Abstract
Antibiotic resistance is an increasing global problem resulting from the pressure of antibiotic usage, greater mobility of the population, and industrialization. Many antibiotic resistance genes are believed to have originated in microorganisms in the environment, and to have been transferred to other bacteria through mobile genetic elements. Among others, β-lactam antibiotics show clinical efficacy and low toxicity, and they are thus widely used as antimicrobials. Resistance to β-lactam antibiotics is conferred by β-lactamase genes and penicillin-binding proteins, which are chromosomal- or plasmid-encoded, although there is little information available on the contribution of other mobile genetic elements, such as phages. This study is focused on three genes that confer resistance to β-lactam antibiotics, namely two β-lactamase genes (blaTEM and blaCTX-M9) and one encoding a penicillin-binding protein (mecA) in bacteriophage DNA isolated from environmental water samples. The three genes were quantified in the DNA isolated from bacteriophages collected from 30 urban sewage and river water samples, using quantitative PCR amplification. All three genes were detected in the DNA of phages from all the samples tested, in some cases reaching 104 gene copies (GC) of blaTEM or 102 GC of blaCTX-M and mecA. These values are consistent with the amount of fecal pollution in the sample, except for mecA, which showed a higher number of copies in river water samples than in urban sewage. The bla genes from phage DNA were transferred by electroporation to sensitive host bacteria, which became resistant to ampicillin. blaTEM and blaCTX were detected in the DNA of the resistant clones after transfection. This study indicates that phages are reservoirs of resistance genes in the environment.
Introduction
Recognized as a global problem [1], antibiotic resistance increases the morbidity and mortality caused by bacterial infections, as well as the cost of treating infectious diseases. The threat from resistance (particularly multiple resistance in bacterial strains that are widely disseminated) is serious. The key factors contributing to this threat are the pressure of increased antibiotic usage (in both human and animal medicine), greater mobility of the population and industrialization [2], [3]. Many potentially life-threatening infections, generally regarded as diseases from the past due to the success of antibiotics and vaccines, have returned as resistance increasingly hampers successful therapy and prophylaxis [4].
Microorganisms produce many antimicrobials in nature [5], [6]. These antibiotic-producing organisms have also become resistant to the antibiotics they produce, and the genes that confer such resistance can be transferred to other non-resistant bacteria. The presence of antibiotics in the environment may provide long-term selective pressure for the emergence and transmission of these resistance-conferring genes in non-producing organisms [5], [7]. Given that many genera found in diverse environments carry resistance determinants [6], it is feasible that antibiotic-resistance genes have originated in the environment and that they could have been transferred from the environment to pathogenic bacteria, which are currently found in clinical settings. [8]. The transfer from the environment to clinical settings might have occurred through horizontal gene transfer, which is the most effective mechanism to accelerate the dispersal of antibiotic-resistance genes. The mobile genetic elements (MGEs) for the horizontal transfer of such genes most commonly studied are plasmids, transposons or, as a few reports suggest, bacteriophages [9]–[11].
Several studies have focused on antibiotic resistance codification in plasmids or transposons, and there is also interesting information about the extent of antibiotic resistance genes in a given environment (the so-called “resistome”) [8], [12]. However, there is less information on the potential contribution of phages to antibiotic resistance-gene transfer, despite calls for research in this field. Recent reports [2], [11] conclude that the horizontal transfer of genetic information by phages is much more prevalent than previously thought, and that the environment plays a crucial role in the phage-mediated transfer of antibiotic-resistance genes [2], [13]. Since many antibiotic resistance genes are plasmid-encoded, much effort has been devoted to the study of plasmids and less to the study of phages carrying genes for antibiotic resistance. However, many reports available suggest that phages can mobilize resistance genes and confer resistance, and some authors suggest that mobilization can occur through generalized transduction [14]–[18]. Only a few reports have analyzed antibiotic resistance genes in phage DNA isolated from wastewater environments [9], [19].
β-lactam antibiotics are characterized by clinical efficacy and low toxicity and they are thus widely used as antimicrobials. One mechanism of resistance to β-lactam antibiotics in Gram-negative bacilli involves the production of β-lactamases [3]. Among other Gram-negative bacteria, members of the family Enterobacteriaceae commonly express plasmid-encoded β-lactamases (e.g. TEM/SHV), which confer resistance to penicillins. More recently, extended-spectrum β-lactamases (ESBLs) evolved, conferring resistance to penicillins and oxymino-cephalosporins. EBSLs are sometimes mutant derivatives of TEM/SHV, but they are also mobilized from environmental bacteria (e.g. CTX-M) [20]. Most β-lactamases are acquired by horizontal gene transfer and the novel β-lactamase genes that emerge dramatically spread worldwide, causing both nosocomial and community-onset infections [3].
Resistance in Gram-positive bacteria is also widely distributed and increasing. This is the case for the emergence of community-associated methicillin-resistant Staphylococcus aureus (MRSA), a development that has blurred the distinction between hospital and community strains [21]. In S. aureus, mecA, a gene encoding for a penicillin-binding protein that confers resistance to methicillin, is located on a mobile genomic island, the Staphylococcal Cassette Chromosome mec (SCCmec) [22], [23]. In addition to the resistance genes carried on SCCmec, S. aureus can also harbor resistance genes on other sites of the genome, such as Tn554, as well as on plasmids [23]. Antibiotic use and environmental factors contribute to the emergence and spread of resistance in S. aureus, which is a common cause of serious and life-threatening infections.
Here we focused on two β-lactamases (bla TEM and bla CTX-M) and a penicillin-binding protein (mecA). bla TEM belongs to class A serine β-lactamases, which have been described in epidemiological studies; bla CTX-M and bla TEM are the most prevalent broad-spectrum β-lactamases and the most widely distributed enzymes worldwide [24]–[26]. mecA was included in this study because of the increasing incidence of infections caused by MRSA. The three genes were quantified by real-time PCR in the viral DNA fraction of water samples contaminated with fecal pollution. Since in most environments studied, phages are the main part of the viral fraction [27], it can be assumed that the DNA isolated from the viral fraction will belong mostly to bacteriophages. We sought to highlight the potential role of phages in the spread of these genes in the aquatic environment.
Results
Microbiological parameters
The numbers of aerobic bacteria and Escherichia coli were relatively homogeneous in all the urban sewage and river water samples tested (Table 1). These values were in accordance with previous water analyses from the same source [28]–[30]. River water samples showed significantly lower numbers (P<0.05) than urban sewage and these differences are attributed to the lower fecal input received by river water. The numbers of resistant bacteria were slightly lower than the total bacteria, as expected. Since bacteria are difficult to recover from the environment because of the stressed conditions of bacterial cells, the method and the low concentration of ampicillin (35 mg/ml) used were intended to prevent the inhibition of growth. Similar concentrations of ampicillin were reported before for the isolation of ampicillin-resistant bacteria [31]. We further tested 10% of all the colonies isolated in LB agar plates (35 mg/l) for sensitivity at higher concentrations of ampicillin (100 mg/l). At this concentration all the isolates were resistant to the antibiotic.
Table 1. Samples analyzed and microbiological parameters.
| Sample | Urban sewage | River | ||
| Average log10 CFU/ml | SD | Average log10 CFU/ml | SD | |
| N | 15 | 15 | ||
| Aerobic bacteria | 6.47 | 0.32 | 3.71 | 0.37 |
| E. coli | 4.75 | 0.64 | 1.22 | 0.56 |
| S. aureus | 2.29 | 0.36 | 1.88 | 0.11 |
| Aerobic bacteria apR | 6.22 | 0.24 | 3.12 | 0.45 |
| E. coli apR | 4.14 | 0.34 | 0.80 | 0.56 |
| S. aureus metR | 1.51 | 0.20 | 0.00 | - |
| Somatic coliphagesa | 4.43 | 0.30 | 2.42 | 0.39 |
PFU/ml.
