Abstract
Objective
To study the potential efficacy and tolerability of a natural multiherbal formula (Immumax) containing Echinacea extract 120 mg, garlic powder 100 mg, Nigella sativa oil 200 mg, and Panax ginseng extract 50 mg plus vitamin C 50 mg and elemental zinc 7.5 mg in the treatment of patients suffering from the common cold.
Design and setting
The study was conducted in a prospective, double-blind, randomized, controlled study design in an outpatient setting.
Patients and methods
Sixty-two eligible patients with symptoms of the common cold were randomized to either Immumax or placebo treatment groups for the duration of their symptoms or a maximum of 14 days. Resolution rates were estimated using Kaplan–Meier analysis, and resolution profiles were compared between groups using the log-rank test. The mean percentage change in total symptom severity scores at days 4 and 8 from baseline were compared between the two groups by one-way analysis of variance (ANOVA).
Results
The median (interquartile range) time to resolution of all symptoms was 8 (5–9) days in the placebo group and 4 (3–6) days in the Immumax group. The results of the log-rank test indicate that symptoms resolved significantly faster in the Immumax group than in the placebo group (P < 0.001). The mean percentage reduction in total symptom severity scores from baseline at days 4 and 8 was significantly greater in the Immumax group than in the placebo group by one-way ANOVA (P < 0.01).
Conclusion
We can conclude from our study that Immumax is helpful in reducing the duration and severity of common cold symptoms.
Keywords: Immumax, common cold, multiherbal
Introduction
The common cold is one of the most prevalent acute illnesses worldwide. It is implicated in about 40% of time lost from employment and 30% of time lost from education.1 Most adults contract two to four colds per year, whereas children can have as many as 10 colds per year, producing substantial expenditure for physician office visits and over-the-counter cold and cough remedies.1,2 The infection is self-limiting. It usually resolves within 7 days, but many colds persist for up to 3 weeks and are due to various viruses. Although it is known that rhinovirus infections cause 10%–40% of colds,1 with coronavirus, parainfluenza virus, adenovirus, echovirus, and coxsackie virus accounting for the remainder of cases,3,4 these viruses produce clinically indistinguishable disease,5,6 making specific viral diagnosis difficult. Available remedies act only to alleviate the cold symptoms (sneezing, nasal stuffiness and discharge, sore or scratchy throat, cough, hoarseness, headache, fever, and myalgia) and have no true therapeutic benefit toward eliminating the viral challenge.2,7,8
Echinacea, a member of the Compositae family, is a herb widely used to treat and prevent common illnesses,9 as it has been shown to have immunostimulatory properties.10 Three out of the nine species in this family are of medicinal interest (Echinacea angustifolia, E. pallida, and E. purpurea). They are commonly used to treat viral upper respiratory tract infections.11 Echinacea causes an increase in numbers of circulating white blood cells, activation of phagocytosis by human granulocytes, and elevation of body temperature,12 resulting primarily from the aerial portion of E. purpurea13 and the root portion of E. pallida.13 Previous research suggests that Echinacea may be most effective at reducing the severity and duration of the common cold when taken early in the illness14,15 but has little to no preventive benefit.16 A review of five randomized, clinical trials investigating the immunomodulatory activity of Echinacea concluded that Echinacea may be an efficacious immune stimulator.17
Garlic (Allium sativum) is one of the oldest medicinal plants used by different cultures. The oldest reports of healthpromoting properties of garlic date back to the16th century BC, when in the Ebers Papyrus from Egypt over 20 ailments were purported to be efficiently cured by garlic.18 Garlic stimulates the immune system and acts as a natural antibiotic not harmful to friendly bacterial flora. Many laboratory studies have confirmed the antibacterial, antifungal, antivirus, immunostimulating, and antioxidant properties of garlic.19–23 In 1990, the US National Cancer Institute initiated the Designer Food Program to determine which foods played an important role in cancer prevention; they concluded that garlic may be the most potent food with cancer-preventive properties.24
