In young obese women with polycystic ovary syndrome, successful treatment of obstructive sleep apnea can provide beneficial cardiometabolic effects.
Abstract
Context:
Women with polycystic ovary syndrome (PCOS) are insulin resistant and have a high risk of early-onset diabetes and cardiovascular disease. Obstructive sleep apnea (OSA) has adverse cardiometabolic consequences and is highly prevalent in women with PCOS. We sought to determine whether continuous positive airway pressure (CPAP) treatment of OSA has beneficial effects on cardiometabolic function in PCOS.
Methods:
Laboratory polysomnography and cardiometabolic measurements including insulin sensitivity and secretion (iv glucose tolerance test); 24-h profiles of plasma catecholamines, cortisol, and leptin; and daytime profiles of blood pressure and cardiac autonomic activity (heart rate variability) were obtained at baseline and again after 8 wk of home CPAP treatment with daily usage monitoring.
Results:
CPAP treatment modestly improved insulin sensitivity after controlling for body mass index (P = 0.013). The change in insulin sensitivity correlated positively with CPAP use (adjusted P = 0.027) and negatively with body mass index (adjusted P = 0.003). Daytime and nighttime norepinephrine levels were decreased after CPAP (P = 0.002), and the reductions were greater with increased CPAP use (P = 0.03). Epinephrine, cortisol, and leptin levels were not changed significantly. Daytime diastolic blood pressure decreased by an average of 2.3 mm Hg after CPAP (P = 0.035). Cardiac sympathovagal balance was 44% lower (P = 0.007) after CPAP, reflecting a shift toward lower sympathetic activity.
Conclusions:
In young obese women with PCOS, successful treatment of OSA improves insulin sensitivity, decreases sympathetic output, and reduces diastolic blood pressure. The magnitude of these beneficial effects is modulated by the hours of CPAP use and the degree of obesity.
Polycystic ovary syndrome (PCOS) is one of the most common hormonal disorders in women with an onset that typically occurs at the time of puberty (1, 2). Insulin resistance is a hallmark of PCOS and plays a major role in its pathogenesis and concurrent cardiometabolic derangements (3). Specifically, women with PCOS are more insulin resistant than weight-matched controls (4) and have an exceptionally high prevalence of early-onset type 2 diabetes (5, 6) as well as a substantial risk for hypertension, dyslipidemia, and coronary and other vascular disorders (1). As such, a diagnosis of PCOS portends lifelong cardiometabolic risks, making it essential to develop further strategies to prevent or delay its cardiovascular and metabolic sequelae.
Obstructive sleep apnea (OSA) is a well-recognized risk factor for cardiovascular disease (7, 8). It is a treatable disorder that has been independently associated with insulin resistance and type 2 diabetes (9–12). OSA is highly prevalent in PCOS at rates far exceeding those observed in women without PCOS who are of similar age and weight (13–15). We (16, 17) as well as others (13, 14) have shown that OSA is an important and perhaps a key determinant of insulin resistance and glucose intolerance in PCOS. Despite the high prevalence of OSA among women with PCOS, it is not known whether treatment of OSA can improve cardiometabolic function and potentially reduce the risk of early-onset diabetes and cardiovascular disease in this population.
The present study was therefore designed to obtain a comprehensive evaluation of multiple components of cardiometabolic function before and after 8 wk of continuous positive airway pressure (CPAP) treatment of OSA (with daily monitoring of adherence to treatment) in young obese women with PCOS.
Patients and Methods
Patients
Women with PCOS were consecutively recruited from the Endocrinology, Diabetes, and Metabolism Clinics at the University of Chicago. A diagnosis of PCOS required 1) the presence of oligo-/amenorrhea; 2) plasma free testosterone level higher than 10 pg/ml; 3) hyperandrogenism, as evidenced by infertility, hirsutism, acne, or androgenetic alopecia; and 4) exclusion of nonclassic 21-hydroxylase deficiency congenital adrenal hyperplasia, Cushing's syndrome, hypothyroidism, or significant elevations in serum prolactin (1). Sleep complaints or symptoms of OSA were not used as selection criteria for the study. Subjects were excluded if they smoked cigarettes; were diabetic or hypertensive; had a history of cardiac, psychiatric, neurological, or endocrine disease; or were taking any medications at the time of the study. PCOS women who were taking sex steroids (oral contraceptives), antiandrogens (e.g. spironolactone), or insulin-lowering medications (metformin or a thiazolidinedione) were removed from their medication for at least 8 wk before and for the duration of the study. All participants gave written informed consent. The study was approved by the University of Chicago Institutional Review Board.
All participants had a medical history and a physical examination. A fasting blood sample was obtained for screening laboratory tests to exclude abnormal liver, renal, or thyroid function. A standard 75-g oral glucose tolerance test was performed to assess glucose tolerance; subjects found to be diabetic were excluded from further study. A full laboratory polysomnogram was performed with bedtimes from 2300–0700 h to screen for sleep disorders. A diagnosis of OSA was made if the apnea-hypopnea index (AHI) was at least five events per hour during sleep. Subjects were excluded from further participation if they did not have OSA.
Design
Metabolic, hormonal, and cardiovascular assessments were obtained at baseline and again after 8 wk of home CPAP (Fisher & Paykel Healthcare Inc., Irvine, CA) treatment. Assessments were obtained over a 2-d inpatient study, which included a morning frequently sampled iv glucose tolerance test (ivGTT) after an overnight fast. Data from the ivGTT were analyzed using Bergman's minimal model (18) to obtain insulin sensitivity and the acute insulin response to glucose, a measure of pancreatic β-cell function. The ivGTT was followed by a 24-h period of blood sampling at 10- to 30-min intervals for the measurement of hormonal profiles during which identical meals (65% carbohydrate, 15% protein, and 20% fat) were ingested at 1400, 1900, and 2100 h. Subjects were required to finish each meal completely within 20 min. No additional sources of calories were allowed. Sleep was recorded during the inpatient studies, and CPAP was used during the postintervention portion.
Before initiating home CPAP treatment, the optimal CPAP pressure setting was determined individually during an overnight laboratory titration study. Compliance with CPAP treatment at home was monitored by a built-in CPAP meter (capturing the mask-on time) and encouraged by telephone follow-ups at 2-wk intervals that also served to troubleshoot any mask-related or other technical problems. Nightly compliance data were downloaded from CPAP machines at the end of 8 wk of treatment. Compliance was defined as the average number of hours for which CPAP was used per night over the 8-wk treatment period. By protocol design, the demanding and labor-intensive postintervention laboratory assessments were not repeated if patients did not fulfill the criteria of a minimum of 4 h of nightly CPAP usage over the 8-wk intervention period, a commonly used cutoff criterion for CPAP compliance (19).
