Juan Capistrano, CA).
Fresh tissue from surgery (1 g) was homogenized in 2 ml of 0.25 m sucrose, 10 mm Tris·HCl (pH 8.0), 5 mm disodium EDTA with 20 strokes of a Dounce homogenizer on ice. Unbroken cells and debris were removed by centrifugation for 3 min at 5,000 × g, and the supernatant was frozen at −20 C in aliquots. Homogenates (0.2–0.4 mg protein) were incubated with [3H]-labeled steroids (PerkinElmer NEN Life Science Products Radiochemicals, Waltham, MA) with unlabeled carrier steroid (Sigma, St. Louis, MO or Steraloids, Providence, RI) in 0.2 ml of 50 mm potassium phosphate buffer (pH 7.4) containing nicotinamide adenine dinucleotide (phosphate) cofactors (NAD[P][H], Sigma). Steroids were extracted with dicholoromethane, concentrated under nitrogen, separated by thin-layer chromatography (TLC) using silica gel plates and 3:1 chloroform:ethyl acetate mobile phase, and imaged using tritium-sensitive screens and a Storm 820 phosphorimager (GE Healthcare Life Sciences, Piscataway, NJ). For HPLC, extracts were dissolved in methanol and separated by reverse phase chromatography on a Breeze 1525 system (Waters, Milford, MA) with in-line radiochemical detector (INUS, Tampa, FL) using a methanol/water gradient as described (17). DHEA sulfotransferase activity was assayed by incubating homogenate (0.4 mg protein) with 1 μm [3H]-DHEA, 1 mm sodium sulfate, and 1 mm ATP in the above phosphate buffer for 60 min at 37C. The incubation was applied to a Sep-Pak C18 cartridge (Waters) and washed with water. DHEAS and DHEA were eluted with 50% or 100% aqueous methanol, respectively, as described (18).
Antisera
The antiserum to human P450c17 has been described (19). To raise an antiserum to P450c21, the 1229-bp human P450c21 cDNA clone phac21-1 encoding amino acids 265-494 plus the entire 3′ untranslated region (20) was excised from pUC18 and subcloned into a pET expression vector. The plasmid was transfected into Escherichia coli strain BL21(DE3), which were induced with isopropyl-thio-β-galactoside for 3 h as described (21). Bacteria were lysed with lysozyme, and DNA was digested with DNaseI (1 μg/ml in 5 mm MgCl2 for 15 min). Bacterial proteins were separated on 10% SDS-PAGE, stained in ice-cold 0.1% Coomassie blue with 0.25 m KCl and 1 mm dithiothreitol. The ∼28-kDa band representing the expressed P450c21 fragment was identified by comparison with similarly prepared proteins from untransfected bacteria. A thin slice of gel containing this protein was excised, crushed, and injected sc into two rabbits. After 6 weeks, one rabbit had a titer of >1;10,000, and this antiserum was aliquotted and used for immunoblots.
Immunoblots
Homogenates of adrenal tissue (15 and 50 μg protein) or yeast microsomes (15 μg protein) containing human P450c21 (22), P450c17 (23, 24), or 3βHSD2 (25) (controls) were resolved on 10% SDS-PAGE. Proteins were transferred to polyvinylidine difluoride membranes (Milipore, Billerica, MA) using a semi-dry electroblotter (Bio-Rad, Richmond, CA), blocked with 5% fat-free milk in Tris-buffered saline with 0.1% Tween-20 (TTBS) overnight, and rinsed with TTBS. Blots were probed with antisera to P450c21 (rabbit, 1:50,000), P450c17 (rabbit, 1:25,000), AKR1C3 (goat, Sigma, 1:3,000), or 3βHSD2 and 3βHSD1 (detects both proteins, mouse monoclonal, Sigma, 1:1,000) diluted in 5% milk-TTBS for 1–12 h. After rinsing with TTBS, blots were incubated in either goat antirabbit IgG-horseradish peroxidase conjugate (Perkin-Elmer, 1:2,500), goat antimouse IgG-horseradish peroxidase conjugate (Thermo Scientific, Pittsburgh, PA, 1:3,000), or donkey antigoat IgG-horseradish peroxidase conjugate (Santa Cruz Biotechnology, Santa Cruz, CA, 1:3,000) in 5% milk-TTBS for 1 h, rinsed thoroughly with TTBS, and imaged with X-Omat, blue XB-1 film (Kodak, Rochester, NY) after saturating with chemiluminescence reagent (ECL Plus, GE Healthcare Life Sciences).
