Lower intermyofibrillar mitochondrial content in skeletal muscle is linked to peripheral insulin resistance, lower reliance on lipid oxidation, and metabolic inflexibility.
Abstract
Context:
Insulin resistance is accompanied by lower lipid oxidation during fasting and metabolic inflexibility. Whether these abnormalities correlate with mitochondrial content in skeletal muscle is unknown.
Objective:
The objective of the study was to investigate whether decreased fasting lipid oxidation, metabolic inflexibility, and impaired glucose disposal correlate with reduced mitochondrial content in intermyofibrillar vs. subsarcolemmal (SS) subpopulations.
Design:
Forty sedentary adults with a wide spectrum of insulin sensitivity were studied: insulin-sensitive lean subjects, insulin-resistant nondiabetic subjects, and subjects with type 2 diabetes mellitus. Glucose disposal was measured by euglycemic clamp and [6,6-D2]-glucose methodology. Fuel oxidation and metabolic flexibility (during clamps) were assessed by indirect calorimetry. Maximum aerobic capacity was assessed by treadmill testing. Intermyofibrillar and SS mitochondrial content were measured by quantitative electron microscopy of muscle biopsy samples.
Results:
Intermyofibrillar mitochondrial content was lower in the insulin-resistant nondiabetic subjects and type 2 diabetes mellitus groups, significantly correlating with glucose disposal in both men (R = 0.72, P < 0.01) and women (R = 0.53, P < 0.01). In contrast, SS mitochondrial content was similar among groups. Lower intermyofibrillar mitochondrial content was not explained by mitochondrial size, altered fiber-type distribution, or differences in maximum aerobic capacity. Intermyofibrillar mitochondrial content was significantly correlated with fasting respiratory quotient (R = −0.46, P = 0.003) and metabolic flexibility (R = 0.38, P = 0.02).
Conclusions:
In obese-insulin-resistant subjects with or without diabetes, intermyofibrillar mitochondrial content is decreased. This is not entirely explained by fitness status or fiber-type composition. SS mitochondrial content is unaffected, suggesting independent mitochondrial pool regulation. Lower mitochondrial content correlates with lower fasting lipid oxidation and metabolic inflexibility, suggesting it may be intrinsically linked to abnormal fuel utilization patterns of obesity-associated insulin resistance.
Skeletal muscle tissue must constantly adapt to rapid changes in nutrient flux and metabolic demands. In concert with glucose disposal, skeletal muscle rapidly switches substrate oxidation patterns in coordination with insulin signals. In the fasting state, skeletal muscle is highly reliant on fatty acid oxidation. Upon stimulation by insulin, glucose oxidation in enhanced and lipid oxidation is suppressed. This flexibility in fuel metabolism has been termed metabolic flexibility (ΔRQ). In insulin-resistant individuals, patterns of fuel oxidation appear to be abnormal. Humans with obesity or type 2 diabetes mellitus (T2DM) show decreased lipid oxidation during fasting and in response to insulin show impaired ΔRQ. The cellular correlates behind those abnormalities remain a mystery but may be related to intrinsic abnormalities in skeletal muscle (1). Conceivably, skeletal muscle mitochondria may be an integral component, given their central role in fuel oxidation.
Although animal models of insulin resistance appear to have intact mitochondrial capacity (2, 3), humans with insulin resistance seem to have reduced mitochondrial capacity in skeletal muscle. Decreased mitochondrial oxidative capacity has been reported as reduced electron transport chain activity (4, 5), mitochondrial fatty acid oxidation (6, 7), and oxidative phosphorylation in vivo (8, 9). These observations suggest that impairments in mitochondrial capacity might contribute to the development of insulin resistance, a hypothesis that has been a matter of growing interest and debate (10, 11). In contrast, a relationship between metabolic inflexibility and mitochondria has been relatively less explored.
Expression of transcription regulators of mitochondrial biogenesis has been reported to be reduced in insulin-resistant subjects (12, 13). An expected consequence would be decreased mitochondrial biogenesis with decreased mitochondrial content. However, it is still not clearly established whether mitochondrial content is reduced in humans with insulin resistance and if so whether it is manifested in those with or without T2DM. Human studies have examined this question with indirect markers of mitochondrial content and results have been conflicting. Studies have typically used citrate-synthase activity or mitochondrial DNA (mtDNA) copy number as surrogate markers of mitochondrial content. In some studies, surrogate markers of mitochondrial content are reduced (7, 9, 14), whereas in others they are not (15, 16). These discrepant observations imply that enzymatic markers and mtDNA may not be reliable indicators of mitochondrial content in skeletal muscle or that reductions in mitochondrial content are present only with more marked degrees of insulin resistance, such as in T2DM. Of note, those markers do not discriminate between intermyofibrillar or subsarcolemmal (SS) mitochondrial subpopulations. This is an important consideration because SS mitochondria may have different metabolic influences on lipid oxidation and respond differently to stimuli. Therefore, a better understanding of how mitochondria distribution in specific subpopulations is related to insulin sensitivity and patterns of fuel use may help in elucidating the etiology of insulin resistance.