Sleep and cardiovascular assessments
In the laboratory, sleep was recorded from 2300–0700 h. Polysomnographic recordings (Neurofax EEG 1100 digital acquisition system, Nihon Kohden, Foothill Ranch, CA) included two central and two occipital electroencephalogram channels, bilateral electrooculograms, submental electromyograms, leg movements by bilateral anterior tibialis electromyogram, electrocardiogram, oronasal airflow by thermistor, chest and abdominal wall motion by piezo electrodes, and arterial oxygen saturation by pulse oximeter. Sleep recordings were visually scored in 30-sec epochs in stages 1, 2, 3, and 4 of non-rapid eye movement (REM) sleep and in REM sleep according to standard criteria (20). Obstructive respiratory events (i.e. apneas and hypopneas) and arousals were scored according to established criteria (21, 22). The AHI was calculated as the total number of obstructive respiratory events per hour of sleep. The arousal index was calculated as the total number of arousals per hour of sleep. The oxygen desaturation index (ODI) was calculated as the total number of oxygen desaturations of at least 3% per hour of sleep. Daytime sleepiness was assessed by a validated scale (Stanford Sleepiness Scale) (23), administered every 2 h during the blood sampling period.
Daytime blood pressure (BP; Dinamap PLUS, Critikon, Tampa, FL) and electrocardiogram (ECG) recordings using two thoracic electrodes were obtained during the 24-h blood sampling period while the subjects were sitting in a comfortable armchair. Systolic and diastolic BP was measured using a cuff on the nondominant arm every 20 min from 1400–2100 h and from 0900–1400 h on the next day. As in previous studies of 24-h BP profiles (24, 25), measurements that corresponded to a pulse pressure less than 15 mm Hg or an isolated increase by more than 50% over the previous measurement were considered as artifacts and were not used in the analysis. In each subject, mean systolic and diastolic BP was calculated by averaging all daytime readings (from all the recordings, the percentage of missing/deleted data were on average 8.1 ± 1.3%). ECG recordings were obtained during the hour preceding each meal and before bedtime (between 2130 and 2200 h). Changes in cardiac autonomic activity were estimated from analyses (PRANA software; Phitools, Strasbourg, France) of heart rate variability (HRV). We simultaneously measured the respiratory effort signal by thoracic belts during ECG recordings to account for changes in HRV due to breathing frequency. The average respiratory rate was similar before (20.2 ± 1.3 breaths/min) and after CPAP treatment (20.1 ± 3.5 breaths/min; P = 0.71). We first performed a spectral analysis of HRV by fast Fourier transform on a 2-min section of each of the four daytime recordings that were free from ectopic beats and artifacts. Data from these four 2-min segments were then averaged to obtain the daytime mean values for markers of cardiac autonomic function in each subject before and after CPAP treatment. Of a total of 72 ECG recordings (i.e. four recordings per subject before and after CPAP in nine subjects), eight were corrupted by artifacts and could not be analyzed. We used the spectral power in the high-frequency band (HF) (0.15–0.40 Hz) as a marker of vagal activity and the spectral power in the low-frequency band (LF) (0.04–0.14 Hz) as a marker of sympathetic activity. We calculated HF and LF in normalized units [normalized HF (HFn) and normalized LF (LFn)], which represent the percentage of power in each band relative to their sum. We used the LF to HF ratio (LF/HF) as an index of cardiac sympathovagal balance (26).
Assays
Plasma glucose was assayed by the glucose oxidase method with a coefficient of variation of less than 2%. Serum insulin and plasma cortisol were measured by chemiluminescence assays using the Immulite immunochemistry system (Diagnostic Products Corp., Los Angeles, CA). Serum leptin was measured by RIA (Linco Research, St. Charles, MO) with a lower limit of sensitivity of 0.5 ng/ml and an intraassay coefficient of variation of 8.3%. We measured plasma catecholamines using a HPLC system (Coulochem MD5001; ESA, Inc., Chelmsford, MA) with a lower limit of sensitivity of 10 pg/ml for norepinephrine and 5 pg/ml for epinephrine and an intraassay coefficient of variation of 6–7.8%. The values were below the detection limit for epinephrine measurement in about 5% of all daytime samples and 33% of all nighttime samples, and these samples were arbitrarily assigned a value equal to 50% of the sensitivity of the assay. Total testosterone was measured using a kit from Diagnostic Products. The free fraction of plasma testosterone was measured by a competitive protein-binding assay as previously described (27). The intra- and interassay coefficients of variation were 3.8 and 8.7%, respectively.
Statistical analysis
Group data were expressed as mean ± sem. Sleep variables and cardiometabolic measures were compared before and after CPAP treatment by the paired Student's t test. Primary outcome variable was the insulin sensitivity. The effect of CPAP treatment on insulin sensitivity and the acute insulin response to glucose was examined by ANOVA for repeated measures with body mass index (BMI) (as well as waist circumference) as covariates. All data from ivGTT were compared in eight of the nine patients because in one subject, a catheter failure occurred during the posttreatment test. Changes in cardiometabolic variables after CPAP treatment are expressed as a percentage of the corresponding baseline values. Associations between hours of CPAP use and changes in cardiometabolic measures were examined using the Pearson coefficient or multivariable linear regression models, as appropriate. All statistical analyses were performed using JMP statistical software for Macintosh (version 8.0.1; SAS Institute, Cary, NC). All reported P values are two sided with significance set at P < 0.05.
Results
Of the 56 women with PCOS who were enrolled and screened, 26 met all inclusion criteria. Seven of 26 patients declined to continue the study after screening procedures. The remaining 19 patients (age, 31.2 ± 1.2 yr, BMI, 46.4 ± 2.4 kg/m2) completed the baseline portion of the study and the entire 8-wk CPAP intervention at home. Of the 19 patients, 10 had an average CPAP use of less than 4 h per night over the 8-wk treatment period; thus, they did not meet the preset criteria to undergo the postintervention laboratory assessments. In the nine women who completed the entire study, the average nightly use of CPAP was 6.6 ± 0.4 h (range, 4.9–8.0). In these compliant women, the average number of days that CPAP was not used was 3.4 ± 1.2 (range, 0–10) over the 8-wk treatment period.
