Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans, which may contribute to cardiac complications and insulin resistance in diabetes.
Abstract
Context:
Insulin recruits microvasculature in both cardiac and skeletal muscle, which increases the endothelial exchange surface area. Plasma concentrations of free fatty acids (FFAs) are elevated in patients with diabetes, which impairs insulin-mediated skeletal muscle microvascular recruitment.
Objective:
The objective of the study was to examine whether elevated FFAs likewise cause insulin resistance in cardiac muscle microvasculature.
Setting:
The study was conducted at the General Clinical Research Center at the University of Virginia.
Methods:
Twenty-two healthy, young adults were studied twice in random order after an overnight fast. Each subject received a 5-h systemic infusion of either saline or Intralipid/heparin with a 1 mU/min · kg euglycemic insulin clamp superimposed for the last 2 h. Cardiac and forearm skeletal muscle microvascular blood volume (MBV) and flow velocity were measured and microvascular blood flow (MBF) calculated before and at the end of the insulin infusion.
Results:
Insulin significantly increased MBV and MBF in both cardiac (P < 0.0001 for both) and skeletal (P = 0.008 and < 0.03, respectively) muscle. Microvascular flow velocity increased slightly but significantly in the skeletal (P = 0.04) but not in cardiac muscle. Lipid infusion lowered insulin-stimulated whole-body glucose disposal and abolished insulin-mediated increases in MBV and MBF in both cardiac and skeletal muscle. Whole-body insulin sensitivity predicted skeletal but not cardiac muscle microvascular responses to insulin. Insulin even decreased skeletal muscle MBV during lipid infusion in subjects who were moderately sensitive to insulin metabolically.
Conclusions:
In conclusion, high plasma concentrations of FFAs cause insulin resistance in cardiac as well as skeletal muscle microvasculature in healthy humans. This may contribute to the association of cardiac complications with metabolic insulin resistance in diabetes.
Patients with type 2 diabetes display metabolic insulin resistance and develop cardiac complications (including diastolic dysfunction, cardiomyopathy, coronary artery disease, cardiac autonomic neuropathy), which contribute significantly to the morbidity and mortality in this patient population. A major function of the microcirculation is to regulate tissue perfusion by providing an endothelial exchange surface area adequate for tissue delivery of nutrients, oxygen, and hormones. Strong evidence has implicated the involvement of microvascular insulin resistance and dysfunction in the pathogenesis of metabolic insulin resistance and cardiovascular complications in diabetes.
Within muscle, microvascular perfusion and surface area are under tonic control of the precapillary terminal arterioles (1, 2) in which insulin regulates vessel tone via stimulating endothelial nitric oxide (NO) synthase (3, 4). In healthy humans, insulin also increases coronary flow reserve by approximately 20–30% (5, 6) and potently recruits cardiac microvasculature by expanding microvascular blood volume (7).
Microvascular insulin resistance is well established in patients and animal models of obesity and/or diabetes (8). Indeed, insulin-stimulated skeletal muscle microvascular recruitment was impaired in obese Zucker rats (an animal model of metabolic syndrome) (9), Zucker diabetic fatty rats (an animal model of type 2 diabetes) (10), and obese humans (4). The mechanisms underlying microvascular insulin resistance are not well understood. However, high plasma concentrations of free fatty acids (FFAs) are thought to play an important role. The plasma levels of FFAs are elevated in patients with type 2 diabetes and acute elevation of plasma FFA concentrations experimentally blunts insulin-mediated skeletal muscle microvascular recruitment in both laboratory animals (11) and healthy humans (3).
Insulin resistance occurs in the coronary microcirculation as well. Insulin increases coronary blood flow and this action is blunted in obese (12) and type 2 diabetic patients (13). Raising plasma insulin concentrations by about 8-fold through ingestion of a mixed meal induces a significant increase in cardiac microvascular perfusion in healthy humans but a paradoxically decrease in patients with diabetes (14). Whether elevated plasma FFAs cause insulin resistance in cardiac muscle microvasculature similar to that seen in skeletal muscle has not been studied.
In the present study, we assessed whether microvascular responses to physiological hyperinsulinemia in both cardiac and skeletal muscle were affected by acute elevation of plasma concentrations of FFAs in healthy humans. Our results demonstrate that high plasma FFA concentrations cause microvascular insulin resistance in both cardiac and skeletal muscle. This may contribute to the common association of cardiac complications and metabolic insulin resistance in humans.
Materials and Methods
Human subjects and study protocols
A total of 22 (10 males, 12 females) young and healthy subjects (aged 22.5 ± 0.8 yr, body mass index 22.5 ± 0.5 kg/m2) with no history of obesity, hypertension, diabetes, or hyperlipidemia were screened and then studied twice in the General Clinical Research Center (GCRC) at the University of Virginia.