To determine the number of S. aureus strains in the samples, 25% of the yellow-pigmented colonies obtained in each plate of agar 110 medium were further confirmed by catalase and with the Slidex Staph Plus kit. Depending on the plates, from 80% to 90% of the colonies were confirmed as S. aureus. The numbers of these bacteria presented (Table 1) are a correction of the percentage of positive colonies among the total number of yellow colonies detected in the agar plate. We detected S. aureus MRSA in sewage but not in river water.
Somatic coliphages, proposed as viral fecal indicators of pollution [29], were analyzed to determine the presence of bacteriophages infecting E. coli in the samples studied. As for bacterial indicators, the numbers of somatic coliphages were relatively homogeneous in all the samples tested (Table 1) and also in accordance with previous analyses of samples from the same source [28]–[30].
Direct observation of bacteriophages in sewage and river water
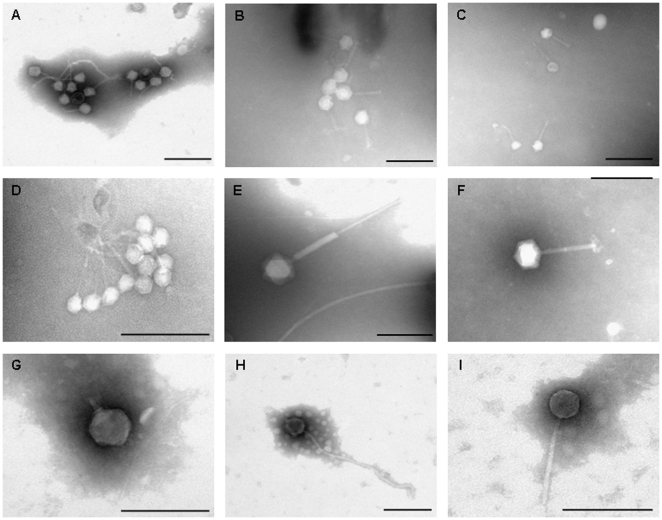
In addition to the evaluation of infectious somatic coliphages in the samples, direct observation of bacteriophages present in the water samples was conducted by electron microscopy. Tailed bacteriophages (Figure 1) belonging to different morphological types were observed, with a greater abundance of phages with contractile tail with Myoviridae morphology and non-contractile tail with Siphoviridae morphology. Variations in capsid and tail size were observed, as expected for bacteriophages that can infect different bacterial genera. Non-tailed virus particles were also observed, although in this case it could not be determined by morphology whether they were bacterial viruses or viruses infecting other hosts.
Figure 1. Electron micrographs of bacteriophages present in sewage and river water.
A–B. Group of phages with Myoviridae and Siphoviridae morphology from sewage. C. Myoviridae phages from river water. D: group of Siphoviridae phages from sewage. E–F. Myoviridae phages from sewage. G: Podoviridae phage from sewage. H–I. Siphoviridae phages from sewage and river water respectively. Bar 200 nm.
Antibiotic resistance genes in the phage and bacterial fraction of sewage and river water bla TEM genes
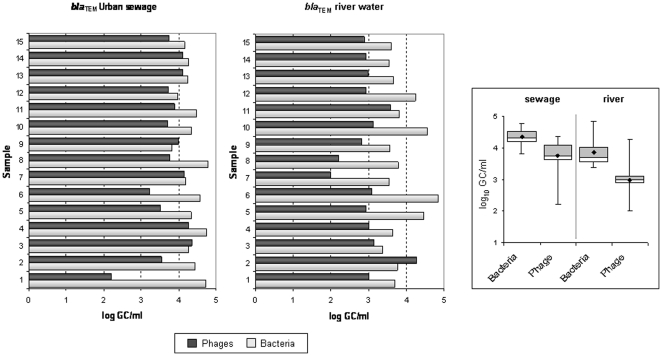
The set of primers and probe used [25], which included amplification of more than 145 TEM variants, allowed efficient screening of bla TEM genes in the environmental samples. From 102 to104 bla TEM gene copies (GC) were detected in the phage DNA fraction of one ml of urban sewage (Figure 2), while in river water the average was one order of magnitude lower. In both types of sample, these values indicate that phage DNA contains a large number of bla TEM gene copies. As explained in the methods section, a careful approach was performed to rule out that DNA from a non-viral origin was amplified in the qPCR, and controls were performed during phage DNA extraction. To this end, controls of the samples, taken after DNase treatment, but before the phage DNA was extracted from the capsid, were used as template for conventional PCR for eubacterial 16S rDNA and for qPCR for the three antibiotic resistance genes. These controls showed negative values for eubacterial 16SrDNA as well as for the three antibiotic resistance genes, which confirmed that the samples were free of bacterial DNA or non-encapsidated DNA, and that our results were due to amplification of DNA located within the viral particles. These controls were performed in all the samples tested.
Figure 2. Number of copies of bla TEM genes (GC/ml) in urban sewage and river water samples in phage and bacterial DNA.
On the left side of the figure, bar chart of the gene copies detected for each sample, dark grey for phage DNA and light grey for bacterial DNA. On the right side of the figure, the box plot chart shows the averaged values obtained from all samples from the same origin. Within the box plot chart, the cross-pieces of each box plot represent (from top to bottom) maximum, upper-quartile, median (black bar), lower-quartile, and minimum values. Black diamond shows the mean value. The grey boxes in the box plot chart include samples showing values within the 75th percentile and white boxes samples showing values within the within the 25th percentile.
The number of copies of bla TEM genes detected in the phage DNA fraction of the samples were, as expected, lower than in bacterial DNA; however, this difference was less than one order of magnitude (Figure 2). Differences in GC/ml found between bacterial and phage DNA were significant (P<0.05). Moreover, a few samples (Samples 3 and 9 in sewage and Sample 2 in river water, Figure 2) showed a higher concentration of bla TEM in phage DNA than in bacterial DNA.
bla CTX-M genes
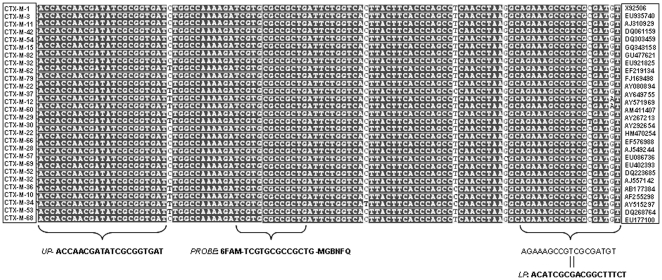
To our knowledge, quantitative real-time PCR probes that are universal for the most common variations of bla CTX-M genes have not previously been reported, and so a primer set for these genes was developed in this study. The nucleotide sequence for diverse bla CTX-M genes was aligned in a search for common sequences. As expected, the five clusters described for the CTX-M family did not share conserved regions (see references [32], [33] for review and presentation of a CTX-M cluster), so it was impossible to design a common qPCR for all the CTX-M variants. We selected Cluster 1 (composed of 31 variants described so far, including CTX-M-1, 3, 10, 11 and 15) [34], which is widespread in Europe and Spain [25], [35]. Alignment of some CTX-M Cluster 1 sequences (Figure 3) showed several regions from which primers and probe can be selected according to the requirements for the design of primers and probes for qPCR, established in the Primer Express Software version 3.0 (Applied Biosystems). The Taqman PCR assay developed was valid for quantitative measurements of all Cluster 1 CTX-M variants assayed, except CTX-M-12, 30 and 60, which did not match the sequence of the lower primer (Figure 3). Standard curves were repeatable and the amplification efficiency (E) of our reactions ranged from 95%-100%. Controls performed with several E. coli strains harboring different CTX-M genes from Cluster 1 confirmed the validity of the qPCR set designed.