There were no significant differences in age (P = 0.66), race (P = 0.09), BMI (P = 0.59), level of sleepiness as quantified by Stanford Sleepiness Scale (P = 0.64), and the degree of severity of OSA (as quantified by AHI; P = 0.85) between the compliant and the noncompliant women (n = 10). In the nine women who were compliant and completed the entire study, the mean age was 30.6 ± 1.7 yr (range, 25–39 yr), and the mean BMI was 48.4 ± 5.0 kg/m2 (range, 33.9–78.8 kg/m2). Weight (134.2 ± 15.2 kg at baseline vs. 135.6 ± 14.8 kg after treatment; P = 0.23) as well as body fat percentage (determined by an impedance technique; 55.6 ± 3.2% at baseline vs. 55.8 ± 2.9% after treatment; P = 0.73) were stable over the treatment period.
Effects of CPAP on sleep characteristics
Sleep characteristics before and after 8 wk of CPAP treatment are summarized in Table 1. Mean scores on the Stanford Sleepiness Scale were lower after CPAP treatment. Markers of OSA severity including the AHI and ODI were markedly decreased. Sleep fragmentation as assessed by the arousal index was 53% lower (P < 0.0001), and the amount of slow wave sleep (i.e. deep non-REM sleep; N3) was 22% higher (P = 0.046) after CPAP treatment. Thus, CPAP treatment was successful in reducing subjective daytime sleepiness and improving nocturnal sleep quality.
Table 1.
Characteristics of sleep and cardiovascular measures before and after 8 wk of CPAP treatment
| Characteristic | Before CPAP treatment | After CPAP treatment | P value |
|---|---|---|---|
| Sleep | |||
| Stanford sleepiness score | 3.0 ± 0.2 | 2.3 ± 0.3 | 0.03 |
| Sleep efficiency (%) | 80.4 ± 3.6 | 85.9 ± 3.2 | 0.16 |
| Wake after sleep onset (min) | 70.5 ± 13.2 | 47.3 ± 10.3 | 0.09 |
| REM sleep (min) | 81.6 ± 10.6 | 94.6 ± 10.4 | 0.27 |
| N1 sleep (min) | 37.1 ± 7.4 | 29.3 ± 3.8 | 0.30 |
| N2 sleep (min) | 211.1 ± 10.8 | 222.7 ± 8.5 | 0.49 |
| N3 (min) | 59.1 ± 5.0 | 72.2 ± 5.5 | 0.046 |
| AHI (per hour of sleep) | 24.3 ± 5.5 | 2.0 ± 1.0 | 0.001 |
| ODI (per hour of sleep) | 12.4 ± 3.4 | 0.9 ± 0.5 | 0.0097 |
| Arousal index (per hour of sleep) | 26.8 ± 3.6 | 12.6 ± 2.6 | <0.0001 |
| BP (mm Hg) | |||
| Systolic | 115.8 ± 4.8 | 112.6 ± 3.8 | 0.19 |
| Diastolic | 65.9 ± 2.6 | 63.5 ± 2.7 | 0.035 |
| HRV indices | |||
| HF (ms2) | 1034 ± 359 | 1629 ± 385 | 0.20 |
| LF (ms2) | 2580 ± 580 | 2470 ± 572 | 0.86 |
| HFn (%) | 27.7 ± 4.7 | 36.1 ± 4.2 | 0.03 |
| LFn (%) | 72.3 ± 4.7 | 63.9 ± 4.2 | 0.03 |
| LF/HF | 4.1 ± 0.8 | 2.3 ± 0.4 | 0.007 |
Data are mean ± sem. N1, N2, and N3 are non-REM-sleep stages.
Effects of CPAP on insulin sensitivity and secretion
Before CPAP treatment, all patients had severe insulin resistance (average insulin sensitivity, 0.87 ± 0.13 mU/liter · min; range, 0.52–1.45 mU/liter · min). In this group of obese PCOS women, 8 wk of CPAP treatment was associated with a modest improvement (on average 7%) in insulin sensitivity (0.87 ± 0.13 vs. 0.93 ± 0.17 mU/liter · min; P = 0.013) after adjusting for BMI. The interaction term (treatment condition × BMI) was significant (P = 0.018), suggesting that the effects of CPAP on insulin sensitivity may vary according to the patient's degree of obesity. When this analysis was repeated using waist circumference (a marker of visceral adiposity) as a covariate (instead of BMI), we found similar improvement in insulin sensitivity after CPAP (adjusted P = 0.014).
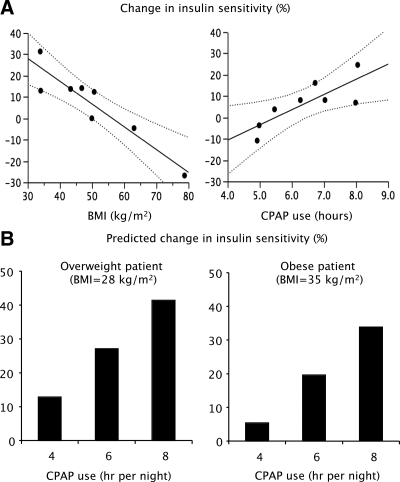
The improvement in insulin sensitivity was positively correlated with hours of CPAP use (adjusted P = 0.027) and negatively correlated with BMI (adjusted P = 0.003; Fig. 1A). For every 1-h increase in CPAP use, the adjusted insulin sensitivity increased by 7.1% after controlling for BMI. Based upon these results, Fig. 1B illustrates the modeled change in insulin sensitivity expected after 4, 6, and 8 h of CPAP use per night in an overweight patient with a baseline BMI of 28 kg/m2 and in an obese patient with a baseline BMI of 35 kg/m2. Predicted improvement in insulin sensitivity after treatment of OSA is more pronounced with longer hours of CPAP use in a dose-dependent manner and is of lesser magnitude in patients with higher degrees of obesity.
Fig. 1.
A, Fitted regression values of the change in insulin sensitivity after CPAP as a function of BMI and hours of CPAP use. The dark line represents the line of fit, and dotted lines represent the 95% confidence bands. Improvement in insulin sensitivity after CPAP was greatest among women with a lower BMI (β-coefficient = −1.068; adjusted P = 0.003) and was greater with more hours of CPAP use (β-coefficient = 7.132; adjusted P = 0.027). B, Modeled change in insulin sensitivity expected after 4, 6, and 8 h of CPAP use per night in an overweight patient with a baseline BMI of 28 kg/m2 and in an obese patient with a baseline BMI of 35 kg/m2. Predicted improvement in insulin sensitivity after treatment of OSA is more pronounced with longer hours of CPAP use in a dose-dependent manner and is of lesser magnitude in patients with higher degrees of obesity.