RNA isolation and cDNA generation
Total RNA was isolated from the hyperplastic adrenal tissue and from five normal human adult adrenals and five human fetal adrenals (HFA) (26) using RNeasy Mini Kit (Qiagen, Germany) as described by the manufacturer. The quantity and purity of the isolated RNA was determined by the NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). Total RNA (2 μg) was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturer's instructions and incubated at 25C for 10 min, then 37 C for 2 h.
Microarray analysis
Total RNA from three normal adult adrenals, four of the fetal adrenals, and the hyperplastic adrenal were subjected to a first- and second-strand RT, followed by a single in vitro transcription amplification that incorporated biotin-labeled nucleotides. The HFA cDNA samples were distributed into two pools, each pool containing two individual fetal adrenal cDNA samples. The labeled RNA was then hybridized to a bead chip containing more than 48,000 probes representing over 25,000 human genes (Illumina, San Diego, CA). The arrays were scanned at high resolution on the iScan system (Illumina) located at the Medical College of Georgia Microarray Core Facility. Results were determined using GeneSpring GX version 11 software (Silicon Genetics, Redwood City, CA) by customizing to the generic single color analysis. A list of steroidogenic enzymes was created to identify the differences among the three groups of adrenal samples.
Real-time quantitative PCR (qPCR)
To confirm the results of the microarray analysis, qPCR analysis was performed using the total RNA isolated from the three groups of adrenal samples using the ABI 7500 Fast Real-Time PCR System (Applied Biosystems) for CYP11A1, CYP11B1, CYP11B2, CYP17A1, CYB5, CYP21A2, HSD3B2, and AKR1C3. Quantitative normalization of cDNA in each tissue-derived sample was performed using 18S rRNA expression as an internal control, using the generated Ct value of each gene divided by its respected Ct value of 18S rRNA (ΔCt). Expression of each gene was further normalized using the average ΔCt value of the normal adrenal samples (ΔΔCt). The final fold expression changes were calculated using the equation: Fold expression difference (F) = 2[exp(−ΔΔCt)]. Results were represented as log2(fold change).
Results
The 24-h urinary free cortisol, serum testosterone, and plasma ACTH were normal, and the serum cortisol rose to >20 μg/dl with normal 17-hydroxyprogesterone and 11-deoxysteroids 45 min after cosyntropin administration (0.25 mg intramuscularly, Table 1). These screening tests excluded ACTH-independent hypercortisolism and congenital adrenal hyperplasia. After 2 mg/d dexamethasone for 4 d, the cortisol fell to <1 μg/dl, but the DHEA, DHEAS, and androstenedione remained markedly elevated (Table 1), confirming selective ACTH-independent production of adrenal 19-carbon steroids.