To more definitely answer whether mitochondrial content is altered in insulin-resistant subjects, we conducted a histological study of skeletal muscle with quantitative electron microscopy. With this approach mitochondria are quantified in situ, which circumvents biochemical and purification artifacts inherent to surrogate markers and allows for discriminating between intermyofibrillar and SS subpopulations. Finally, we tested the novel hypothesis that lower skeletal muscle mitochondrial content may be connected to two patterns of abnormal fuel oxidation seen in obese, insulin-resistant individuals: lower reliance on lipid oxidation during fasting and metabolic inflexibility.
Materials and Methods
Subjects characteristics, aerobic capacity, and body composition
The protocol was approved by the University of Pittsburgh Institutional Review Board. All subjects gave informed consent. Subjects were categorized into three groups according to their insulin sensitivity phenotype. The first group consisted of lean, insulin-sensitive controls (IS-Lean). The second group included insulin-resistant, nondiabetic subjects (IR-ND), with a body mass index (BMI) of 28 kg/m2 or greater and either impaired glucose tolerance and/or the metabolic syndrome (National Cholesterol Education Program Adult Treatment Panel III definition). The third group consisted of subjects with T2DM who were on nonpharmacological management only, metformin and/or sulfonylurea. Six subjects in this group and two in the IR-ND group were on statin therapy. All participants had a screening medical history, physical examination, and laboratory tests including a 75-g oral glucose challenge. All subjects were required to be sedentary, defined as less than 20 min of exercise/week, and had their maximum aerobic capacity (VO2max) measured by treadmill testing. Subjects were weight stable (<3 kg change in the past month) and otherwise healthy. They were asked not to exercise or consume alcohol for 48 h before study visits. Body composition was assessed by dual-energy x-ray absorptiometry.
Euglycemic clamps and indirect calorimetry
Subjects were admitted on the evening before clamps. A standardized dinner (7 kcal/kg; 50% carbohydrates, 20% protein, and 30% fat) was provided, oral hypoglycemic agents discontinued, and fasting initiated for 12 h overnight. In the following morning, a primed (200 mg/m2), continuous (2 mg/min−1 · m−2) [6,6-D2]-glucose infusion was started 150 min before insulin. Euglycemic clamps were initiated with an insulin infusion (40 mU/m−2 · min−1) and glycemia maintained at 90 ± 5 mg/dl (∼5 mmol/liter) during 4 h with a variable dextrose infusion. Insulin sensitivity was determined by the glucose disposal rate (Rd) during the last 20 min of steady state and corrected for endogenous glucose appearance calculated from [6,6-D2]-glucose tracer dilution (17). An open-circuit spirometry system (DeltaTrac, Anaheim, CA) was used to measure resting metabolic rate, respiratory quotient (RQ), and substrate oxidation before the clamp and during steady state. ΔRQ was calculated as the difference between the steady state and fasting RQ.
Muscle biopsies
Samples of vastus lateralis muscle were obtained by percutaneous needle biopsy after local anesthesia with 2% lidocaine. Any adipose or connective tissue was promptly removed by dissection under a microscope. Tissue was saved for light microscopy (fiber typing) and mounted in Cryomatrix (Shandon, Pittsburgh, PA) and then frozen directly in isopentane cooled in liquid nitrogen. For electron microscopy, 1- × 1- × 2-mm pieces were saved in 2.5% glutaraldehyde for subsequent fixing and embedding (17, 18).
Muscle fiber typing
Immunohistochemistry with antimyosin monoclonal antibodies was performed to identify fiber types. From tissue blocks, serial transverse sections (8 μm) were cut using a cryostat at −20 C and then mounted on Superfrost/Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were incubated overnight at room temperature with antibodies against antihuman myosin heavy chain-7 (type I fibers) and myosin heavy chain-2 (type IIa fibers). Sections were subsequently incubated with secondary antibodies conjugated with fluorescein (type IIa fibers) and rhodamine (type I fibers) (Santa Cruz Biotechnology, Santa Cruz, CA). Type IIx fibers remained unstained. Images were acquired with an optical microscope (Nikon Microphot-FXL, Tokyo, Japan). Fiber types were counted based on their specific staining and the percent proportion of each was calculated.
Quantitative transmission electron microscopy
Mitochondrial content was determined by the parameter of mitochondrial volume density measured by standard stereological methods (19, 20) as in our previous studies (17, 18, 21). This parameter expresses the percentage of cell volume occupied by mitochondria and reflects mitochondrial content (19, 20). For each biopsy specimen, random longitudinal sections were obtained and imaged at ×36,000 magnification. For each specimen, 15–20 random micrographs were acquired and downloaded into digital image analytical software (Metamorph 6.3; Molecular Devices Corp., Sunnyvale, CA). A 144-point quadratic grid for point counting was overlapped for quantification of mitochondrial density (19, 20). SS mitochondrial density was similarly measured by classical stereology in random micrographs (20 per specimen) acquired at ×23,000 near the cell surface. The reference space was the cytoplasmic space between the sarcolemma and the first layer of myofibrils. The average mitochondrial size was calculated as the mean of mitochondrial profiles from all micrographs (mean 105 profiles/specimen).
