Abstract
EBV infection is associated with development of the autoimmune disease systemic lupus erythematosus (SLE), and EBV can reactivate during SLE flares. Latent membrane protein-1 (LMP1) is an EBV-encoded, oncogenic mimic of CD40 that can be re-expressed in PBMCs during SLE flares, as >90% of humans are latently EBV-infected. Whether LMP1 signaling exacerbates SLE is unknown. The phenotype of mice expressing a chimeric molecule with the mouse CD40 extracellular domain and the LMP1 intracellular signaling regions (mCD40-LMP1tg) includes enhanced autoreactivity, yet these mice do not develop fatal autoimmune disease. We hypothesized that LMP1-mediated activation signals cooperate with and/or amplify events that predispose individuals to development of autoimmunity. To determine which aspects of autoimmunity may be exacerbated by LMP1, we bred mCD40-LMP1tg mice to two lupus-prone strains, B6.Sle1 and B6.Sle3, and analyzed autoimmunity parameters. LMP1+Sle1+/+ mice developed enlarged lymphoid organs containing increased frequencies of germinal center, B cells, CD86+ B cells, and activated and memory T cells compared to non-transgenic littermates. Anti-histone antibodies were elevated in serum of LMP1+Sle1+/+ mice, and they had signs of kidney pathology. LMP1+Sle1+/+ B cells produced increased IL-6 and upregulated CD86 to a higher degree following CD40 stimulation in vitro, suggesting that the in vivo autoimmune exacerbation is B-cell intrinsic. In contrast, the LMP1 transgene has no additional effects on autoimmunity on the B6.Sle3 background. These data indicate that LMP1-induced effects can cooperate with distinct subsets of host genes that predispose to autoimmunity, and can thus be an exacerbating factor in autoimmune disease via multiple mechanisms.
Keywords: B cells, systemic lupus erythematosus, autoimmunity, autoantibodies
Introduction
Epstein-Barr virus (EBV) is a B-lymphotropic γ-herpesvirus that establishes a lifelong latent infection in >90% of humans (1, 2). Despite the high seroprevalence of EBV in the normal human population, EBV infection is associated with increased incidence of childhood and adult systemic lupus erythematosus (SLE), where EBV seroprevalence is >99% (3, 4). Very few EBV-encoded proteins are expressed during latent infection of memory B cells. However, EBV can reactivate during conditions of profound immunosuppression and during flares of autoimmune diseases including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (5, 6). SLE patients have increased EBV viral loads in peripheral blood mononuclear cells (PBMCs) relative to healthy controls, suggesting that SLE patients do not effectively control EBV latency (7–9). EBV-encoded proteins, including latent membrane protein 1 (LMP1), are detected in PBMCs of SLE patients only during flares of disease, suggesting that these proteins may contribute to disease pathogenesis or severity (10).
LMP1 is a viral mimic of the TNF receptor family member, CD40, and signals in an amplified and sustained manner relative to CD40 (11). LMP1 possesses a six transmembrane (TM) spanning domain that mediates ligand-independent activation, and two cytoplasmic C-terminal activating regions that transduce intracellular signals by binding to TNF receptor associated factors (TRAFs) (12). However, the ability of LMP1 to signal constitutively is not required for its amplified signaling to B cells, as signaling by domain-swapped molecules which replace the N-terminal and TM domains with that of human(h) or mouse(m) CD40 still deliver sustained and amplified signals to B cells (13, 14). Signaling by mCD40-LMP1 or hCD40-LMP1 in B cells results in increased pro-inflammatory cytokine and Ig production, as well as CD80/86 upregulation compared to CD40 (14, 15). Transgenic mice expressing a chimeric mCD40-LMP1 molecule under control of the mouse MHC class II Eα promoter and bred onto a CD40-deficient background, so the transgene is the only CD40 molecule expressed, were previously generated by our laboratory (15). Previous work with this mouse demonstrates that the LMP1 cytoplasmic tail can substitute for CD40 signaling in many respects, restoring isotype switching, germinal center (GC) formation, and Ig affinity maturation in response to immunization with T-dependent antigens (15). However, mCD40-LMP1tg mice on a CD40−/− background also have an unexpected autoimmune phenotype, consisting of spontaneous GC formation, increased frequency of splenic immature/activated B cells and plasmacytoid dendritic cells (DC), elevated IL-6, IL-12, and TNF-α in serum, and elevated levels of anti-dsDNA and anti-phospholipid Abs (15, 16). Additionally, these mice develop exacerbated disease in a mouse model of rheumatoid arthritis, collagen-induced arthritis (17). Yet mCD40-LMP1tg mice live a normal lifespan, suggesting that if LMP1 exacerbates autoimmunity, as suggested in human studies cited above, it must cooperate with the host genetic background to do so. However, the aspects of autoimmunity exacerbated by LMP1 expression and which sets of lupus-prone genes cooperate with LMP1 are unknown and are the focus of the present study.
Several spontaneous mouse models of SLE were previously created by various laboratories (18). The NZM2410 strain develops fully penetrant, fatal glomerulonephritis by six months of age (18). In order to determine the individual genes responsible for this phenotype, congenic strains on the C57BL/6 background were created (19). The B6.Sle1, B6.Sle2, and B6.Sle3 strains of mice bear a segment from chromosome (chr) 1, chr4, or chr7 of NZM2410, respectively, and display various aspects of the parental autoimmune pathology (18–20). B6.Sle2 mice have a phenotype which is most consistent with that of the mCD40-LMP1tg mouse, displaying polyclonal B cell activation and elevated serum IgM (21). B6.Sle1 mice do not display lymphosplenomegaly and rarely develop kidney disease with age (20). Rather, this strain displays spontaneous T and B cell activation and produces high titers of anti-chromatin and anti-histone antibodies (22, 23). The T and B cell activation defects appear to be distinct and independent, as autoreactivity still occurs in B6.Sle1 mice with defects in T or B cell development (24). B6.Sle3 mice have normal lymphoid organ size, but display spontaneous T and B cell activation in older (9–12 month old) mice, resulting from hyperactive DC (25, 26), and develop mild immune complex-mediated kidney disease by 12 months of age (20). Genetic combination of these strains reveals that the B6.Sle1 locus is required for breaching tolerance to chromatin, and that B6.Sle1Sle3 is the most potent dual loci combination, reconstituting most of the autoimmune phenotypes of the NZM2410 strain (27–29).
LMP1 signals strongly activate B cells both in vitro and in vivo, resulting in increased secretion of pro-inflammatory cytokines including IL-6, upregulation of CD80/86, spontaneous GC formation, autoantibody production, and enlarged lymphoid organs, as discussed above. Thus, we hypothesized that the presence of these LMP1-mediated events would exacerbate disease in autoimmune-prone strains of mice, by enhancing and/or cooperating with genetic factors predisposing to immune hyperactivation (13, 15, 30). To test this hypothesis, and determine which aspects of autoimmunity may be exacerbated by LMP1, we bred mCD40-LMP1tg (LMP1+) mice to B6.Sle1 and B6.Sle3 mice, as the B6.Sle2 phenotype appears redundant with the effects of LMP1. We characterized the phenotype of mice generated by these crosses, which include LMP1+Sle1+/+, LMP1+Sle3+/+, and non-tg littermates (LM). All mice analyzed are homozygous for endogenous CD40. In this study, we observe that LMP1 cooperated with genes in the B6.Sle1, but not the B6.Sle3 strain. As the effects of the Sle3 genes are primarily on DC, this suggests that LMP1 exerts its influence on autoimmunity mainly via the mechanism of B lymphocyte dysregulation. LMP1-Sle1 cooperation resulted in increased lymphoid organ size, and evidence of kidney pathology characteristic of autoimmune disease. Multiple mechanisms likely contribute to this outcome, including demonstrated increased frequency of activated T and B cells, spontaneous GCs, and elevated levels of autoantibodies. Additionally, LMP1+Sle1+/+ B cells produced increased IL-6 and upregulated CD86 to a higher degree following CD40 stimulation in vitro, further supporting a B-cell intrinsic mechanism for the in vivo autoimmune exacerbation. We conclude that LMP1 induces gene activation that is nonredundant with the Sle1 phenotype, and instead cooperates with the products of autoimmunity-predisposing genes affecting B and T cell activation to exacerbate autoimmunity.
Materials and Methods
Mice
mCD40-LMP1 transgenic (LMP1+) (15), B6.Sle1+/+, and B6.Sle3+/+ mice (20) have been described in the references cited above. LMP1+ mice were bred with B6.Sle1+/+ or B6.Sle3+/+ mice to generate the following strains: LMP1+Sle1+/+, LMP1+Sle3+/+ and non-transgenic LMP1−Sle1+/+ or LMP1−Sle3+/+ littermates (LM). All mice were homozygous for endogenous CD40. Mice were age- and sex-matched, and analyzed at 4–6 months, 9 months, or 12 months of age. Mice were housed in a specific pathogen-free barrier facility with restricted access, and all procedures were performed as approved by the University of Iowa Animal Care and Use Committee.
Antibodies and Reagents
The following antibodies were used for flow cytometry: FITC anti-PNA, unconjugated anti-CD16, FITC- or PE-conjugated anti-CD3, anti-B220, anti-CD86, anti-CD40, anti-CD154, anti-CD23, anti-CD21/35, anti-IgM, anti-IgD, anti-CD44, anti-CD62L, anti-CD4, anti-CD8, anti-MHCII, and APC-conjugated anti-CD25 (eBioscience, San Diego, CA); PerCP-conjugated anti-B220, anti-CD4, and anti-CD8 and isotype control Ab (BD Biosciences, San Diego, CA).
The following antibodies were used for cell stimulation: goat anti-mouse IgM F(ab)’2 µ-chain specific (anti-IgM; Jackson Immunoresearch, West Grove, PA), hamster anti-mCD40 (HM40.3; eBioscience). Anti-Thy1.2 (HO13.4) mAb was purified by 50% SAS precipitation from serum-free cultures.
Hi5 insect cells infected with wild-type baculovirus (Hi5-WTBV) or a baculovirus encoding mCD154 (Hi5-mCD154) have been described previously (31). Hi5 cells normally grow at room temperature, lyse at 37°C, and do not overgrow cell cultures, while providing a membrane-bound trimeric form of mCD154.
