ERα and CYP19 polymorphisms affect the therapeutic outcome of docetaxel in patients with castrationresistant prostate cancer (CRPC) and are associated with CRPC risk.
Abstract
Context:
Reactive estrogen species cause genotoxicity and interfere with docetaxel-mediated tubulin polymerization resulting in shortened survival in men with castrate-resistant prostate cancer (CRPC).
Objective:
We hypothesized that polymorphisms in estrogen synthesis and estrogen targets (i.e., CYP19 and ERα) would be linked to interindividual variation in CRPC risk, docetaxel response, and overall survival in men with CRPC.
Materials and Methods:
Patients with CRPC (n=115) treated with docetaxel, single-agent thalidomide (n=42), or healthy controls (n=289) were genotyped for the CYP19 R264C (rs700519) and the ERα PvuII T>C (rs2234693) and XbaI A>G (rs9340799) polymorphisms.
Results:
Patients carrying two copies of ERα polymorphisms had shorter progression-free survival on docetaxel than other patients (median survival difference ≥3.1 months; P ≤ 0.036). When the analysis was limited to nonobese patients, the relationship between the ERα XbaI A>G polymorphism and PFS improved (median survival difference = 3.5 months; P = 0.0078). The CYP19 R264C variant was related to the duration of survival after docetaxel in patients who were >70 years old (median survival difference =10.6 months; P=0.041). Both ERα polymorphisms were also associated with increases in CRPC risk [P ≤ 0.032; double variants vs. wild-type odds ratio ≥ 2.6], and the association with the ERα PvuII T>C also improved in those men who were <70 years old (P = 0.0073; odds ratio = 3.0).
Conclusions:
This study demonstrates that estrogen-related genetic variation affects docetaxel clinical response and that this relationship is dependent on age and body-type in men with CRPC. Moreover, this study suggests ERα polymorphisms confer risk of developing prostate cancer, especially in men under 70 years of age.
Estrogen disposition might influence docetaxel therapy as estrogen metabolites inhibit docetaxel-mediated tubulin polymerization and covalently adduct docetaxel (1–3). These metabolites are produced by CYP1B1 when intratumoral levels of CYP19 (CYP19A1, aromatase) are increased (3). CYP1B1 is also regulated, in part, by the most abundant estrogen receptor in CRPC (ERα) (3, 4). Therefore, genetic markers ERα and CYP19 may have predictive power to ascertain the effectiveness of docetaxel-based therapy, and this information may be useful to inform therapeutic decisions regarding use of docetaxel in men with castrate-resistant prostate cancer (CRPC).
CYP19 is expressed in several tissues within the male genitourinary system including the testes, and prostate stromal cells; benign prostatic hyperplasia and prostate tumors often overexpress CYP19 (4). Transcript levels of CYP19 also increase with advancing age (5). Although it remains controversial, several studies found a relationship between the CYP19 R264C variant (rs700519) and prostate cancer risk (6).
The ERα has also been related to the prostate response toward estrogens. ERα is expressed in prostate stromal cells where it is thought to stimulate growth factor release and cause epithelial cell proliferation (4). ERα expression has been observed in many prostate tumors and is negatively correlated with prognosis (4). Several investigations have demonstrated prostate cancer risk associations with ERα polymorphisms, especially the PvuII T>C (rs2234693) and XbaI A>G (rs9340799) transitions, although not consistently (7, 8). Because ERα acts as a transcription factor for CYP1B1, polymorphisms in ERα may alter the concentration of reactive estrogens in the prostate (3).
We sought to determine whether polymorphisms in CYP19 and ERα were related to survival characteristics after treatment with docetaxel in patients with CRPC. As a secondary aim, we evaluated whether these polymorphisms were related to CRPC risk.