Figure 3. Primers and TaqMan assay probe resulting from the alignment of bla CTX-M genes from Cluster 1.
Conserved nucleotides are marked in bold, non-conserved nucleotides in white. Sequence reverse and complementary is shown for lower primer. Right column indicate the GenBank accession number of each gene.
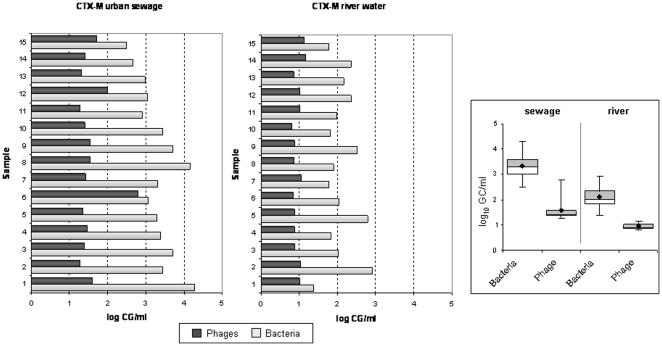
The number of copies of bla CTX-M detected in phage DNA in sewage ranged from 1.5 to 3 log10 units, while fewer than one log10 units were still detected in one ml of river water (Figure 4). Differences between the number of copies of the bla CTX-M genes in phage and bacterial DNA were significant (P<0.05) in both sewage and river water. The number of copies of the gene detected in phage DNA were from <1 to 2.5 log10 units lower than in bacterial DNA, with few exceptions (sewage sample 6). The number of copies of the bla CTX-M genes in bacterial DNA was as high as 4 log10 units in 1 ml of sewage and almost 3 log10 units in 1 ml of river water.
Figure 4. Number of copies of bla CTX-M genes (GC/ml) in urban sewage and river water samples in phage and bacterial DNA and box plot of averaged values.
mecA
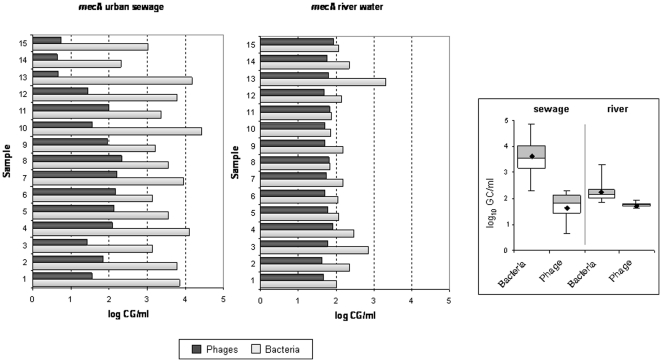
All samples showed the presence of mecA in either bacterial or phage DNA. While values in bacterial DNA were higher in sewage, in phage DNA the average and also inter-sample comparison showed that some samples of sewage presented lower values than river water samples. The variability of the mecA content in phage and bacterial DNA in urban sewage samples was greater than in river samples (Figure 5).
Figure 5. Number of copies of mecA (GC/ml) in urban sewage and river water samples in phage and bacterial DNA and box plot of averaged values.
Ability of phage-encoded genes to confer antibiotic resistance in bacterial strains
To evaluate whether the antibiotic resistance sequences in phage DNA correspond to potential active genes able to confer resistance in a bacterial background, phage DNA from sewage samples 6, 7 and 15, was transfected in two E. coli recipient hosts (C600nalR and WG5), both of which nalidixic acid-resistant and ampicillin-sensitive. After transfection, E. coli colonies were selectively grown in Chromocult ap/nal plates (Table 2). 25% of the ap/nal resistant E. coli colonies in each plate were randomly selected and analyzed for bla genes using conventional PCR with the respective primers (Table 2) and confirmed by sequencing. More ap-resistant clones were detected using WG5 as recipient than C600nalR. Analysis of the bla genes located in each clone showed from 0–10% of the clones harbouring bla TEM or bla CTX-M. Among these, more clones harboring bla TEM and bla CTX-M were also found with WG5. bla TEM was detected in a greater percentage of colonies than bla CTX-M in both host strains and no clones were detected for bla CTX-M in C600nalR on two of the three samples assayed (Table 2). Accordingly, the densities of bla TEM genes in the sewage samples used were greater than densities of bla CTX-M (Figs. 2 and 4 respectively). Both genes were never detected simultaneously in a single clone. Other clones showing nal/ap resistance were not harboring the two bla genes analyzed, suggesting that other gene conferring ampicillin resistance could have been transferred.
Table 2. Transfection of phage DNA isolated from sewage in E. coli WG5 and C600 strains.
| Sample number | ||||
| Sewage 6 | Sewage 7 | Sewage 15 | ||
| µg of phage DNA transfected | 2.60 | 1.14 | 1.74 | |
| Ampicillin/Nal WG5 | N° of ap/nal resistant clonesa | 552 | 422 | 310 |
| % bla TEM b | 10.0 | 13.6 | 16.6 | |
| %bla CTX-M b | 6.8 | 1.7 | 13.3 | |
| Ampicillin/Nal C600nalR | N° of ap/nal resistant clonesa | 89 | 101 | 42 |
| % bla TEM | 3.6 | 7.7 | 6.2 | |
| %bla CTX-M | 0 | 1.2 | 0 | |
Averaged number of colonies per plate after transduction.
Percentage of colonies where these genes have been detected by PCR and confirmed by sequencing.
Discussion
Genes of antibiotic resistance are present in bacterial chromosomes and they are detected in plasmids when analyzed in clinical settings, but there is controversy as to how these genes originate and how they reach the pathogenic strains found in hospitals. Several authors indicate a plausible environmental origin of these genes, and we suggest here that phages could be suitable candidates as intermediates between the original bacteria and the clinical isolate.
The genes examined in the present study are the most widely distributed. TEM has been reported worldwide [36] and CTX-M is currently the most widespread and threatening mechanism of antibiotic resistance, particularly in community-acquired infections [25]. The qPCR set designed for CTX-M detected one of the five main clusters described for bla CTX-M genes [20]. Cluster 1 is one of the most diversified groups, which is of particular interest because of the recently described international spread and changing epidemiology of clones carrying the CTX-M-15 variant [3], [5], [34], [37]. Although the qPCR set detected other types in addition to type 15, the prevalence of bla CTX-M-1 in phage DNA (Figure 4) indicates this cluster is abundant in environmental phage DNA. Our results may be applicable to other CTX-M clusters, and it is feasible that the other clusters would also be detectable in phage DNA. Recent studies suggest that the CTX-M-type derives from chromosomal genes from several Kluyvera species and that it is rapidly mobilized from these species to a number of genetic platforms [20], such as insertion sequences, integrons, transposons and plasmids.