Cell Isolation
T-depleted splenocytes (TDS) used in the CD86 upregulation and IL-6 production experiments were prepared by treatment with anti-Thy1.2 (HO13.4) mAb and complement (Pel-Freez, Brown Deer, WI) as described (15).
Flow Cytometry
Single cell suspensions were made from spleen and LN by dissociating tissue between two frosted glass microscope slides. Erythrocytes were lysed using hypotonic ACK buffer. Cells were washed several times in ice cold media. 1×106 cells were stained with directly conjugated fluorescent Abs as described (15, 32), washed, and fixed using BD Cytofix/Cytoperm (BD Biosciences) according to manufacturer’s instructions. Data were collected on a FACSCalibur (Becton Dickinson, Mountain View, CA) using Cell Quest Softward. The results were analyzed using FlowJo software (TreeStar, San Carlos, CA), gating first on live cells.
In vitro CD86 upregulation and IL-6 production
T-depleted splenocytes were stimulated in triplicate at 1×106 cells/well in a 24-well plate for 48h with the following stimuli: medium only, goat anti-mouse IgM F(ab)’2 µ-chain specific (anti-IgM) or hamster anti-mCD40 (HM40.3) Abs at 2 µg/ml, WTBV-Hi5 or Hi5-mCD154 cells at 1 Hi5 per 10 B cells. Supernatants were analyzed for IL-6 by ELISA as described (31). Cells were collected and stained with FITC-CD86 or FITC-Rat IgG1 as an isotype control Ab and analyzed by flow cytometry.
Serum autoantibody and IL-6 detection
Serum α-dsDNA IgG and α-dsDNA/histone IgG antibody titers were measured by ELISA as described (23), n=9–11 female mice per genotype at 4–6 or 12 month ages. IL-6 was detected using a commercial kit (R+D Systems, Minneapolis, MN) according to manufacturer’s instructions.
Kidney histology
10 kidneys from 4–6 month old or 12 month old female LMP+Sle1+/+ and non-tg LM (1 kidney/mouse) were fixed in 10% neutral formalin, then processed and embedded in paraffin. 4–5 µm sections were prepared with a microtome (HM 355, Microm) then stained with hematoxylin and eosin with an automated slide stainer (Sakura DRS 601 Diversified Stainer, Sakura). Stained slides were mounted with Solvent 100 mounting media, and bright-field micrographs of stained sections were taken with a microscope (Olympus BX-51, Olympus America) fitted with an Olympus DP70 camera (Olympus America). Slides were examined by a pathologist who was blinded to the study groups. Morphometric assessment was made on 5 random glomeruli per kidney selected for large size to best represent a mid cross-section of the glomerulus, and those with distinct separation of the glomerulus from Bowman’s capsule for accuracy.
Results
Lymphoid organ size in LMP1+Sle1+/+ mice
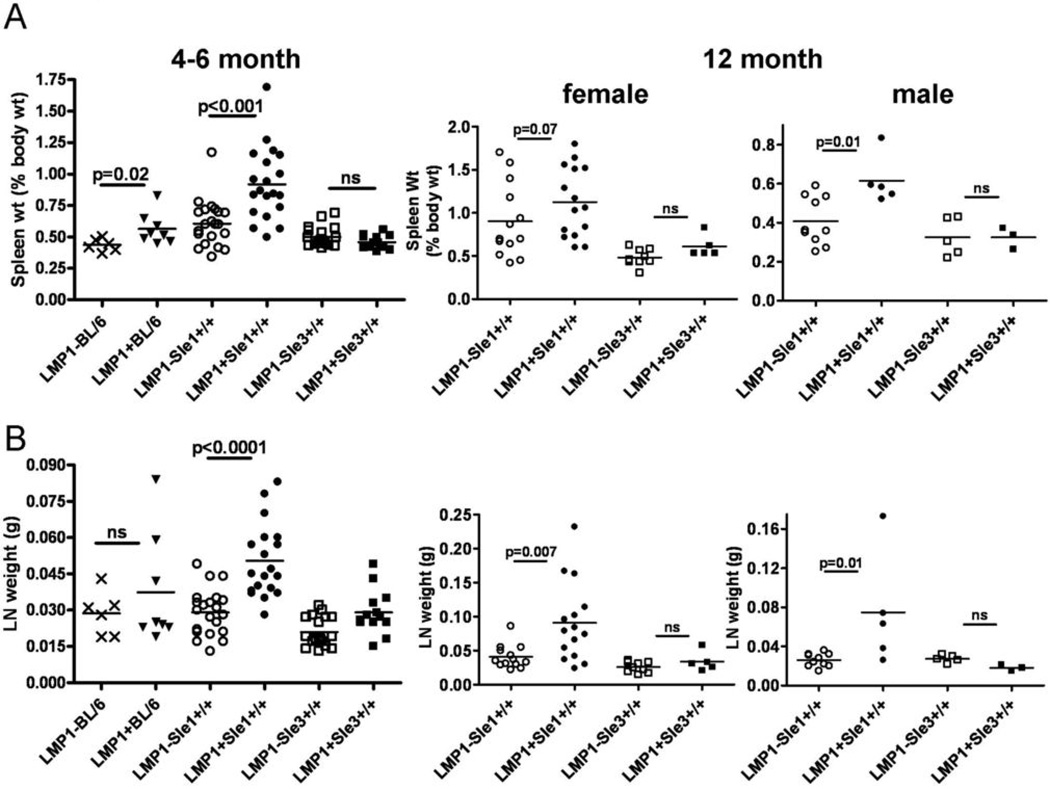
As previously reported, B6.Sle1 and Sle3 mice had normal spleen and lymph node weights in mice aged up to 1 year (20, 25); Figure 1). However, LMP1+Sle1+/+ mice showed enlarged spleens and lymph nodes at 4–6 months of age relative to LM. Interestingly, this increase in lymphoid organ size was only observed in female mice. Once mice reached 12 months of age, both male and female LMP1+Sle1+/+ mice had enlarged lymphoid organs compared to LMP1+Sle3srsid3700896 and LM. There was a small but statistically significant increase in spleen weight of LMP1+ mice on a C57BL/6 background (LMP1+BL/6) compared to LMP1−BL/6, but no difference in LN weight between LMP1+BL/6 and LMP1−BL/6 mice, suggesting that the difference in spleen and LN size in LMP1+Sle1+/+ mice was due to cooperation between LMP1 and genes in the Sle1 interval. The increased LN weight was accompanied by an increase in total cell number, which included a statistically significant increase in the number of B and T cells recovered from LMP1+Sle1+/+ LN (Tables I and II). However, there was no effect of the LMP1 transgene on lymphoid organ size or cell number on the B6.Sle3 background. Thus, LMP1 did not cooperate with genes in the Sle3 interval to enhance autoimmunity. The lack of significantly increased lymph node and spleen weight in LMP1+ vs. LMP1−BL/6 mice in this study could be due to the presence of endogenous CD40, which can ameliorate some of the effects of LMP1 in vivo (L.L. Stunz, unpublished data).
Figure 1. Lymphoid organ size in LMP1+Sle1+/+ vs. LMP1+Sle3+/+ and non-tg LM mice.
A. Spleen weights of 4–6 month old female (left panel) or 12 month old male and female (center and right panels) LMP1+Sle1+/+ mice vs. LMP1+Sle3+/+, LMP1+BL/6, and non-tg LM mice. Each symbol represents the spleen weight of 1 mouse.
B. Combined lymph node (LN) weights of 4 cervical and 2 axillary LN of 4–6 month old female (left panel) or 12 month old male and female (center and right panels) LMP1+Sle1+/+ mice vs. LMP1+Sle3+/+ and non-tg LM mice. p values were determined by two-tailed Mann-Whitney U test; ns=not significant.
Table I.
Total cell numbers in lymphoid organs of 4–6 month old LMP1+Sle1 vs. LMP1+Sle3 mice and non-transgenic littermates
| LMP1−Sle1 | LMP1+SIe1 | LMP1−Sle3 | LMP1+Sle3 | |||
|---|---|---|---|---|---|---|
|
Spleen | ||||||
|
Total cells (x 106) |
204.6 ± 81 | 329.5 ± 201 | 89.1 ± 30.7 | 75.8 ± 15.9 | ||
| B cells | 65.6 ± 28.8 | 100.3 ± 55 | 25.3 ± 9.8 | 22.6 ± 3.7 | ||
| T cells | 58.7 ± 29.8 | 85.6 ± 50.6 | 25.8 ± 10.2 | 22.8 ± 3.4 | ||
| CD4+ T cells | 31.5 ± 11.2 | 48.6 ± 33.1 | 16 ± 7.5 | 13.8 ± 3.7 | ||
| naive | 10.4 ± 5.2 | 7.2 ± 5.3 | 7.2 ± 3.3 | 5.4 ± 0.4 | ||
| activated | 14.4 ± 8.1 | 30.5 ± 23.7* | 5.4 ± 2.8 | 5.4 ± 2.4 | ||
| CD8+ T cells | 14.9 ± 6.5 | 15.1 ± 10.8 | 8.1 ± 2.1 | 7.2 ± 1.04 | ||
| naive | 8.8 ± 4.9 | 8.3 ± 8.3 | 5 ± 1.7 | 4.4 ± 0.6 | ||
| activated | 1.5 ± 0.6 | 3.1 ± 2.5 | 0.61 ± 0.2 | 0.81 ± 0.05 | ||
| memory | 1.8 ± 0.61 | 2.48 ± 2.9 | 0.66 ± 0.21 | 0.87 ± 0.15 | ||
| LN | ||||||
| Total cells | 50.6 ± 29.3 | 82.8 ± 50.1* | 40.6 ± 42.8 | 38.4 ± 2.3 | ||
| B cells | 15.4 ± 9.2 | 26.6 ± 15.4* | 11.9 ± 14.2 | 11.2 ± 2.6 | ||
| T cells | 20.9 ± 13.3 | 27.5 ± 18.7* | 17 ± 17.4 | 17.7 ± 0.4 | ||
| CD4+ T cells | 10.4 ± 6.3 | 15.3 ± 10.5 | 8.4 ± 7.9 | 8 ± 0.3 | ||
| naive | 7 ± 4.2 | 9.1 ± 7.2 | 5.7 ± 5.2 | 8.1 ± 4.5 | ||
| activated | 1.3 ± 0.9 | 3.1 ± 1.8* | 1 ± 1.02 | 2.1 ± 1.2 | ||
| CD8+ T cells | 9.3 ± 4.8 | 13 ± 9.9 | 7.4 ± 7.1 | 7.04 ± 0.9 | ||
| naive | 6.8 ± 3.8 | 9.2 ± 7.4 | 5.7 ± 5.2 | 5.1 ± 2 | ||
| activated | 0.33 ± 0.3 | 0.65 ± 0.3* | 0.23 ± 0.28 | 0.26 ± 0.03 | ||
| memory | 0.81 ± 0.54 | 1.5 ± 1.4 | 0.51 ± 0.5 | 0.6 ± 0.004 | ||
= p < 0.05 by Mann-Whitney U test
Table II.