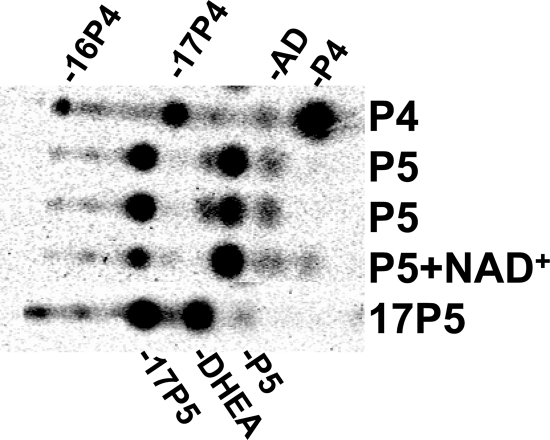
After surgical removal of the adrenal tissue, tissue homogenates were incubated with [3H]-labeled pregnenolone, progesterone, and 17-hydroxypregnenolone. Product analysis showed robust 17-hydroxylase activity and somewhat lower 17,20-lyase activity (P450c17), based on accumulation of intermediate 17-hydroxypregnenolone. In contrast, 21-hydroxylase (P450c21) and 3β-hydroxysteroid dehydrogenase/isomerase (3βHSD) activities were low or absent (Figure 2). DHEA sulfotransferase (SULT2A1) activity was 0.3 pmol/min/mg protein (not shown).
Fig. 2.
Steroidogenic enzyme assay of adrenal tissue. Phosphorimage shows products from [3H]-labeled pregnenolone (P5), progesterone (P4), and 17-hydroxypregnenolone (17P5) after incubation with adrenal homogenates, extraction, and separation by TLC. All incubations contained NADPH, to test P450 activities, except P5 + NAD+ as labeled, to test 3βHSD activity. Incubation with P4 shows metabolism to 17α- and 16α-hydroxyprogesterone (17P4, 16P4) and a trace of androstenedione (AD), but no 11-deoxycortisol (migrating slightly above 16P4), indicating high 17-hydroxylase but low 21-hydroxylase activities. Incubation with P5 + NADPH yields 17α-hydroxypregnenolone (17P5) and some DHEA, and incubation with 17P5 yields DHEA, confirming robust 17-hydroxylase and good 17,20-lyase activities. In contrast, incubation with P5 and NAD+ yields only a trace of P4, documenting very low 3βHSD activity. The identity of all products was confirmed by HPLC analysis on separate incubations, including incubations starting with 17P4 (not shown).
Microarray analysis allowed the comparison of all transcripts needed for steroid hormone biosynthesis between the tumor tissue and fetal adrenal as a model of the zona reticularis, both normalized to values obtained with normal adult adrenal (Figure 3). Tumor tissue was found to express high levels of the transcripts required for the synthesis of DHEA and DHEAS. Consistent with the enzyme assay data, expression of the cognate transcripts encoding 3βHSD2, 3βHSD1, P450c21, and P450c11β (11-hydroxylase) was much lower than seen in normal adult adrenal. Expression of b5 and AKR1C3, markers of the zona reticularis, were profoundly elevated compared with that seen in normal adult or fetal adrenals. Expression data from microarrays were confirmed by qPCR (Table 2). Immunoblots (Figure 4) confirmed high P450c17 and AKR1C3 protein but low or absent HSD3B1, HSD3B2, and P450c21 expression.
Fig. 3.
Heat maps comparing steroidogenic enzyme expression in four pathways vs. other adrenal specimens. A, Aldosterone pathway (zona glomerulosa). B, Cortisol pathway (zona fasciculata). C, DHEAS pathway (zona reticularis). D, Gonadal pathways to androgens and estrogens. Expression pattern is consistently highest for enzymes required for DHEAS synthesis.
Table 2.
Fold change in steroidogenic gene expression by quantitative real-time PCR
| Steroidogenic enzyme gene | Difference [log2 (fold change)] |
||
|---|---|---|---|
| Normal adrenal | Fetal adrenal | Patient's adrenal tissue | |
| CYP11A1 | 0 ± 0.30 | 1.723 ± 0.159 | −0.04 |
| CYP21A2 | 0 ± 0.52 | −0.343 ± 0.41 | −1.734 |
| CYP17A1 | 0 ± 0.37 | 1.07 ± 0.10 | 0.02 |
| CYB5 | 0 ± 0.46 | 0.98 ± 0.20 | 4.18 |
| HSD3B2 | 0 ± 0.73 | −9.59 ± 0.43 | −9.11 |
| CYP11B1 | 0 ± 0.54 | −1.18 ± 0.50 | −4.49 |
| CYP11B2 | 0 ± 0.63 | ND | −8.68 |
| AKR1C3 | 0 ± 0.76 | −6.82 ± 0.28 | 4.73 |
Results expressed as the log2 (fold change) are compared to the gene expression seen in fetal adrenal (n = 5), both normalized to normal adult adrenal tissue (n = 5). Data are represented as mean ± sem, normalized to normal adult adrenal, for which values are defined as 0 (using log scale) with sem given to indicate variance.