Materials and Methods
Patients and treatment
Subjects with CRPC (n = 111) were treated with docetaxel on the following clinical trials: estramustine, docetaxel, and thalidomide [n = 20; (9)], ketoconazole and docetaxel [KD; n = 21; (10)], docetaxel and thalidomide [DT; n = 50; (11)], and docetaxel alone [n = 24; (11)]. Some patients participated in two trials (KD and D n = 1; KD and DT n = 1; KD and estramustine, docetaxel, and thalidomide n = 2). The subjects' median age [95% confidence interval (CI)] was 69.2 years (65.0–68.3 years). Patients with CRPC, of similar age [median (95%CI) = 68.4 (65.0–69.6) years], treated with thalidomide alone were used as survival controls and were combined with patients receiving docetaxel therapy in risk assessments [THD; n = 42; (12)]. A cohort of healthy men [median age (95%CI) = 40.0 (39.1–42.0)] served as controls for risk analyses (n = 289). The NCI Institutional Review Board approved all studies and genotyping.
Genotyping
PCR amplification was conducted on germline DNA using Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA) per the manufacturer's instructions using the following primers: ERα (rs2234693 and rs9340799) 5′-ATCCAGGGTTATGTGGCAATGAC-3′, and 5′-CCCTGGCGTCGATTATCTGA-3′; and CYP19 (rs700519) 5′-CGCTAGATGTCTAAACTGAG-3′, and 5′-CATATGTGGCATGGGAATA-3′. Direct sequencing was conducted using the Big Dye Terminator Cycle Sequencing Ready Reaction kit V3.1 on an ABI Prism 3130xl Genetic Analyzer (Applied BioSystems, Foster City, CA). The primer sequences were as follows: ERα (rs2234693 and rs9340799) 5′-GGGTTATGTGGCAATGACGTA-3′, and 5′-GGCGTCGATTATCTGAATTTG-3′; and CYP19 (rs700519) 5′-GATGTCTAAACTGAGTAAAAATAG-3, and 5′- ATGTGGCATGGGAATTACAGT-3′.
Statistical considerations
A comparison was made between CRPC cases and controls according to ERα T>C, and ERα A>G genotypes via a χ2 test. CYP19 CT and TT genotypes were compared with the CYP19 CC genotype a priori using Fisher's Exact test. When genotypes were pooled into two categories a posteriori, the resulting P value was corrected with a Bonferroni adjustment. Homogeneity of genotypes among trials was tested using the exact Fisher-Freeman-Halton test. All risk analyses were restricted to Caucasians due to the low numbers of African-American and Asian patients.
Survival characteristics were estimated by Kaplan–Meier curves, and the associations between genotype and these outcomes were determined using the log-rank test with P values from the permutation distribution of the log-rank statistic in analyses with small group sizes. Cox modeling was conducted to justify the pooling of survival data from four docetaxel trials together; analyses were also stratified by trial as further justification. When genotypes were pooled, a Bonferroni-type adjustment was made to correct for the number of implicit tests performed which led to the selection of the final grouping. All P values are two-tailed.
Results
Genotyping
Genotyping data in men with CRPC from different racial backgrounds are reported in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). No association was found between any ERα or CYP19 alleles and age, Gleason score, or body mass index (BMI) (P > 0.05). The allele frequencies in Caucasian controls were the same as previously reported in older non-Hispanic Caucasians without prostate disease (P ≥ 0.73) for CYP19 (13) and ERα T>C (14); thus, while our control population consists of young men, many of whom will develop CRPC in their lifetime, the allele frequencies are similar to older healthy Caucasian populations.
Genotype related to survival characteristics in docetaxel trials
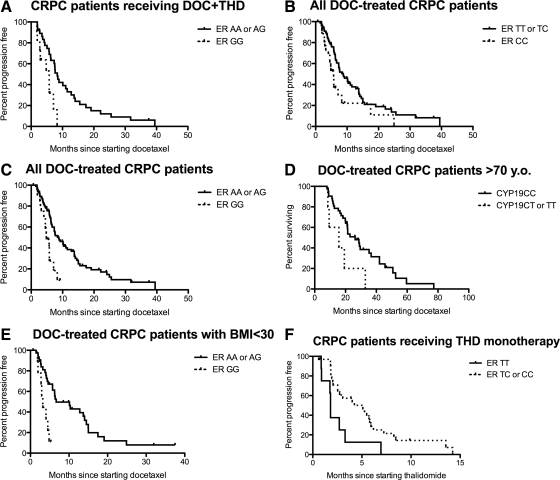
No polymorphism was related to any survival characteristics of the four docetaxel trials considered individually (P > 0.05), with two exceptions. Those carrying CYP19 variants who received the KD combination had a lower median overall survival (OS) following the regimen (9.0 vs. 23.3 months respectively; n = 2 and n = 19 respectively; P = 0.0095); however, this relationship depended on small numbers of patients. Individuals carrying only variants at ERα A>G receiving DT therapy had lower median PFS (P = 0.015; Fig. 1A).