Total cell numbers in lymphoid organs of 12 month old LMP1+Sle1 vs. LMP1+Sle3 mice and non-transgenic littermates
| LMP1−Sle1 | LMP1+Sle1 | LMP1−Sle3 | LMP1+Sle3 | |||
|---|---|---|---|---|---|---|
| Spleen | ||||||
|
Total cells (x 106) |
163 ± 70.4 | 157 ± 78.7 | 112 ± 67.3 | 127 ± 82.8 | ||
| B cells | 66.8 ± 31.1 | 51.4 ± 25.7 | 46.8 ± 30.6 | 54.6 ± 39.2 | ||
| T cells | 26.8 ± 14.6 | 29.8 ± 15.6 | 23 ± 14.7 | 23.7 ± 18.4 | ||
| CD4+ T cells | 22 ± 12.7 | 25 ± 12.8 | 14.9 ± 8 1 | 14.7 ± 10 | ||
| naive | 2.04 ± 2.78 | 0.72 ± 0.53* | 4.86 ± 4.3 | 2.31 ± 1.42 | ||
| activated | 13 7 ± 8.3 | 18.1 ± 10.6 | 6.17 ± 2 8 | 7.87 ± 7 | ||
| CDS+ T cells | 8.75 ± 6.6 | 7.7 ± 4.8 | 10.8 ± 8.5 | 10.7 ± 9.8 | ||
| naive | 2.47 ± 3.3 | 0.74 ± 0.53 | 4.8 ± 4.1 | 3.6 ± 3.7 | ||
| activated | 2.19 ± 0.99 | 3.09 ± 1.8 | 1.9 ± 1.4 | 2.16 ± 1.58 | ||
| memory | 1.83 ± 2.09 | 1.18 ± 1.09 | 2.27 ± 1.9 | 3.01 ± 2.8 | ||
| LN | ||||||
| Total cells | 28.1 ± 13 | 58.6 ± 49.4* | 14 ± 4.47 | 29.2 ± 41.3 | ||
| B cells | 10.6 ± 4.8 | 21 ± 18.3* | 4.52 ± 1.4 | 11.7 ± 17.5 | ||
| T cells | 6.8 ± 3.6 | 16.1 ± 15.8* | 4.7 ± 1.9 | 7.7 ± 10.8 | ||
| CD4+ T cells | 3 ± 2.6 | 7.54 ± 9.1* | 1.7 ± 1.2 | 3.6 ± 5.5 | ||
| naive | 0.67 ± 0.64 | 1.47 ± 2 | 0.72 ± 0.56 | 1.1 ± 1.4 | ||
| activated | 1.77 ± 1.6 | 4.43 ± 5* | 0.59 ± 0.63 | 1.6 ± 2.6 | ||
| CD8+ T cells | 1.9 ± 1.5 | 4.2 ± 5.1 | 1.3 ± 0.8 | 2.5 ± 4 | ||
| naive | 0.85 ± 0.78 | 1.35 ± 1.7* | 0.76 ± 0.58 | 1.35 ± 2.1 | ||
| activated | 0.34 ± 0.25 | 1 ± 1.2* | 0.15 ± 0.11 | 0.32 ± 0.52 | ||
| memory | 0.29 ± 0.23 | 1.06 ± 1.6* | 0.19 ± 0.07 | 0.35 ± .45 | ||
= p < 0.05 by Mann-Whitney U test
Spontaneous GC formation in LMP1+Sle1+/+ mice
Given the enlarged lymphoid organs observed in LMP1+Sle1+/+ mice, we further evaluated the spleen and LN to determine whether spontaneous lymphocyte activation was the mechanism of this enlargement. The frequencies of B cells, CD4+T, CD8+T, and myeloid cells were normal in all mouse strains, regardless of the presence of the LMP1 transgene. The splenic marginal zone, follicular, and immature B cell compartments, identified by the expression of CD23 and CD21/35, were also examined. The frequency of splenic MZ and FO B cell populations was not significantly different among the mouse strains (Supplementary Figure 1). The CD23loCD21/35loB220+ population (“population L”) (15, 16) was occasionally increased in some B6.Sle1 and B6.Sle3 mice, relative to LMP1-BL/6 mice (Supplementary Figure 1). However, no additional increase in the frequency of this population was observed in LMP1+Sle1+/+ or LMP1+Sle3+/+ mice (Supplementary Figure 1). Germinal centers (GC) are specialized lymphoid structures within the splenic white pulp which only form after immunization. In the GC, B cells receive survival signals, undergo Ig isotype switching and affinity maturation, and differentiate into antibody-secreting plasma cells and memory B cells (33). Spontaneous GC formation occurs in several mouse models of SLE (18, 33, 34).
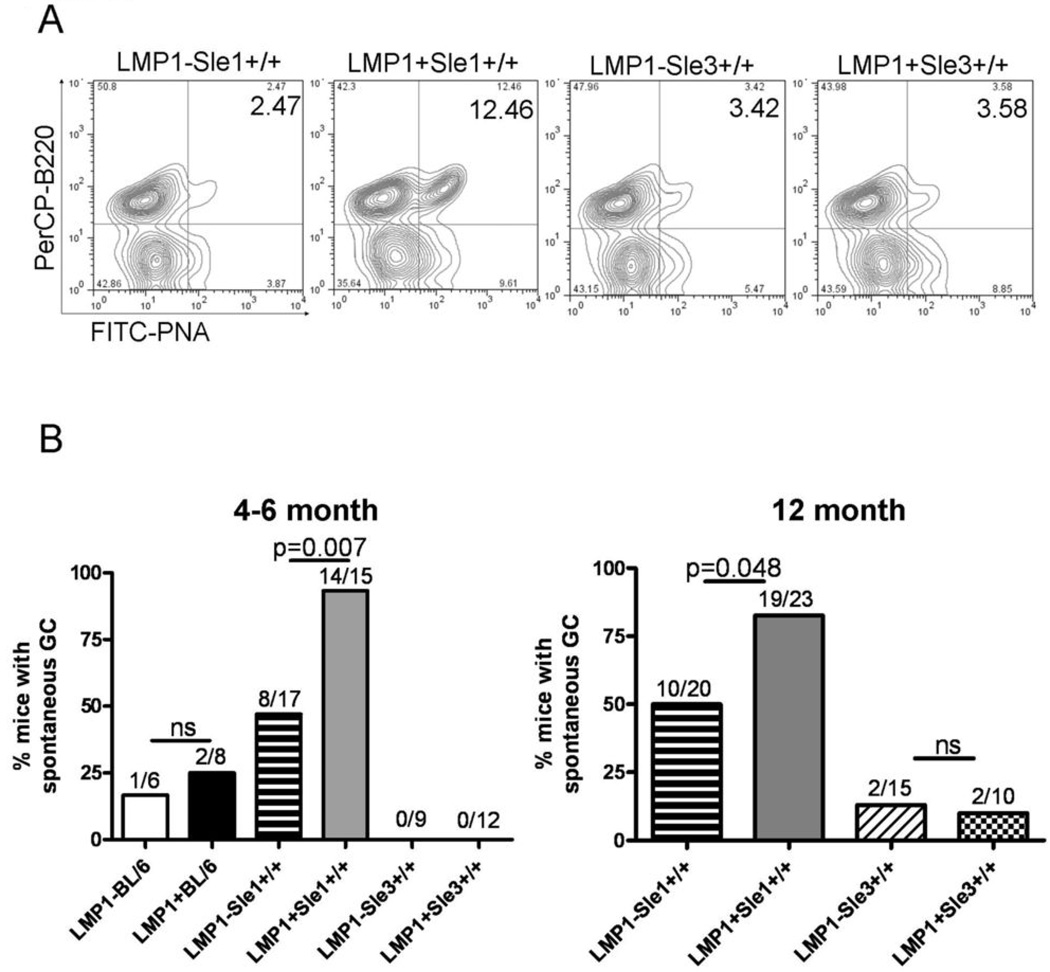
An increase in spontaneous GC could provide a mechanism for increased production of autoantibodies. To test whether GC B cells were forming in the enlarged spleens of LMP1+Sle1+/+ mice, the presence of spontaneous GC in LMP1+Sle1+/+ mice was examined and seen by immunofluorescence staining of frozen splenic sections (data not shown). However, quantification of splenic GC by this method is technically difficult. Thus, we analyzed LMP1+Sle1+/+ mice for the presence of spontaneous splenic GC B cells by flow cytometry using a reliable, accepted quantitative method (35). Single-cell splenocyte suspensions were examined for expression of B220 and PNA, a lectin which binds sugar residues highly expressed on GC B cells (15). As shown in Figure 2A, significantly increased frequencies of GC B cells were consistently identified in female LMP1+Sle1+/+ mice, but only rarely in LM or LMP1+Sle3+/+ mice, even at 12 months of age (Figure 2B). These data demonstrate that LMP1 and Sle1, but not Sle3 gene products cooperate to enhance the formation of GC B cells in unimmunized mice, an indicator of, and contributor to the mechanism of autoimmunity.
Figure 2. Frequency of spontaneous GC B cells in LMP1+Sle1+/+ vs. LMP1+Sle3+/+ and non-tg LM mice.