ND, not detected.
Fig. 4.

Immunoblots of adrenal tissues for steroidogenic enzymes. Blots were prepared with microsomes (MS, 15 μg protein) from yeast expressing human 3βHSD2 (3β), P450c21 (c21), or P450c17 (c17) and homogenates (15 or 50 μg protein) of macronodular adrenocortical hyperplasia of the zona fasciculata (producing cortisol, MH ZF) or zona reticularis (producing DHEA and DHEAS, MH ZR). Two blots were probed with antibodies to P450c17 (A), P450c21 (B), 3βHSD2 and 3βHSD1 (C), and AKR1C3 (D). P450c17 is somewhat more abundant in MH ZR tissue than MH ZF tissue, whereas P450c21 is abundant in MH ZF tissue but undetectable in MH ZR tissue. AKR1C3 is barely detectable in MH ZF tissue but high in MH ZR tissue. We were unable to convincingly demonstrate 3βHSD2 in MH ZF tissue, although higher oligomers might be present (arrow), which are absent in MH ZR tissue.
Discussion
The biochemical machinery of the zona reticularis is ideally engineered for efficient DHEAS production, with high expression of P450c17, POR, and b5 to favor 17-hydroxylation and 17,20-lyase cleavage of nascent pregnenolone (2). Genetic defects in any of the genes encoding these proteins can cause isolated 17,20-lyase deficiency (24, 27–29), attesting to the requirement of all three components for this reaction. In addition, efficient DHEAS production requires suppression of alternative pathways such as conversion to Δ4-steroid hormones. The adrenal tissue from this patient met all these characteristics, with high expression of the P450c17-POR-b5 catalytic system, with low P450c21, 3βHSD1, and 3βHSD2. In addition, AKR1C3, which is a marker of the zona reticularis within the adrenal cortex (8), was also highly expressed with SULT2A1 (Figure 5).
Fig. 5.
Expression of steroidogenic genes in the DHEAS secreting tumor vs. normal adrenal. This graphic comparison derives from data determined by microarray and confirmed by qPCR.
The 17,20-lyase activity of the adrenal homogenates appeared lower than its 17-hydroxylase activity, despite abundant b5 expression. Using normal adrenal homogenates or yeast microsomes containing P450c17 and b5, pregnenolone is readily metabolized to DHEA with little 17-hydroxypregenolone accumulating (23). Expression of POR in this tissue, however, was reduced compared with normal adrenal, rendering the b5/POR ratio relatively high. Addition of a 30-fold molar excess of b5 relative to P450c17 or reduction of POR content paradoxically lowers 17,20-lyase activity, perhaps by sequestering POR in POR·b5 complexes (23, 30). Consequently, POR might be limiting for P450c17 activity in this unique adrenal tissue.
ACTH-independent steroid production was demonstrated by prolonged dexamethasone suppression. In fact, the 19-carbon steroids appeared to rise with dexamethasone, which is reminiscent of the paradoxical cortisol rise with dexamethasone seen in micronodular hyperplasia (31). These changes were less than a factor of two, and the samples were not obtained at the exact same time of day, precluding definitive conclusions about the influence of dexamethasone. In addition, normal cortisol dynamics were preserved, even though we could not find any areas with normal adrenal zonation. Although our data characterize the steroidogenic activity of this tissue, the molecular mechanisms responsible for adrenal growth in most cases of adrenocortical hyperplasia, including our case, remain unknown. Our microarray experiments found that LH/CG receptor expression was only 3.5-fold higher than in normal adult adrenal (not shown), which is low compared with amounts in aldosteronomas, where these ectopic receptors might contribute to their pathogenesis (15).