At the screening visit, blood samples were taken for pregnancy (for women), prothrombin time, partial thromboplastin time, complete blood counts with differential analysis, cholesterol, triglycerides, insulin, and glucose measurements. Subjects were excluded from the study if they were pregnant, were smokers, were anemic, had a bleeding tendency, or were taking any medications or supplements known to affect either endothelial function or glucose metabolism. Subjects with a family history of diabetes in a first-degree relative were also excluded from the study. The study protocol was approved by the Institutional Review Board and the GCRC Advisory Committee at the University of Virginia. All subjects provided written informed consent at the screening visit.
All studies were done after a 12-h overnight fast. Subjects were admitted to the GCRC the evening before the study. On the morning of the study, a catheter was placed in the antecubital vein of the right arm and used for the infusion of normal saline or Intralipid (with heparin, insulin, glucose, and microbubbles. Another catheter was placed in a peripheral vein for blood sampling of glucose, FFAs, and insulin. Subjects were then studied under the following two protocols in random order, with at least 2 wk in between the admissions.
Saline (control) protocol
After obtaining baseline blood samples, baseline skeletal and cardiac muscle microvascular parameter measurements including microvascular blood volume (MBV), microvascular blood flow velocity (MFV), and microvascular blood flow (MBF) were determined using the contrast-enhanced ultrasound (CEU)/myocardial contrast echocardiography (MCE) technique. Each subject then received a 2-h iv infusion of regular insulin (2 mU/min · kg × 10 min and 1 mU/min · kg × 110 min). Blood samples were taken for plasma glucose measurements every 5 min throughout the 120-min insulin infusion and 20% dextrose was infused at variable rates to maintain euglycemia. At the end of the clamp, skeletal and cardiac muscle MBV and MFV were again measured and MBF calculated. Plasma insulin and FFAs were measured at 0, 30, 60, 90, and 120 min. Venous plasma glucose was clamped at a level approximately 10 mg/dl below the basal level to avoid arterial hyperglycemia because venous glucose concentrations decline progressively with time when arterial glucose is maintained at a constant rate during insulin clamp.
Lipid protocol
The study was carried out in the exact same fashion as the saline (control) protocol except all subjects received an iv infusion of Intralipid (20%, 45 ml/h) and heparin (0.2 U/min · kg) for a total of 5 h. Heparin was given to activate endothelial lipoprotein lipase, which facilitates the conversion of circulating lipids to FFAs in the body. The insulin clamp started 3 h after beginning the Intralipid/heparin infusion (time 0) and the CEU/MCE measurements were done before and after 120 min of insulin infusion.
CEU/MCE procedure
In the current study, the term CEU is used for contrast ultrasonographic examination of skeletal muscle, whereas the term MCE is used for cardiac muscle. The skeletal (CEU) and cardiac (MCE) muscle microvascular parameters were measured using a SONOS 7500 ultrasound system and an S3 phased array transducer (Philips Medical Systems, Andover, MA) while the subject was in the left decubitus position, as previously described (3, 7, 15). In brief, the contrast agent (Definity microbubbles; Lantheus Medical Imaging, North Billerica, MA) was delivered iv (1.5 ml diluted in normal saline to a total volume of 30 ml and infused at 90–120 ml/h). After the systemic microbubble concentrations reached steady-state (∼3 min), intermittent imaging of the forearm was performed in a transaxial plane 5 cm distal to the antecubital fossa using ultraharmonic imaging, with pulsing intervals ranging from 1 to 20 sec. Three images were captured at each pulsing interval, with a mechanical index of 1.5, which is capable of destroying all microbubbles within the ultrasound beam. Immediately after the forearm skeletal muscle CEU measurements, myocardial microvascular parameters were assessed using MCE by using intermittent ultraharmonic imaging from the apical four-, two-, and three-chamber windows, with ultrasound transmitted at 1.3 MHz and received at 3.6 MHz and a mechanical index of 1.5. Intermittent imaging was performed at pulse intervals of one, two, three, four, five, and eight cardiac cycles. Depth, focus, and gains (overall gain, time-gain compensation, and lateral-gain compensation) were optimized at the beginning of each study and held constant throughout each study.
All muscle CEU images and cardiac MCE images were blindly analyzed using the QLAB software (Philips Medical Systems), as described previously (3, 7). The videointensity in the region of interest was determined at each pulsing interval, and the pulsing interval (time) vs. video-intensity curve was generated and fitted to an exponential function: y = A (1 − e−βt), where y is the video intensity at a pulsing interval t, A is the plateau video intensity representing MBV, and β is the rate constant reflecting the rate of rise of video intensity (i.e. MFV) (15, 16). MBF is derived from the product of MBV and MFV (i.e. MBF = MBV × MFV).