Nigella sativa is the black seed referred to by the prophet Mohammed as having healing powers. It is also identified as the curative black cumin in the Holy Bible and is described as the Melanthion of Hippocrates and Discroides and as the Gith of Pliny.25
The effect of N. sativa on immune responses was evaluated in human volunteers. The results showed that black seed enhanced by 55% the ratio between helper T cells (CD4) and the suppressor T cells (CD8), and a 30% average enhancement of the natural killer (NK) cell activity.26 N. sativa has also established efficacy against several species of pathogenic bacteria (Staphylococcus aureus, Pseudomonas aeruginosa) and pathogenic yeast (Candida albicans).27
Extracts of American ginseng (Panax quinquefolium) have been shown to have immunomodulatory effects.28–34 These extracts have been shown to enhance immune responses such as immunoglobulin production by lymphocytes and natural immune responses by peritoneal exudate macrophages.28 They have also been found to enhance anticomplementary and reticuloendothelial system activities,29 enhance macrophage Fc receptor expression,30 increase the phagocytosis index along with phagocytosis fraction,31 and induce messenger RNA expression of interleukin-2 (IL-2), interferon-gamma (IFN-γ), interleukin-1, and granulocyte-macrophage colony-stimulating factor as well as lymphokine-activated killer cells and CD8+ cells.32 In addition, these extracts appear to stimulate cell-mediated immune response and NK cell cytotoxicity,33 as well as to have cytotoxic effects on a wide range of tumor cell lines without major histocompatibility complex restriction.34 Ginseng extract was found to effectively prevent acute respiratory illness due to influenza and respiratory syncytial virus by 89% in a clinical trial involving elderly people living in institutions.35
Vitamin C, (ascorbic acid), is a water-soluble vitamin found in fruit and vegetables, particularly citrus fruit. It is necessary for iron absorption, wound healing, and collagen formation.36 Vitamin C is also recognized as being important to the successful production of neurotransmitters and improvement of glucose metabolism; its deficiency results in the neurological disease of scurvy.37 Vitamin C’s association with immune strengthening is derived from its ability to enhance the function of the immune system, including antimicrobial and NK cell activities, macrophages, lymphocyte proliferation, chemotaxis, and delayed-type hypersensitivity.38
Zinc salts have been found to inhibit rhinovirus replication in vitro at concentrations of <0.1 mmol/L,39 possibly by interfering with rhinovirus protein cleavage.38 Alternatively, it has been suggested that zinc salts may protect plasma membranes against lysis by cytotoxic agents such as microbial toxins and complement.40 The proposed protective mechanism is either via immunomodulation41,42 or via the binding of zinc ions to rhinovirus surface canyons, thus inhibiting viral interactions with intercellular adhesion molecule-1 (ICAM-1), the site of rhinovirus binding to cells.43 Because ICAM-1 is also the binding site for leukocyte function associated antigen-1 (LFA-1), the block of LFA-1/ICAM-1 binding has been postulated to possibly suppress inflammation.43 Several randomized, controlled clinical studies showed a beneficial effect of using zinc for treating the common cold, particularly when zinc is started within the first 24 hours of onset of symptoms.44–47
Immumax in management of common cold Immumax, a product of Beovita-Safe Pharma, an Egyptian– German pharmaceutical company, is a combination of natural herbal extracts, including Echinacea extract 120 mg, garlic powder 100 mg, Nigella sativa oil 200 mg, and Panax ginseng extract 50 mg plus vitamin C 50 mg and elemental zinc 7.5 mg.
Objective
The multiple immunomodulatory activities at different levels and the proven in vitro antiviral activities have encouraged us to study the potential efficacy and tolerability of this multi-ingredient formula in the treatment of human patients suffering from the common cold.
Design and setting
The study was conducted in a prospective, double-blind, randomized, controlled manner in an outpatient setting.
Patients and methods
To detect a difference of 3 days between the mean duration of symptoms in the two treatment groups with a standard deviation of 4 days, given a two-sided P value of 0.05 and an approximate power of 80%, we calculated the sample size for each group to be 28 patients.