A. RBC-depleted splenocytes from unimmunized 4–6 month old female LMP1+Sle1+/+, LMP1+Sle3+/+, and non-tg LM mice were stained with PNA and anti-B220 to identify spontaneous GC B cells by flow cytometry after gating out dead cells. Representative flow cytometry plots are shown.
B. Composite data showing the percentage of 4–6 month old female (left panel) and 12 month old male and female (right panel) LMP1+Sle1+/+ mice with elevated spontaneous %GC B cells, compared to LMP1+Sle3+/+, LMP1+BL/6, and non-tg LM. A mouse was considered to have elevated %GC B cells if the frequency was larger than the LMP1−Sle3+/+ mice analyzed in the same experiment. The numbers above each column represent the number of mice of each genotype with elevated %GC B cells out of all mice analyzed. p-values were determined by Chi-square test; ns=not significant.
CD4+ and CD8+ T cell activation in lymphoid organs of LMP1+Sle1+/+ mice
GC formation is typically a T cell-dependent event (36), and LMP1+Sle1+/+ mice displayed evidence of spontaneous GC formation. Additionally, as discussed earlier, the Sle1 gene cluster predominantly affects activation of T and B lymphocytes, as compared to the principal effects of Sle3 genes on myeloid cells. Thus, we investigated whether T cells were also spontaneously activated in LMP1+Sle1+/+ mice. Although the frequencies of CD4+ and CD8+ T cells were normal in LMP1+Sle1+/+ mice compared to control strains, we observed increases in the total numbers of these cell types in LN (Tables I and II). The frequency and total number of CD3+CD4−CD8− cells in lymphoid organs of LMP1+Sle1+/+ mice was not significantly different from LMP1−Sle1+/+ LM (data not shown). We further characterized the phenotype of CD4+ and CD8+ T cells by expression of CD44 and CD62L, cell surface receptors which are differentially expressed on naïve (CD44loCD62Lhi), activated (CD44hiCD62Llo), and memory (CD44hiCD62Lhi) T cell populations. Consistent with previously published results, increased frequencies of activated CD4+ and CD8+ T cells were observed in spleen and LN of B6.Sle3 and B6.Sle1 mice at 4–6 months of age, with a compensatory decrease in the frequency of naïve T cells in these organs ((23, 25); Table III). Interestingly, 4–6 month old LMP1+Sle1+/+ mice displayed an even more dramatic increase in the frequency of activated CD4+ and CD8+ T cells in the spleen, with a corresponding decrease in the frequency of naïve cells. This difference was reflected by increased total numbers of activated splenic and LN CD4+ T cells, as well as increased numbers of activated CD8+ T cells in LN of LMP1+Sle1+/+ mice (Table I). LMP1+Sle3+/+ mice had no additional changes in naïve or activated T cell frequency or cell number at 4–6 months of age. No differences in memory CD8+ T cell frequency were observed in spleen or LN at 4–6 months of age in any of the mouse strains (Table III). The increased frequency and total number of activated/memory T cells in lymphoid organs of LMP1+Sle1+/+ mice was even more pronounced in 12 month old mice (Table II and IV). These mice had very few naïve CD4+ and CD8+ T cells in spleen and LN compared to LM and LMP1+Sle3+/+ mice (Table II and IV). Additionally, memory CD8+T cell frequency and total numbers were increased in the LN, but not the spleen, of LMP1+Sle1+/+ mice relative to LMP1+Sle3+/+ and LM (Table II and IV). These data highlight another mechanism by which LMP1 cooperates with genes in the Sle1 interval to enhance CD4 and CD8 T cell activation and memory cell formation, which in turn can enhance B cell activation and autoantibody production.
Table III.
Frequency of Activated T cells and B220+CD86+ cells in lymphoid organs of 4–6 month old LMP1+Sle1 vs. LMP1+Sle3 mice and non-transgenic littermates
| LMP1−Sle1 | LMPI+Sle1 | LMP1−Ste3 | LMP1+Sle3 | |||
|---|---|---|---|---|---|---|
|
Spleen | ||||||
| B220+CD86+ | 25.2 ± 3.5 | 29.4 ± 3.7* | 23.2 ± 1.1 | 23.6 ± 5.5 | ||
| CD4+ T cells | ||||||
| naive | 36.1 ± 8.8 | 27.2 ± 10* | 53.9 ± 3.3 | 44.1 ± 4* | ||
| activated | 42.3 ± 9 | 51.3 ± 10* | 24.7 ± .7 | 33.6 ± 3.8* | ||
| CD8+T cells | ||||||
| naive | 52.2 ± 5 | 39.2 ± 11.8* | 61.2 ± 4.1 | 55.6 ± 7 | ||
| activated | 12.4 ± 3.6 | 23.6 ± 12.8* | 9.9 ± 2.3 | 13.8 ± 5 3 | ||
| memory | 15 ± 5 | 16.1 ± 5.9 | 12.1 ± 1.6 | 13.8 ± 0.6 | ||
| LN | ||||||
| B220+CD86+ | 20.6 ± 2.7 | 30.6 ± 7.1* | 18.1 ± 2.1 | 22.2 ± 5.6 | ||
| CD4+ T cells | ||||||
| naive | 59.2 ± 4.6 | 57.8 ± 5.8 | 63.2 ± 8.2 | 63.7 ± 4 | ||
| activated | 17.8 ± 4 | 18.1 ± 4.2 | 11.8 ± 1.4 | 12.4 ± 1.8 | ||
| CD8+ T cells | ||||||
| naive | 64.4 ± 9 | 55.4 ± 10* | 67.4 ± 4.9 | 64 ± 5.5 | ||
| activated | 7.8 ± 2.2 | 9.5 ± 2.9 | 4.6 ± 1 | 5.8 ± 1 | ||
| memory | 12.8 ± 5.4 | 15.5 ± 4.9 | 8.8 ± 2.3 | 11.1 ± 2.8 | ||
= p < 0.05 by two-taited Student’s t-test
Table IV.
Frequency of Activated T cells and B220+CD86+ cells in lymphoid organs of 12 month old LMP1+Sle1 vs. LMP1+Sle3 mice and non-transgenic littermates
| LMP1−Sle1 | LMP1+Sle1 | LMP1−Sle3 | LMP1+Sle3 | |||
|---|---|---|---|---|---|---|
|
Spleen | ||||||
| B220+CD86+ | 35.5 ± 3.1 | 39.4 ± 3.7 | 28.6 ± 2.9 | 29.8 ± 4.1 | ||
| CD4+ T cells | ||||||
| naive | 17.9 ± 11.8 | 1.8 ± 1.1* | 36 ± 7.8 | 17.9 ± 7.1* | ||
| activated | 57 ± 13 | 81.6 ± 1.8* | 40 ± 6.3 | 57 ± 7* | ||
| CD8+ T cells | ||||||
| naive | 36.4 ± 5.8 | 12.4 ± 6.8* | 44.5 ± 4.4 | 33.8 ± 9.3 | ||
| activated | 16.9 ± 4 | 30.4 ± 5* | 16.6 ± 2.6 | 17.5 ± 3.7 | ||
| memory | 26.6 ± 3.1 | 19.9 ± 11.3 | 23 ± 4 | 32.7 ± 10 | ||
| LN | ||||||
| B220+CD86+ | 30.2 ± 3.1 | 39.5 ± 4.1* | 21 ± 2.6 | 26.1 ± 7.4 | ||