This man retained normal testicular function, fathering two children before adrenalectomy and one after the surgery, despite circulating DHEA concentrations two orders of magnitude above normal. This observation emphasizes the minor contribution of adrenal 19-carbon steroid production to total testosterone synthesis in men. Rare instances of gonadal suppression have been observed in men with poorly controlled 21-hydroxylase deficiency (32, 33). In contrast, half or more of circulating testosterone in women derives from adrenal precursors (34), and women are more vulnerable to symptoms from adrenal-derived androgen excess, as in nonclassical 21-hydroxylase deficiency (35, 36). Consequently, we speculate that women with macronodular hyperplasia of the zona reticularis would show prominent signs of androgenization, including hirsutism, acne, and possibly also virilization. A related but somewhat different case of a woman with virilization and macronodular adrenocortical hyperplasia has been described previously (37). In that case, a dominant nodule or adenoma of one adrenal appeared to be the major source of testosterone. Although the contralateral adrenal was minimally enlarged, the residual hyperandrogenemia after unilateral adrenalectomy suppressed with leuprolide acetate. The evidence from that report indicated that testosterone production was driven by ectopic LH/CG receptor expression, but the presence of steroidogenic enzyme activity and/or gene expression in the tissue was not characterized in detail (37).
The description of this case should encourage evaluation of other patients for this condition, particularly asymptomatic men with bilateral adrenal enlargement and normal cortisol dynamics and women with androgen excess and adrenal hyperplasia but normal cortisol and 17-hydroxyprogesterone production. With further study, the genetic and molecular bases for this condition might be elucidated. This knowledge, in turn, will further our understanding of adrenarche and adrenal zonation.
Acknowledgments
We thank C. David Vance for assistance with specimen handling and HPLC.
This work was supported by a Clinician Scientist Award in Translational Research #1005954 from the Burroughs-Wellcome Fund and the Charles A. and Elizabeth Ann Sanders Chair in Translational Research (to R.J.A.) and grant R01-DK069950 (to W.E.R.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- 3βHSD
- 3β-Hydroxysteroid dehydrogenase/isomerase
- 17βHSD5
- 17β-hydroxysteroid dehydrogenase type 5
- CT
- computed tomography
- DHEA
- dehydroepiandrosterone
- DHEAS
- dehydroepiandrosterone sulfate
- HFA
- human fetal adrenals
- HLRCC
- hereditary leiomyomatosis and renal cell cancer
- NAD[P][H]
- nicotinamide adenine dinucleotide (phosphate)
- TLC
- thin-layer chromatography.
