Abstract
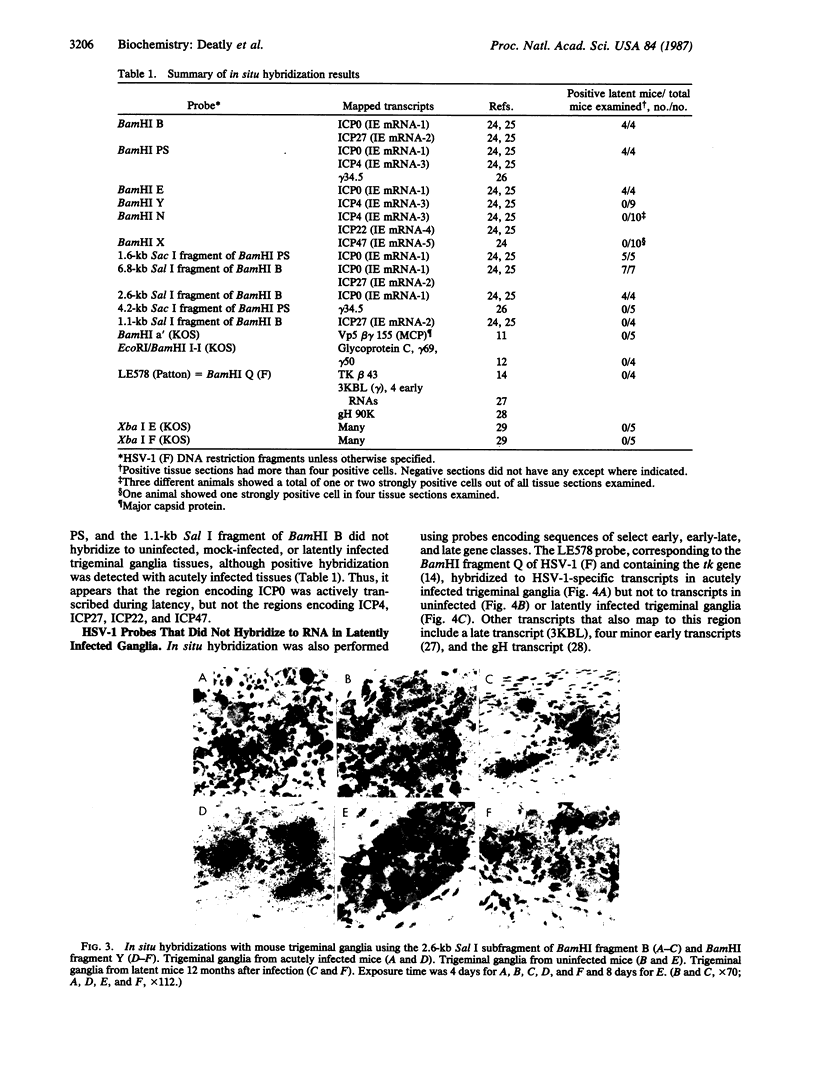
Transcription of the type 1 herpes simplex virus (HSV-1) genome in trigeminal ganglia of latently infected mice was studied using in situ hybridization. Probes representative of each temporal gene class were used to determine the regions of the genome that encode the transcripts present in latently infected cells. Probes encoding HSV-1 sequences of the five immediate early genes and representative early (thymidine kinase), early-late (major capsid protein), and late (glycoprotein C) genes were used in these experiments. Of the probes tested, only those encoding the immediate early gene product infected-cell polypeptide (ICP) 0 hybridized to RNA in latently infected tissues. Probes containing the other immediate early genes (ICP4, ICP22, ICP27, and ICP47) and the representative early, early-late, and late genes did not hybridize. Two probes covering approximately equal to 30% of the HSV-1 genome and encoding over 20 early and late transcripts also did not hybridize to RNA in latently infected tissues. These results, with probes spanning greater than 60% of the HSV-1 genome, suggest that transcription of the HSV-1 genome is restricted to one region in latently infected mouse trigeminal ganglia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Chou J., Sarmiento M., Lerner R. A., Roizman B. Identification by antibody to a synthetic peptide of a protein specified by a diploid gene located in the terminal repeats of the L component of herpes simplex virus genome. J Virol. 1986 Jun;58(3):843–850. doi: 10.1128/jvi.58.3.843-850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. B., McLauchlan J., McGeoch D. J. Orientation of herpes simplex virus type 1 immediate early mRNA's. Nucleic Acids Res. 1979 Sep 11;7(1):77–91. doi: 10.1093/nar/7.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Devi B. G., Anderson K. P., Gaylord B. H., Wagner E. K. Characterization of a major late herpes simplex virus type 1 mRNA. J Virol. 1981 May;38(2):483–496. doi: 10.1128/jvi.38.2.483-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist L. W., Madden M. J., Schiop-Stanley P., Vande Woude G. F. Cloning of herpes simplex type 1 DNA fragments in a bacteriophage lambda vector. Science. 1979 Feb 9;203(4380):541–544. doi: 10.1126/science.216076. [DOI] [PubMed] [Google Scholar]
- Frink R. J., Anderson K. P., Wagner E. K. Herpes simplex virus type 1 HindIII fragment L encodes spliced and complementary mRNA species. J Virol. 1981 Aug;39(2):559–572. doi: 10.1128/jvi.39.2.559-572.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C. M., McDougall J. K. Limited transcription of the herpes simplex virus genome when latent in human sensory ganglia. J Virol. 1982 Feb;41(2):686–691. doi: 10.1128/jvi.41.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Gelman I. H., Silverstein S. Co-ordinate regulation of herpes simplex virus gene expression is mediated by the functional interaction of two immediate early gene products. J Mol Biol. 1986 Oct 5;191(3):395–409. doi: 10.1016/0022-2836(86)90135-x. [DOI] [PubMed] [Google Scholar]
- Gomez-Marquez J., Puga A., Notkins A. L. Regions of the terminal repetitions of the herpes simplex virus type 1 genome. Relationship to immunoglobulin switch-like DNA sequences. J Biol Chem. 1985 Mar 25;260(6):3490–3495. [PubMed] [Google Scholar]
- Green M. T., Courtney R. J., Dunkel E. C. Detection of an immediate early herpes simplex virus type 1 polypeptide in trigeminal ganglia from latently infected animals. Infect Immun. 1981 Dec;34(3):987–992. doi: 10.1128/iai.34.3.987-992.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Hyman R. W. Sequences in the proximal IRL of herpes simplex virus DNA hybridize to human DNA. Virus Res. 1986 Jun;4(4):369–375. doi: 10.1016/0168-1702(86)90083-3. [DOI] [PubMed] [Google Scholar]