Biochemical analysis
Plasma cholesterol, high-density lipoprotein cholesterol, and triglycerides were assayed by the University of Virginia clinical chemistry laboratories. Plasma glucose was measured using an YSI glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma insulin was determined using the Immulite 2000 automated immunoassay analyzer (Siemens Healthcare Diagnostics Inc., Deerfield, IL). Plasma FFAs were quantified using an in vitro enzymatic colorimetric assay with a Wako HR Series NEFA-HR kit (Wako Diagnostics, Richmond, VA).
Statistical analysis
Data are presented as mean ± sem. Statistical analyses were performed using SigmaStat 3.1 software (Systat Software, Inc., Chicago, IL). Each subject served as his/her own control. Comparisons between measurements made before and after insulin clamp within each study protocol and between the two studies were done using a two-way, repeated-measures ANOVA with Holm-Sidak post hoc analysis. A P < 0.05 was considered statistically significant.
Results
Subject characteristics
At the screening visit, all subjects had normal blood pressure (systolic 125 ± 2 mm Hg, diastolic 73 ± 2 mm Hg) and normal lipid profile (total cholesterol 161 ± 7, triglycerides 64 ± 5, low density lipoprotein cholesterol 97 ± 5, and high density lipoprotein cholesterol 53 ± 3 mg/dl). During the saline (control) study, insulin infusion increased plasma insulin concentrations within the physiological range (from 23 ± 3 to 214 ± 9 pm, P < 0.0001) and markedly suppressed plasma FFA concentrations (from 0.69 ± 0.07 to 0.19 ± 0.03 mm, P < 0.0001). Although all subjects were healthy volunteers with no personal or family history of diabetes and obesity, insulin-stimulated, whole-body glucose disposal rates ranged from 1.98 to 11.43 mg/min · kg, suggesting a wide range of metabolic insulin sensitivity. Lipid infusion raised plasma concentrations of FFAs by more than 5-fold (to 4.07 ± 0.31 mm), which was associated with a significant increase in plasma concentrations of insulin (to 34 ± 4 pm, P < 0.0002) but not those of plasma glucose (5.1 ± 0.1 mm). Insulin decreased plasma FFA concentrations to 2.8 ± 0.3 mm, and insulin-stimulated whole-body glucose disposal rates were greater than 20% lower during lipid infusion (5.6 ± 0.5 vs. 4.4 ± 0.4 mg/min · kg, P < 0.0002), confirming acute metabolic insulin resistance induced by lipid infusion.
Effects of insulin and lipid infusion on cardiac microvascular parameters
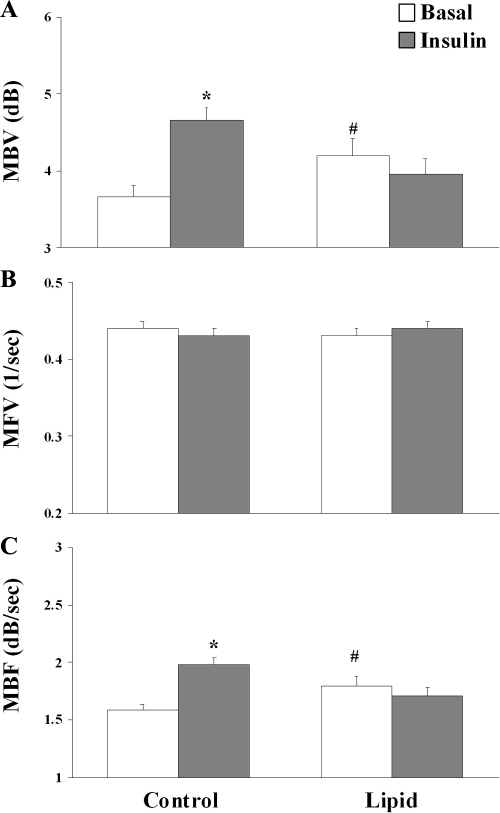
As shown in Fig. 1, 2 h of insulin infusion significantly increased cardiac MBV (from 3.66 ± 0.16 to 4.66 ± 0.16 dB, P < 0.0001) but did not affect cardiac MFV. This led to a 25% increase in cardiac MBF (from 1.58 ± 0.06 to 1.98 ± 0.06 dB/sec, P < 0.0001). Lipid infusion caused a slight but significant increase in basal MBV (to 4.20 ± 0.21 dB, P < 0.02) without affecting MFV. As a result, basal cardiac MBF was significantly higher during lipid infusion (1.80 ± 0.08 dB/sec, P < 0.02) than during saline infusion. Consistent with decreased insulin response in whole-body glucose disposal during lipid infusion, insulin infusion did not significantly alter either cardiac MBV or MFV. Thus, lipid infusion acutely blocked insulin-induced increase in cardiac MBF.
Fig. 1.