We detected MRSA in sewage. although in other studies Staphylococcus was not detected in municipal wastewater [38], or it was detected but not quantified [39]. The results of mecA in phage DNA showed a lack of correlation with fecal pollution in the samples, since averaged values of sewage and river water were similar. This suggests that the mecA detected came from phages other than those found in human fecal pollution. Although the sewage samples analyzed contain exclusively human fecal pollution river samples in this study carried mostly human fecal pollution but also some animal fecal pollution [29], as well as autochthonous freshwater bacteria. Since previous experiments with these urban sewage samples indicated that the values of fecal pollutants are highly consistent over time [30], the variability in the number of copies of the mecA detected in phages supports the hypothesis of an origin other than the human fecal load. Our results do not allow us to discern whether the gene derives from animals or autochthonous microorganisms.
S. aureus can mobilize fragments of its chromosome, the pathogenicity islands, or with helper phages [40]. The transfer of S. aureus phages into and out of isolates may occur in nature or during the course of colonization or infection of patients [23]. The number of copies of mecA detected in phage DNA supports our hypothesis that, regardless of its origin, mecA is located in phages in aquatic environments. This wide spread of mecA could have influenced the emergence of community-acquired strains, which are responsible for serious diseases in healthy individuals [41].
The occurrence of antibiotic resistance genes in the viral DNA fraction of water samples provides new insights into the extent to which ecosystems serve as pools of resistance genes and suggests that phage DNA can act as reservoirs of these genes. However, our results do not indicate whether these genes confer resistance in a given bacterial host. To elucidate this point, a set of experiments aiming to transduce the genes from phage particles isolated from the samples in E. coli was attempted. Unfortunately, as shown in other studies [42], this approach might need to identify a suitable and sensitive host strain (E. coli or others) that would support infection with these phages and subsequent transduction. The search for the suitable host and the right conditions for transduction to occur is likely a complicated task. Moreover, the phages in which antibiotic resistance genes were detected are not necessarily infectious particles. We were therefore unable to achieve transduction of the antibiotic resistance to a bacterial host strain (data not shown), although more efforts will be made to pursue this objective.
However, we were able to demonstrate that the sequences corresponding to resistance genes detected in phage DNA can confer resistance to a recipient bacteria. Using E. coli as a Gram-negative host we generated resistant clones after transfection of phage DNA. This approach avoids the requirement of a suitable host strain and the need for phage infectivity, and only requires a suitable genetic background in which the genes can be expressed. The bla TEM, bla CTX-M genes were transferred into the host strains, which then became resistant to the respective antibiotics after transfection of environmental phage DNA. This demonstrates that these genes can be expressed in a bacterial genetic background.
A similar experimental approach was attempted with environmental phage DNA carrying mecA in an S. aureus mecA− strain, although no methicillin-resistant colonies were obtained (data not shown). This is not surprising since methicillin resistance is conferred by acquisition of the SCCmec element, which includes a type-specific ccr complex, and the mec complex, which includes mecA and its regulatory genes [22], [43]. Although a complete SCCmec element may not be needed, at least a complete mec complex seems to be necessary for the expression of methicillin-resistance. mecA is always localized within mec complexes in all reported MRSA isolates and it is never transferred alone. The various SCCmec elements are between 21 and 67 kb, so it is unlikely that a phage would carry such a long, active SCCmec element, which could then be transferred and confer resistance.
Several reports relate wastewater and antibiotic resistance [39], [44], [45]. Many characteristics of wastewater make it a highly suspect medium for the spread of antibiotic resistance genes, i.e., the presence of antibacterials from household products (soaps, detergents, etc.), the presence of antibiotics that have been excreted by humans or disposed of down the drain, and a high bacterial load. The evolution of MGEs, which allow horizontal gene transfer, depends on the selective forces operating on them, independently of the host strain. However, these elements often encode products with a selective value for the host, and bacteria increase their fitness and diversity when they acquire these elements. In this case, the incorporation of antibiotic resistance in environments with high antibacterial concentrations would guarantee the survival of the bacterial host.
There are only a few examples of antibiotic resistance genes identified as elements of phage chromosomes. However, phages mobilize antibiotic resistance genes through generalized transduction, as reported in several bacterial genera [14]–[16]. Other “phage-like particles” may also be responsible for the spread of antibiotic resistance genes [46]. In vitro, phages transduce resistance to imipenem, aztreonam and ceftazidime in Pseudomonas aeruginosa by generalized transduction [12]. The epidemic strain Salmonella enterica serovar Typhimurium DT104, characterized by various multiresistance patterns, transduces some of the resistance genes [14]. Bacillus anthracis temperate phage encodes demonstrable fosfomycin resistance [47]. Since 1970s evidence has been presented that prophages participate in the dissemination of erythromycin-resistance phenotype Streptococcus infections [17], [48]. The mefA gene, encoding macrolide resistance, is associated with a 58.8-kb chimeric genetic element composed of a transposon inserted into a prophage in S. pyogenes [18]. The ermA gene, a erythromycin resistance determinant, is located on an integrated conjugative element present in Streptococcus strain GAS [49]. These mobile elements identified for macrolide transfer can contribute to mobilization of the genes studied here. However experimental identification of the resistance determinant within a phage is needed.
Other indirect evidence for beta-lactam antibiotics mobilized by phages has been reported. CTX-M-10 was linked to a phage-related element which disseminates among Enterobacteriacea in a hospital [46]. We agree with these authors that the transfer of bla CTX-M-10 from the chromosome of Kluyvera spp. to a transferable plasmid may have been mediated by transduction by a phage. Genetic analyses of Kluyvera phages revealed high homology with phages infecting E. coli [50]. This observation indicates that recombination between the two phages facilitated gene exchange between these bacterial genera. In 1972, Smith [51] reported ampicillin resistance conferred by phage infection, but these studies were not pursued. We previously described the presence of phages encoding sequences of bla OXA-2, bla PSE-1 or bla PSE-4 and bla PSE-type genes in sewage. This was the first report of the contribution of phages to the spread of β-lactamase genes in the environment [9], although the genes detected were not quantified.
Phages, either lytic or temperate, usually persist better in water environments than their bacterial hosts do [28], [29]. This higher survival makes them suitable candidates for transferring genes among bacteria. Due to the structural characteristics of phages, their persistence in the environment is higher than free DNA (either linear fragments or plasmids), which is more sensitive to nucleases, temperature, predation and radiation [52]–[54]. This observation supports the notion that the contribution of phages to gene transfer in natural extra-intestinal environments and in human-generated environments is greater than that of plasmids or transposons. Plasmids and transposons may be the main routes for antibiotic resistance transfer In clinical settings. However, the fact that they are degraded faster than phages limits their role as MGEs in the environment.
The presents study shows that phages carry antibiotic resistance genes able to confer resistance to a bacterial strain. The possibility of transfer of these genes that lead to the emergence of new clones will depend on the susceptibility of infection of the recipient strains as well as the environmental conditions, but it could be assumed that it is likely to occur, although probably at a low frequency. In-depth analysis of the environmental dissemination of phages carrying antibiotic resistance genes outside the clinical setting could increase information about the antibiotic resistance genes circulating among the healthy human population, and their influence on the generation of resistance in the environment. Antibiotic resistance will continue to develop more rapidly than the new antimicrobial agents generated to treat infections, and mobilization through MGEs ensures dissemination of these genes. In many examples, the presence of antibiotics will increase SOS responses, which allows the mobilization of MGEs carrying antibiotic resistance genes, thereby ensuring their own dissemination [55]. It is, therefore, crucial to determine the mechanisms behind the spread of antibiotic resistance genes and to identify the new genes before they become a public health problem.