Statistics
Data are presented as mean ± sem unless otherwise indicated. A P < 0.05 defined statistical significance. Variables were preexamined for normal distribution. For comparisons of group means, one-way ANOVA tests were used. Analysis of covariance was used to adjust mitochondrial content for VO2max/fat-free mass as a covariate. Gender distribution was analyzed by χ2 test.
Results
Characteristics of subjects (Table 1)
Table 1.
Characteristics of subjects and glucose metabolism
| IS-Lean (n = 12) | IR-ND (n = 17) | T2DM (n = 11) | |
|---|---|---|---|
| Subject characteristics | |||
| Age (yr) | 47 ± 2.1 | 44 ± 1.7 | 44 ± 2.7 |
| Female (%) | 75 | 65 | 55 |
| Body composition | |||
| Height (cm) | 166 ± 2 | 167 ± 2 | 170 ± 3 |
| Body weight (kg) | 65.6 ± 2.2 | 91.5 ± 3.0a | 99.6 ± 3.4a |
| BMI (kg/m2) | 23.7 ± 0.6 | 32.9 ± 0.8a | 34.3 ± 0.8a |
| Fat mass (kg) | 21.2 ± 1.3 | 39.8 ± 1.6b | 38.2 ± 2.5a |
| Fat mass (percent body fat) | 32.6 ± 2.0 | 43.8 ± 1.8a | 38.9 ± 2.8 |
| Lean mass (kg) | 43.4 ± 2.5 | 53.4 ± 2.6a | 58.6 ± 4.0a |
| Waist circumference (cm) | 81.8 ± 1.7 | 110.7 ± 2.8a | 109.4 ± 2.4a |
| Maximal aerobic capacity | |||
| VO2max (ml/min) | 2158 ± 156 | 2408 ± 152a | 2567 ± 86a |
| VO2max (ml/min · kg FFM) | 49.5 ± 1.6 | 44.8 ± 1.2a | 44.1 ± 1.5a |
| Glucose tolerance and laboratory values | |||
| Plasma glucose, fasting (mg/dl) | 90.5 ± 1.8 | 98.4 ± 2.1 | 160.0 ± 13.9a,c |
| Plasma glucose, 2 h after challenge (mg/dl) | 105.9 ± 9.5 | 151.1 ± 6.4a | 255.0 ± 15.2a,c |
| HbA1c (%) | 5.47 ± 0.11 | 5.49 ± 0.09 | 7.65 ± 0.51a,c |
| Fasting serum insulin (μIU/ml) | 4.6 ± 0.5 | 17.7 ± 1.5a | 19.6 ± 3.0a |
| Fasting plasma FFA (mmol/liter) | 0.38 ± 0.02 | 0.50 ± 0.03a | 0.55 ± 0.04a |
| Euglycemic clamps | |||
| Glucose infusion rate (mg/min · kg FFM) | 12.1 ± 0.9 | 5.6 ± 0.5a | 2.9 ± 0.8a,c |
| Endogenous glucose appearance rate (mg/min · kg FFM) | 0 ± 0 | 0.2 ± 0.2 | 1.2 ± 0.3a,c |
| Rd (mg/min · kg FFM) | 12.1 ± 0.9 | 5.9 ± 0.5a | 4.1 ± 0.6a,d |
| Steady-state plasma glucose (mg/dl) | 91 (90–96) | 90 (88–92) | 92 (89–97) |
| Steady-state serum insulin (μIU/ml) | 60 ± 2 | 89 ± 5b | 82 ± 4b |
Results are expressed as mean ± sem. Plasma glucose during steady state is expressed as median with interquartile range. FFM, Fat-free mass; HbA1c, glycosylated hemoglobin.
P < 0.01 vs. IS-Lean.
P < 0.05 vs. IS-Lean.
P < 0.01 for T2DM vs. IR-ND.
0.058 for T2DM vs. IR-ND.
Mean age was similar in the three groups. There were slightly fewer women in the insulin-resistant groups, but the trend was not statistically significant. Subjects in the IR-ND and T2DM groups were overweight or obese, showing clearly increased measures of adiposity (BMI, fat mass, and waist circumference). Maximal aerobic capacity, when corrected for lean body mass, was slightly lower in the insulin-resistant groups. Oral glucose tolerance tests revealed obvious intergroup differences in plasma glucose but most notably postglucose challenge. In the T2DM group, the mean glycosylated hemoglobin was consistent with modest glycemic control before study enrollment. Fasting insulin and free fatty acid (FFA) concentrations were elevated in the IR-ND and T2DM groups, suggesting insulin resistance. To more directly determine skeletal muscle insulin sensitivity, we used euglycemic clamps with [6,6-D2]-glucose dilution methodology. The systemic glucose Rd was used as a measure of insulin sensitivity because it accounts for rates of endogenous glucose appearance. In the IR-ND and T2DM groups, Rd was less than half of the IS-Lean group, indicative of significant insulin resistance in skeletal muscle.