Myocardial MBV (A), MFV (B), and MBF (C) at baseline and at the end of the 120-min insulin infusion. The P values were derived with two-way, repeated-measures ANOVA with Holm-Sidak post hoc analysis. P < 0.001 for MBV, P = 0.101 for MFV, and P < 0.001 for MBF. *, P < 0.0001 compared with control baseline; #, P < 0.02 compared with control baseline.
Effect of insulin and lipid infusion on forearm skeletal muscle microvascular parameters
Figure 2 shows forearm skeletal muscle microvascular parameter changes in response to insulin infusion in the absence or presence of lipid infusion. Similar to changes in cardiac microvascular parameters, insulin infusion significantly increased skeletal muscle MBV (from 5.42 ± 0.49 to 6.39 ± 0.59 dB, P = 0.008), which was accompanied with a slight but significant decrease in MFV (P = 0.04). This led to a significant increase in skeletal muscle MBF (from 2.23 ± 0.16 to 2.55 ± 0.20 dB/sec, P < 0.03). Lipid infusion did not alter either basal muscle MBV or MFV. However, in the presence of lipid and insulin stimulation, MBV, instead of increasing, decreased significantly (P < 0.02), which was accompanied by a slight but significant increase in MFV (0.42 ± 0.01 vs. 0.44 ± 0.01, P = 0.006). The net changes in MBV induced by insulin infusion between saline infusion study and lipid infusion study was highly significant (21 ± 8% vs. −13 ± 7%, P < 0.0002).
Fig. 2.
Skeletal muscle MBV (A), MFV (B), and MBF (C) at baseline and at the end of the 120-min insulin infusion. The P values were derived with two-way, repeated-measures ANOVA with Holm-Sidak post hoc analysis. P < 0.001 for MBV, P <0.001 for MFV, and P = 0.001 for MBF. *, P = 0.008 compared with control baseline; **, P = 0.04 compared with control baseline; ***, P < 0.03 compared with control baseline; #, P < 0.02 compared with lipid baseline; ##, P = 0.006 compared with lipid baseline.
Correlation analysis of insulin-induced changes in MBV between cardiac and skeletal muscle
Because MFV either did not change (cardiac muscle) or even decreased slightly (skeletal muscle) during insulin infusion in the control study, insulin-induced MBF increases in both the cardiac and skeletal muscle microvascular beds were solely from changes in MBV. This was consistent with our previous observations in humans (3, 4, 7). To examine whether cardiac and skeletal muscle microvascular beds responded to insulin similarly, we next correlated insulin-induced changes in MBV during the control (saline) study between the cardiac and skeletal muscle. As shown in Fig. 3, there was no correlation between the changes in MBV between the two microvascular beds (r = 0.27, P > 0.1).
Fig. 3.
Regression analysis of insulin-induced changes in MBV between cardiac and skeletal muscle microvascular beds.
Effects of whole-body insulin sensitivity on insulin and lipid responses in the cardiac and skeletal muscle microcirculation
To further assess the microvascular responses to insulin, we divided subjects into three subgroups based on insulin-stimulated, steady-state glucose infusion rates (GIRs) during control admission: lower tertile (GIR < 4 mg/min · kg, n = 7), middle tertile (GIR ≥ 4 but ≤ 7 mg/min · kg, n = 9), and upper tertile (GIR > 7 mg/min · kg, n = 6). As expected and shown in Fig. 4, lipid infusion blunted insulin-stimulated, whole-body glucose disposal only in the middle and upper tertiles, not in the lowest tertile. As expected, the effect was more pronounced in the upper tertile subjects.
Fig. 4.
Effect of lipid infusion on steady-state, whole-body glucose disposal based on whole-body metabolic insulin sensitivity. L, Lower tertile (GIR < 4 mg/min · kg, n = 7); M, middle tertile (GIR ≥ 4 but ≤ 7 mg/min · kg, n = 9); U, upper tertile (GIR > 7 mg/min · kg, n = 6). *, P < 0.0001 compared with control L; **, P = 0.001 compared with control M; #, P = 0.05 compared with control M; ##, P = 0.001 compared with control H; @, P < 0.003 compared with lipid L; @@, P < 0.0002 compared with lipid L; @@, P < 0.0002 compared with lipid M.
Figure 5 compares the cardiac muscle MBV insulin responses in the absence or presence of high-plasma FFA concentrations among subjects in the three tertiles of whole-body insulin metabolic sensitivity. Insulin infusion significantly increased cardiac MBV in subjects in both upper and middle tertiles, and these effects were completely blocked by lipid infusion. In contrast, insulin infusion increased forearm skeletal muscle MBV only in subjects in the upper tertile (5.59 ± 1.09 vs. 7.38 ± 1.12 dB, P < 0.03), not in subjects in the lower and middle tertiles (Fig. 6). In the presence of high plasma concentrations of FFAs, the insulin-induced skeletal muscle MBV increase in the upper tertile subjects disappeared. Although skeletal muscle MBV did not change in the lower tertile subjects, it significantly decreased in the middle tertile subjects (5.56 ± 0.59 vs. 3.89 ± 0.93 dB, P = 0.009) after insulin infusion in the presence of lipid infusion.