Sixty-two consecutive patients presenting to two outpatient clinics in Alexandria, Egypt, suffering from common cold symptoms and meeting the eligibility criteria (stated in Inclusion criteria) in the period between 5 September 2009 and 5 March 2010 were recruited to the study. The local research ethical committee approved the study protocol, and all participants signed informed consents at the time of enrollment.
Inclusion criteria
Patients were included if they had had cold symptoms for 36 hours or less. Patients must have had at least two of the following 10 symptoms: cough, headache, hoarseness, muscle aches, nasal discharge, nasal congestion, scratchy throat, sore throat, sneezing, or an oral temperature >37.7°C.
Exclusion criteria
Patients were excluded if they were pregnant; had a known immune deficiency, cancer, severe liver/renal dysfunction, or critical illness; or had had symptoms of the common cold for more than 36 hours.
Interventions
Immumax capsules and identical placebo capsules containing beeswax were packed and coded by a research assistant blinded to the study participants. Patients fulfilling the inclusion/exclusion criteria were randomly divided into two treatment groups: an experimental group who started treatment with Immumax at a dose of one capsule twice daily until recovery of symptoms or a maximum of 14 days, and a control group who took one placebo capsule twice daily with the same regimen. Randomization was done using a software-generated block randomization technique, and patients and investigators were blinded to the allocated drug. The randomization list and the drug codes were locked in sealed envelopes until the end of the follow-up period, final assessment, and statistical analysis. Each patient was given a similar pack of 30 capsules and was asked to swallow one capsule twice daily for as long as he/she had cold symptoms. The first capsule was administered just after enrollment at the clinic to assess initial tolerability. Participants were asked to take no other cold preparations during the study period apart from acetaminophen for symptomatic relief of pain or fever on an as-needed basis. Oral thermometers were given to the patients at the time of enrollment. All patients were asked to revisit at day 1 after the start of treatment and within 1 day of noting that their cold symptoms had resolved. At this visit, they returned unused capsules so that adherence to the protocol could be checked through capsule counts and the treating physician could confirm that cold symptoms had resolved.
Patients were asked to complete a daily record documenting the severity of symptoms and the medications taken throughout the duration of their cold or for as long as 14 days.
Every day, patients graded each symptom as 0 for none, 1 for mild, 2 for moderate, or 3 for severe. Total symptom scores were calculated by summing the scores of all symptoms for each patient each day. Cold resolution was defined as resolution of all symptoms (a total symptom score of 0) or resolution of all but one mild symptom (a total symptom score of 1).
Statistical analysis
The time to cold resolution was calculated as the number of days from study entry and summarized as median (interquartile range [IQR]) for each treatment group. Resolution rates were estimated using the Kaplan–Meier method, and resolution profiles were compared between groups using the log-rank test. The mean percentage change in total symptom severity scores at days 4 and 8 from baseline were compared between the two groups by one-way analysis of variance (ANOVA). Chi-square test (or Fisher’s exact test) was used to analyze associations between the side effects and assigned groups. Patients were considered adherent if they took an average of two capsules per day for the first 4 days of the study (eight capsules) and if they took no antibiotic agents.
Results
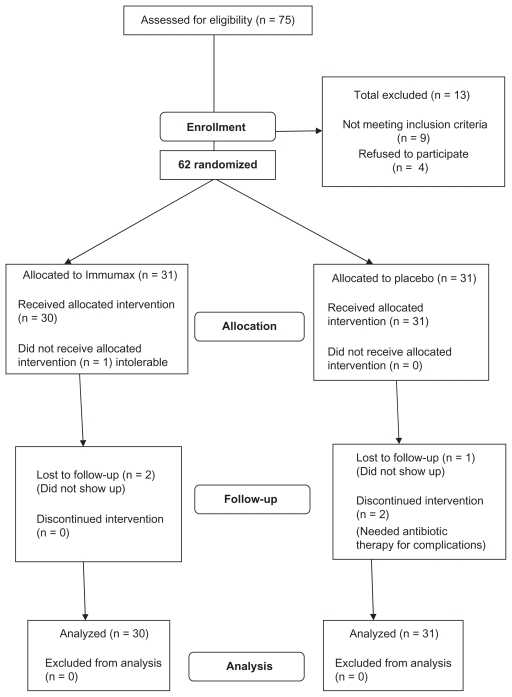
Seventy-five patients presenting with common cold symptoms were assessed for eligibility criteria. Nine of them were excluded, as they did not fulfill the eligibility criteria, whereas another four refused to sign the informed consent. Sixty-two patients were randomized to two equal groups: 31 patients were assigned to the Immumax group, and the remaining 31 were assigned to the placebo group. One patient in the Immumax group withdrew from the study on the first day because he could not tolerate the capsules (see Figure 1). All other patients, as directly observed by the study nurse, indicated that they had good tolerance of the first dose.