References
- 1. Orentreich N, Brind JL, Rizer RL, Vogelman JH. 1984. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59:551–555 [DOI] [PubMed] [Google Scholar]
- 2. Auchus RJ, Rainey WE. 2004. Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 60:288–296 [DOI] [PubMed] [Google Scholar]
- 3. Cutler GB, Glenn M, Bush M, Hodgen GD, Graham CE, Loriaux DL. 1978. Adrenarche: a survey of rodents, domestic animals and primates. Endocrinology 103:2112–2118 [DOI] [PubMed] [Google Scholar]
- 4. Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. 2009. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol 203:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Remer T, Boye KR, Hartmann MF, Wudy SA. 2005. Urinary markers of adrenarche: reference values in healthy subjects, aged 3–18 years. J Clin Endocrinol Metab 90:2015–2021 [DOI] [PubMed] [Google Scholar]
- 6. Miller WL. 2009. Androgen synthesis in adrenarche. Rev Endocr Metab Disord 10:3–17 [DOI] [PubMed] [Google Scholar]
- 7. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. 2009. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord 10:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nakamura Y, Hornsby PJ, Casson P, Morimoto R, Satoh F, Xing Y, Kennedy MR, Sasano H, Rainey WE. 2009. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab 94:2192–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Auchus RJ, Nwariaku FE. 2007. Primary aldosteronism. Curr Cardiol Rep 9:447–452 [DOI] [PubMed] [Google Scholar]
- 10. Mitchell IC, Auchus RJ, Juneja K, Chang AY, Holt SA, Snyder WH, 3rd, Nwariaku FE. 2007. “Subclinical Cushing's syndrome” is not subclinical: improvement after adrenalectomy in 9 patients. Surgery 142:900–905 [DOI] [PubMed] [Google Scholar]
- 11. Bertherat J, Horvath A, Groussin L, Grabar S, Boikos S, Cazabat L, Libe R, Rene-Corail F, Stergiopoulos S, Bourdeau I, Bei T, Clauser E, Calender A, Kirschner LS, Bertagna X, Carney JA, Stratakis CA. 2009. Mutations in regulatory subunit type 1A of cyclic adenosine 5′-monophosphate-dependent protein kinase (PRKAR1A): phenotype analysis in 353 patients and 80 different genotypes. J Clin Endocrinol Metab 94:2085–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, Robinson-White AJ, Nesterova M, Lacroix A, Stratakis CA. 2009. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab 94:2930–2937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bourdeau I, D'Amour P, Hamet P, Boutin JM, Lacroix A. 2001. Aberrant membrane hormone receptors in incidentally discovered bilateral macronodular adrenal hyperplasia with subclinical Cushing's syndrome. J Clin Endocrinol Metab 86:5534–5540 [DOI] [PubMed] [Google Scholar]
- 14. Lacroix A, Bourdeau I, Lampron A, Mazzuco TL, Tremblay J, Hamet P. 2010. Aberrant G-protein coupled receptor expression in relation to adrenocortical overfunction. Clin Endocrinol (Oxf) 73:1–15 [DOI] [PubMed] [Google Scholar]
- 15. Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. 2007. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol 195:39–48 [DOI] [PubMed] [Google Scholar]
- 16. Reznik Y, Lefebvre H, Rohmer V, Charbonnel B, Tabarin A, Rodien P, Lecomte P, Bardet S, Coffin C, Mahoudeau J. 2004. Aberrant adrenal sensitivity to multiple ligands in unilateral incidentaloma with subclinical autonomous cortisol hypersecretion: a prospective clinical study. Clin Endocrinol (Oxf) 61:311–319 [DOI] [PubMed] [Google Scholar]
- 17. Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 2003. 5α-androstane-3α,17β-diol is formed in tammar wallaby pouch young testes by a pathway involving 5α-pregnane-3α,17α-diol-20-one as a key intermediate. Endocrinology 144:575–580 [DOI] [PubMed] [Google Scholar]
- 18. Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjovall J, Baulieu EE. 2004. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res 45:2287–2302 [DOI] [PubMed] [Google Scholar]
- 19. Lin D, Black SM, Nagahama Y, Miller WL. 1993. Steroid 17α-hydroxylase and 17,20 lyase activities of P450c17: Contributions of serine106 and P450 reductase. Endocrinology 132:2498–2506 [DOI] [PubMed] [Google Scholar]