Skeletal muscle mitochondrial content and size
Mitochondria are heterogeneously distributed in skeletal muscle cells. About 85% of the mitochondrial mass in human skeletal muscle is accounted for by intermyofibrillar mitochondria (22). The remainder is accounted for by SS mitochondria, which tend to be larger and more densely clustered. An electron micrograph illustrating these subpopulations is shown in Fig. 1.
Fig. 1.
Micrograph of a skeletal muscle cell illustrating intermyofibrillar and SS mitochondria. IMF mitochondria are located between parallel bundles of myofibrils. The SS mitochondria are located between the sarcolemma and the most superficial myofibrils. Micrograph (×11,400 magnification) was obtained from a biopsy of vastus lateralis muscle from a lean, insulin-sensitive subject.
Mitochondrial content in both intermyofibrillar and SS subpopulations were measured in all three groups (Fig. 2) Nondiabetic, insulin-resistant subjects displayed 23.5% lower intermyofibrillar mitochondrial content relative to insulin-sensitive lean subjects. Lower intermyofibrillar mitochondrial content was also present in T2DM (39.7% lower than IS-Lean). When intermyofibrillar mitochondrial content was analyzed by analysis of covariance with VO2max per kilogram fat-free mass as a covariate, differences in mitochondrial content persisted. The respective adjusted means were: IS-Lean, 3.75%; IR-ND, 3.03% (P < 0.05 vs. IS-Lean); and T2DM, 2.51% (P < 0.01 vs. IS-Lean). In contrast, mitochondrial content within the SS space was similar among groups, revealing that specific subpopulations of mitochondria are affected differently in insulin resistance. The mitochondrial size was statistically similar across groups in both the intermyofibrillar and SS subpopulations (Fig. 3).
Fig. 2.
IMF and SS mitochondrial content in skeletal muscle. In the insulin-resistant groups (IR-ND and T2DM), IMF mitochondrial content, reflected by the mitochondrial volume density parameter, was significantly reduced. In contrast, SS mitochondrial content was statistically similar across groups. *, P < 0.01 vs. the IS-Lean group.
Fig. 3.
Mean mitochondrial size and insulin resistance. Mitochondrial size was statistically similar in the insulin-sensitive and insulin-resistant groups. This observation was confirmed in both the IMF and SS subpopulations.
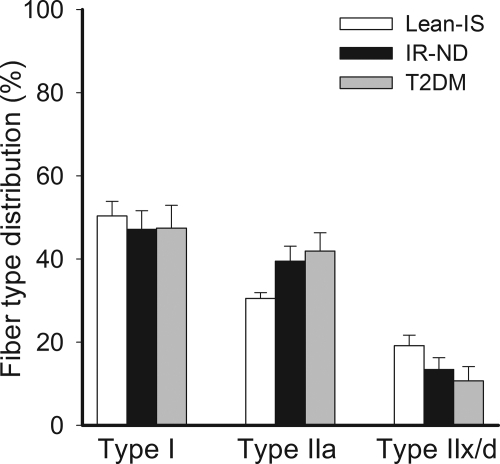
Fiber-type composition
Human skeletal muscle is composed of a mixture of fiber types with types I and IIa fibers having greater oxidative capacity than type IIx/d fibers. For this reason, fiber-type composition was examined. As shown in Fig. 4, proportions of type I or IIa fibers were not diminished in the insulin-resistant groups. In fact, there was a modest trend for a greater proportion of type IIa fibers, although not statistically significant. These results indicate that lower mitochondrial content in insulin resistance does not seem to be due to altered fiber-type distribution.
Fig. 4.
Skeletal muscle fiber-type composition across groups. Type I fibers were equally abundant among groups. Although not statistically significant, type IIa fibers were slightly more common in the insulin-resistant groups.
Physiological correlates
Next, we sought to understand which physiological differences among groups were associated with mitochondria. First, mitochondrial content in each subpopulation was examined for a correlation with the severity of insulin resistance. As shown in Fig. 5 (upper panel), intermyofibrillar mitochondrial content was robustly correlated with insulin resistance, a relationship that was replicated in both genders. In contrast, SS mitochondrial content did not correlate with insulin resistance (Fig. 5, bottom panel). The intermyofibrillar mitochondrial content did not correlate with VO2max in women (P = NS) and was tenuously correlated with VO2max in men (r = 0.20, P = 0.054). The intermyofibrillar mitochondrial content correlated with BMI (r = −0.58, P < 0.01) and waist circumference (r = − 0.46, P < 0.01) but not with percent body fat (r = −0.29, P = NS). It also correlated with plasma fasting FFAs (r = −0.33, P < 0.05), fasting glucose before (r = −0.44, P < 0.01) and after an oral glucose challenge (r = −0.45, P < 0.01) but not with glycosylated hemoglobin (r = −0.22, P = NS). SS mitochondrial content did not correlate with any of those parameters.