Materials and Methods
Bacterial strains, bacteriophages and media
E. coli strain C600 containing pGEM vector was used as a control for bla TEM. E. coli strains isolated from sewage during this study were used as controls for bla CTX-M genes carrying types CTX-M 1, 3, 10, 11, 15 and 34. S. aureus MRSA isolated from a human patient was used as a positive control for mecA. E. coli strain WG5 (a nalidixic acid-resistant mutant) (ATCC 700078) (anonymous) and strain C600nalR [56] were used as host for transfection experiments.
Luria-Bertani (LB) agar or broth was used for routine bacterial propagation. Chromocult® Coliform Agar (Merck, Darmstadt, Germany) and Staphylococcus Medium 110 (Difco Laboratories, France) were used to evaluate background flora. When necessary, media were supplemented with ampicillin (35 mg/l or 100 mg/l), 10 mg/l methicillin, or nalidixic acid (25 mg/l) (Sigma-Aldrich, Steinheim, Germany).
Samples
Urban sewage
We used 15 sewage samples collected from the influent of an urban sewage plant that serves the urban area of Barcelona, including a number of cities and towns, of approximately 500 000 inhabitants. Samples were collected regularly approximately every 15 days over six months.
River sample
Fifteen samples were collected from the Llobregat river, near Barcelona, a watercourse that receives mixed human and animal contamination. Samples were collected regularly approximately every 15 days over six months.
Microbiological parameters
Aerobic bacteria present in the samples and grown in TSA were evaluated by performing decimal dilutions of the sample in PBS, plating 0.1 ml of each dilution in TSA and incubating plates in aerobic conditions at 37°C for 18 h. E. coli was detected using Chromocult as an indicator of bacterial fecal pollution by the membrane filtration method, as described elsewhere [57]. Somatic coliphages, proposed as indicators of viral fecal pollution [58], were enumerated using the ISO method [59]. The estimation of total bacteria and E. coli resistant to β-lactam antibiotics was performed as described above but using TSA and Chromocult respectively supplemented with 35 mg/l of ampicillin.
Estimation of S. aureus in the same samples was done with Staphylococcus Medium 110 (Difco Laboratories, France), which was incubated at 37°C for 48 h for the isolation of staphylococci. For the estimation of methicillin-resistant S. aureus, agar plates supplemented with 10 mg/l methicillin (Sigma-Aldrich. Spain) were used. Colonies grown in this medium that showed yellow-orange pigment were suspected of being S. aureus. This was confirmed with the Slidex Staph Plus (Biomerieux España, Madrid. Spain).
Standard PCR procedures
PCRs were performed with a GeneAmp PCR system 2700 (Applied Biosystems, Barcelona, Spain). The DNA template was prepared directly from two colonies of each strain suspended in 50 µl of double-distilled water and heated to 96°C for 10 min prior to the addition of the reaction mixture. Purified bacterial or phage DNA was diluted 1:20 in double-distilled water. The oligonucleotides used to amplify mecA, bla TEM or bla CTX-M are described in Table 3. Five µl of each PCR product was analyzed by agarose (1.5%) gel electrophoresis and bands were visualized by ethidium bromide staining. When necessary, PCR products were purified using a PCR Purification Kit (Qiagen Inc., Valencia, USA).
Table 3. Oligonucleotides used in this study.
| Target gene | PCR | Sequence | Conditions | Amplimer (bp) | Reference |
| 16SrDNA | UP | AAGAGTTTGATCCTGGCTCAG | 95°C 5 min (1 cycle); 95°C 1 min, 42°C 0.5 min, and 72°C 2 min (35 cycles), 72°C 2 min (1 cycle). | 1503 | [61] |
| LP | TACGGCTACCTTGTTACGACTT | ||||
| TEM PCR | UP | CTCACCCAGAAACGCTGGTG | 95°C 5 min (1 cycle). 94°C, 15 s, 63°C 1 min, 72°C, 1.3 min (30 cycles). 72°C, 4 min (1 cycle). | 569 | This study |
| LP | ATCCGCCTCCATCCAGTCTA | ||||
| TEM qPCR | UP | CACTATTCTCAGAATGACTTGGT | 50°C 2 min (1 cycle). 95°C 15 min (1 cycle) 94°C for 15 s and 60°C 1 min (45 cycles). | 85 | [36] |
| LP | TGCATAATTCTCTTACTGTCATG | ||||
| Probe | 6FAM-CCAGTCACAGAAAAGCATCTTACGG-MGBNFQ | ||||
| CTX-M-1 PCR | UP | ACGTTAAACACCGCCATTCC | 95°C 5 min (1 cycle). 94°C, 15 s, 60°C 1 min, 72°C, 1.3 min (30 cycles) 72°C, 4 min (1 cycle). | 356 | This study |
| LP | TCGGTGACGATTTTAGCCGC | ||||
| CTX-M-1 qPCR | UP CTX-M | ACCAACGATATCGCGGTGAT | 50°C 2 min (1 cycle). 95°C 15 min (1 cycle) 94°C for 15 s and 60°C 1 min (45 cycles). | 101 | This study |
| LP CTX-M | ACATCGCGACGGCTTTCT | ||||
| Probe | 6FAM – TCGTGCGCCGCTG- MGBNFQ | ||||
| MecA PCR | UP | ATACTTAGTTCTTTAGCGAT | 95°C 5 min (1 cycle). 94°C, 15 s; 48°C 1 min, 72°C, 1.3 min (30 cycles). 72°C, 4 min (1 cycle). | 434 | This study |
| LP | GATAGCAGTTATATTTCTA | ||||
| MecA qPCR | UP | CGCAACGTTCAATTTAATTTTGTTAA | 50°C 2 min (1 cycle). 95°C 10 min (1 cycle). 95°C for 15 s and 60°C 1 min (40 cycles) | 92 | [38] |
| LP | TGGTCTTTCTGCATTCCTGGA | ||||
| Probe | FAM-AATGACGCTATGATCCCAATCTAACTTCCACA-TAMRA |
qPCR procedures
Preparation of standard curves
For the generation of standards for the qPCR assays, a plasmid construct was used. The 569-bp fragment of TEM, the 356-bp fragment of CTX-M, and the 434-bp fragment of mecA, all obtained by conventional PCR with the primers described in Table 3, and purified as described above, were cloned with a pGEM-T Easy vector for insertion of PCR products, following the manufacturer's instructions (Promega, Barcelona, Spain). The construct was transformed by electroporation into E. coli DH5α electrocompetent cells. Cells were electroporated at 2.5 kV, 25 F capacitance and 200 Ω resistance.
Colonies containing the vector were screened by conventional PCR to evaluate the presence of the vector containing each insert. The presence of the insert in the vector and its orientation was assessed by conventional PCR and sequencing, as described above, using the primers in Table 3. The vector containing the insert was purified from the positive colonies using the Qiagen Plasmid Midi purification kit (Qiagen Inc., Valencia, CA, USA) and the concentration of the vector was quantified by a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies. Thermoscientifics. Wilmington. USA). The reaction product was linearized by digestion with XmnI restriction endonuclease (Promega Co., Madison, USA). The restricted product was purified and quantified again.