Figure 1.
Flowchart of patients.
Baseline characteristics of the two groups are given in Table 1. The incidence and severity of individual symptoms at baseline, as well as other demographic characteristics, were almost similar in the two groups.
Table 1.
Baseline characteristics of the 62 randomized patients
| Variable | Placebo | Immumax |
|---|---|---|
| Number randomized | 31 | 31 |
| Age mean (SD) | 38.6 (9.4) | 37.9 (7.5) |
| Sex (male/female) | 19/12 | 17/14 |
| Smokers | 5 | 4 |
| History of allergy | 12 | 14 |
| Baseline symptom scores: | ||
| Mean (±SD) | 8.6 (±3.1) | 8.1 (±3.6) |
| Median | 8 | 8 |
Abbreviation: SD, standard deviation.
Seven patients (five in the placebo group and two in the Immumax group) had colds that were not reported to resolve during the follow-up period of the study (censored). Two of these patients (both were placebo recipients) completed the 14 days of the study with remaining symptoms. Another two patients of the placebo group reported that their conditions had worsened and needed to be treated with antibiotics for lower respiratory tract bacterial infection. The remaining three of the seven censored patients were lost to follow-up, two from the Immumax group and one from the placebotreated group.
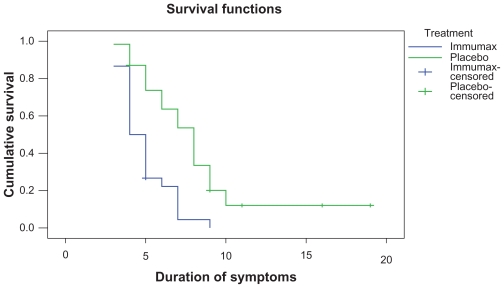
We used Kaplan–Meier survival analysis to estimate the percentage of patients whose colds resolved (a total symptom score of ≤1) on each day of the study (Figure 2).
Figure 2.
Kaplan–Meier curve for the duration of colds in 61 patients. The blue line (lower curve) represents Immumax, and the green line represents the placebo group.
The median (IQR) time to resolution of all symptoms was 8 (5–9) days in the placebo group and 4 (3–6) days in the Immumax group. The results of the log-rank test indicate that symptoms resolved significantly faster in the Immumax group than in the placebo group (P < 0.001). There was no significant difference in compliance between groups during the first 4 days of therapy (P < 0.05). The mean percentage reduction in total symptom severity scores from baseline at days 4 and 8 was significantly greater in the Immumax group than in the placebo group by one-way ANOVA (Table 2).
Table 2.
Comparison of mean percentage reduction in total symptom severity scores
| Variable | Group | N | Mean | 95% CI |
F | P | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| Percentage reduction at day 4 | Immumax | 30 | 56.79 | 52.26 | 61.32 | 8.45 | 0.005 |
| Placebo | 31 | 47.99 | 43.77 | 52.21 | |||
| Percentage reduction at day 8 | Immumax | 30 | 95.07 | 92.96 | 97.17 | 15.83 | 0.0002 |
| Placebo | 31 | 86.40 | 82.52 | 90.27 | |||
Abbreviation: CI, confidence interval.