| CD4+ T cells | ||||||
| naive | 31.5 ± 10 | 13.8 ± 2* | 51.3 ± 3.1 | 32 ± 9* | ||
| activated | 45 ± 10 | 60 ± 2* | 24.1 ± 2.3 | 43.1 ±7.4* | ||
| CD8+ T cells | ||||||
| naive | 52.2 ± 7.5 | 28 ± 6.9* | 63.4 ± 3.8 | 46 ± 5.1* | ||
| activated | 15.1 ± 4.5 | 23.6 ± 7.3 | 8.1 ± 1.6 | 17.1 ± 3.4* | ||
| memory | 22.5 ± 5 | 38.5 ± 3.7* | 19.6 ± 2.6 | 25.5 ± 3* | ||
= p < 0.05 by two-tailed Student’s t-test
CD86 expression and upregulation on B220+ cells in LMP1+Sle1+/+ mice
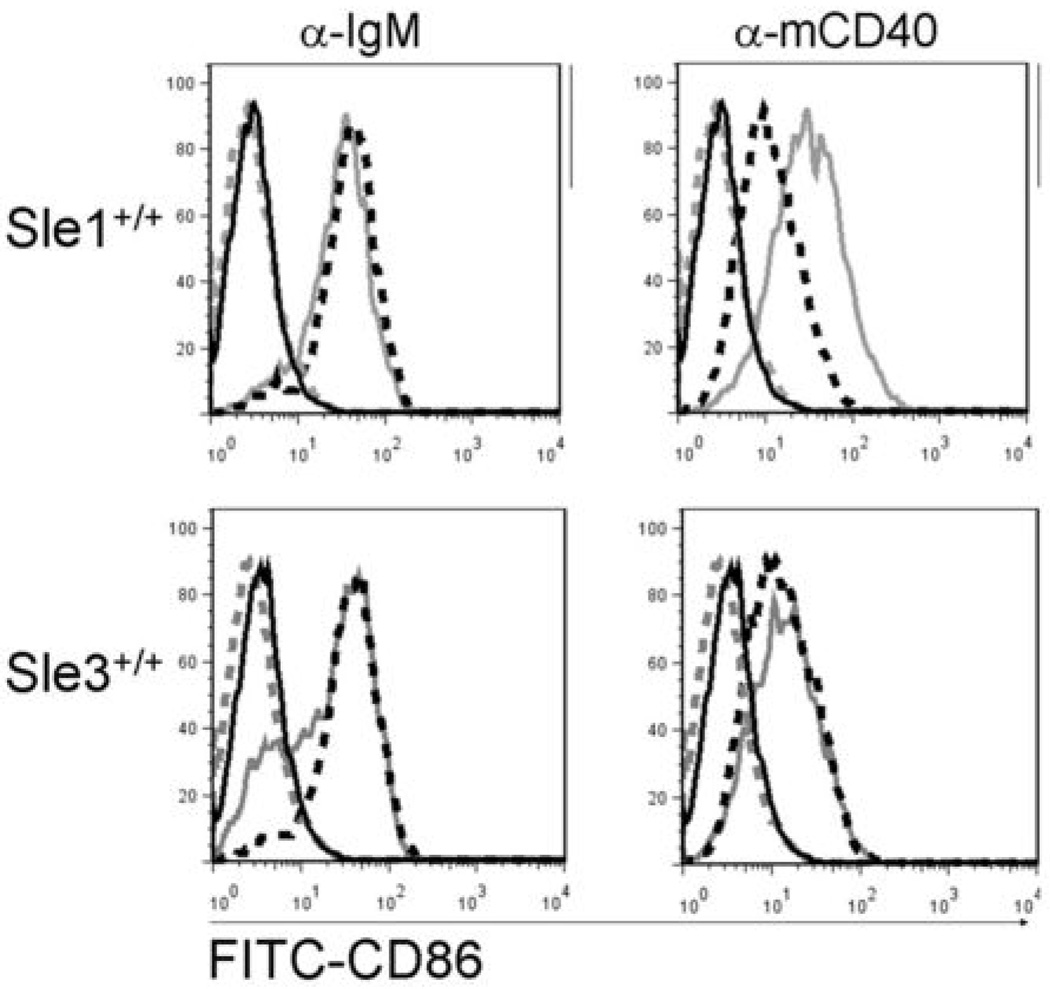
CD86 is upregulated on B cells following exposure to a variety of stimuli, including CD40 agonists, and is an important costimulatory molecule for T cells. CD86 expression is increased on splenic B cells from B6.Sle1 and B6.Sle3 mice at 6–9 months of age (22, 25). The spontaneous GC formation and T cell activation in LMP1+Sle1+/+ mice, together with a lack of effect of the DC-affecting Sle3 genes suggested that B cells expressing LMP1 may act as more potent APC by virtue of enhanced cytokine production and costimulatory molecule expression. Splenic and LN B cells expressing CD86 were present at a higher frequency in either 4–6 month old or 12 month old LMP1+Sle1+/+ mice, compared to LMP1+Sle3+/+ mice and LM (Table III and IV). The increased percentage of B220+CD86+ cells correlated with increased frequency of activated CD4+ and CD8+ T cells in spleen and LN of LMP1+Sle1+/+ mice, relative to LMP1+Sle3+/+ mice (Table III and IV). To test whether LMP1 signaling was the mechanism for directly enhancing upregulation of CD86 in splenic B cells from LMP1+Sle1+/+ mice, T-depleted splenocytes (TDS) from LMP1+Sle1+/+, LMP1+Sle3+/+, and LM were stimulated for 48h via CD40 or the BCR and analyzed for CD86 expression. Unstimulated TDS from all mouse strains expressed very low levels of CD86 (Figure 3; gray dashed and black solid lines), and all upregulated CD86 to the same degree following BCR stimulation (gray solid and black dashed lines; left upper and lower panels). However, stimulation via CD40 induced a higher degree of CD86 expression on LMP1+Sle1+/+ TDS than on LMP1−Sle1+/+ TDS (upper right panel; compare gray solid and black dashed lines). Consistent with the in vivo data, LMP1+Sle3+/+ and LMP1−Sle3+/+ TDS upregulated CD86 equivalently in response to endogenous mCD40 signaling (lower right panel; compare gray solid and black dashed lines). These data indicate that LMP1 signaling in B cells enhanced CD86 expression only in the Sle1 genetic background, which is consistent with enhanced CD86 expression on B cells directly ex vivo and correlates with enhanced T cell activation in vivo. These findings support the concept that B cells could act as more efficient APC when stimulated by LMP1.
Figure 3. CD86 expression on B cells from LMP1+Sle1+/+ vs. LMP1+Sle3+/+ and non-tg LM mice.
T-depleted splenocytes were isolated from 4–6 month old female LMP1+Sle1+/+, LMP1+Sle3+/+, and non-tg littermates. Cells were stimulated in triplicate with agonistic anti-IgM (left panel) or anti-mCD40 Abs for 48h, stained with FITC-anti-CD86, and analyzed by flow cytometry by first gating on live cells. Histograms shown are representative of three independent experiments. Gray dashed line= unstimulated LMP1+, black dashed line= unstimulated LMP1, gray solid line= stimulated LMP1+, black dashed line= stimulated LMP1−. Cells placed at 4°C and stained 48h later with either isotype control or anti-CD86 Abs overlapped with the unstimulated histograms shown.
Serum anti-dsDNA and anti-dsDNA/histone antibodies in LMP1+Sle1+/+ mice
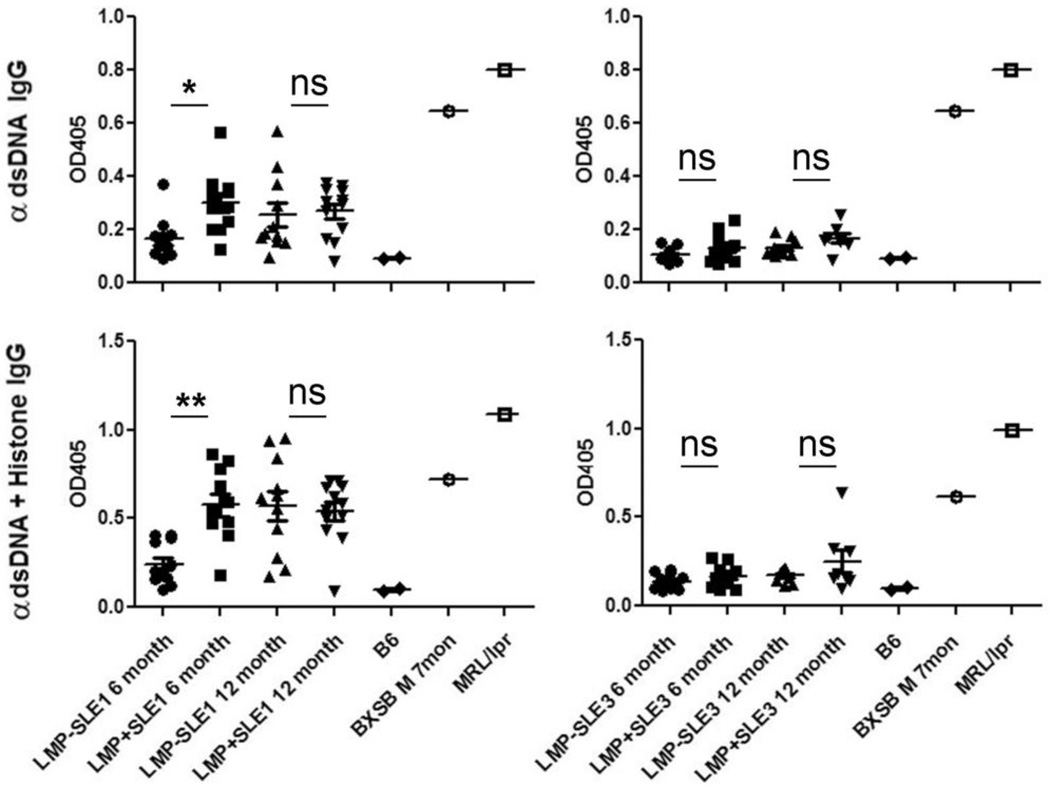
B6.Sle1 mice produce high titers of antibodies reactive against nuclear antigens, particularly chromatin and histones (20, 23), and LMP1 signaling in mice enhances serum anti-dsDNA Abs, as discussed above. Thus, enhanced spontaneous T and B cell activation in LMP1+Sle1+/+ mice led us to evaluate serum of female mice at 4–6 months or 12 months of age for the presence and levels of anti-dsDNA and anti-histone/dsDNA IgG(23). Levels of total IgM, IgA, IgG1, IgG2b, and IgG3 were measured by ELISA and were not significantly different between B6.Sle1 and B6.Sle3 mice or their LMP1+ LM (data not shown). As shown in Figure 4, LMP1+Sle1+/+ female mice had significantly higher serum levels of anti-dsDNA and anti-histone/dsDNA IgG at 6 months of age than LMP1−Sle1+/+ mice. At 12 months of age, the difference was no longer detectable in serum. In contrast, even very low titers of anti-dsDNA and anti-histone/dsDNA IgG were not detected in serum of LMP1+Sle3+/+ mice or their non-tg LM, even at 12 months of age (Figure 4). These data, together with those in Figures 2 and 3, indicate that not only are B cells more highly activated in LMP1+Sle1+/+ mice compared to the non-tg LM, but this increased B cell activation leads to production of autoantibodies which may contribute to kidney pathology.
Figure 4. Serum autoantibodies present in LMP1+Sle1+/+ vs. LMP1+Sle3+/+ and non-tg LM mice.
Serum from 4–6 month old female or 12 month old female LMP1+Sle1+/+(left panels) LMP1+Sle3+/+ (right panels), and non-tg LM, 9–11 mice per group, was analyzed for the presence of anti-dsDNA IgG (top panels) and anti-dsDNA/histone IgG (bottom panels) by ELISA. Serum from C57Bl/6 mice (n=2) was included as a non-autoimmune negative control, while serum from autoimmune BXSB and MRL/lpr mice was a positive control for the assay. * = p<0.005, ** = p-value < 0.0005 as determined by two-tailed Student’s t-test. ns = not significant.