To calculate the number of construct gene copies (GC), the following formula was used: [concentration of the pGEM-T- Easy::insert (ng/µl)/molecular weight (ng/mol)] ×6.022×1023 molecules/mol = n° molecules pGEM-T-Easy::insert/µl. The number of GC/µl of the stock prepared for each gene was calculated. Serial decimal dilutions of this stock were made in double-distilled water to prepare the standard curve for qPCR. The standard dilutions were then aliquoted and stored at −80°C until use. Three replicates of each dilution were added to each qPCR reaction.
blaCTX-M primers and probe set
Using the software tool Primer Express 3.0 (Applied Biosystems), primers and probes were selected for use in a standardized TaqMan amplification protocol. All primers and FAM-labeled fluorogenic probes were commercially synthesized by Applied Biosystems (Spain). CTX-M probe was a Minor groove binding probe with a FAM reporter (FAM: 6-carboxyfluorescein) and a non-fluorescent quencher (NFQ). Primers and probes were used under standard conditions in a Step One Real Time PCR System (Applied Biosystems, Spain). Primer and probe specificity was determined with sequence alignments using BLAST and NCBI data entries. The primers and probe set was tested for cross-reactions with the respective sensitive strains. Amplification was performed in a 20 µl reaction mixture with the TaqMan Environmental Real Time PCR Master Mix 2.0 (Applied Biosystems, Spain). The mixture contained 2 µl of the DNA sample or quantified plasmid DNA. Thermal cycler conditions were as follows: an initial setup of 10 min at 95°C, and forty cycles of 15 s of denaturation at 95°C, and 1 min of annealing/extension at 60°C. All samples were run in triplicate, as well as the standards, and positive and negative controls. The number of GC was defined as the average of the triplicate data obtained.
To screen for PCR inhibition, dilutions of the standard were spiked with environmental DNA and the experimental difference was compared to the true copies of the target genes in the standards. Inhibition of the PCR by environmental DNA was not detected.
Purification of phage DNA
Fifty ml of sewage and 100 ml of river water samples were passed through low protein-binding 0.22-µm-pore-size membrane filters (Millex-GP, Millipore, Bedford, MA). When necessary, several filter units were used to filter the whole volume. This allowed us to partially purify viral particles from the samples- The viruses were then 100-fold concentrated by means of protein concentrators (100 kDa Amicon Ultra centrifugal filter units, Millipore, Bedford, MA), following the manufacturer's instructions. The total volume was reduced to 0.5 ml. The centrifugation time varied depending on the sample and ranged from 10–90 min. The viral concentrate was recovered from the tube and the volume was adjusted to 2 ml with double distilled sterile water. Samples were treated with DNase (100 units/ml of the viral concentrate) to eliminate free DNA outside the phage particles.
Control of non-phage DNA
An aliquot of the sample at this stage was evaluated to rule out the presence of bacterial or non-encapsidated DNA. After DNase treatment, but before desencapsidation, the samples were used as template for conventional PCR of eubacterial 16SrDNA (Table 3) and for qPCR of the three antibiotic resistance genes (Table 3). This control was to ensure that the DNase treatment had removed all the non-encapsidated DNA from the samples.
DNA from the viral particles was isolated by proteinase K digestion and phenol/chloroform (1∶1) (v∶v) treatment [59]. The mixture phenol/chloroform/phage lysate was added to Phase Lock Gel tubes (5- Prime, VWR International, Madrid, Spain) and centrifuged following the manufacturer's instructions. The DNA from the supernatant was precipitated using 100% ethanol and 3M sodium acetate, and the volume was adjusted to 250 µl. DNA was further purified by using Microcon YM-100 centrifugal filter units (Millipore, Bedford, MA), following the manufacturer's instructions. Purified DNA was eluted in a final volume of 50 µl and evaluated by agarose (0.8%) gel electrophoresis. The bands were then viewed by ethidium bromide staining. The concentration and purity of the phage DNA extracted was determined by a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies. Thermoscientifics. Wilmington. USA).
Purification of bacterial DNA
Fifty ml of sewage and 100 ml of river water samples were passed through 0.45 µm polyvinylidene fluoride (PVDF) DURAPORE® membrane filters (Millipore, Bedford, Massachusetts), described by the manufacturer as low protein-binding membranes. These allowed the phages to pass through whilst bacteria were retained on the surface of the filter. To remove phages retained on the filters, 10 ml of PBS was added to the surface of the filter, gently agitated and removed by filtration. Two washing steps allowed high (99%) phage reduction without significant loss of bacteria [60]. The membrane containing retained bacteria was recovered in 4 ml of LB. The suspension was centrifuged at 3000 g for 10 min. To recover DNA from both Gram-positive and Gram-negative bacteria, the pellet was suspended in 180 µl of enzymatic solution (20 mg/ml lysozyme; 25 mg/ml lisostaphine, 20 mM Tris-HCl, pH = 8.0; 2 mM EDTA; 1,2% Triton) and incubated for 30 min at 37°C. DNA was then extracted using a QIAamp DNA Stool Mini Kit (Qiagen Inc., Valencia, USA), following the manufacturer's instructions.
Transfection with antibiotic resistance genes
Twenty µl of phage DNA prepared as described above from three sewage samples (samples 6, 7 and 15) was transfected by electroporation into ap-sensitive, nal-resistant E. coli WG5 and C600nalR strains (each culture containing 5×108 CFU/ml). Electrocompetent cells were prepared and phage DNA was electroporated as described above and incubated for 2 h in LB at 37°C. The clones were selected on Chromocult plates supplemented with ap/nal. A 25% of the ap/nal-resistant colonies were randomly selected and screened for the presence of bla TEM and bla CTX-M genes with the corresponding primers for conventional PCR (Table 3). Positive amplification of the genes was confirmed by sequencing.
Electron microscopy
The sewage and river samples were used as a source of bacteriophages. Viruses from the samples were partially purified by filtration and 100-fold concentrated (sewage) or 1000-fold concentrated (river water), by means of protein concentrators (100 kDa Amicon Ultra centrifugal filter units, Millipore, Bedford, MA), following the manufacturer's instructions. Ten-µl of each virus suspension was deposited on copper grids with carbon-coated Formvar films and stained with 2% KOH phosphotungstic acid (pH 7.2) for 2.0 min. Samples were examined in a JEOL JEM-1010 electron microscope operating at 80 kV.
Sequencing and sequence analyses
The amplified DNA of each resistance gene cloned into the pGEM-T-Easy vector used to generate the standard was confirmed by sequencing. Amplicons of bla TEM, bla CTX-M and mecA, generated by conventional PCR with primers described in Table 3, were electrophoretically analyzed in a 1% agarose gel, and bands were viewed by ethidium bromide staining. The bands were excised from the agarose gel and purified using a QIAquick Gel Extraction Kit (Qiagen Inc., Valencia, CA, USA), following the manufacturer's instructions. The purified amplicons were used as a template for sequencing. Sequencing was performed with an ABI PRISM Big Dye 3.1 Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Spain) in an ABI PRISM 3730 DNA Analyzer (Applied Biosystems, Spain), following the manufacturer's instructions. All sequences were performed at least in duplicate.
Nucleotide sequence analysis searches for homologous DNA sequences in the EMBL and GenBank database libraries were carried out using Wisconsin Package Version 10.2, Genetics Computer Group (GCG), (Madison, WI). BLAST analyses were performed with the tools available on the National Institutes of Health (NIH) webpage: http://www.ncbi.nlm.nih.gov. Sequences were assembled with the MultAlin program available on the web page: http://bioinfo.genotoul.fr/multalin/multalin.html.