Fig. 5.
Cardiac muscle MBV based on whole-body metabolic insulin sensitivity in the absence or presence of lipid infusion. A, Lower tertile (GIR < 4 mg/min · kg, n = 7). B, Middle tertile (GIR ≥ 4 but ≤ 7 mg/min · kg, n = 9). C, Upper tertile (GIR > 7 mg/min · kg, n = 6). P values were derived with two-way, repeated-measures ANOVA with Holm-Sidak post hoc analysis. P = 0.172 for lower tertile, P < 0.001 for middle tertile, and P < 0.03 for upper tertile. *, P = 0.005 compared with control baseline; **, P = 0.05 compared with control baseline; #, P = 0.007 compared with control baseline.
Fig. 6.
Skeletal muscle MBV based on whole-body metabolic insulin sensitivity in the absence or presence of lipid infusion. A, Lower tertile (GIR < 4 mg/min · kg, n = 7). B, Middle tertile (GIR ≥ 4 but ≤ 7 mg/min · kg, n = 9). C. Upper tertile (GIR > 7 mg/min · kg, n = 6). P values were derived with two-way, repeated-measures ANOVA with Holm-Sidak post hoc analysis. P = 0.15 for lower tertile, P < 0.02 for middle tertile, and P < 0.01 for upper tertile. *, P < 0.03 compared with control baseline; #, P = 0.009 compared with control baseline.
Discussion
In the current study, we found that insulin at physiological concentrations potently recruits microvasculature in both cardiac and skeletal muscle, and acute elevation of plasma concentrations of FFAs via systemic infusion of lipid blocks these responses in both cardiac and skeletal muscle microvascular beds in healthy humans. The microvascular response to insulin differed between the cardiac and skeletal muscle microvasculature in that insulin increased cardiac MBV, irrespective of whole-body insulin metabolic sensitivity, but skeletal muscle MBV increased most in subjects who were metabolically insulin sensitive. Insulin even decreased skeletal muscle MBV during lipid infusion in subjects who were moderately sensitive to insulin metabolically. These findings confirm that FFAs produce microvascular insulin resistance in human cardiac muscle as well as in skeletal muscle.
Contrast ultrasonography is widely used clinically to enhance ultrasound images and experimentally to noninvasively quantify blood flow and volumes within the microvasculature in skeletal muscle, heart, and kidneys (17–20). Using this technique, we previously reported that insulin potently recruits microvasculature in both cardiac and skeletal muscle by expanding MBV. In the current study, for the first time we compared insulin responses in both cardiac and skeletal muscle microvascular beds in the same subjects. Our results are clearly consistent with prior reports in that insulin only increases MBV in both tissues. These results suggest that insulin plays an important role to enhance nutrient delivery to cardiac and skeletal muscle by increasing perfusion volume. It is in the microcirculation that substrate exchange takes place, and this process is dependent on microvascular blood flow, exchange surface area, and vascular permeability (2).
We recently reported that acute elevation of plasma FFA concentrations causes skeletal muscle microvascular insulin resistance by blunting insulin-induced expansion of MBV in healthy humans (3). Results from the current study confirmed our previous findings and are in line with other reports showing that high plasma concentrations of FFAs decrease insulin receptor substrate-1-associated phosphatidylinositol 3 (PI3)-kinase activity and glucose transport in human skeletal muscle (21), induce oxidative stress, and blunt insulin-mediated vasodilation and NO production in humans (22–24). Although insulin can acutely suppress lipolysis, as evidenced by a significant decrease in plasma FFA concentrations during insulin infusion in the current study, and exert antiinflammatory effect including a reduction in intranuclear nuclear factor-κB, an increase in inhibitor of nuclear factor-κB, and decreases in reactive oxygen species generation (25), it is likely that the effects resulting from these actions of insulin were overcome by lipid infusion. Inasmuch as insulin-mediated microvascular recruitment precedes insulin-stimulated glucose uptake in skeletal muscle (26) and blockade of insulin-mediated capillary recruitment with NO synthase inhibitor NG-nitro-l-arginine methyl ester decreases glucose disposal by 40% (26, 27), skeletal muscle microvascular insulin resistance induced by FFAs could contribute significantly to the pathogenesis of glucose intolerance and muscle insulin resistance in diabetes.