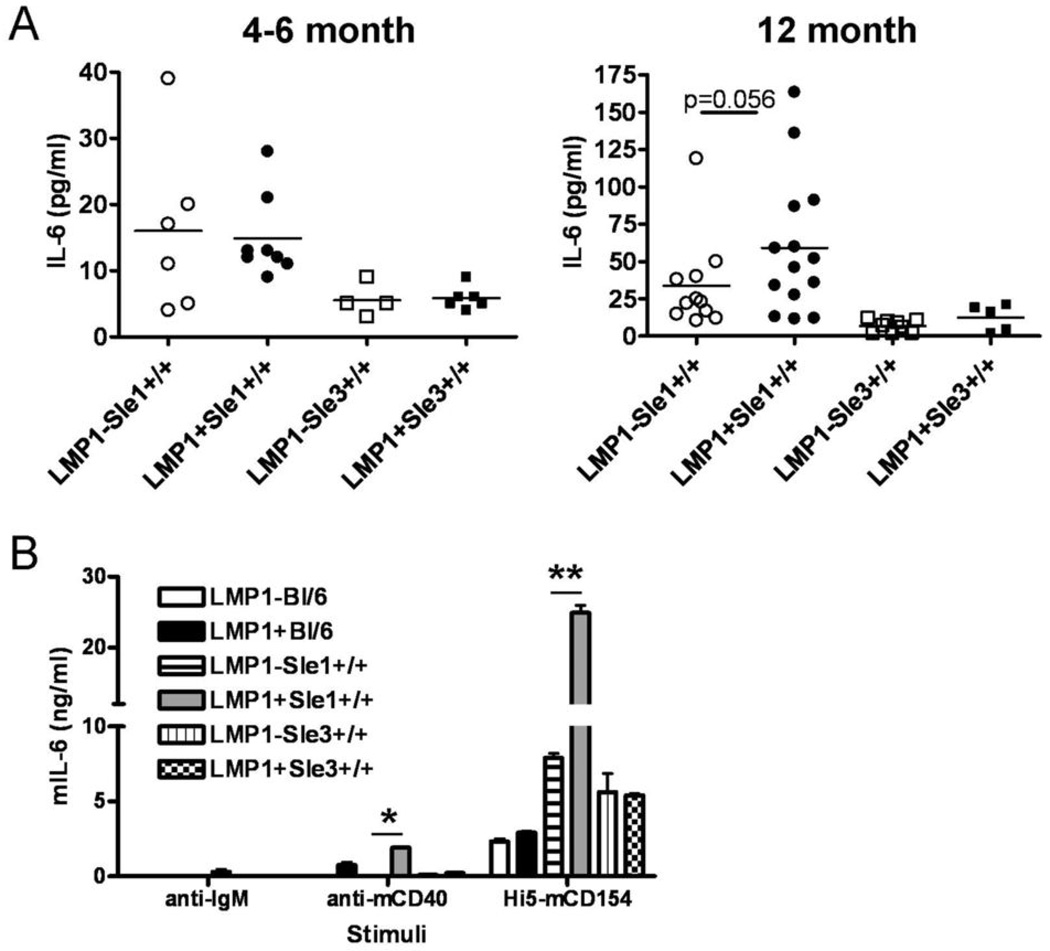
IL-6 production by LMP1+Sle1+/+ B cells
IL-6 is a pro-inflammatory cytokine which contributes to antibody production and is pathogenic in autoimmune responses (37). IL-6 is elevated in serum of mCD40-LMP1tg mice, as well as in serum of B6.Sle1 mice (15, 38). Elevated IL-6 production by B6.Sle1 myeloid cells inhibits suppression of T cell activation by T regulatory cells (39). The enhanced autoimmunity observed in LMP1+Sle1+/+ mice, as measured by multiple parameters, was also reflected by IL-6 production. Serum IL-6 levels in 4–6 month old LMP1+Sle1+/+ mice were not increased by statistically significant amounts over LMP1−Sle1+/+ levels (Figure 5A). 12 month old LMP1+Sle1+/+ mice, however, did show a trend toward elevated serum IL-6, which correlated with autoantibody production. There was no effect of the LMP1 transgene on serum IL-6 levels in B6.Sle3 mice, even in 12 month old animals (Figure 5A). Serum levels of other pro-inflammatory cytokines including IFN-γ, IL-12, and IL-17, were analyzed with no significant differences observed between LMP1+Sle1+/+, LMP1+Sle3+/+, and the respective non-tg LM (data not shown). To test whether LMP1 signaling was the mechanism responsible for enhanced IL-6 production by LMP1-expressing B6.Sle1 B cells, TDS from LMP1+BL/6, LMP1+Sle1+/+, LMP1+Sle3+/+ and LM were stimulated for 24 hours with insect cells expressing mCD154, the ligand for mCD40 and for the transgenic mCD40-LMP1, or anti-IgM Ab as a control. CD154 stimulation was used to measure this parameter because, unlike most CD40-mediated B cell effects, IL-6 production requires the membrane-bound ligand for CD40, and agonistic Ab cannot act as a substitute (31). CD40 expression on B cells from LMP1+BL/6, LMP1+Sle1+/+ and LMP1+Sle3+/+ mice was equivalent, although slightly elevated relative to LM (data not shown). B6.Sle1 and B6.Sle3 TDS produced increased amounts of IL-6 relative to BL/6 controls, consistent with previously published results (26, 38, 39). There was no effect of the LMP1 transgene on IL-6 production by either BL/6 or B6.Sle3 TDS. However, LMP1+Sle1+/+ TDS produced ∼2 fold more IL-6 following CD40 signaling relative to non-tg LM (Figure 5B), consistent with the concept that LMP1 signals to B cells effectively cooperate with genes in the B6.Sle1 interval. Levels of other pro-inflammatory cytokines including TNF-α, IFN-γ, IL-12, and IL-17, were analyzed within these same culture supernatants with no significant differences observed between LMP1+Sle1+/+, LMP1+Sle3+/+, and the respective non-tg LM (data not shown).
Figure 5. IL-6 levels in serum and culture supernatants of LMP1+Sle1+/+ vs. LMP1+Sle3+/+ and non-tg LM mice.
A. Serum from 4–6 month old female (left panel) or 12 month old female (right panel) LMP1+Sle1+/+, LMP1+Sle3+/+ and non-tg LM was analyzed for IL-6 by ELISA.
B. T-depleted splenocytes were isolated from 4–6 month old female LMP1+Sle1+/+, LMP1+Sle3+/+, and non-tg littermates. Cells were stimulated in triplicate with agonistic anti-IgM or anti-mCD40 Abs, or Hi5-mCD154 cells for 24h. Culture supernatants were analyzed for IL-6 by quantitative ELISA. Data are representative of three separate experiments. ** = p< 0.0001 * = p< 0.05 by two-tailed Student’s t-test.
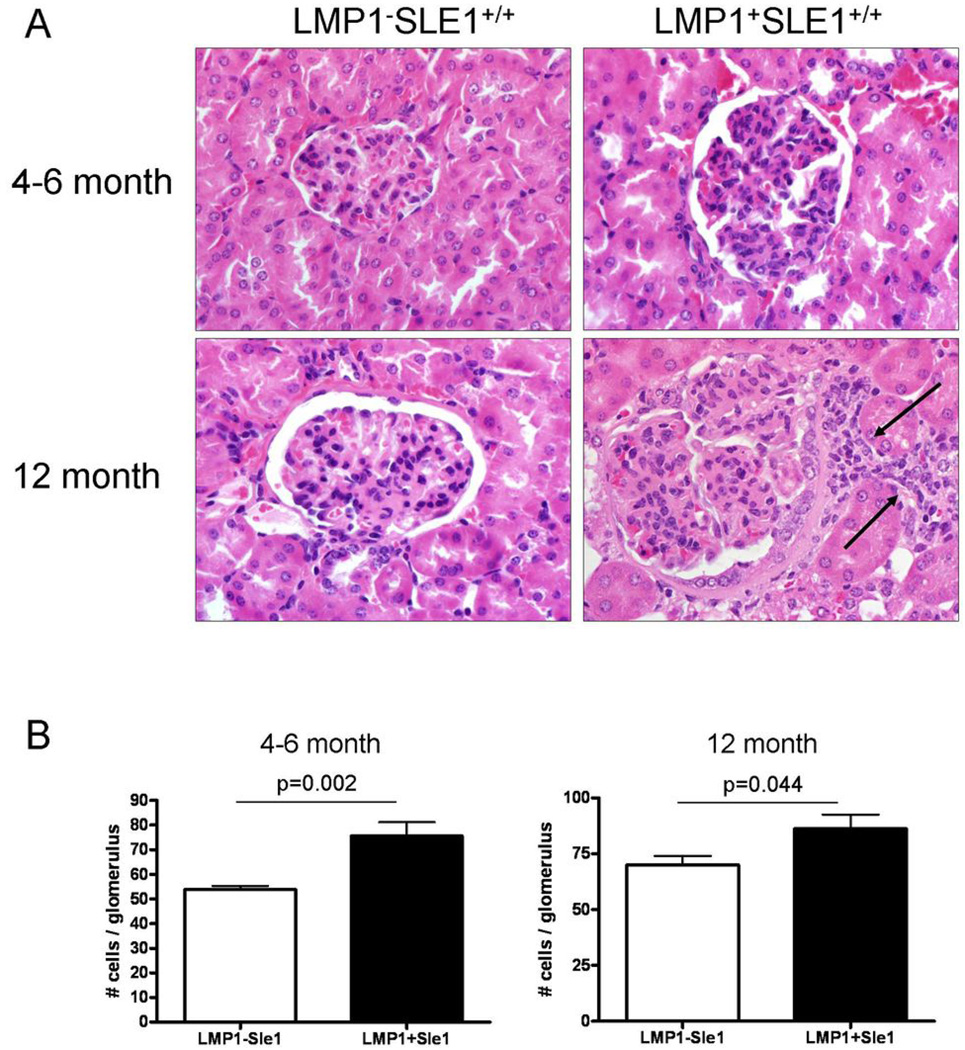
Kidney pathology in LMP1+Sle1+/+ mice
While B6.Sle1 mice have high levels of anti-histone and anti-chromatin Ab, neither proteinuria nor immune complex deposition is noted even in kidneys of aged mice, and less than 20% of B6.Sle1 mice display evidence of mild glomerulonephritis (20). LMP1 signals clearly enhance various events contributing to autoimmune disease in B6.Sle1 mice, so we next examined LMP1+Sle1+/+ female mice for evidence of enhanced nephritis compared to non-tg LM at 4–6 months and 12 months of age. While consistent differences in proteinuria were not observed (not shown), histopathologic examination showed differences between the LMP1+SLE1+/+ and non-tg LM groups at both time points. Kidneys from 4–6 month old and 12 month old LMP1+SLE1+/+ groups had glomeruli with noticeably increased cellularity (Figure 6A) confirmed by morphometric analysis (Figure 6B). Additional lesions preferentially detected in the LMP1+SLE1+/+ groups included mild to moderate interstitial lymphoplasmacytic infiltrates (Figure 6A, arrows), glomerular hypertrophy, periglomerular fibrosis, and increased eosinophilic mesangial matrix.