Fig. 1.
Survival characteristics of patients on the studied trials vs. genetic variants in ERα and CYP19. A, PFS of docetaxel plus thalidomide in patients carrying only ERα A>G variants (5.8 months; n = 11) and those carrying at least a single wild-type allele (8.3 months; n = 37, P = 0.015). B, PFS of combined docetaxel-based trials in patients carrying only ERα T>C variants (5.7 months; n = 26) and those carrying at least a single wild-type allele (8.8 months; n = 81; P = 0.036). C, Patients carrying only ERα A>G variants (4.8 months; n = 20) and those carrying at least a single wild-type allele (8.8 months; n = 88; P = 0.0012). D, Survival after docetaxel treatment of combined docetaxel trials in patients who were >70 years of age carrying CYP19 variant alleles (15.7 months; n = 5) and those carrying only wild-type alleles (26.3 months; n = 42; P = 0.041). E, PFS of docetaxel-based trials in individuals who were not obese (BMI < 30) carrying only ERα A>G variant alleles (3.2 months; n = 9) and those carrying at least one copy of a wild-type allele (6.7 months, n = 40; P = 0.0078). F, PFS of thalidomide monotherapy in patients carrying ERα T>C homozygous and heterozygous genotypes (5.3 and 3.1 months, respectively; n = 9 and n = 23, respectively) and those carrying wild-type alleles (1.8 months; n = 8; P = 0.040).
When genotyping data were analyzed in pooled docetaxel-based trials, there was no relationship between any of the ERα or CYP19 alleles and OS after diagnosis, or OS after docetaxel-based therapy (both P > 0.05). No differences in OS after diagnosis, or OS after thalidomide were noted (P > 0.05).
Interestingly, each of the ERα alleles was associated with PFS in pooled docetaxel-based trials. Patients who were double variant for either ERα T>C or ERα A>G had lower median PFS than other individuals (P = 0.036 and P = 0.0012, respectively; Fig. 1, B and C). CYP19 genotype status was not related to PFS (P = 0.11). A Cox-model accounting for individual docetaxel trials resulted in smaller P values than those reported above and did not indicate a genotype effect in any particular trial was driving the association with PFS. Moreover, when the analysis was stratified by trial, this also resulted in smaller P-values.
Because CYP19 expression increases with older age (5), we compared genotypes and survival characteristics only in those men who were ≥70 years of age at the start of therapy. A significant difference in OS after docetaxel treatment was noted based on CYP19 genotype status (P = 0.041; Fig. 1D). Similar analyses of ER variants did not improve the association with docetaxel outcome (P ≥ 0.17).
Because more estrogen is also produced in men with increasing BMI (15), we also evaluated the relationship between CYP19 and ERα alleles in normal to overweight (BMI = 18.5–29.9) and in obese men (BMI ≥ 30). No associations were found among obese patients (n = 46; P ≥ 0.15). However, in patients who were not obese, the ERα A>G polymorphism was related to PFS (P = 0.0078; Fig. 1E). No other relationship was determined between any survival characteristic and genotypes in ERα or CYP19 (P ≥ 0.06).