- 20. Matteson KJ, Phillips JA, III, Miller WL, Chung B, Orlando PJ, Frisch H, Ferrandez A, Burr IM. 1987. P450XXI (steroid 21-hydroxylase) gene deletions are not found in family studies of congenital adrenal hyperplasia. Proc Natl Acad Sci USA 84:5858–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Black SM, Szklarz GD, Harikrishna JA, Lin D, Wolf CR, Miller WL. 1993. Regulation of proteins of the cholesterol side-chain cleavage system in Y-1 and JEG-3 cells. Endocrinology 132:539–545 [DOI] [PubMed] [Google Scholar]
- 22. Auchus RJ, Kumar AS, Boswell CA, Gupta MK, Bruce K, Rath NP, Covey DF. 2003. The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21. Arch Biochem Biophys 409:134–144 [DOI] [PubMed] [Google Scholar]
- 23. Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20 lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165 [DOI] [PubMed] [Google Scholar]
- 24. Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. 2003. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569 [DOI] [PubMed] [Google Scholar]
- 25. Lee TC, Miller WL, Auchus RJ. 1999. Medroxyprogesterone acetate and dexamethasone are competitive inhibitors of different human steroidogenic enzymes. J Clin Endocrinol Metab 84:2104–2110 [DOI] [PubMed] [Google Scholar]
- 26. Xing Y, Nakamura Y, Rainey WE. 2009. G protein-coupled receptor expression in the adult and fetal adrenal glands. Mol Cell Endocrinol 300:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geller DH, Auchus RJ, Mendonça BB, Miller WL. 1997. The genetic and functional basis of isolated 17,20 lyase deficiency. Nat Genet 17:201–205 [DOI] [PubMed] [Google Scholar]
- 28. Hershkovitz E, Parvari R, Wudy SA, Hartmann MF, Gomes LG, Loewental N, Miller WL. 2008. Homozygous mutation G539R in the gene for P450 oxidoreductase in a family previously diagnosed as having 17,20-lyase deficiency. J Clin Endocrinol Metab 93:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. 2010. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab 95:994–999 [DOI] [PubMed] [Google Scholar]
- 30. Auchus RJ, Miller WL. 2002. Human 17α-hydroxylase/17,20-lyase. In Mason JI. ed. Genetics of steroid biosynthesis and function. New York: Taylor and Francis; 259–286 [Google Scholar]
- 31. Stratakis CA, Sarlis N, Kirschner LS, Carney JA, Doppman JL, Nieman LK, Chrousos GP, Papanicolaou DA. 1999. Paradoxical response to dexamethasone in the diagnosis of primary pigmented nodular adrenocortical disease. Ann Intern Med 131:585–591 [DOI] [PubMed] [Google Scholar]
- 32. Reisch N, Flade L, Scherr M, Rottenkolber M, Pedrosa Gil F, Bidlingmaier M, Wolff H, Schwarz HP, Quinkler M, Beuschlein F, Reincke M. 2009. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab 94:1665–1670 [DOI] [PubMed] [Google Scholar]
- 33. Tiitinen A, Välimäki M. 2002. Primary infertility in 45-year-old man with untreated 21-hydroxylase deficiency: successful outcome with glucocorticoid therapy. J Clin Endocrinol Metab 87:2442–2445 [DOI] [PubMed] [Google Scholar]
- 34. Arlt W, Justl H-G, Callies F, Reincke M, Hubler D, Oettel M, Ernst M, Schulte HM, Allolio B. 1998. Oral dehydroepiandrosterone for adrenal androgen replacement: pharmacokinetics and peripheral conversion to androgens and estrogens in young healthy females after dexamethasone suppression. J Clin Endocrinol Metab 83:1928–1934 [DOI] [PubMed] [Google Scholar]
- 35. Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. 2004. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab 89:453–462 [DOI] [PubMed] [Google Scholar]
- 36. Speiser PW, Knochenhauer ES, Dewailly D, Fruzzetti F, Marcondes JA, Azziz R. 2000. A multicenter study of women with nonclassical congenital adrenal hyperplasia: relationship between genotype and phenotype. Mol Genet Metab 71:527–534 [DOI] [PubMed] [Google Scholar]
- 37. Goodarzi MO, Dawson DW, Li X, Lei Z, Shintaku P, Rao CV, Van Herle AJ. 2003. Virilization in bilateral macronodular adrenal hyperplasia controlled by luteinizing hormone. J Clin Endocrinol Metab 88:73–77 [DOI] [PubMed] [Google Scholar]