Figure 6. Kidney pathology in female LMP1+Sle1+/+ vs. non-tg LM mice.
A. Representative 5 µm H+E stained kidney sections from 4–6 month old (top panels) and 12 month old (lower panels) LMP1−Sle1+/+ (left column) and LMP1+Sle1+/+ (right column) female mice at 60X magnification. Arrows denote mild to moderate interstitial lymphoplasmacytic infiltrates preferentially detected in LMP1+Sle1+/+ kidneys.
B. Morphometric analysis of average glomerular cellularity in 4–6 month and 12 month old LMP1+Sle1+/+ and non-tg LM was performed as described in Methods. 10 kidneys per genotype were analyzed per time point by a pathologist in a blinded fashion. P-values were obtained by two-tailed Student’s t-test.
Discussion
Greater than 90% of humans are latently infected with EBV, but of course this does not in itself lead to autoimmune disease. However, transient re-expression of LMP1 has been associated with flares of human SLE, and may exacerbate the disease, particularly in genetically predisposed individuals (10). Thus, to examine the potential ability of LMP1 signaling to cooperate with the products of other autoimmune disease-related gene clusters in exacerbating autoimmunity, we studied its effects in two strains of SLE-prone mice, B6.Sle1 and B6.Sle3, which express specific subsets of autoimmunity-predisposing genes. LMP1+Sle1+/+ mice displayed evidence of enhanced autoreactivity, including increased lymphoid organ size, and kidney pathology. Mechanisms contributing to this enhanced autoimmunity include number and frequency of activated T and B cells, spontaneous GC formation, anti-histone Ab production, and enhanced IL-6 production and CD86 upregulation following LMP1 signaling to B cells. We did not detect any significant differences in cytokine production by CD4+ T cells following stimulation with anti-CD3 + anti-CD28 Abs in vitro, including IL-2, IL-4, IL-6, IL-17, or IFN-γ by LMP1−Sle1+/+ vs. LMP1+Sle1+/+ (data not shown). These data support the conclusion that the effect of LMP1 on autoimmunity is primarily B cell-intrinsic, with LMP1-activated Sle1+/+ B cells up-regulating CD86 to a higher degree than their non-tg counterparts, which in turn drives increased activation of CD4+ T cells in vivo. These activated T cells can then further amplify dysregulated B cell activation, enhancing GC formation and autoantibody production. In sharp contrast to the B6.Sle1 strain, there was no effect of LMP1 signaling on autoimmunity in vitro or in vivo in the B6.Sle3 strain. Thus LMP1 cooperated with the products of genes in the B6.Sle1, but not Sle3 interval to enhance autoimmunity.
Many genes of immunologic interest reside within the B6.Sle3 locus, including CD22, tristetraprolin, TGF-β, and Bcl3 (20, 25). However, to date no allelic differences between C57BL/6 and NZM2410 have been found. Thus the genes responsible for the B6.Sle3 autoimmune phenotype have yet to be identified. Our studies show that LMP1 signaling does not cooperate with Sle3 interval gene products in vivo or in vitro. One interpretation of our results is that LMP1 cannot signal at all in the B6.Sle3 background, due to mutations in required yet unidentified genes. However, in vitro activation of mitogen- and stress-activated protein kinases by LMP1 signaling was similar between LMP1+Sle1+/+ and LMP1+Sle3+/+ B cells (data not shown), suggesting that early events in LMP1 signaling to B cells are intact in both strains. An alternative explanation is that the Sle3 gene products mainly alter the function of DC, and LMP1 signals predominantly alter B cells, and their interactions with T cells.
Several candidate genes within the B6.Sle1 interval that predispose to autoimmunity have been identified, and include polymorphisms in complement receptor 2 (CR2) as well as the SAP/SLAM family member Ly108 (40, 41). The NZM2410 alleles are thought to contribute to decreased strength of BCR signaling during B cell development in the bone marrow, allowing autoreactive cells to escape apoptosis and populate peripheral lymphoid tissues (40, 41). Ly108 in particular is expressed on both B and T cells, where it plays a role in cell adhesion and costimulation of TCR signaling (41–43). Although expression of Ly108 was increased in B6.Sle1 B cells ex vivo, there was no additional detectable increase of Ly108 expression on LMP1+Sle1+/+ B cells (data not shown). Furthermore, stimulation with CD40 agonists (which will signal through both endogenous CD40 and the chimeric mCD40-LMP1 protein) in vitro did not further increase Ly108 expression in LMP1+Sle1+/+ vs. LMP1−Sle1+/+ B cells (data not shown). Based on these results, we conclude that LMP1 signaling did not enhance activation either in vitro or in vivo by affecting Ly108 expression in B6.Sle1 B cells.
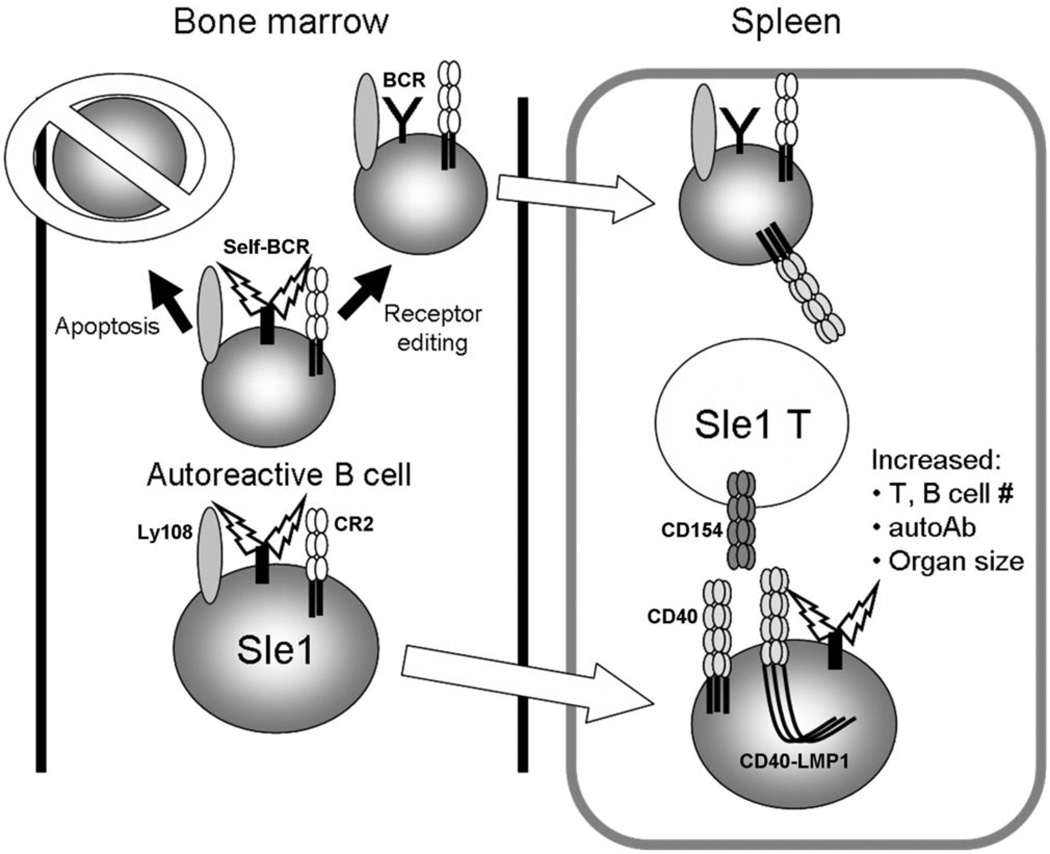
Therefore, LMP1 and B6.Sle1 genes may be acting in distinct yet cooperative pathways as proposed in the following model (Figure 7). Ly108 and CR2 are thought to function during B cell development in the bone marrow where these receptors fine-tune BCR signaling (40, 41). During B cell selection, a strong BCR signal is indicative of an autoreactive cell and leads to apoptosis. The NZM2410 alleles of Ly108 and CR2 lead to weaker signals through the BCR, which may allow autoreactive B cells to escape deletion and populate peripheral lymphoid tissues (40, 41). Once in the periphery, signaling by LMP1, whose re-expression from latency may be transiently triggered by inflammation, enhances upregulation of CD86, which further costimulates T cells. LMP1 signaling in the B6.Sle1 background also leads to enhanced B cell activation, which aids in plasma cell formation and contributes to autoimmunity (15). LMP1 cooperates with Sle1 interval genes in a quantitative manner as outlined above, including the production of anti-dsDNA/histone and anti-dsDNA IgG, yet is unable to induce kidney disease resulting in consistent, severe proteinuria (data not shown). These events may require other SLE genes (i.e. Sle3) or EBV-encoded genes expressed during viral reactivation (i.e. LMP2A, BZLF1)(10).
Figure 7. Model of LMP1 cooperation with Sle1 interval genes.
During B cell development, autoreactive B cells receiving strong signals through the BCR (aided by Ly108 and CR2) are deleted by apoptosis or undergo receptor editing prior to populating peripheral lymphoid tissues. However, B6.Sle1 B cells carry mutations in CR2 and Ly108. These receptors send a weaker BCR signal, allowing potentially autoreactive cells to escape deletion and populate lymphoid tissues without undergoing receptor editing. Once in the periphery, constitutive signals through transiently expressed LMP1 provide additional stimulatory effects, resulting in enhanced increases in organ size, number of activated T and B cells, spontaneous GC formation, and autoantibody production
This study demonstrates that the EBV-encoded CD40 mimic, LMP1, can exacerbate autoimmunity in vivo by cooperating with the products of specific subsets of predisposing genes, whose specific identification is an important future goal. Our results suggest that LMP1 expression during a SLE flare exacerbates autoimmunity via multiple mechanisms, including direct effects on B cell activation, enhancement of B cell costimulatory properties, and resultant enhanced T cell activation, which can in turn further activate B cells. Because SLE susceptibility genes within the B6.Sle1 interval have also been identified in humans, inhibiting LMP1 signaling during disease flares may serve as a potent therapeutic target for SLE treatment.
Acknowledgments
The authors wish to thank Drs. John Colgan and Bruce S. Hostager for helpful discussions during preparation of this manuscript.