We did not detect any significant impact of CPAP treatment on insulin secretion, as quantified by the acute insulin response to iv glucose (1711 ± 370 vs. 1614 ± 385 mU/liter · min; P = 0.64, adjusted for BMI).
Effects of CPAP on 24-h hormonal profiles
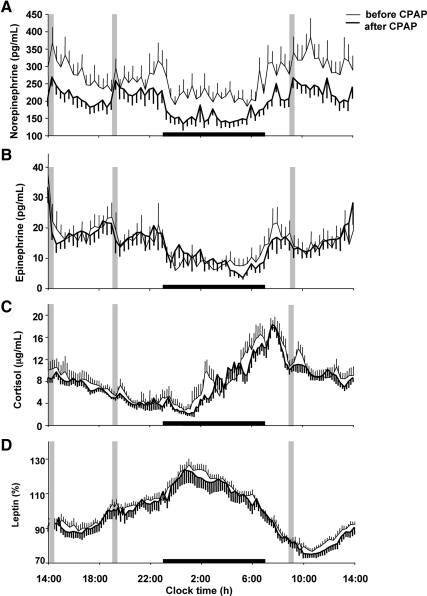
CPAP treatment resulted in a 25% reduction (267 ± 28 vs. 199 ± 20 pg/ml; P = 0.002) in mean 24-h plasma norepinephrine levels (Fig. 2A), whereas plasma epinephrine levels remained unchanged (Fig. 2B). Average daytime norepinephrine levels (from 0700–2300 h) decreased by 24% (290 ± 32 vs. 221 ± 22 pg/ml; P = 0.003), whereas average nighttime levels (from 2300–0700 h) decreased by 29% (221 ± 23 vs. 157 ± 17 pg/ml; P = 0.006). The magnitude of the decrease in mean 24-h norepinephrine levels was strongly associated with increasing hours of CPAP use (Pearson r = −0.72; P = 0.03).
Fig. 2.
Mean (± sem) 24-h profiles of norepinephrine (A), epinephrine (B), cortisol (C), and leptin (D) before and after 8 wk of CPAP treatment. Gray bars indicate identical carbohydrate-rich meals (served at 1400, 1900, and 0900 h.) Black bars indicate time in bed. Leptin profiles before and after CPAP are expressed as the percentage of mean 24-h values before CPAP in each individual.
On average, the 24-h mean cortisol level was not significantly different after CPAP (8.6 ± 0.9 vs. 7.8 ± 0.5 μg/ml; P = 0.35), although early nighttime levels (from 2300–0300 h) tended to decrease (5.2 ± 1.2 vs. 3.6 ± 0.4 μg/ml; P = 0.075; Fig. 2C). In an exploratory analysis, when only the women who had an average CPAP use of more than 5 h per night were considered (n = 7), the early nighttime cortisol levels were significantly lower after CPAP (6.2 ± 1.3 vs. 3.8 ± 0.6 μg/ml; P = 0.023).
There was no difference in mean 24-h leptin levels before and after CPAP (67.6 ± 10.5 vs. 65.8 ± 9.9 ng/ml; P = 0.59; Fig. 2D). Total and free testosterone levels were measured at 2-h intervals during the 24-h period and did not change after CPAP treatment [67.1 ± 6.5 vs. 68.8 ± 7.6 ng/dl (P = 0.82), and 20.1 ± 2.2 vs. 19.7 ± 1.7 pg/ml (P = 0.84), respectively].
Effects of CPAP on daytime blood pressure and cardiac autonomic activity
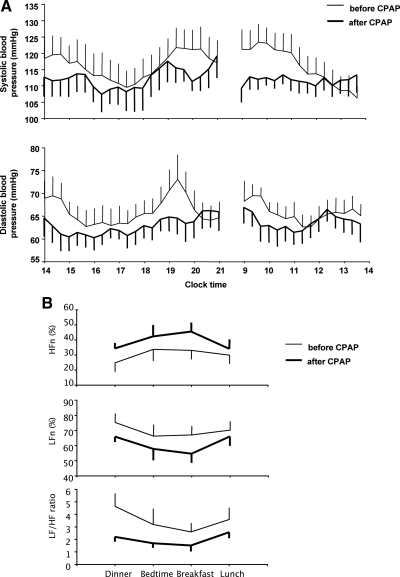
In this group of normotensive obese PCOS women, mean daytime diastolic blood pressure decreased on average by 2.3 mm Hg after CPAP (Table 1 and Fig. 3A). Although there was no significant change in mean daytime systolic blood pressure, morning levels (from 0900–1100 h) tended to decrease (121.1 ± 4.8 vs. 112.4 ± 2.7 mm Hg; P = 0.109) after CPAP (Table 1 and Fig. 3A). HFn was increased by 30%, LFn was decreased by 12%, and the ratio of LF to HF (a marker of cardiac sympathovagal balance) was 44% lower after CPAP treatment, reflecting a shift in cardiac autonomic activity toward lower sympathetic and higher parasympathetic tone (Table 1 and Fig. 3B). There was no significant correlation between hours of CPAP use and changes in blood pressure or cardiac autonomic activity.
Fig. 3.
A, Mean (± sem) daytime (from 1400–2100 and from 0900–1400) profiles of systolic and diastolic blood pressure before and after 8 wk of CPAP treatment. The profiles are illustrated as smoothed data using a three-point moving average (i.e. a window width of 1 h). Mean daytime diastolic blood pressure decreased on average by 2.3 mm Hg after CPAP, whereas there was no significant change in mean daytime systolic blood pressure. B, Mean (± sem) daytime profiles of cardiac autonomic activity derived from HRV analysis of ECG recordings that were obtained during the hour preceding each meal (dinner, breakfast, and lunch) and before bedtime (between 2130 and 2200 h) during the 24-h blood sampling period. HFn is the normalized spectral power in the HF (0.15–0.40 Hz; used as a marker of vagal activity), and LFn is the normalized spectral power in the LF (0.04–0.14 Hz; used as a marker of sympathetic activity). LF to HF ratio (LF/HF; a marker of cardiac sympathovagal balance) was 44% lower after CPAP treatment, reflecting a shift toward lower sympathetic and higher parasympathetic activity.