Fig. 5.
Relationship between mitochondrial content in the IMF and SS subpopulations vs. systemic insulin sensitivity (Rd). Upper panel, IMF mitochondrial volume density was positively correlated with the severity of systemic insulin resistance. This observation was confirmed in both genders. Lower panel, In contrast to IMF mitochondria, SS mitochondrial content was not correlated with insulin resistance (P = NS).
Mitochondria and patterns of whole-body fuel oxidation
As shown in Table 2, resting energy expenditure normalized for lean body mass was similar across groups. Fasting RQ was higher in the insulin-resistant groups, albeit not quite reaching statistical significance. ΔRQ, however, was significantly lower in the insulin-resistant groups as demonstrated by their lower ΔRQ.
Table 2.
Fuel oxidation and ΔRQ
| IS-Lean | IR-ND | T2DM | |
|---|---|---|---|
| Basal metabolism | |||
| Resting energy expenditure (kcal/d) | 1420 ± 50 | 1770 ± 60a | 1939 ± 128a |
| Resting energy expenditure (kcal/d · kg FFM) | 33.22 ± 1.03 | 33.62 ± 0.85 | 33.39 ± 1.32 |
| RQ | |||
| Before clamp | 0.78 ± 0.01 | 0.80 ± 0.01 | 0.80 ± 0.01 |
| During clamp steady state | 0.93 ± 0.02 | 0.88 ± 0.01b | 0.85 ± 0.01a |
| ΔRQ | 0.15 ± 0.02 | 0.07 ± 0.01a | 0.05 ± 0.01a |
FFM, Fat-free mass.
P < 0.01 vs. IS-Lean.
P < 0.05 vs. IS-Lean.
As shown in Fig. 6 (upper panel), there was a significant correlation between intermyofibrillar mitochondrial content and fasting RQ, suggesting that lower mitochondrial content in skeletal muscle has a relationship with decreased reliance on lipid oxidation during fasting. This observation was replicated in both genders. A relationship between intermyofibrillar mitochondrial content and metabolic inflexibility was observed as well (Fig. 6, bottom panel). This finding was confirmed in both genders. In contrast to intermyofibrillar mitochondria, SS mitochondria were not correlated with fasting RQ or ΔRQ (P = NS, data not shown).
Fig. 6.
Relationship between mitochondrial content and patterns of lipid oxidation. Upper panel, In the IMF subpopulation, mitochondrial volume density was inversely related to whole-body RQ in the fasting state, suggesting that lower mitochondrial content is associated with lower reliance on lipid oxidation. This relationship was evident in both genders. Lower panel, Lower IMF mitochondrial content was also associated with impaired ΔRQ in response to insulin, a relationship that was replicated in men and women.
Discussion
Mitochondria content in insulin resistance
A number of studies have consistently demonstrated lower oxidative capacity in insulin-resistant subjects (5, 7, 8, 14, 23). The etiology of this abnormality is still unknown. In contrast to humans, rodent models of insulin resistance show intact mitochondrial capacity in skeletal muscle (2, 3), suggesting important interspecies differences. Expression of regulators of mitochondrial biogenesis is lower in humans with insulin resistance (12, 13) and therefore impaired mitochondrial biogenesis resulting in decreased mitochondrial content may be one explanation. However, definitive evidence for this postulation has been insufficient. Studies examining surrogate markers of mitochondrial content in insulin-sensitive vs. insulin-resistant subjects have produced contradictory findings. For instance, citrate synthase activity was found to be lower in obesity in some studies (7, 13, 24). Other studies, however, have shown no significant reductions in citrate synthase in obesity (4, 5, 25), T2DM (26), or nondiabetic subjects with a family history of T2DM (16). The reason for these discrepancies among studies is not clear but may be related to methodological limitations. Enzyme activity measurements are sensitive to variations in assay protocols and rely on assumptions of enzyme activity being uniformly proportional to mitochondrial content. In skeletal muscle, mitochondria can be difficult to isolate from the cytoskeleton, and differences in the purity or yields of mitochondrial isolates add variability to measurements. Likewise, mtDNA content has produced conflicting findings. Insulin resistance was associated with less mtDNA in some studies (9, 14) but not in others (15). In summary, surrogate markers of mitochondrial content have not yet produced a conclusive picture of whether mitochondrial content is reduced in insulin resistance. In our study, we sought to resolve this question by directly quantifying mitochondrial content in situ.