Statistical analyses
Computation of data and statistical tests were performed using the Statistical Package for Social Science software (SPSS). One-way analysis of variance (ANOVA) tests were used to evaluate the differences between microbiological parameters in sewage and river samples and the differences between the resistance genes detected in bacterial and phage DNA. Evaluations were based on a 5% significance level in both cases (P 0.05). The box-plot graph used to compare the number of detected copies of the genes was done using EXCEL software (Microsoft® EXCEL 2000). The calculations performed to generate the box-plot graph included mean, standard deviation, media, quartiles and minimum and maximum values for each group of samples.
Acknowledgments
We thank F. Navarro for providing us with the S. aureus MRSA strain. We thank L. Imamovic for advice on the qPCR experiments and A. Garcia-Vilanova for excellent technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Generalitat de Catalunya (2009SGR1043), the Spanish Ministry of Education and Science (AGL2009-07576 and SOSTAQUA-CENIT) and the Xarxa de Referència en Biotecnologia (XRB). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Geneva, Switzerland.: 1996. The world health report. [Google Scholar]
- 2.American Academy of Microbiology. ASM. Washington; 2009. Antibiotic Resistance: An Ecological Perspective on an Old Problem. [Google Scholar]
- 3.Hawkey PM, Jones AM. The changing epidemiology of resistance. J Antimicrob Chemother. 2009;64S1:i3–10. doi: 10.1093/jac/dkp256. [DOI] [PubMed] [Google Scholar]
- 4.Jansen WTM, Van Der Bruggen JT, Verhoef J, Fluit AC. Bacterial resistance: a sensitive issue. Complexity of the challenge and containment strategy in Europe. Drug Res Updates. 2006;9:123–133. doi: 10.1016/j.drup.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canton R. Antibiotic resistance genes from the environment: a perspective through newly identified antibiotic resistance mechanisms in the clinical setting. Clin Microbiol Infect. 2009;1:20–25. doi: 10.1111/j.1469-0691.2008.02679.x. [DOI] [PubMed] [Google Scholar]
- 6.Davies J. Inactivation of antibiotics and the dissemination of resistance genes. Science. 1994;264:375–382. doi: 10.1126/science.8153624. [DOI] [PubMed] [Google Scholar]
- 7.Murray BE. Problems and dilemmas of antimicrobial resistance. Pharmacotherapy. 1992;12(6 Pt 2):86S–93S. [PubMed] [Google Scholar]
- 8.Wright GD. Antibiotic resistance in the environment: a link to the clinic? Curr Opin Microbiol. 2010;13:589–594. doi: 10.1016/j.mib.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Muniesa M, García A, Miró E, Mirelis B, Prats G, et al. Bacteriophages and diffusion of β-lactamase genes. Emerg Infect Dis. 2004;10:1134–1137. doi: 10.3201/eid1006.030472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witte W. International dissemination of antibiotic resistant strains of bacterial pathogens. Infect Genet Evol. 2004;4:187–191. doi: 10.1016/j.meegid.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Brabban AD, Hite E, Callaway TR. Evolution of foodborne pathogens via temperate bacteriophage-mediated gene transfer. Foodborne Pathog Dis. 2005;2:287–303. doi: 10.1089/fpd.2005.2.287. [DOI] [PubMed] [Google Scholar]
- 12.D'Costa VM, McGrann KM, Hughes DW, Wright GD. Sampling the antibiotic resistome. Science. 2006;311:374–377. doi: 10.1126/science.1120800. [DOI] [PubMed] [Google Scholar]
- 13.Cangelosi GA, Freitag NE, Buckley MR. Environmental Microorganisms as Human Pathogens. American Academy of Microbiology Report. ASM. Washington; 2004. From outside to Inside. [Google Scholar]
- 14.Blahova J, Hupkova M, Babalova M, Krcmery V, Schafer V. Transduction of resistance to imipenem, aztreonam and ceftazidime in nosocomial strains of Pseudomonas aeruginosa by wild-type phages. Acta Virol. 1993;37:429–436. [PubMed] [Google Scholar]
- 15.Willi K, Sandmeier H, Kulik EM, Meyer J. Transduction of antibiotic resistance markers among Actinobacillus actinomycetemcomitans strains by temperate bacteriophages Aaphi 23. Cell Mol Life Sci. 1997;53:904–910. doi: 10.1007/s000180050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmieger H, Schicklmaier P. Transduction of multiple drug resistance of Salmonella enterica serovar typhimurium DT104. FEMS Microbiol Lett. 1999;170:251–256. doi: 10.1111/j.1574-6968.1999.tb13381.x. [DOI] [PubMed] [Google Scholar]
- 17.Hyder SL, Streitfeld MM. Transfer of erythromycin resistance from clinically isolated lysogenic strains of Streptococcus pyogenes via their endogenous phage. J Infect Dis. 1978;138:281–286. doi: 10.1093/infdis/138.3.281. [DOI] [PubMed] [Google Scholar]
- 18.Banks DJ, Porcella SF, Barbian KD, Beres SB, Philips LE, et al. Progress toward Characterization of the Group A Streptococcus Metagenome: Complete Genome Sequence of a Macrolide-Resistant Serotype M6 Strain. J Infect Dis. 2004;190:727–738. doi: 10.1086/422697. [DOI] [PubMed] [Google Scholar]
- 19.Parsley LC, Consuegra EJ, Kakirde KS, Land AM, Harper WF, Jr, et al. Identification of Diverse Antimicrobial Resistance Determinants Carried on Bacterial, Plasmid, or Viral Metagenomes from an Activated Sludge Microbial Assemblage. Appl Environ Microbiol. 2010;76:3753–3757. doi: 10.1128/AEM.03080-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow M, Reik RA, Jacobs SD, Medina M, Meyer MP, et al. High rate of mobilization for bla CTX-M. Emerging Infectious Diseases. 2008;14:423–428. doi: 10.3201/eid1403.070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, et al. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–1336. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC, Drug Resist Updat. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay JA, Holden MT. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct Integr Genomics. 2006;6:186–201. doi: 10.1007/s10142-005-0019-7. [DOI] [PubMed] [Google Scholar]
- 24.Patterson JE. Extended-spectrum beta-lactamases (2003) Semin Respir Crit Care Med. 2003;24:79–88. doi: 10.1055/s-2003-37919. [DOI] [PubMed] [Google Scholar]
- 25.Rodríguez-Baño J, Alcalá JC, Cisneros JM, Grill F, Oliver A, et al. Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897–1902. doi: 10.1001/archinte.168.17.1897. [DOI] [PubMed] [Google Scholar]
- 26.Díaz MA, Hernández-Bello JR, Rodríguez-Baño J, Martínez-Martínez L, Calvo J, et al. The Diversity of Escherichia coli Producing Extended-Spectrum β-lactamases in Spain: Second Nationwide Study. J Clin Microbiol. 2010;48:2840–2845. doi: 10.1128/JCM.02147-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinsdale EA, Edwards RA, Hall D, Angly F, Breitbart M, et al. Functional metagenomic profiling of nine biomes. Nature. 2008;452:629–632. doi: 10.1038/nature06810. [DOI] [PubMed] [Google Scholar]
- 28.Muniesa M, Lucena F, Jofre J. Comparative survival of free shiga toxin 2-encoding phages and Escherichia coli strains outside the gut. Appl Environ Microbiol. 1999;65:5615–5618. doi: 10.1128/aem.65.12.5615-5618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durán AE, Muniesa M, Méndez X, Valero F, Lucena F, et al. Removal and inactivation of indicator bacteriophages in fresh waters. J Appl Microbiol. 2002;92:338–347. doi: 10.1046/j.1365-2672.2002.01536.x. [DOI] [PubMed] [Google Scholar]