Discussion
We found that in young obese women with PCOS, successful treatment of OSA with CPAP results in improved measures of cardiometabolic function. To our knowledge, this is the first study to investigate the impact of treatment of OSA in women with PCOS. Despite the presence of morbid obesity in the majority of our participants, we were able to detect a positive impact of CPAP on multiple components of cardiometabolic function including insulin sensitivity, daytime and nighttime sympathetic activity, and daytime diastolic BP in the absence of any change in weight. Thus, our findings indicate that successful treatment of OSA may offer potential benefits to young PCOS women who face a lifelong risk of cardiovascular disease and type 2 diabetes. Nonetheless, it is important to note that our observations must be interpreted cautiously; our sample size was small, this was not a placebo-controlled study, and we investigated only the short-term effects (8 wk) of CPAP. Despite these limitations, we believe that our study provides an important first step to our understanding how treatment of OSA with CPAP affects cardiometabolic function in high-risk individuals, such as women with PCOS.
We found dose-response relationships between the number of hours of CPAP use and the changes in both insulin sensitivity and 24-h norepinephrine levels, indicating that the duration of CPAP use may be a key determinant of its cardiometabolic effects. Previous studies have demonstrated a graded relationship between nightly CPAP use and improvements in vigilance and cognitive function (28). However, the optimal use of CPAP, which appears to be outcome specific, is yet to be determined for cardiovascular and metabolic changes. Our study was not designed to follow up the subjects who declined participation after the screening procedures, and thus we do not have CPAP compliance data in the seven women who declined further participation after the screening. In the remaining 19 women, only about 50% achieved the commonly used target CPAP use of more than 4 h per night. Indeed, CPAP compliance continues to be a major challenge to the successful treatment of OSA, and despite its demonstrated high efficacy, a significant proportion of individuals with OSA (ranging from 46–83%) are noncompliant with treatment when compliance is defined as greater than 4 h of nightly CPAP use (29). Individual patient characteristics, the degree of OSA severity, technical aspects, and psychological and social factors have all been examined as potential predictors of CPAP compliance (19, 29). Our study was not designed to assess these factors, all of which could potentially affect the CPAP compliance. All subjects were provided with the same information regarding the outcome expectancies associated with CPAP treatment, but we did not collect specific data on subject motivation, which could be a potential confounder in our findings. However, we did not find any significant differences in age, race, BMI, the level of sleepiness, or the degree of OSA severity between compliant and noncompliant women.
We observed a modest (overall 7%) improvement in insulin sensitivity after 8 wk of CPAP treatment in PCOS women with profound obesity (BMI ranging from 34–79 kg/m2) in five of the eight subjects (Fig. 1A). Our analysis model predicted that in PCOS women who are overweight or moderately obese, the improvement in insulin sensitivity after CPAP would be considerably larger, averaging 30–40% (as illustrated in Fig. 1B in two hypothetical patients), i.e. within the range reported for treatment with metformin, where the improvement in insulin sensitivity varied between 18 and 80% (30–32). In one study involving morbidly obese PCOS women, the insulin sensitivity was not affected by metformin treatment (33). There is also evidence to indicate that metformin administration has no additional benefit on insulin levels beyond those achieved through diet-induced weight loss (34). Indeed, lifestyle interventions (with diet and exercise) in obese women with PCOS result in marked improvements in insulin resistance accompanying weight losses of 5–10% (35). We did not collect data on diet and exercise measures over the 8-wk intervention period, but the subjects were instructed not to deviate from their usual dietary and exercise habits throughout the study. Notably, we did not observe any significant change in weight or percent body fat accompanying the improvement in insulin sensitivity after CPAP. Our findings thus suggest that CPAP treatment of OSA in women with PCOS can provide additional benefits and, where appropriate, may be a useful adjunctive treatment strategy to more traditional insulin-lowering therapies.
For every 1-h increase in CPAP use, the insulin sensitivity increased by 7.1% after controlling for BMI, corresponding to a 34% improvement in insulin sensitivity in an obese PCOS woman whose BMI is 35 kg/m2 and who uses CPAP for 8 h per night. By comparison, one study using euglycemic clamp in 40 nondiabetic OSA patients (34 males) with an average BMI of approximately 32 kg/m2 reported 31% improvement in insulin sensitivity after 3 months of CPAP in the absence of weight change (36). There is also evidence from the general population to suggest that the degree of obesity (36) and the amount of CPAP use (37) may be important predictors of metabolic response to CPAP. Although some studies (36, 38–40) have reported reductions in insulin resistance after CPAP, others were negative (41–44). Notably, compared with our young study population in whom we observed a positive effect, the majority of these CPAP studies were conducted in the middle-aged and older individuals, who might have had OSA for longer periods of time and thus suffering from potentially less reversible metabolic derangements.
The sympathetic nervous system plays an essential role in the regulation of cardiovascular and metabolic responses. Although obesity alone can lead to region-specific alterations in sympathetic activity (45), the presence of OSA has been associated with a markedly elevated muscle sympathetic nerve activity, independently of body weight (46–48), as well as an increased cardiac sympathetic drive, in the absence of existing cardiovascular disease (46). High sympathetic output is thought to be an important mediator of the increased cardiovascular risk associated with OSA (7), and treatment with CPAP results in marked reductions in sympathetic activity (49). We demonstrated that in morbidly obese women with PCOS, in the absence of any change in weight, CPAP treatment of OSA results in a 25% reduction in mean daytime and nighttime levels of plasma norepinephrine, the primary peripheral neurotransmitter of sympathetic activity. Importantly, the decrease in 24-h norepinephrine levels occurred in all subjects with greater reductions being observed with increasing hours of CPAP use. Sympathetic neural mechanisms have also been implicated in the development of insulin resistance. In an exploratory analysis, we found that the change in insulin sensitivity after CPAP was positively correlated with the magnitude of decrease in norepinephrine levels (between 2300 and 1400 h) after controlling for BMI (adjusted P = 0.044). Daytime cardiac sympathovagal balance was approximately 44% lower after CPAP treatment, reflecting a shift toward lower sympathetic activity. Taken together, our findings are in agreement with previous reports in non-PCOS populations showing that CPAP treatment of OSA reduces circulating norepinephrine levels (measured at a single time point) (50–54) and cardiac sympathetic activity (55, 56).