The observation that insulin-mediated microvascular responses in the myocardium did not correlate with those observed in the skeletal muscle is not entirely surprising. Insulin increased cardiac MBV, irrespective of whole-body metabolic insulin sensitivity. On the contrary, insulin only increased skeletal muscle MBV in subjects with high glucose infusion rates (>7 mg/min · kg). Several factors might have contributed to this difference. First, cardiac muscle was constantly contracting while skeletal muscle was at rest during the study. It has been reported that muscle contraction improves skeletal muscle insulin sensitivity, and even modest exercise/muscle contraction markedly recruits muscle microvasculature (28, 29). Second, evidence points to more sympathetic involvement in insulin-mediated blood flow increase in myocardium than in skeletal muscle (30). Finally, on a per-gram tissue basis, the endothelial surface area in the myocardium is much larger than that in the skeletal muscle, which may enable more robust response to insulin (19).
Our finding of insulin decreasing muscle MBV in the presence of lipid infusion in moderately insulin sensitive subjects is particularly interesting and might be explained by the concept of selective insulin resistance. In the insulin-sensitive state, insulin fine-tunes vascular tone and tissue perfusion through balancing its vasodilatory actions via the PI3-kinase/Akt/endothelial NO synthase signaling pathway with its vasoconstrictive signals through the MAPK pathway. It has been well documented that with insulin resistance there is a selective impairment of insulin's vasodilatory action, whereas its vasoconstrictive effect remains intact (31). In isolated resistance arterioles, insulin actually causes vasoconstriction in the presence of PI3-kinase inhibition (32) or during TNF-α infusion (33). However, it is puzzling that this was not observed in the least insulin-sensitive subjects. It is likely that lipid infusion does not further acutely accentuate insulin resistance in subjects with a certain degree of insulin resistance. Indeed, lipid infusion did not significantly decrease the whole-body glucose disposal in those subjects (Fig. 4).
In human hearts, coronary blood volume is distributed almost equally among the arterial, microcirculatory, and venous compartments, and most of the arterial and venous blood volumes are located on the epicardial surface of the heart (19). Insulin clearly causes coronary vasodilation and increases coronary blood flow (34, 35), and we have previously confirmed that insulin at physiological concentrations increases cardiac microvascular blood flow by expanding cardiac MBV (7). Our current finding that high FFA concentrations abolish insulin-mediated increases in cardiac MBV confirms that FFAs also induce insulin resistance in cardiac microvasculature in humans. This finding is of particular physiological and clinical significance. Patients with obesity and diabetes have an increased risk for coronary artery stenosis, which limits blood supply to the cardiac microcirculation. Microvascular blood flow is determined by both MBV and MFV (MBF = MBV × MFV). Adenosine, the major coronary vasodilator in humans, causes coronary vasodilation in a predominantly flow-mediated fashion (36, 37), and it increases MBF entirely via increasing MFV without affecting MBV (15). In the presence of coronary arterial stenosis, both MBV and MFV, especially MFV, are depressed during hyperemia, which severely limits microvascular blood flow (38, 39). Because insulin exerts vasodilatory effect via a nitric oxide-dependent mechanism (2, 31) and it appears to increase MBF by expanding MBV but not MFV, in the presence of coronary artery stenosis with severely depressed MFV, a relatively small increase in the MBV (i.e. microvascular surface area) induced by insulin could markedly increase substrate extraction by the cardiac muscle. Thus, the presence of insulin resistance in the coronary microvasculature could further limit the capability of cardiac muscle to extract oxygen, nutrients, and anabolic hormones, which may explain partly why patients with diabetes tend to develop cardiac complications including cardiomyopathy and heart failure.
Our observation of lipid infusion acutely increasing cardiac but not skeletal muscle MBV is of interest and the underlying mechanisms remain unclear. A previous study demonstrated that lipid infusion increases total leg blood flow by nearly 30% (24). Although we did not observe significant changes in blood pressure and heart rate, lipid infusion is associated with increases in blood pressure, heart rate, and myocardial oxygen demand, which may have contributed to the increased myocardial MBV. Inasmuch as triglycerides contribute to increased blood viscosity, which impacts on myocardial vascular resistance, we did not observe significant changes in MFV with lipid infusion. Using the current lipid infusion rates, plasma triglycerides increase on average only to about 300 mg/dl in our GCRC, which likely does not contribute significantly to blood viscosity and thus flow velocity.
In the current study, we used high concentrations of FFAs because the major purpose was to compare the cardiac microvascular bed with that of the skeletal muscle, and we have previously reported that FFAs at these concentrations blocked insulin- and mixed-meal-induced muscle microvascular recruitment in healthy humans (3). Whether FFAs at lower plasma concentrations, as seen in patients with obesity and/or diabetes, can exert similar effects in either cardiac or skeletal muscle microvasculature remains to be examined. Another weakness of the design is that we did not use arterialized blood during the insulin clamp. We opted not to arterialize venous blood because any effort to warm the hands or feet would significantly increase arterial blood flow, including the flow in the microvasculature, which was our primary end point. We clamped venous blood glucose concentrations at approximately 10 mg/dl (from 89.8 ± 1.2 to 78.7 ± 1.4 mg/dl during the saline study and from 90.9 ± 1.2 to 79.1 ± 1.4 mg/dl during the lipid study) based on data from similar human volunteers in our GCRC (40) to avoid hypoglycemia and arterial hyperglycemia.