At the end of the study, eight (39%) of the placebo recipients and 18 (60%) of the Immumax recipients reported that the study medication had helped improve their cold symptoms (P = 0.01). The frequency of reported adverse effects, including nausea, vomiting, abdominal pain, diarrhea, fatigue, and dizziness, did not differ significantly between the two groups.
Discussion
To our knowledge, this is the first study to test the effect of this multiherbal preparation with vitamin C and zinc (already consumed as a dietary supplement) on the duration and severity of common cold symptoms. As we have indicated in our introduction, a plethora of data in the literature, either basic or clinical studies, addresses the immunostimulatory and antiviral activities for each of the components.10–47 We opted to conduct this simple pilot study as a pragmatic hypothesis rather than as a confirmatory or explanatory study.
In our opinion, alternative medicine is in need of much translational research and clinical trials on human subjects to be performed and published in scientific literature, especially for those products that are already being consumed as over-the-counter dietary supplements. The authors admit that there are many limitations in this study, including its small size, the short follow-up period, the dependence mainly on symptoms, and subjective scoring as outcome measures without correlating findings with more objective laboratory data. These limitations can be explained by the perception that studies on alternative medicine, due to lack of patent protections, unlike those on patented new chemical entities funded from the pharmaceutical industry, usually suffer financial and logistic constraints.
Conclusion
We can conclude from our study that Immumax is helpful in reducing the duration and severity of common cold symptoms. More confirmatory and explanatory randomized studies are needed to confirm this.
Acknowledgment
We acknowledge sincerely the help and support of Dr Medhat Kassem and Dr Abdullah Abbass from Beovita- Safe Pharma in the preparation and coding of the drug packs.
Footnotes
Disclosure
Beovita-Safe Pharma freely supplied us with the tested drug and placebo.
References
- 1.Kirkpatrick GL. The common cold. Prim Care. 1996;23:657–675. doi: 10.1016/S0095-4543(05)70355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turner RB. Epidemiology, pathogenesis, and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78:531–539. doi: 10.1016/S1081-1206(10)63213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel JP. Viral upper respiratory infections. Semin Respir Infect. 1995;10:3–13. [PubMed] [Google Scholar]
- 4.Lowenstein SR, Parrino TA. Management of the common cold. Adv Intern Med. 1987;32:207–233. [PubMed] [Google Scholar]
- 5.Tyrrell DA, Cohen S, Schlarb JE. Signs and symptoms in common colds. Epidemiol Infect. 1993;111:143–156. doi: 10.1017/s0950268800056764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gwaltney JM., Jr Rhinovirus infection of the normal human airway. Am J Respir Crit Care Med. 1995;152(2):S36–S39. doi: 10.1164/ajrccm/152.4_Pt_2.S36. [DOI] [PubMed] [Google Scholar]
- 7.Lorber B. The common cold. J Gen Intern Med. 1996;11:229–236. doi: 10.1007/BF02642480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossad SB. Treatment of the common cold. BMJ. 1998;317:33–36. doi: 10.1136/bmj.317.7150.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chichon PG. Herbs and the common cold. Adv Nurse Pract. 2000;8:31–32. [PubMed] [Google Scholar]
- 10.Pepping J. Echinacea. Am J Health Syst Pharm. 1999;56:121–122. doi: 10.1093/ajhp/56.2.121. [DOI] [PubMed] [Google Scholar]