We did not observe any significant change in 24-h profiles of epinephrine, suggesting a differential sympathetic response with decreased peripheral, but not adrenomedullary, activity after CPAP treatment of OSA in PCOS. Elevated leptin levels, observed in most obese individuals, have been associated with sympathetic activation and increased cardiovascular risk (57). OSA is typically associated with hyperleptinemia, independently of BMI (58). In our study, 24-h leptin profiles were not affected by 8 wk of CPAP treatment, which could be partly due to extreme adiposity in the majority of our participants and the relatively shorter duration of intervention. In general, studies reporting a positive effect of CPAP use on leptin levels have enrolled individuals with a lesser degree of obesity who were treated for a period of time that exceeded 8 wk (42, 59–61). Excess cortisol levels can lead to the development of insulin resistance, glucose intolerance, hypertension, and other cardiovascular diseases (62). We did not detect a significant difference in 24-h profiles of cortisol levels in response to CPAP treatment. However, early nighttime cortisol levels tended to decrease after CPAP, and in an exploratory analysis, this difference became statistically significant when only the seven women who used the CPAP more than 5 h per night were considered. Our finding of decreased early nighttime cortisol levels after CPAP is highly consistent with the known physiology of sleep-related inhibition of cortisol levels (63–65). Other studies have shown beneficial effects of 3 months of CPAP treatment on 24-h profiles of cortisol in obese men with OSA (66, 67).
We found a positive effect of CPAP on daytime diastolic BP in women with PCOS who were normotensive at baseline. Mean daytime diastolic BP decreased on average by 2.3 mm Hg, and there was a tendency for lower systolic BP in the morning. Previous studies examining the effects of CPAP treatment on blood pressure suggest that the reduction in blood pressure is either modest or absent in normotensive individuals with OSA (7, 68).
PCOS women face lifelong risk of cardiometabolic disease with nearly half developing type 2 diabetes before the end of their fourth decade of life (5, 6). OSA is highly prevalent in PCOS and is independently associated with adverse cardiovascular and metabolic outcomes. Our findings suggest that CPAP treatment of OSA has favorable effects on cardiometabolic function in young obese women with PCOS and thus may offer benefits adjunctive to those provided by more traditional insulin-lowering therapies. More rigorous CPAP studies with a randomized placebo-controlled design will be necessary to confirm these findings. CPAP compliance is generally suboptimal and highly variable among patients with OSA; thus, more research is also needed to find ways to maximize compliance with CPAP and to investigate, in PCOS, the effects of alternative OSA treatment strategies such as weight loss or use of oral appliances.
Acknowledgments
We thank Dr. Eve Van Cauter for her expertise, intellectual contributions, and careful review of this manuscript. We thank Jackie Imperial for performing iv glucose tolerance tests, Theresa Marcinkowski for performing the catecholamine assays, and Abby Rue, Katie Nitsche, and Karla Temple for assistance with subject recruitment, study coordination, and data collection. We also thank the subjects for participating in this demanding study and the nursing and dietary staff of the University of Chicago General Clinical Research Center for their expert assistance.
This work was supported by grants from the National Institutes of Health (RR024999, PO1-AG11412, RO1-HL-075079, P50-HD057796, and P60-DK20595) and a gift from the Blum-Kovler Foundation.
The www.ClinicalTrials.gov identifier is NCT00696111.
Disclosure Summary: E.T., R.L., H.W., and D.A.E. having nothing to declare. F.C. is the designer and a codeveloper of the PRANA software used in the analysis of heart rate variability in this study and receives royalties from the distribution of this software.
Footnotes
- AHI
- Apnea-hypopnea index
- BMI
- body mass index
- BP
- blood pressure
- CPAP
- continuous positive airway pressure
- ECG
- electrocardiogram
- HF
- high-frequency band
- HFn
- normalized HF
- HRV
- heart rate variability
- ivGTT
- iv glucose tolerance test
- LF
- low-frequency band
- LFn
- normalized LF
- ODI
- oxygen desaturation index
- OSA
- obstructive sleep apnea
- PCOS
- polycystic ovary syndrome
- REM
- rapid eye movement.
References
- 1. Ehrmann DA. 2005. Polycystic ovary syndrome. N Engl J Med 352:1223–1236 [DOI] [PubMed] [Google Scholar]
- 2. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R. 1998. Prevalence of polycystic ovary syndrome in unselected Black and White women of the southeastern United States: a prospective study. J Clin Endocrinol Metab 83:3078–3082 [DOI] [PubMed] [Google Scholar]
- 3. Hoffman LK, Ehrmann DA. 2008. Cardiometabolic features of polycystic ovary syndrome. Nat Clin Pract Endocrinol Metab 4:215–222 [DOI] [PubMed] [Google Scholar]
- 4. Dunaif A, Segal KR, Futterweit W, Dobrjansky A. 1989. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38:1165–1174 [DOI] [PubMed] [Google Scholar]
- 5. Ehrmann DA, Barnes RB, Rosenfield RL, Cavaghan MK, Imperial J. 1999. Prevalence of impaired glucose tolerance and diabetes in women with polycystic ovary syndrome. Diabetes Care 22:141–146 [DOI] [PubMed] [Google Scholar]
- 6. Legro RS, Kunselman AR, Dodson WC, Dunaif A. 1999. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab 84:165–169 [DOI] [PubMed] [Google Scholar]
- 7. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. 2008. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. J Am Coll Cardiol 52:686–717 [DOI] [PubMed] [Google Scholar]
- 8. Hla KM, Young T, Finn L, Peppard PE, Szklo-Coxe M, Stubbs M. 2008. Longitudinal association of sleep-disordered breathing and nondipping of nocturnal blood pressure in the Wisconsin Sleep Cohort Study. Sleep 31:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tasali E, Ip MS. 2008. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc 5:207–217 [DOI] [PubMed] [Google Scholar]