In conclusion, our data demonstrate that high plasma concentrations of FFAs cause insulin resistance in cardiac as well as skeletal muscle microvasculature in healthy humans by blunting insulin-induced increase in microvascular recruitment and endothelial surface exchange area. This could result in decreased delivery of nutrients, hormones, and oxygen and removal of toxic metabolic wastes in the cardiac and skeletal muscle and may explain the association of cardiac complications and metabolic insulin resistance in human diabetes.
Acknowledgments
This work was supported by American Diabetes Association Grants 7-07-CR-34 and 9-09-NOVO-11 (to Z.L.), National Institutes of Health Grants R01HL094722 (to Z.L.), R01DK057878 and R01DK073759 (to E.J.B.), and RR-00847 (to the University of Virginia General Clinical Research Center).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CEU
- Contrast-enhanced ultrasound
- FFA
- free fatty acid
- GCRC
- General Clinical Research Center
- GIR
- glucose infusion rate
- MBF
- microvascular blood flow
- MBV
- microvascular blood volume
- MCE
- myocardial contrast echocardiography
- MFV
- microvascular blood flow velocity
- NO
- nitric oxide
- PI3
- phosphatidylinositol 3.
References
- 1. Segal SS. 2005. Regulation of blood flow in the microcirculation. Microcirculation 12:33–45 [DOI] [PubMed] [Google Scholar]
- 2. Barrett E, Eggleston EM, Inyard AC, Wang H, Li G, Chai W, Liu Z. 2009. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 52:752–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. 2009. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab 94:3543–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clerk LH, Vincent MA, Jahn LA, Liu Z, Lindner JR, Barrett EJ. 2006. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 55:1436–1442 [DOI] [PubMed] [Google Scholar]
- 5. Sundell J, Nuutila P, Laine H, Luotolahti M, Kalliokoski K, Raitakari O, Knuuti J. 2002. Dose-dependent vasodilating effects of insulin on adenosine-stimulated myocardial blood flow. Diabetes 51:1125–1130 [DOI] [PubMed] [Google Scholar]
- 6. Laine H, Nuutila P, Luotolahti M, Meyer C, Elomaa T, Koskinen P, Ronnemaa T, Knuuti J. 2000. Insulin-induced increment of coronary flow reserve is not abolished by dexamethasone in healthy young men. J Clin Endocrinol Metab 85:1868–1873 [DOI] [PubMed] [Google Scholar]
- 7. Liu Z. 2007. Insulin at physiological concentrations increases microvascular perfusion in human myocardium. Am J Physiol Endocrinol Metab 293:E1250–E1255 [DOI] [PubMed] [Google Scholar]
- 8. Jonk AM, Houben AJ, de Jongh RT, Serné EH, Schaper NC, Stehouwer CD. 2007. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology 22:252–260 [DOI] [PubMed] [Google Scholar]
- 9. Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark AD, Clark MG. 2002. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51:3492–3498 [DOI] [PubMed] [Google Scholar]
- 10. Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. 2007. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab 293:E1804–E1809 [DOI] [PubMed] [Google Scholar]
- 11. Clerk LH, Rattigan S, Clark MG. 2002. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 51:1138–1145 [DOI] [PubMed] [Google Scholar]
- 12. Sundell J, Laine H, Luotolahti M, Kalliokoski K, Raitakari O, Nuutila P, Knuuti J. 2002. Obesity affects myocardial vasoreactivity and coronary flow response to insulin. Obes Res 10:617–624 [DOI] [PubMed] [Google Scholar]
- 13. Jagasia D, Whiting JM, Concato J, Pfau S, McNulty PH. 2001. Effect of non-insulin-dependent diabetes mellitus on myocardial insulin responsiveness in patients with ischemic heart disease. Circulation 103:1734–1739 [DOI] [PubMed] [Google Scholar]
- 14. Scognamiglio R, Negut C, De Kreutzenberg SV, Tiengo A, Avogaro A. 2005. Postprandial myocardial perfusion in healthy subjects and in type 2 diabetic patients. Circulation 112:179–184 [DOI] [PubMed] [Google Scholar]
- 15. Wei K, Ragosta M, Thorpe J, Coggins M, Moos S, Kaul S. 2001. Noninvasive quantification of coronary blood flow reserve in humans using myocardial contrast echocardiography. Circulation 103:2560–2565 [DOI] [PubMed] [Google Scholar]
- 16. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. 1998. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 97:473–483 [DOI] [PubMed] [Google Scholar]
- 17. Lindner JR, Wei K. 2002. Contrast echocardiography. Curr Probl Cardiol 27:454–519 [DOI] [PubMed] [Google Scholar]