- 30.Lucena F, Duran AE, Moron A, Calderon E, Campos C, et al. Reduction of bacterial indicators and bacteriophages infecting faecal bacteria in primary and secondary wastewater treatments. J Appl Microbiol. 2004;97:1069–1076. doi: 10.1111/j.1365-2672.2004.02397.x. [DOI] [PubMed] [Google Scholar]
- 31.Edge TA, Hill S. Occurrence of antibiotic resistance in Escherichia coli from surface waters and fecal pollution sources near Hamilton, Ontario. Can J Microbiol. 2005;51:501–505. doi: 10.1139/w05-028. [DOI] [PubMed] [Google Scholar]
- 32.Bonnet R. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48(1):1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walther-Rasmussen J, Høiby N. Cefotaximases (CTX-M-ases), an expanding family of extended-spectrum beta-lactamases. Can J Microbiol. 2004;50(3):137–65. doi: 10.1139/w03-111. [DOI] [PubMed] [Google Scholar]
- 34.Novais A, Comas I, Baquero F, Cantón R, Coque TM, et al. Evolutionary trajectories of beta-lactamase CTX-M-1 cluster enzymes: predicting antibiotic resistance. PLoS Pathog. 2010;6(1):e1000735. doi: 10.1371/journal.ppat.1000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coque TM, Baquero F, Canton R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Euro Surveill. 2008;13(47):pii: 19044. [PubMed] [Google Scholar]
- 36.Lachmayr KL, Kerkhof LJ, Dirienzo AG, Cavanaugh CM, Ford TE. Quantifying nonspecific TEM beta-lactamase (bla TEM) genes in a wastewater stream. Appl Environ Microbiol. 2009;75:203–211. doi: 10.1128/AEM.01254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitout JD, Laupland KB. Extended-spectrum β-lactamase producing Enterobacteriaceae: an emerging public-health problem. Lancet Infect Dis. 2008;8:150–166. doi: 10.1016/S1473-3099(08)70041-0. [DOI] [PubMed] [Google Scholar]
- 38.Volkmann H, Schwartz T, Bischoff P, Kirchen S, Obst U. Detection of clinically relevant antibiotic-resistance genes in municipal wastewater using real-time PCR (TaqMan). J Microbiol Methods. 2004;56:277–286. doi: 10.1016/j.mimet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 39.Börjesson S, Melin S, Matussek A, Lindgren PE. A seasonal study of the mecA gene and Staphylococcus aureus including methicillin-resistant S. aureus in a municipal wastewater treatment plant. Water Res. 2009;43:925–932. doi: 10.1016/j.watres.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Tormo-Más MA, Mir I, Shrestha A, Tallent SM, Campoy S, et al. Moonlighting bacteriophage proteins derepress staphylococcal pathogenicity islands. Nature. 2010;465:779–782. doi: 10.1038/nature09065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chambers HF. Community-associated MRSA-resistance and virulence converge. N Engl J Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 42.Muniesa M, Mocé-Llivina L, Katayama H, Jofre J. Bacterial host strains that support replication of somatic coliphages. Antonie Van Leeuwenhoek. 2003;83:305–315. doi: 10.1023/a:1023384714481. [DOI] [PubMed] [Google Scholar]
- 43.Berger-Bachi B, Rohrer S. Factors influencing methicillin resistance in Staphylococci. Arch Microbiol. 2002;178:165–171. doi: 10.1007/s00203-002-0436-0. [DOI] [PubMed] [Google Scholar]
- 44.Cooke MD. Antibiotic resistance among coliform and fecal coliform bacteria isolated from the freshwater mussel Hydridella menziesii. Antimicrob Agents Chemother. 1976;9:885–888. doi: 10.1128/aac.9.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz T, Kohnen W, Jansen B, Obst U. Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms. FEMS Microbiol Ecol. 2003;43:325–335. doi: 10.1111/j.1574-6941.2003.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 46.Oliver A, Coque TM, Alonso D, Valverde A, Baquero F, et al. CTX-M-10 linked to a phage-related element is widely disseminated among Enterobacteriaceae in a Spanish hospital. Antimicrob Agents Chemother. 2005;49:1567–1571. doi: 10.1128/AAC.49.4.1567-1571.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schuch R, Fischetti VA. Detailed genomic analysis of the Wbeta and gamma phages infecting Bacillus anthracis: implications for evolution of environmental fitness and antibiotic resistance. J Bacteriol. 2006;188:3037–3051. doi: 10.1128/JB.188.8.3037-3051.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McShan WM. The bacteriophages of group A streptococci. In: Fischetti VA, editor. Gram-Positive Pathogens. Washington DC: ASM Press; 2000. pp. 105–116. [Google Scholar]
- 49.Beres SB, Musser JM. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PLoS One. 2007;2(8):e800. doi: 10.1371/journal.pone.0000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lingohr EJ, Villegas A, She YM, Ceyssens PJ, Kropinski AM. The genome and proteome of the Kluyvera bacteriophage Kvp1—another member of the T7-like Autographivirinae. Virol J. 2008;5:122. doi: 10.1186/1743-422X-5-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith HW. Ampicillin resistance in Escherichia coli by phage infection. Nat New Biol. 1972;238:205–206. doi: 10.1038/newbio238205a0. [DOI] [PubMed] [Google Scholar]
- 52.Lorenz MG, Wackernagel W. Bacterial gene transfer by natural genetic transformation in the environment. Microbiol Rev. 1994;58:563–602. doi: 10.1128/mr.58.3.563-602.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupray E, Caprais MP, Derrien A, Fach P. Salmonella DNA persistence in natural seawaters using PCR analysis. J Appl Microbiol. 1997;82:507–510. doi: 10.1046/j.1365-2672.1997.00143.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhu B. Degradation of plasmid and plant DNA in water microcosms monitored by natural transformation and real-time polymerase chain reaction (PCR). Water Res. 2006;40:3231–3238. doi: 10.1016/j.watres.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 55.Beaber JW, Hochhut B, Waldor MK. SOS response promotes horizontal dissemination of antibiotic resistance genes. Nature. 2003;427:72–74. doi: 10.1038/nature02241. [DOI] [PubMed] [Google Scholar]
- 56.Imamovic L, Jofre J, Schmidt H, Serra-Moreno R, Muniesa M. Phage-mediated Shiga toxin 2 gene transfer in food and water. Appl Environ Microbiol. 2009;75:1764–1768. doi: 10.1128/AEM.02273-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anonymous. American Public Health Association, American Works Association and Water Environmental Federation. Washington, D.C..: 1998. Standard methods for the examination of water and wastewater. 20th Edition.1200 pp. [Google Scholar]
- 58.Anonymous. International Organisation for Standardisation. Geneva: Switzerland; 2000. ISO 10705-2: Water quality. Detection and enumeration of bacteriophages -part 2: Enumeration of somatic coliphages. [Google Scholar]
- 59.Sambrook J, Russell DW. Cold Spring Harbor Laboratory. Cold Spring Harbor, N.Y.: 2001. Molecular cloning: a laboratory manual, 3rd ed. [Google Scholar]
- 60.Muniesa M, Blanch AR, Lucena F, Jofre J. Bacteriophages may bias outcome of bacterial enrichment cultures. Appl Environ Microbiol. 2005;71:4269–4275. doi: 10.1128/AEM.71.8.4269-4275.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sander M, Schmieger H. Method for host-independent detection of generalized transducing bacteriophages in natural habitats. Appl Environ Microbiol. 2001;67:1490–1493. doi: 10.1128/AEM.67.4.1490-1493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]