Footnotes
This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Health, Services Research and Development, Merit Review Award 383 (to G.A.B.). G.A.B. is supported by grants from the National Institutes of Health (AI28847, AI49993, CA099997). A.L.P. is supported by a predoctoral fellowship from the American Heart Association (0815735G).
Abbreviations used: chromosome (chr), Latent Membrane Protein 1 (LMP1), T-depleted splenocytes (TDS), lymph node (LN), littermate (LM), germinal center (GC), not significant (ns).
References
- 1.Thorley-Lawson DA. EBV the prototypical human tumor virus--just how bad is it? J Allergy Clin Immunol. 2005;116:251–261. doi: 10.1016/j.jaci.2005.05.038. quiz 262. [DOI] [PubMed] [Google Scholar]
- 2.James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–467. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- 3.James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997;100:3019–3026. doi: 10.1172/JCI119856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James JA, Neas BR, Moser KL, Hall T, Bruner GR, Sestak AL, Harley JB. Systemic lupus erythematosus in adults is associated with previous Epstein-Barr virus exposure. Arthritis Rheum. 2001;44:1122–1126. doi: 10.1002/1529-0131(200105)44:5<1122::AID-ANR193>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.Katz BZ, Salimi B, Kim S, Nsiah-Kumi P, Wagner-Weiner L. Epstein-Barr virus burden in adolescents with systemic lupus erythematosus. Pediatr Infect Dis J. 2001;20:148–153. doi: 10.1097/00006454-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Chen CJ, Lin KH, Lin SC, Tsai WC, Yen JH, Chang SJ, Lu SN, Liu HW. High prevalence of immunoglobulin A antibody against Epstein-Barr virus capsid antigen in adult patients with lupus with disease flare: case control studies. J Rheumatol. 2005;32:44–47. [PubMed] [Google Scholar]
- 7.Tsokos GC, Magrath IT, Balow JE. Epstein-Barr virus induces normal B cell responses but defective suppressor T cell responses in patients with systemic lupus erythematosus. J Immunol. 1983;131:1797–1801. [PubMed] [Google Scholar]
- 8.Moon UY, Park SJ, Oh ST, Kim WU, Park SH, Lee SH, Cho CS, Kim HY, Lee WK, Lee SK. Patients with systemic lupus erythematosus have abnormally elevated Epstein-Barr virus load in blood. Arthritis Res Ther. 2004;6:R295–R302. doi: 10.1186/ar1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang I, Quan T, Nolasco H, Park SH, Hong MS, Crouch J, Pamer EG, Howe JG, Craft J. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol. 2004;172:1287–1294. doi: 10.4049/jimmunol.172.2.1287. [DOI] [PubMed] [Google Scholar]
- 10.Gross AJ, Hochberg D, Rand WM, Thorley-Lawson DA. EBV and SLE: A new perspective. J Immunol. 2005;174:6599–6607. doi: 10.4049/jimmunol.174.11.6599. [DOI] [PubMed] [Google Scholar]
- 11.Bishop GA. The multifaceted roles of TRAFs in the regulation of B cell function. Nat Rev Immunol. 2004;4:775–786. doi: 10.1038/nri1462. [DOI] [PubMed] [Google Scholar]
- 12.Thorley-Lawson DA. EBV: exploiting the immune system. Nature Rev. Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- 13.Busch LK, Bishop GA. The EBV transforming protein, LMP1, mimics and cooperates with CD40 signaling in B lymphocytes. J. Immunol. 1999;162:2555–2561. [PubMed] [Google Scholar]
- 14.Brown KD, Hostager BS, Bishop GA. Differential signaling and TRAF degradation by CD40 and the EBV oncoprotein LMP1. J. Exp. Med. 2001;193:943–954. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stunz LL, Busch LK, Munroe ME, Tygrett L, Sigmund C, Waldschmidt TW, Bishop GA. Expression of the LMP1 cytoplasmic tail in mice induces hyperactivation of B lymphocytes and disordered lymphoid architecture. Immunity. 2004;21:255–266. doi: 10.1016/j.immuni.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Kraus ZJ, Nakano H, Bishop GA. TRAF5 is a critical mediator of in vitro signals and in vivo functions of LMP1, the viral oncogenic mimic of CD40. Proc Natl Acad Sci U S A. 2009;106:17140–17145. doi: 10.1073/pnas.0903786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Munroe ME, Arbiser JL, Bishop GA. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J Immunol. 2007;179:753–763. doi: 10.4049/jimmunol.179.2.753. [DOI] [PubMed] [Google Scholar]
- 18.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 19.Morel L, Rudofsky UH, Longmate JA, Schiffenbauer J, Wakeland EK. Polygenic control of susceptibility to murine systemic lupus erythematosus. Immunity. 1994;1:219–229. doi: 10.1016/1074-7613(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 20.Morel L, Mohan C, Yu Y, Croker BP, Tian N, Deng A, Wakeland EK. Functional dissection of systemic lupus erythematosus using congenic mouse strains. J Immunol. 1997;158:6019–6028. [PubMed] [Google Scholar]
- 21.Mohan C, Morel L, Yang P, Wakeland EK. Genetic dissection of systemic lupus erythematosus pathogenesis: Sle2 on murine chromosome 4 leads to B cell hyperactivity. J Immunol. 1997;159:454–465. [PubMed] [Google Scholar]
- 22.Sobel ES, Mohan C, Morel L, Schiffenbauer J, Wakeland EK. Genetic dissection of SLE pathogenesis: adoptive transfer of Sle1 mediates the loss of tolerance by bone marrow-derived B cells. J Immunol. 1999;162:2415–2421. [PubMed] [Google Scholar]
- 23.Mohan C, Alas E, Morel L, Yang P, Wakeland EK. Genetic dissection of SLE pathogenesis. Sle1 on murine chromosome 1 leads to a selective loss of tolerance to H2A/H2B/DNA subnucleosomes. J Clin Invest. 1998;101:1362–1372. doi: 10.1172/JCI728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobel ES, Satoh M, Chen Y, Wakeland EK, Morel L. The major murine systemic lupus erythematosus susceptibility locus Sle1 results in abnormal functions of both B and T cells. J Immunol. 2002;169:2694–2700. doi: 10.4049/jimmunol.169.5.2694. [DOI] [PubMed] [Google Scholar]
- 25.Mohan C, Yu Y, Morel L, Yang P, Wakeland EK. Genetic dissection of Sle pathogenesis: Sle3 on murine chromosome 7 impacts T cell activation, differentiation, and cell death. J. Immunol. 1999;162:6492–6502. [PubMed] [Google Scholar]
- 26.Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, Wakeland EK, Mohan C. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005;115:1869–1878. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morel L, Croker BP, Blenman KR, Mohan C, Huang G, Gilkeson G, Wakeland EK. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci U S A. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohan C, Morel L, Yang P, Watanabe H, Croker B, Gilkeson G, Wakeland EK. Genetic dissection of lupus pathogenesis: a recipe for nephrophilic autoantibodies. J Clin Invest. 1999;103:1685–1695. doi: 10.1172/JCI5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wakui M, Morel L, Butfiloski EJ, Kim C, Sobel ES. Genetic dissection of systemic lupus erythematosus pathogenesis: partial functional complementation between Sle1 and Sle3/5 demonstrates requirement for intracellular coexpression for full phenotypic expression of lupus. J Immunol. 2005;175:1337–1345. doi: 10.4049/jimmunol.175.2.1337. [DOI] [PubMed] [Google Scholar]
- 30.Xie P, Hostager BS, Bishop GA. Requirement for TRAF3 in signaling by LMP1, but not CD40, in B lymphocytes. J Exp Med. 2004;199:661–671. doi: 10.1084/jem.20031255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baccam M, Bishop GA. Membrane-bound CD154, but not anti-CD40 mAbs, induces NF-κB independent B cell IL-6 production. Eur. J. Immunol. 1999;29:3855–3866. doi: 10.1002/(SICI)1521-4141(199912)29:12<3855::AID-IMMU3855>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Waldschmidt TJ, Panoskaltsis-Mortari A, McElmurry RT, Tygrett LT, Taylor PA, Blazar BR. Abnormal T cell-dependent B-cell responses in SCID mice receiving allogeneic bone marrow in utero. Severe combined immune deficiency. Blood. 2002;100:4557–4564. doi: 10.1182/blood-2002-04-1232. [DOI] [PubMed] [Google Scholar]
- 33.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 34.Liu K, Mohan C. What do mouse models teach us about human SLE? Clin Immunol. 2006;119:123–130. doi: 10.1016/j.clim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 36.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired Ig class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 37.Kishimoto T. Interleukin-6: from basic science to medicine--40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 38.Liu K, Liang C, Liang Z, Tus K, Wakeland EK. Sle1ab mediates the aberrant activation of STAT3 and Ras-ERK signaling pathways in B lymphocytes. J Immunol. 2005;174:1630–1637. doi: 10.4049/jimmunol.174.3.1630. [DOI] [PubMed] [Google Scholar]
- 39.Wan S, Xia C, Morel L. IL-6 produced by DC from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J. Immunol. 2007;178:271–279. doi: 10.4049/jimmunol.178.1.271. [DOI] [PubMed] [Google Scholar]
- 40.Boackle SA, Holers VM, Chen X, Szakonyi G, Karp DR, Wakeland EK, Morel L. Cr2, a candidate gene in the murine Sle1c lupus susceptibility locus, encodes a dysfunctional protein. Immunity. 2001;15:775–785. doi: 10.1016/s1074-7613(01)00228-x. [DOI] [PubMed] [Google Scholar]
- 41.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 42.Zhong MC, Veillette A. Control of T lymphocyte signaling by Ly108, a signaling lymphocytic activation molecule family receptor implicated in autoimmunity. J Biol Chem. 2008;283:19255–19264. doi: 10.1074/jbc.M800209200. [DOI] [PubMed] [Google Scholar]
- 43.Chan AY, Westcott JM, Mooney JM, Wakeland EK, Schatzle JD. The role of SAP and the SLAM family in autoimmunity. Curr Opin Immunol. 2006;18:656–664. doi: 10.1016/j.coi.2006.09.010. [DOI] [PubMed] [Google Scholar]