- 10. Lévy P, Bonsignore MR, Eckel J. 2009. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 34:243–260 [DOI] [PubMed] [Google Scholar]
- 11. Punjabi NM, Beamer BA. 2009. Alterations in glucose disposal in sleep-disordered breathing. Am J Respir Crit Care Med 179:235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. 2010. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med 181:507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fogel RB, Malhotra A, Pillar G, Pittman SD, Dunaif A, White DP. 2001. Increased prevalence of obstructive sleep apnea syndrome in obese women with polycystic ovary syndrome. J Clin Endocrinol Metab 86:1175–1180 [DOI] [PubMed] [Google Scholar]
- 14. Vgontzas AN, Legro RS, Bixler EO, Grayev A, Kales A, Chrousos GP. 2001. Polycystic ovary syndrome is associated with obstructive sleep apnea and daytime sleepiness: role of insulin resistance. J Clin Endocrinol Metab 86:517–520 [DOI] [PubMed] [Google Scholar]
- 15. Gopal M, Duntley S, Uhles M, Attarian H. 2002. The role of obesity in the increased prevalence of obstructive sleep apnea syndrome in patients with polycystic ovarian syndrome. Sleep Med 3:401–404 [DOI] [PubMed] [Google Scholar]
- 16. Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. 2008. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab 93:3878–3884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tasali E, Van Cauter E, Ehrmann DA. 2006. Relationships between sleep disordered breathing and glucose metabolism in polycystic ovary syndrome. J Clin Endocrinol Metab 91:36–42 [DOI] [PubMed] [Google Scholar]
- 18. Bergman RN. 2005. Minimal model: perspective from 2005. Horm Res 64(Suppl 3):8–15 [DOI] [PubMed] [Google Scholar]
- 19. Weaver TE. 2006. Adherence to positive airway pressure therapy. Curr Opin Pulm Med 12:409–413 [DOI] [PubMed] [Google Scholar]
- 20. Iber C, Ancoli-Israel S, Chesson A, Quan SF. 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specification. Darien, IL: American Academy of Sleep Medicine [Google Scholar]
- 21. 1992. EEG arousals: scoring rules and examples. A preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep 15:173–184 [PubMed] [Google Scholar]
- 22. 1999. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22:667–689 [PubMed] [Google Scholar]
- 23. Hoddes E, Zarcone V, Smythe H, Phillips R, Dement WC. 1973. Quantification of sleepiness: a new approach. Psychophysiology 10:431–436 [DOI] [PubMed] [Google Scholar]
- 24. Degaute JP, van de Borne P, Linkowski P, Van Cauter E. 1991. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 18:199–210 [DOI] [PubMed] [Google Scholar]
- 25. Biston P, Van Cauter E, Ofek G, Linkowski P, Polonsky KS, Degaute JP. 1996. Diurnal variations in cardiovascular function and glucose regulation in normotensive humans. Hypertension 28:863–871 [DOI] [PubMed] [Google Scholar]
- 26. 1996. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 93:1043–1065 [PubMed] [Google Scholar]
- 27. VanHelder T, Symons JD, Radomski MW. 1993. Effects of sleep deprivation and exercise on glucose tolerance. Aviat Space Environ Med 64:487–492 [PubMed] [Google Scholar]
- 28. Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, Kader G, Mahowald M, Younger J, Pack AI. 2007. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep 30:711–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weaver TE, Grunstein RR. 2008. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc 5:173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Unlühizarci K, Kele°timur F, Bayram F, Sahin Y, Tutu° A. 1999. The effects of metformin on insulin resistance and ovarian steroidogenesis in women with polycystic ovary syndrome. Clin Endocrinol (Oxf) 51:231–236 [DOI] [PubMed] [Google Scholar]
- 31. Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, Zanolin E, Muggeo M. 2000. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab 85:139–146 [DOI] [PubMed] [Google Scholar]
- 32. Diamanti-Kandarakis E, Kouli C, Tsianateli T, Bergiele A. 1998. Therapeutic effects of metformin on insulin resistance and hyperandrogenism in polycystic ovary syndrome. Eur J Endocrinol 138:269–274 [DOI] [PubMed] [Google Scholar]
- 33. Ehrmann DA, Cavaghan MK, Imperial J, Sturis J, Rosenfield RL, Polonsky KS. 1997. Effects of metformin on insulin secretion, insulin action, and ovarian steroidogenesis in women with polycystic ovary syndrome. J Clin Endocrinol Metab 82:524–530 [DOI] [PubMed] [Google Scholar]
- 34. Crave JC, Fimbel S, Lejeune H, Cugnardey N, Déchaud H, Pugeat M. 1995. Effects of diet and metformin administration on sex hormone-binding globulin, androgens, and insulin in hirsute and obese women. J Clin Endocrinol Metab 80:2057–2062 [DOI] [PubMed] [Google Scholar]
- 35. Moran LJ, Pasquali R, Teede HJ, Hoeger KM, Norman RJ. 2009. Treatment of obesity in polycystic ovary syndrome: a position statement of the Androgen Excess and Polycystic Ovary Syndrome Society. Fertil Steril 92:1966–1982 [DOI] [PubMed] [Google Scholar]
- 36. Harsch IA, Schahin SP, Radespiel-Tröger M, Weintz O, Jahreiss H, Fuchs FS, Wiest GH, Hahn EG, Lohmann T, Konturek PC, Ficker JH. 2004. Continuous positive airway pressure treatment rapidly improves insulin sensitivity in patients with obstructive sleep apnea syndrome. Am J Respir Crit Care Med 169:156–162 [DOI] [PubMed] [Google Scholar]
- 37. Babu AR, Herdegen J, Fogelfeld L, Shott S, Mazzone T. 2005. Type 2 diabetes, glycemic control, and continuous positive airway pressure in obstructive sleep apnea. Archives of internal medicine 165:447–452 [DOI] [PubMed] [Google Scholar]
- 38. Lam JC, Lam B, Yao TJ, Lai AY, Ooi CG, Tam S, Lam KS, Ip MS. 2010. A randomised controlled trial of nasal continuous positive airway pressure on insulin sensitivity in obstructive sleep apnoea. Eur Respir J 35:138–145 [DOI] [PubMed] [Google Scholar]
- 39. Dorkova Z, Petrasova D, Molcanyiova A, Popovnakova M, Tkacova R. 2008. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 134:686–692 [DOI] [PubMed] [Google Scholar]
- 40. Henley DE, Buchanan F, Gibson R, Douthwaite JA, Wood SA, Woltersdorf WW, Catterall JR, Lightman SL. 2009. Plasma apelin levels in obstructive sleep apnea and the effect of continuous positive airway pressure therapy. J Endocrinol 203:181–188 [DOI] [PubMed] [Google Scholar]