Genotype related to survival characteristics in the THD trial
Opposite results were observed for those receiving single-agent thalidomide where variant allele carriers survived longer than wild-type allele carriers (P = 0.040; Fig. 1F). Those with CYP19 variants (n = 4) also had a shorter PFS on thalidomide than those carrying wild-type genotypes (n = 33; 1.8 vs. 4.2 months, respectively; P = 0.022). The ERα A>G polymorphism was not related to PFS on the THD trial (P = 0.33). Restrictions based on age or BMI did not improve the relationship between ERα or CYP19 alleles and survival characteristics (P > 0.05).
Genotypes related to CRPC risk
Genotypes among Caucasian patients with CRPC were compared with genotypes among race-matched controls. Genotype distributions among Caucasians did not vary between any of the clinical trials (P ≥ 0.17), thus analyses comparing patients with cancer to controls were conducted without regard to trial. The ERα T>C and ERα A>G transitions were both related to the risk of developing CRPC; individuals carrying variant alleles had a higher risk of CRPC (P = 0.010 and P = 0.032, respectively; Table 1). No significant differences were observed for CYP19 C>T (P = 0.30). The ERα T>C polymorphism is much more frequent in cases than controls in men <70 years of age before undergoing therapy for CRPC (P = 0.0073; Table 1). However, age at diagnosis data were not available in these men. Consideration of other alleles (i.e., CYP19 C>T or ERα T>C) based on age or BMI did not increase the statistical significance of the findings for any of the alleles tested.
Table 1.
Comparison of genotypic frequencies of ER and CYP19 polymorphisms in Caucasian patients diagnosed with CRPC and healthy volunteers (control)
| Polymorphism | Genotype frequencies |
OR (95% CI) | P value | |
|---|---|---|---|---|
| Cases | Controls | |||
| ERα (rs2234693) | ||||
| TT | 25 | 46 | 1.0 (N/A) | 0.010a |
| TC | 75 | 60 | 2.3 (1.3–4.2) | |
| CC | 28 | 20 | 2.6 (1.2–5.5) | |
| ERα (rs2234693) in men <70 yr old | ||||
| TT | 13 | 46 | 1.0 (N/A) | 0.0073a |
| TC | 50 | 60 | 2.9 (1.4–6.1) | |
| CC | 17 | 20 | 3.0 (1.2–7.3) | |
| ERα (rs9340799) | ||||
| AA | 42 | 58 | 1.0 (N/A) | 0.032a |
| AG | 69 | 61 | 1.6 (0.9–2.6) | |
| GG | 18 | 8 | 3.1 (1.2–7.8) | |
| CYP19 (rs700519) | ||||
| CC | 125 | 139 | 1.0 (N/A) | 0.30b |
| CT or TT | 5 | 11 | 0.5 (0.2–1.5) | |
P value determined using a χ2 test.
P value determined using a two-tailed Fisher's exact test.
Discussion
There are several possible explanations for men with CRPC having both increased risk and poor response to docetaxel based on variation in ERα and CYP19. Both genes regulate intraprostatic concentrations of 3,4-QE2, which result in estrogen-purine DNA lesions, thus increasing the likelihood of prostate cancer (3). Genotoxic 3,4-QE2 metabolites also oppose the therapeutic effects of docetaxel by destabilizing tubulin polymerization reactions and covalently binding docetaxel (2). Moreover, CYP1B1 siRNA did not alter cytotoxicity in MCF-7 cells but did decrease cell survival when these cells were treated with docetaxel (16). We have also verified that genetic variation in CYP1B1, responsible for converting estradiol into 3,4-QE2, is related to the efficacy of docetaxel therapy (1, 2). Therefore, variation in factors that regulate the intraprostatic concentration of 3,4-QE2 may be related to docetaxel therapy outcome and to CRPC risk. Other possibilities may include: 1) increased estrogen concentrations promote survival in the presence of docetaxel, but not thalidomide; 2) because docetaxel induces apoptosis, in part, by inactivating the antiapoptotic protein Bcl-2 (17), estrogens may decrease the sensitivity of cells toward docetaxel by increasing levels of Bcl-2 through ERα-related pathways (18, 19); and 3) the androgen/estrogen balance is different in men with certain CYP19 or ERα variants (20), affecting docetaxel treatment but not thalidomide treatment. It should be noted that the above pathways are not mutually exclusive with the reactive estrogen hypothesis, and environmental factors (e.g., environmental carcinogens and xenobiotics with estrogen agonist effects) may also play a role.