- 18. Lindner JR. 2004. Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 3:527–532 [DOI] [PubMed] [Google Scholar]
- 19. Wei K, Kaul S. 2004. The coronary microcirculation in health and disease. Cardiol Clin 22:221–231 [DOI] [PubMed] [Google Scholar]
- 20. Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. 2001. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol 37:1135–1140 [DOI] [PubMed] [Google Scholar]
- 21. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. 1999. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest 103:253–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tripathy D, Mohanty P, Dhindsa S, Syed T, Ghanim H, Aljada A, Dandona P. 2003. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes 52:2882–2887 [DOI] [PubMed] [Google Scholar]
- 23. Steinberg HO, Paradisi G, Hook G, Crowder K, Cronin J, Baron AD. 2000. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 49:1231–1238 [DOI] [PubMed] [Google Scholar]
- 24. Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. 1997. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest 100:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. 2001. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab 86:3257–3265 [DOI] [PubMed] [Google Scholar]
- 26. Vincent MA, Clerk LH, Lindner JR, Klibanov AL, Clark MG, Rattigan S, Barrett EJ. 2004. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 53:1418–1423 [DOI] [PubMed] [Google Scholar]
- 27. Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. 2003. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab 285:E123–E129 [DOI] [PubMed] [Google Scholar]
- 28. Vincent MA, Clerk LH, Lindner JR, Price WJ, Jahn LA, Leong-Poi H, Barrett EJ. 2006. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290:E1191–E1197 [DOI] [PubMed] [Google Scholar]
- 29. Inyard AC, Clerk LH, Vincent MA, Barrett EJ. 2007. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56:2194–2200 [DOI] [PubMed] [Google Scholar]
- 30. Liang C, Doherty JU, Faillace R, Maekawa K, Arnold S, Gavras H, Hood WB., Jr 1982. Insulin infusion in conscious dogs. Effects on systemic and coronary hemodynamics, regional blood flows, and plasma catecholamines. J Clin Invest 69:1321–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim JA, Montagnani M, Koh KK, Quon MJ. 2006. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 32. Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. 2002. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res 56:464–471 [DOI] [PubMed] [Google Scholar]
- 33. Eringa EC, Stehouwer CD, Walburg K, Clark AD, van Nieuw Amerongen GP, Westerhof N, Sipkema P. 2006. Physiological concentrations of insulin induce endothelin-dependent vasoconstriction of skeletal muscle resistance arteries in the presence of tumor necrosis factor-α dependence on c-jun N-terminal kinase. Arterioscler Thromb Vasc Biol 26:274–280 [DOI] [PubMed] [Google Scholar]
- 34. Iozzo P, Chareonthaitawee P, Di Terlizzi M, Betteridge DJ, Ferrannini E, Camici PG. 2002. Regional myocardial blood flow and glucose utilization during fasting and physiological hyperinsulinemia in humans. Am J Physiol Endocrinol Metab 282:E1163–E1171 [DOI] [PubMed] [Google Scholar]
- 35. Lautamaki R, Airaksinen KE, Seppänen M, Toikka J, Härkönen R, Luotolahti M, Borra R, Sundell J, Knuuti J, Nuutila P. 2006. Insulin improves myocardial blood flow in patients with type 2 diabetes and coronary artery disease. Diabetes 55:511–516 [DOI] [PubMed] [Google Scholar]
- 36. Wilson RF, Wyche K, Christensen BV, Zimmer S, Laxson DD. 1990. Effects of adenosine on human coronary arterial circulation. Circulation 82:1595–1606 [DOI] [PubMed] [Google Scholar]
- 37. Lupi A, Buffon A, Finocchiaro ML, Conti E, Maseri A, Crea F. 1997. Mechanisms of adenosine-induced epicardial coronary artery dilatation. Eur Heart J 18:614–617 [DOI] [PubMed] [Google Scholar]
- 38. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. 1998. Basis for detection of stenosis using venous administration of microbubbles during myocardial contrast echocardiography: bolus or continuous infusion? J Am Coll Cardiol 32:252–260 [DOI] [PubMed] [Google Scholar]
- 39. Pacella JJ, Villanueva FS. 2006. Effect of coronary stenosis on adjacent bed flow reserve: assessment of microvascular mechanisms using myocardial contrast echocardiography. Circulation 114:1940–1947 [DOI] [PubMed] [Google Scholar]
- 40. Eggleston EM, Jahn LA, Barrett EJ. 2007. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 56:2958–2963 [DOI] [PubMed] [Google Scholar]