- 11.Giles JT, Palat CT, 3rd, Chien SH, Chang ZG, Kennedy DT. Evaluation of Echinacea for treatment of the common cold. Pharmacotherapy. 2000;20:690–697. doi: 10.1592/phco.20.7.690.35173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumenthal M, Riggins C. Popular Herbs in the US Market: Therapeutic Monographs. Austin, Texas: American Botanical Council; 1997. pp. 1–68. [Google Scholar]
- 13.Blumenthal M. The Complete German Commission E Monographs. Boston, Mass: Integrative Medicine Communications; 1998. [Google Scholar]
- 14.Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schäfer M. Echinagard treatment shortens the course of the common cold: a double-blind, placebo-controlled clinical trial. Eur J Clin Res. 1997;9:261–268. [Google Scholar]
- 15.Schulten B, Bulitta M, Ballering-Bruhl B, Koster U, Schafer M. Efficacy of Echinacea purpurea in patients with a common cold: a placebocontrolled, randomised, double-blind clinical trial. Arzneimittelforschung. 2001;51:563–568. doi: 10.1055/s-0031-1300080. [DOI] [PubMed] [Google Scholar]
- 16.Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections: a double-blind, placebo-controlled, randomized trial. Arch Fam Med. 1998;7:541–545. doi: 10.1001/archfami.7.6.541. [DOI] [PubMed] [Google Scholar]
- 17.Melchart D, Linde K, Worku F, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. J Altern Complement Med. 1995;1:145–160. doi: 10.1089/acm.1995.1.145. [DOI] [PubMed] [Google Scholar]
- 18.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 19.Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994;60:17–20. doi: 10.1055/s-2006-959522. [DOI] [PubMed] [Google Scholar]
- 20.O’Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol. 2000;66:2269–2273. doi: 10.1128/aem.66.5.2269-2273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsao S, Yin M. In vitro activity of garlic oil and four diallyl sulphides against antibiotic-resistant Pseudomonas aeruginosa and Klebsiella pneumoniae. J Antimicrob Chemother. 2001;47:665–670. doi: 10.1093/jac/47.5.665. [DOI] [PubMed] [Google Scholar]
- 23.Corzo-Martinez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol. 2007;18:609–625. [Google Scholar]
- 24.Caragay AB. Cancer-preventive foods and ingredients. Food Technol. 1992;4:65–68. [Google Scholar]
- 25.Attar-ur-Rahman, Malik S, Cunheng H, Clardy J. Isolation and structure of determination of nigellicine, a novel alkaloid from the seeds ofNigella sativa. Tetrahedron Lett. 1985;26:2759–2762. [Google Scholar]
- 26.E1-Kadi, Kandil O. The 1st International Conference on Scientific Miracles of Quran and Sunnah; Islamabad, Pakistan. 1987. [Google Scholar]
- 27.Hanafy MSM, Hatem ME. Studies on the antimicrobial activity of Nigella sativa seed (black cumin) J Ethnopharmacol. 1991;34:275–278. doi: 10.1016/0378-8741(91)90047-h. [DOI] [PubMed] [Google Scholar]
- 28.Wang M, Guilbert LJ, Ling L, Li J, Wu Y, Xu S, et al. Immunomodulating activity of CVT-E002, a proprietary extract from North American ginseng (Panax quinquefolium) J Pharm Pharmacol. 2001;53:1515–1523. doi: 10.1211/0022357011777882. [DOI] [PubMed] [Google Scholar]
- 29.Tomoda M, Hirabayashi K, Shimizu N, Gonda R, Ohara N, Takada K. Characterization of two novel polysaccharides having immunological activities from the root of Panax ginseng. Biol Pharm Bull. 1993;16:1087–1090. doi: 10.1248/bpb.16.1087. [DOI] [PubMed] [Google Scholar]
- 30.Shin KS, Kiyohara H, Matsumo T, Yamada H. Rhamnogalactouronan II from the leaves of Panax ginseng C.A. Meyer as macophage Fcreceptor expression enhancing polysaccharide. Carbohydr Res. 1997;300:239–249. doi: 10.1016/s0008-6215(97)00055-4. [DOI] [PubMed] [Google Scholar]