Consideration of both age and BMI improved the association between CYP19 and the ERα A>G variant. Both advanced age and increased BMI have been related to increased concentrations of estrogens (15), and our results suggest that genetic variation in estrogen disposition affects the treatment outcome of individuals with CRPC differently based on age and BMI. While it could be expected that polymorphic variation in these genes would be more frequent in CRPC cases based on age and BMI covariates, we did not observe this interaction. Rather, variation in ERα was an independent risk factor, affecting all patients in this study.
Because this study is limited by a relatively small sample size and was not able to ascertain serum and intraprostatic estrogen concentrations, it should be considered a pilot experience warranting future confirmation. Nonetheless, we provide further validation that estrogen may significantly interact with docetaxel-based therapy (1, 2). It is hoped that such data will provide a rationale for clinical trials that ascertain estrogen biosynthesis as a guide for pharmacotherapy with docetaxel.
Supplementary Material
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Bethesda, Maryland. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government.
www.ClinicalTrials.gov Identifiers: NCT00083005, NCT00020046, NCT00039221, NCT00001446.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- BMI
- Body mass index
- CI
- confidence interval
- CRPC
- castrate-resistant prostate cancer
- DT
- docetaxel and thalidomide
- ER
- estrogen receptor
- KD
- ketoconazole and docetaxel
- OS
- overall survival.
References
- 1. Pastina I, Giovannetti E, Chioni A, Sissung TM, Crea F, Orlandini C, Price DK, Cianci C, Figg WD, Ricci S, Danesi R. 2010. Cytochrome 450 1B1 (CYP1B1) polymorphisms associated with response to docetaxel in Castration-Resistant Prostate Cancer (CRPC) patients. BMC Cancer 10:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sissung TM, Danesi R, Price DK, Steinberg SM, de Wit R, Zahid M, Gaikwad N, Cavalieri E, Dahut WL, Sackett DL, Figg WD, Sparreboom A. 2008. Association of the CYP1B1*3 allele with survival in patients with prostate cancer receiving docetaxel. Mol Cancer Ther 7:19–26 [DOI] [PubMed] [Google Scholar]
- 3. Sissung TM, Price DK, Sparreboom A, Figg WD. 2006. Pharmacogenetics and regulation of human cytochrome P450 1B1: implications in hormone-mediated tumor metabolism and a novel target for therapeutic intervention. Mol Cancer Res 4:135–150 [DOI] [PubMed] [Google Scholar]
- 4. Williams GP. 2010. The role of oestrogen in the pathogenesis of obesity, type 2 diabetes, breast cancer and prostate disease. Eur J Cancer Prev 19:256–271 [DOI] [PubMed] [Google Scholar]
- 5. Ellem SJ, Risbridger GP. 2007. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer 7:621–627 [DOI] [PubMed] [Google Scholar]
- 6. Travis RC, Schumacher F, Hirschhorn JN, Kraft P, Allen NE, Albanes D, Berglund G, Berndt SI, Boeing H, Bueno-de-Mesquita HB, Calle EE, Chanock S, Dunning AM, Hayes R, Feigelson HS, Gaziano JM, Giovannucci E, Haiman CA, Henderson BE, Kaaks R, Kolonel LN, Ma J, Rodriguez L, Riboli E, Stampfer M, Stram DO, Thun MJ, Tjønneland A, Trichopoulos D, Vineis P, Virtamo J, Le Marchand L, Hunter DJ. 2009. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev 18:2734–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cunningham JM, Hebbring SJ, McDonnell SK, Cicek MS, Christensen GB, Wang L, Jacobsen SJ, Cerhan JR, Blute ML, Schaid DJ, Thibodeau SN. 2007. Evaluation of genetic variations in the androgen and estrogen metabolic pathways as risk factors for sporadic and familial prostate cancer. Cancer Epidemiol Biomarkers Prev 16:969–978 [DOI] [PubMed] [Google Scholar]