- 41. Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Collins B, Basta M, Pejovic S, Chrousos GP. 2008. Selective effects of CPAP on sleep apnoea-associated manifestations. Eur J Clin Invest 38:585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Trenell MI, Ward JA, Yee BJ, Phillips CL, Kemp GJ, Grunstein RR, Thompson CH. 2007. Influence of constant positive airway pressure therapy on lipid storage, muscle metabolism and insulin action in obese patients with severe obstructive sleep apnoea syndrome. Diabetes Obes Metab 9:679–687 [DOI] [PubMed] [Google Scholar]
- 43. Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. 2007. Cardiovascular and metabolic effects of CPAP in obese men with OSA. Eur Respir J 29:720–727 [DOI] [PubMed] [Google Scholar]
- 44. Murri M, Alcázar-Ramírez J, Garrido-Sánchez L, Linde F, Alcaide J, Cardona F, Tinahones FJ. 2009. Oxidative stress and metabolic changes after continuous positive airway pressure treatment according to previous metabolic disorders in sleep apnea-hypopnea syndrome patients. Transl Res 154:111–121 [DOI] [PubMed] [Google Scholar]
- 45. Davy KP, Orr JS. 2009. Sympathetic nervous system behavior in human obesity. Neurosci Biobehav Rev 33:116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. 1998. Altered cardiovascular variability in obstructive sleep apnea. Circulation 98:1071–1077 [DOI] [PubMed] [Google Scholar]
- 47. Narkiewicz K, Somers VK. 2003. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol Scand 177:385–390 [DOI] [PubMed] [Google Scholar]
- 48. Grassi G, Facchini A, Trevano FQ, Dell'Oro R, Arenare F, Tana F, Bolla G, Monzani A, Robuschi M, Mancia G. 2005. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension 46:321–325 [DOI] [PubMed] [Google Scholar]
- 49. Somers VK, Dyken ME, Clary MP, Abboud FM. 1995. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Phillips BG, Narkiewicz K, Pesek CA, Haynes WG, Dyken ME, Somers VK. 1999. Effects of obstructive sleep apnea on endothelin-1 and blood pressure. J Hypertens 17:61–66 [DOI] [PubMed] [Google Scholar]
- 51. Mills PJ, Kennedy BP, Loredo JS, Dimsdale JE, Ziegler MG. 2006. Effects of nasal continuous positive airway pressure and oxygen supplementation on norepinephrine kinetics and cardiovascular responses in obstructive sleep apnea. J Appl Physiol 100:343–348 [DOI] [PubMed] [Google Scholar]
- 52. Hedner J, Darpö B, Ejnell H, Carlson J, Caidahl K. 1995. Reduction in sympathetic activity after long-term CPAP treatment in sleep apnoea: cardiovascular implications. Eur Respir J 8:222–229 [DOI] [PubMed] [Google Scholar]
- 53. Fletcher EC. 2003. Sympathetic over activity in the etiology of hypertension of obstructive sleep apnea. Sleep 26:15–19 [DOI] [PubMed] [Google Scholar]
- 54. Bratel T, Wennlund A, Carlström K. 1999. Pituitary reactivity, androgens and catecholamines in obstructive sleep apnoea. Effects of continuous positive airway pressure treatment (CPAP). Respir Med 93:1–7 [DOI] [PubMed] [Google Scholar]
- 55. Shiina K, Tomiyama H, Takata Y, Yoshida M, Kato K, Saruhara H, Hashimura Y, Matsumoto C, Asano K, Usui Y, Yamashina A. 2010. Effects of CPAP therapy on the sympathovagal balance and arterial stiffness in obstructive sleep apnea. Respir Med 104:911–916 [DOI] [PubMed] [Google Scholar]
- 56. Roche F, Court-Fortune I, Pichot V, Duverney D, Costes F, Emonot A, Vergnon JM, Geyssant A, Lacour JR, Barthélémy JC. 1999. Reduced cardiac sympathetic autonomic tone after long-term nasal continuous positive airway pressure in obstructive sleep apnoea syndrome. Clin Physiol 19:127–134 [DOI] [PubMed] [Google Scholar]
- 57. Sweeney G. 2010. Cardiovascular effects of leptin. Nat Rev Cardiol 7:22–29 [DOI] [PubMed] [Google Scholar]
- 58. Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. 2000. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol 279:H234–H237 [DOI] [PubMed] [Google Scholar]
- 59. Saarelainen S, Lahtela J, Kallonen E. 1997. Effect of nasal CPAP treatment on insulin sensitivity and plasma leptin. J Sleep Res 6:146–147 [DOI] [PubMed] [Google Scholar]
- 60. Ip MS, Lam KS, Ho C, Tsang KW, Lam W. 2000. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest 118:580–586 [DOI] [PubMed] [Google Scholar]
- 61. Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH. 2003. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J 22:251–257 [DOI] [PubMed] [Google Scholar]
- 62. Whitworth JA, Williamson PM, Mangos G, Kelly JJ. 2005. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag 1:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Born J, Muth S, Fehm HL. 1988. The significance of sleep onset and slow wave sleep for nocturnal release of growth hormone (GH) and cortisol. Psychoneuroendocrinology 13:233–243 [DOI] [PubMed] [Google Scholar]
- 64. Follenius M, Brandenberger G, Bandesapt JJ, Libert JP, Ehrhart J. 1992. Nocturnal cortisol release in relation to sleep structure. Sleep 15:21–27 [DOI] [PubMed] [Google Scholar]
- 65. Gronfier C, Luthringer R, Follenius M, Schaltenbrand N, Macher JP, Muzet A, Brandenberger G. 1997. Temporal relationships between pulsatile cortisol secretion and electroencephalographic activity during sleep in man. Electroencephalogr Clin Neurophysiol 103:405–408 [DOI] [PubMed] [Google Scholar]
- 66. Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bentley CM, Bixler EO, Sarrigiannidis A, Basta M, Chrousos GP. 2007. Hypothalamic-pituitary-adrenal axis activity in obese men with and without sleep apnea: effects of continuous positive airway pressure therapy. J Clin Endocrinol Metab 92:4199–4207 [DOI] [PubMed] [Google Scholar]
- 67. Henley DE, Russell GM, Douthwaite JA, Wood SA, Buchanan F, Gibson R, Woltersdorf WW, Catterall JR, Lightman SL. 2009. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J Clin Endocrinol Metab 94:4234–4242 [DOI] [PubMed] [Google Scholar]
- 68. Alajmi M, Mulgrew AT, Fox J, Davidson W, Schulzer M, Mak E, Ryan CF, Fleetham J, Choi P, Ayas NT. 2007. Impact of continuous positive airway pressure therapy on blood pressure in patients with obstructive sleep apnea hypopnea: a meta-analysis of randomized controlled trials. Lung 185:67–72 [DOI] [PubMed] [Google Scholar]