The first major finding from our study is the novel observation that mitochondrial content is lower across the continuum of insulin sensitivity and is not limited to T2DM. Furthermore, we describe the novel observation that this phenomenon occurs with subcellular specificity, affecting mostly intermyofibrillar mitochondria, which account for 85–90% of the total mitochondrial mass (22). This observation was explained by neither altered fiber-type composition nor mitochondrial size. Scanning electron microscopy studies have revealed that intermyofibrillar mitochondria are not always individual entities but interconnect as a reticulum (27). Therefore, similar mitochondrial size across groups indicates that mitochondrial content was reduced because of an atrophy of the mitochondrial reticulum. It also reveals that mitochondrial size may not be relevant in relationship to insulin resistance, contrasting with a former report of smaller mitochondria in insulin resistance (5). However, in that study a sedentary lifestyle was not a prerequisite for insulin-sensitive control subjects and aerobic capacity was not controlled for. In the present investigation, we carefully selected only sedentary subjects to avoid confounding effects of well-fit trained individuals among controls. This is an important consideration because mitochondrial size increases with training (18, 28).
The correlation between insulin resistance and mitochondria was strong, but what mediates this relationship is not clear. Diminished insulin action on mitochondrial biogenesis is an imperfect explanation because long-term sustained improvements in insulin sensitivity do not enhance mitochondrial content in insulin resistance (21). In the present study, hyperglycemia emerged as a correlate of mitochondria, a notable finding because high intracellular glucose can promote mitochondrial oxidative stress in susceptible cells. In skeletal muscle cells, however, mitochondria are relatively protected from hyperglycemia because impaired transmembrane glucose uptake is the chief manifestation of insulin resistance in this tissue. When hyperglycemia is significantly reversed by several weeks of insulin therapy, skeletal muscle mitochondrial content and respiration are not enhanced (29). Therefore, the apparent relationships with glycemia were arguably spurious and caused by underlying factors. Obesity might be a determining factor, although there are data in favor and against this possibility. In the present study, mitochondrial content correlated with BMI and waist circumference but not with percent body fat, thus showing inconsistent associations with obesity. Other studies have reported correlations between mitochondria and insulin resistance in the absence of obesity (8, 9), and mitochondrial content does not improve after clinically significant weight loss (21). These observations argue against obesity as a simple explanation. On the other hand, experimental interventions show that nutrient excess can be linked to changes in mitochondria. In human subjects, high-fat feeding down-regulates genes required for oxidative phosphorylation (30), and contemporary studies place mitochondrial oxidative stress induced by lipid overload as a prominent factor in insulin resistance (31). Therefore, in insulin-resistant subjects the underlying factor explaining the relationship with lower mitochondrial content might be intramyocellular lipid overload and attendant mitochondrial adaptations, rather than either whole-body obesity or insulin resistance per se.
SS mitochondria and insulin resistance
Compared with intermyofibrillar mitochondria, SS mitochondria respond differently to stimuli and have different characteristics that may influence fuel metabolism. However, we found that SS mitochondrial content appears to be intact in insulin-resistant subjects. Our data suggest that mitochondrial subpopulations are regulated differently in insulin-resistant subjects, an observation that draws a parallel with differential regulation of mitochondrial subpopulations in diabetic (Zucker diabetic fatty) rats (2). A former study reported SS mitochondrial deficiency in T2DM (14), a conclusion reached in part by demonstration of a thinner SS layer. The SS space thickness may not be the best assessment of mitochondrial content though. It is not a direct measure of mitochondria and the SS space can be influenced by lipid droplets and nuclei occupying it. Instead, we used more rigorous methodology (quantitative stereology) to measure mitochondrial content. Furthermore, in comparison with that study, we studied a considerably greater number of subjects and placed emphasis on selecting only sedentary subjects for controls to diminish the confounding effects of differences in physical activity habits between groups. Each or all of these factors could have contributed to the difference in findings between studies.
The second new finding from this study is that muscle disuse does not seem to be the major factor explaining the association between insulin resistance and mitochondria. The specific down-regulation of intermyofibrillar mitochondria with sparing of the SS subpopulation diverges from expected patterns after muscle use or disuse. In comparison with intermyofibrillar mitochondria, SS mitochondria respond to a greater degree, or earlier in time, to muscle disuse (32, 33) and training (22, 33, 34). In the insulin-resistant groups, the observed pattern was the opposite, arguing against muscle disuse as a major etiological factor. In agreement with this notion, differences in intermyofibrillar mitochondrial content among groups persisted after adjustment for minimal differences in aerobic capacity. On the other hand, it is important to emphasize that these findings do not negate the notion that physical activity is an important co-modulator of mitochondrial content. In fact, we have shown that even in the insulin-resistant state, skeletal muscle mitochondria respond to training (17, 18, 21).