- 31.Scaglione F, Ferrara F, Dugnani S, Falchi M, Santoro G, Fraschini F. Immunomodulatory effects of two extracts of Panax ginseng C.A. Meyer. Drugs Exp Clin Res. 1990;16:537–542. [PubMed] [Google Scholar]
- 32.Kim JY, Germolec DR, Luster MI. Panax ginseng as a potential immunomodulator: studies in mice. Immunopharmacol Immunotoxicol. 1990;12:257–276. doi: 10.3109/08923979009019672. [DOI] [PubMed] [Google Scholar]
- 33.Kim KH, Lee YS, Jung IS, Park SY, Chung HY, Lee IR, et al. Acidic polysaccharide from Panax ginseng, ginsan induces Th1 cell and macrophage cytokines and generates LAK cells in synergy with rIL-2. Plant Med. 1998;64:110–115. doi: 10.1055/s-2006-957385. [DOI] [PubMed] [Google Scholar]
- 34.Lee YS, Chung IS, Lee IR, Kim KH, Hong WS, Yun YS. Activation of multiple effector pathways of immune system by the antineoplastic immunostimulator acidic polysaccharide ginseng isolated fromPanax ginseng. Anticancer Res. 1997;17:323–331. [PubMed] [Google Scholar]
- 35.McElhaney JE, Gravenstein S, Cole S, et al. A placebo-controlled trial of a proprietary extract of North American ginseng (CVT-E002) to prevent acute respiratory illness in institutionalized older adults. J Am Geriatr Soc. 2004;52:13–19. doi: 10.1111/j.1532-5415.2004.52004.x. [DOI] [PubMed] [Google Scholar]
- 36.Natural Standard. Vitamin C. 2006. [Accessed 2008 Feb 15]. Available from: http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-vitaminc.html.
- 37.Harvard Medical School. Vitamin C and your health: C for crucial, C for controversial. Harvard Men’s Health Watch. 2006;10(11):1–5. [PubMed] [Google Scholar]
- 38.Wintergerst ES, Maggini S, Hornig DH. Immune-enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. doi: 10.1159/000090495. [DOI] [PubMed] [Google Scholar]
- 39.Korant BD, Kauer JE, Butterworth BE. Zinc ions inhibit replication of rhinoviruses. Nature (Lond) 1974;248:588–590. doi: 10.1038/248588a0. [DOI] [PubMed] [Google Scholar]
- 40.Korant BD, Butterworth BE. Inhibition by zinc of rhinovirus protein cleavage: interaction of zinc with capsid polypeptides. J Virol. 1976;18:298–306. doi: 10.1128/jvi.18.1.298-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly RW, Abel MH. Copper and zinc inhibit the metabolism of prostaglandin by the human uterus. Biol Reprod. 1983;28:883–889. doi: 10.1095/biolreprod28.4.883. [DOI] [PubMed] [Google Scholar]
- 42.Geist FC, Bateman JA, Hayden FG. In vitro activity of zinc salts against human rhinoviruses. Antimicrob Agents Chemother. 1987;31:622–624. doi: 10.1128/aac.31.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novick SG, Godfrey JC, Godfrey NJ, Wilder HR. How does zinc modify the common cold? Med Hypoth. 1996;46:295–302. doi: 10.1016/s0306-9877(96)90259-5. [DOI] [PubMed] [Google Scholar]
- 44.Petrus EJ, Lawson KA, Bucci LR, Blum K. Randomized, doublemasked, placebo-controlled clinical study of the effectiveness of zinc acetate capsules on common cold symptoms in allergy-tested subjects. Curr Ther Res. 1998;59:595–607. doi: 10.1016/S0011-393X(98)85058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eby GA, Davis DR, Halcomb WW. Reduction in duration of common cold by zinc gluconate lozenges in a double-blind study. Antimicrob Agents Chemother. 1984;25:20–24. doi: 10.1128/aac.25.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Nakib W, Higgins PG, Barrow I, Batstone G, Tyrrell DA. Prophylaxis and treatment of rhinovirus colds with zinc gluconate lozenges. J Antimicrob Chemother. 1987;20:893–901. doi: 10.1093/jac/20.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godfrey JC, Conant Sloane B, Smith DS, Turco JH, Mercer N, Godfrey NJ. Zinc gluconate and the common cold: a controlled clinical study. J Int Med Res. 1992;20:234–246. doi: 10.1177/030006059202000305. [DOI] [PubMed] [Google Scholar]