- 8. Nicolaiew N, Cancel-Tassin G, Azzouzi AR, Grand BL, Mangin P, Cormier L, Fournier G, Giordanella JP, Pouchard M, Escary JL, Valeri A, Cussenot O. 2009. Association between estrogen and androgen receptor genes and prostate cancer risk. Eur J Endocrinol 160:101–106 [DOI] [PubMed] [Google Scholar]
- 9. Figg WD, Li H, Sissung T, Retter A, Wu S, Gulley JL, Arlen P, Wright JJ, Parnes H, Fedenko K, Latham L, Steinberg SM, Jones E, Chen C, Dahut W. 2007. Pre-clinical and clinical evaluation of estramustine, docetaxel and thalidomide combination in androgen-independent prostate cancer. BJU Int 99:1047–1055 [DOI] [PubMed] [Google Scholar]
- 10. Figg WD, Liu M, Acharya MR, Gulley JL, Arlen PM, Lewis HL, Parnes CC, Jones E, Dahut W. 2003. A phase I trial of high dose ketoconazole plus weekly docetaxel in metastatic androgen independent prostate cacner. Proc Am Soc Clin Oncol 22:1731 (Abstract) [Google Scholar]
- 11. Dahut WL, Gulley JL, Arlen PM, Liu Y, Fedenko KM, Steinberg SM, Wright JJ, Parnes H, Chen CC, Jones E, Parker CE, Linehan WM, Figg WD. 2004. Randomized phase II trial of docetaxel plus thalidomide in androgen-independent prostate cancer. J Clin Oncol 22:2532–2539 [DOI] [PubMed] [Google Scholar]
- 12. Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, Jones E, Premkumar A, Linehan WM, Floeter MK, Chen CC, Dixon S, Kohler DR, Krüger EA, Gubish E, Pluda JM, Reed E. 2001. A randomized phase II trial of thalidomide, an angiogenesis inhibitor, in patients with androgen-independent prostate cancer. Clin Cancer Res 7:1888–1893 [PubMed] [Google Scholar]
- 13. Beuten J, Gelfond JA, Franke JL, Weldon KS, Crandall AC, Johnson-Pais TL, Thompson IM, Leach RJ. 2009. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 18:1869–1880 [DOI] [PubMed] [Google Scholar]
- 14. Low YL, Taylor JI, Grace PB, Mulligan AA, Welch AA, Scollen S, Dunning AM, Luben RN, Khaw KT, Day NE, Wareham NJ, Bingham SA. 2006. Phytoestrogen exposure, polymorphisms in COMT, CYP19, ESR1, and SHBG genes, and their associations with prostate cancer risk. Nutr Cancer 56:31–39 [DOI] [PubMed] [Google Scholar]
- 15. Jankowska EA, Rogucka E, Medraœ M, Welon Z. 2000. Relationships between age-related changes of sex steroids, obesity and body fat distribution among healthy Polish males. Med Sci Monit 6:1159–1164 [PubMed] [Google Scholar]
- 16. Martinez VG, O'Connor R, Liang Y, Clynes M. 2008. CYP1B1 expression is induced by docetaxel: effect on cell viability and drug resistance. Br J Cancer 98:564–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haldar S, Basu A, Croce CM. 1997. Bcl2 is the guardian of microtubule integrity. Cancer Res 57:229–233 [PubMed] [Google Scholar]
- 18. Friedman AE. 2007. Can a single model explain both breast cancer and prostate cancer? Theor Biol Med Model 4:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kandouz M, Siromachkova M, Jacob D, Chretien Marquet B, Therwath A, Gompel A. 1996. Antagonism between estradiol and progestin on Bcl-2 expression in breast-cancer cells. Int J Cancer 68:120–125 [DOI] [PubMed] [Google Scholar]
- 20. Henderson BE, Feigelson HS. 2000. Hormonal carcinogenesis. Carcinogenesis 21:427–433 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.