Mitochondria and patterns of fuel oxidation
The third novel finding from this study is that skeletal muscle mitochondrial content is a marker of abnormal patterns of systemic fuel oxidation. Skeletal muscle tissue accounts for up to 40% of total body mass and is an important determinant of whole-body metabolism (35). Skeletal muscle is highly reliant on fatty acid oxidation during fasting conditions but, more importantly, displays considerable plasticity in fuel oxidation, which is best exemplified by rapid switching between carbohydrate and lipid in response to insulin. This plasticity has been termed ΔRQ (36). There has been considerable published evidence for lower fasting lipid oxidation and lower ΔRQ in obesity and T2DM, at both the whole-body level and the tissue level (36–38). This characteristic appears to be intrinsic to skeletal muscle because primary muscle cell cultures from insulin-resistant subjects retain abnormal patterns of whole-body fuel use (1). In the present investigation, we report the novel observation that skeletal muscle mitochondrial content in the intermyofibrillar subpopulation has a strong relationship with altered patterns of fuel metabolism in obesity and T2DM. These observations reveal a new context in which reduced mitochondrial capacity may be linked to insulin resistance. Consequently, an important question should be raised: how could mitochondrial content influence fuel metabolism in insulin-resistant subjects? Mitochondrial content is biologically modulated to meet demands for ATP generation associated with periods of contractile activity. Therefore, mitochondrial content in skeletal muscle exceeds the energetic demands of this tissue in the resting state, and a reduction in mitochondrial content may simply represent a loss of functional reserve rather than a state of mitochondrial insufficiency. Therefore, alternative explanations must be pursued. One potential explanation is that lower mitochondrial density may be a marker of mitochondria with qualitative functional characteristics of lower efficiency for lipid oxidation and more inflexibility in fuel switching. Another hypothesis to consider is that the degree of expansion of the skeletal muscle mitochondrial apparatus may confer properties to muscle that influence fuel use. For instance, expansion of the mitochondrial network endows muscle cells with an architecture that favors more rapid access of fatty acids into the enzymatic machinery of mitochondria. Precedents in the exercise physiology literature support this notion. Exercise training increases mitochondrial content, an adaptation that supports greater ATP production but is paralleled by greater capacity for lipid oxidation (39, 40). Gollnick and Saltin (41) proposed that the amount of mitochondrial protein per cell may be a prime factor supporting increased preference for lipid oxidation. Therefore, although mitochondrial content primarily adapts to ATP production demands, it may have a parallel influence on the propensity of skeletal muscle to oxidize lipid and switch substrates in response to metabolic signals. In support of this idea, our findings demonstrate that lower mitochondrial content in skeletal muscle tracks with altered patterns of fuel oxidation in insulin-resistant individuals.
Acknowledgments
We thank Donna Stolz, Ph.D., and Simon Watkins, Ph.D., for superb technical support at the University of Pittsburgh's Center for Biological Imaging.
This work was supported in part by the University of Pittsburgh General Clinical Research Center (Grant 5 M01RR00056), the Obesity and Nutrition Research Center (Grant P30DK462), and National Institutes of Health Grant 5R01-DK49200-08. Z.R. was a visiting postdoctoral fellow from the Institute of Experimental Endocrinology, Slovak Academy of Sciences, Slovakia.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- FFA
- free fatty acid
- IR-ND
- insulin-resistant, nondiabetic subjects
- IS-Lean
- insulin-sensitive controls
- mtDNA
- mitochondrial DNA
- Rd
- glucose disposal rate
- RQ
- respiratory quotient
- ΔRQ
- metabolic flexibility
- SS
- subsarcolemmal
- T2DM
- type 2 diabetes mellitus
- VO2max
- maximum aerobic capacity.
References
- 1. Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, Smith SR. 2005. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest 115:1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holloway GP, Gurd BJ, Snook LA, Lally J, Bonen A. 2010. Compensatory increases in nuclear PGC1α protein are primarily associated with subsarcolemmal mitochondrial adaptations in ZDF rats. Diabetes 59:819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. 2008. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105:7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simoneau JA, Kelley DE. 1997. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol 83:166–171 [DOI] [PubMed] [Google Scholar]
- 5. Kelley D, He J, Menshikova EV, Ritov VB. 2002. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes mellitus. Diabetes 51:2944–2950 [DOI] [PubMed] [Google Scholar]
- 6. Berggren JR, Boyle KE, Chapman WH, Houmard JA. 2008. Skeletal muscle lipid oxidation and obesity: influence of weight loss and exercise. Am J Physiol Endocrinol Metab 294:E726–E732 [DOI] [PubMed] [Google Scholar]
- 7. Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. 2000. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- 8. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. 2004. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350:664–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ukropcova B, Sereda O, de Jonge L, Bogacka I, Nguyen T, Xie H, Bray GA, Smith SR. 2007. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 56:720–727 [DOI] [PubMed] [Google Scholar]
