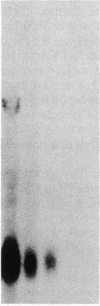
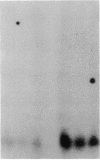
Abstract
Cellular retinol-binding protein (CRBP) may be an important mediator of vitamin A action. We report here the identification of a cDNA clone corresponding to the rat CRBP gene. The cDNA is 695 nucleotides long, with an open reading frame corresponding to a protein of 134 amino acids. The deduced amino acid sequence is identical with that of rat CRBP. The nucleotide sequence shows 90.5% similarity with the human CRBP cDNA sequence. Genomic DNA analysis indicates that CRBP is present in one, or at most two, copies within the rat genome. Analysis of mRNA reveals a single species in every tissue tested and suggests that the isolated cDNA is full-length. Finally, when retinol-deficient rats are fed retinyl acetate for 4 hr, about 4-fold accumulation of CRBP-specific mRNA is observed in the lungs. This rapid effect suggests that the micronutrient retinol may directly influence the expression of its specific intracellular binding protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appling D. R., Chytil F. Evidence of a role for retinoic acid (vitamin A-acid) in the maintenance of testosterone production in male rats. Endocrinology. 1981 Jun;108(6):2120–2124. doi: 10.1210/endo-108-6-2120. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashor M. M., Toft D. O., Chytil F. In vitro binding of retinol to rat-tissue components. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3483–3487. doi: 10.1073/pnas.70.12.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Chytil F. Retinoic acid: biochemistry, pharmacology, toxicology, and therapeutic use. Pharmacol Rev. 1984 Jun;36(2 Suppl):93S–100S. [PubMed] [Google Scholar]
- Colantuoni V., Cortese R., Nilsson M., Lundvall J., Båvik C. O., Eriksson U., Peterson P. A., Sundelin J. Cloning and sequencing of a full length cDNA corresponding to human cellular retinol-binding protein. Biochem Biophys Res Commun. 1985 Jul 16;130(1):431–439. doi: 10.1016/0006-291x(85)90435-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C., Gouy M., Jacobzone M., Mercier R. Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res. 1981 Jan 10;9(1):r43–r74. doi: 10.1093/nar/9.1.213-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984 Mar 15;308(5956):241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Lamb A. J., Apiwatanaporn P., Olson J. A. Induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr. 1974 Sep;104(9):1140–1148. doi: 10.1093/jn/104.9.1140. [DOI] [PubMed] [Google Scholar]
- Liau G., Ong D. E., Chytil F. Interaction of the retinol/cellular retinol-binding protein complex with isolated nuclei and nuclear components. J Cell Biol. 1981 Oct;91(1):63–68. doi: 10.1083/jcb.91.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Omori M., Chytil F. Mechanism of vitamin A action. Gene expression in retinol-deficient rats. J Biol Chem. 1982 Dec 10;257(23):14370–14374. [PubMed] [Google Scholar]
- Ong D. E., Crow J. A., Chytil F. Radioimmunochemical determination of cellular retinol- and cellular retinoic acid-binding proteins in cytosols of rat tissues. J Biol Chem. 1982 Nov 25;257(22):13385–13389. [PubMed] [Google Scholar]
- Porter S. B., Fraker L. D., Chytil F., Ong D. E. Localization of cellular retinol-binding protein in several rat tissues. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6586–6590. doi: 10.1073/pnas.80.21.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sundelin J., Anundi H., Trägårdh L., Eriksson U., Lind P., Ronne H., Peterson P. A., Rask L. The primary structure of rat liver cellular retinol-binding protein. J Biol Chem. 1985 May 25;260(10):6488–6493. [PubMed] [Google Scholar]
- THOMPSON J. N., HOWELL J. M., PITT G. A. VITAMIN A AND REPRODUCTION IN RATS. Proc R Soc Lond B Biol Sci. 1964 Feb 18;159:510–535. doi: 10.1098/rspb.1964.0017. [DOI] [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Cellular retinol-binding protein allows specific interaction of retinol with the nucleus in vitro. Proc Natl Acad Sci U S A. 1979 May;76(5):2204–2208. doi: 10.1073/pnas.76.5.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takase S., Ong D. E., Chytil F. Cellular retinol-binding protein allows specific interaction of retinol with the nucleus in vitro. Proc Natl Acad Sci U S A. 1979 May;76(5):2204–2208. doi: 10.1073/pnas.76.5.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]