- Jones T. R., Parks C. L., Spector D. J., Hyman R. W. Hybridization of herpes simplex virus DNA and human ribosomal DNA and RNA. Virology. 1985 Jul 30;144(2):384–397. doi: 10.1016/0042-6822(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Mackem S., Roizman B. Regulation of herpesvirus macromolecular synthesis: transcription-initiation sites and domains of alpha genes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7122–7126. doi: 10.1073/pnas.77.12.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N. J., Kinross J. H., Busuttil A., Ludgate S. M., Smart G. E., Jones K. W. The detection of DNA tumour virus-specific RNA sequences in abnormal human cervical biopsies by in situ hybridization. J Gen Virol. 1981 Jul;55(Pt 1):123–137. doi: 10.1099/0022-1317-55-1-123. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J., Davison A. J. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 1986 May 27;14(10):4281–4292. doi: 10.1093/nar/14.10.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Post L. E., Conley A. J., Mocarski E. S., Roizman B. Cloning of reiterated and nonreiterated herpes simplex virus 1 sequences as BamHI fragments. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4201–4205. doi: 10.1073/pnas.77.7.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Cantin E. M., Notkins A. L. Homology between murine and human cellular DNA sequences and the terminal repetition of the S component of herpes simplex virus type 1 DNA. Cell. 1982 Nov;31(1):81–87. doi: 10.1016/0092-8674(82)90407-x. [DOI] [PubMed] [Google Scholar]
- Rock D. L., Fraser N. W. Detection of HSV-1 genome in central nervous system of latently infected mice. Nature. 1983 Apr 7;302(5908):523–525. doi: 10.1038/302523a0. [DOI] [PubMed] [Google Scholar]
- Sears A. E., Meignier B., Roizman B. Establishment of latency in mice by herpes simplex virus 1 recombinants that carry insertions affecting regulation of the thymidine kinase gene. J Virol. 1985 Aug;55(2):410–416. doi: 10.1128/jvi.55.2.410-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. A., Wagner M. J., Summers W. C. Transcription of herpes simplex virus genes in vivo: overlap of a late promoter with the 3' end of the early thymidine kinase gene. J Virol. 1983 Jan;45(1):10–17. doi: 10.1128/jvi.45.1.10-17.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop W. G., Rock D. L., Fraser N. W. Localization of herpes simplex virus in the trigeminal and olfactory systems of the mouse central nervous system during acute and latent infections by in situ hybridization. Lab Invest. 1984 Jul;51(1):27–38. [PubMed] [Google Scholar]
- Tenser R. B., Dawson M., Ressel S. J., Dunstan M. E. Detection of herpes simplex virus mRNA in latently infected trigeminal ganglion neurons by in situ hybridization. Ann Neurol. 1982 Mar;11(3):285–291. doi: 10.1002/ana.410110309. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Umene K., Sakaki Y., Mori R., Takagi Y. Isolation of human DNAs homologous to the BamHI-Z fragment of herpes simplex virus type 1 DNA. Gene. 1984 Nov;31(1-3):9–16. doi: 10.1016/0378-1119(84)90189-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Walz M. A., Notkins A. L. Viral-specific thymidine kinase in sensory ganglia of mice infected with herpes simplex virus. Virology. 1977 Feb;76(2):866–869. doi: 10.1016/0042-6822(77)90267-7. [DOI] [PubMed] [Google Scholar]