- 10. Morino K, Petersen KF, Shulman GI. 2006. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55:S9–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lanza IR, Nair KS. 2009. Muscle mitochondrial changes with aging and exercise. Am J Clin Nutr 89:467S–471S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. 2003. Coordinated reduction in genes of oxidative metabolism in humans with insulin resistance and diabetes: potential roles of PGC1 and NRF-1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heilbronn LK, Gan SK, Turner N, Campbell LV, Chisholm DJ. 2007. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab 92:1467–1473 [DOI] [PubMed] [Google Scholar]
- 14. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. 2005. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes 54:8–14 [DOI] [PubMed] [Google Scholar]
- 15. Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. 2006. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes 55:3309–3319 [DOI] [PubMed] [Google Scholar]
- 16. Østergard T, Andersen JL, Nyholm B, Lund S, Nair KS, Saltin B, Schmitz O. 2006. Impact of exercise training on insulin sensitivity, physical fitness, and muscle oxidative capacity in first-degree relatives of type 2 diabetic patients. Am J Physiol Endocrinol Metab 290:E998–E1005 [DOI] [PubMed] [Google Scholar]
- 17. Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. 2007. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56:2142–2147 [DOI] [PubMed] [Google Scholar]
- 18. Toledo FG, Watkins S, Kelley DE. 2006. Changes induced by physical activity and weight loss in the morphology of intermyofibrillar mitochondria in obese men and women. J Clin Endocrinol Metab 91:3224–3227 [DOI] [PubMed] [Google Scholar]
- 19. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A. 1988. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 96:379–394 [DOI] [PubMed] [Google Scholar]
- 20. Weibel ER. 1979. Stereological methods. Practical methods for biological morphometry. London: Academic Press [Google Scholar]
- 21. Toledo FG, Menshikova EV, Azuma K, Radikova Z, Kelley CA, Ritov VB, Kelley DE. 2008. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes 57:987–994 [DOI] [PubMed] [Google Scholar]
- 22. Hoppeler H, Howald H, Conley K, Lindstedt SL, Claassen H, Vock P, Weibel ER. 1985. Endurance training in humans: aerobic capacity and structure of skeletal muscle. J Appl Physiol 59:320–327 [DOI] [PubMed] [Google Scholar]
- 23. Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. 2003. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 300:1140–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holloway GP, Thrush AB, Heigenhauser GJ, Tandon NN, Dyck DJ, Bonen A, Spriet LL. 2007. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab 292:E1782–E1789 [DOI] [PubMed] [Google Scholar]
- 25. Simoneau JA, Veerkamp JH, Turcotte LP, Kelley DE. 1999. Markers of capacity to utilize fatty acids in human skeletal muscle: relation to insulin resistance and obesity and effects of weight loss. FASEB J 13:2051–2060 [DOI] [PubMed] [Google Scholar]
- 26. Mogensen M, Sahlin K, Fernström M, Glintborg D, Vind BF, Beck-Nielsen H, Hojlund K. 2007. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes 56:1592–1599 [DOI] [PubMed] [Google Scholar]
- 27. Ogata T, Yamasaki Y. 1997. Ultra-high-resolution scanning electron microscopy of mitochondria and sarcoplasmic reticulum arrangement in human red, white, and intermediate muscle fibers. Anat Rec 248:214–223 [DOI] [PubMed] [Google Scholar]
- 28. Kiessling KH, Piehl K, Lundquist CG. 1970. Effect of physical training on ultrastructural features in human skeletal muscle. In: Pernow B., Saltin B. eds. New York: Plenum Press; 97–101 [Google Scholar]
- 29. Rabol R, Højberg PM, Almdal T, Boushel R, Haugaard SB, Madsbad S, Dela F. 2009. Effect of hyperglycemia on mitochondrial respiration in type 2 diabetes. J Clin Endocrinol Metab 94:1372–1378 [DOI] [PubMed] [Google Scholar]
- 30. Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. 2005. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54:1926–1933 [DOI] [PubMed] [Google Scholar]
- 31. Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. 2009. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119:573–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. 2007. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 102:1143–1151 [DOI] [PubMed] [Google Scholar]
- 33. Krieger DA, Tate CA, McMillin-Wood J, Booth FW. 1980. Populations of rat skeletal muscle mitochondria after exercise and immobilization. J Appl Physiol 48:23–28 [DOI] [PubMed] [Google Scholar]
- 34. Hoppeler H, Fluck M. 2003. Plasticity of skeletal muscle mitochondria: structure and function. Med Sci Sports Exerc 35:95–104 [DOI] [PubMed] [Google Scholar]
- 35. Zurlo F, Larson K, Bogardus C, Ravussin E. 1990. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest 86:1423–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelley DE, Mandarino LJ. 2000. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 49:677–683 [DOI] [PubMed] [Google Scholar]
- 37. Kelley DE, Goodpaster B, Wing RR, Simoneau JA. 1999. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 38. Kelley DE, Simoneau JA. 1994. Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeukendrup AE, Saris WH, Wagenmakers AJ. 1998. Fat metabolism during exercise: a review—part II: regulation of metabolism and the effects of training. Int J Sports Med 19:293–302 [DOI] [PubMed] [Google Scholar]
- 40. Horowitz JF, Klein S. 2000. Lipid metabolism during endurance exercise. Am J Clin Nutr 72:558S–563S [DOI] [PubMed] [Google Scholar]
- 41. Gollnick PD, Saltin B. 1982. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin Physiol 2:1–12 [DOI] [PubMed] [Google Scholar]