Abstract
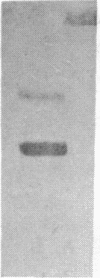
Apolipoprotein(a) [apo(a)] is a glycoprotein with Mr approximately equal to 280,000 that is disulfide linked to apolipoprotein B in lipoprotein(a) particles. Elevated plasma levels of lipoprotein(a) are correlated with atherosclerosis. Partial amino acid sequence of apo(a) shows that it has striking homology to plasminogen. Plasminogen is a plasma serine protease zymogen that consists of five homologous and tandemly repeated domains called kringles and a trypsin-like protease domain. The amino-terminal sequence obtained for apo(a) is homologous to the beginning of kringle 4 but not the amino terminus of plasminogen. Apo(a) was subjected to limited proteolysis by trypsin or V8 protease, and fragments generated were isolated and sequenced. Sequences obtained from several of these fragments are highly (77-100%) homologous to plasminogen residues 391-421, which reside within kringle 4. Analysis of these internal apo(a) sequences revealed that apo(a) may contain at least two kringle 4-like domains. A sequence obtained from another tryptic fragment also shows homology to the end of kringle 4 and the beginning of kringle 5. Sequence data obtained from two tryptic fragments show homology with the protease domain of plasminogen. One of these sequences is homologous to the sequences surrounding the activation site of plasminogen. Plasminogen is activated by the cleavage of a specific arginine residue by urokinase and tissue plasminogen activator; however, the corresponding site in apo(a) is a serine that would not be cleaved by tissue plasminogen activator or urokinase. Using a plasmin-specific assay, no proteolytic activity could be demonstrated for lipoprotein(a) particles. These results suggest that apo(a) contains kringle-like domains and an inactive protease domain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albers J. J., Adolphson J. L., Hazzard W. R. Radioimmunoassay of human plasma Lp(a) lipoprotein. J Lipid Res. 1977 May;18(3):331–338. [PubMed] [Google Scholar]
- Armstrong V. W., Walli A. K., Seidel D. Isolation, characterization, and uptake in human fibroblasts of an apo(a)-free lipoprotein obtained on reduction of lipoprotein(a). J Lipid Res. 1985 Nov;26(11):1314–1323. [PubMed] [Google Scholar]
- Berg K., Dahlén G., Frick M. H. Lp(a) lipoprotein and pre-beta1-lipoprotein in patients with coronary heart disease. Clin Genet. 1974;6(3):230–235. doi: 10.1111/j.1399-0004.1974.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Cool D. E., Edgell C. J., Louie G. V., Zoller M. J., Brayer G. D., MacGillivray R. T. Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa. J Biol Chem. 1985 Nov 5;260(25):13666–13676. [PubMed] [Google Scholar]
- Dahlen G. H., Guyton J. R., Attar M., Farmer J. A., Kautz J. A., Gotto A. M., Jr Association of levels of lipoprotein Lp(a), plasma lipids, and other lipoproteins with coronary artery disease documented by angiography. Circulation. 1986 Oct;74(4):758–765. doi: 10.1161/01.cir.74.4.758. [DOI] [PubMed] [Google Scholar]
- Dahlén G., Berg K., Gillnäs T., Ericson C. Lp(a) lipoprotein/pre-beta1-lipoprotein in Swedish middle-aged males and in patients with coronary heart disease. Clin Genet. 1975 Apr;7(4):334–341. doi: 10.1111/j.1399-0004.1975.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Dahlén G., Ericson C., Furberg C., Lundkvist L., Svärdsudd K. Studies on an extra pre-beta lipoprotein fraction. Acta Med Scand Suppl. 1972;531:1–29. [PubMed] [Google Scholar]
- Fless G. M., Rolih C. A., Scanu A. M. Heterogeneity of human plasma lipoprotein (a). Isolation and characterization of the lipoprotein subspecies and their apoproteins. J Biol Chem. 1984 Sep 25;259(18):11470–11478. [PubMed] [Google Scholar]
- Fless G. M., ZumMallen M. E., Scanu A. M. Isolation of apolipoprotein(a) from lipoprotein(a). J Lipid Res. 1985 Oct;26(10):1224–1229. [PubMed] [Google Scholar]
- Fless G. M., ZumMallen M. E., Scanu A. M. Physicochemical properties of apolipoprotein(a) and lipoprotein(a-) derived from the dissociation of human plasma lipoprotein (a). J Biol Chem. 1986 Jul 5;261(19):8712–8718. [PubMed] [Google Scholar]
- Floren C. H., Albers J. J., Bierman E. L. Uptake of Lp (a) lipoprotein by cultured fibroblasts. Biochem Biophys Res Commun. 1981 Sep 30;102(2):636–639. doi: 10.1016/s0006-291x(81)80179-9. [DOI] [PubMed] [Google Scholar]
- Gaubatz J. W., Chari M. V., Nava M. L., Guyton J. R., Morrisett J. D. Isolation and characterization of the two major apoproteins in human lipoprotein [a]. J Lipid Res. 1987 Jan;28(1):69–79. [PubMed] [Google Scholar]
- Gaubatz J. W., Heideman C., Gotto A. M., Jr, Morrisett J. D., Dahlen G. H. Human plasma lipoprotein [a]. Structural properties. J Biol Chem. 1983 Apr 10;258(7):4582–4589. [PubMed] [Google Scholar]
- Gilmore N. J., Moroz L. A. Plasminogen-binding lipoprotein: isolation and characterization of a plasma very low density lipoprotein which co-chromatographs with plasminogen on lysine-sepharose. Thromb Res. 1983 Sep 15;31(6):863–874. doi: 10.1016/0049-3848(83)90117-2. [DOI] [PubMed] [Google Scholar]
- Günzler W. A., Steffens G. J., Otting F., Kim S. M., Frankus E., Flohé L. The primary structure of high molecular mass urokinase from human urine. The complete amino acid sequence of the A chain. Hoppe Seylers Z Physiol Chem. 1982 Oct;363(10):1155–1165. doi: 10.1515/bchm2.1982.363.2.1155. [DOI] [PubMed] [Google Scholar]
- Hajjar K. A., Harpel P. C., Jaffe E. A., Nachman R. L. Binding of plasminogen to cultured human endothelial cells. J Biol Chem. 1986 Sep 5;261(25):11656–11662. [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Rall S. C., Jr, Innerarity T. L., Jacobson S. F., Urdea M. S., Levy-Wilson B., Powell L. M., Pease R. J., Eddy R., Nakai H. Human apolipoprotein B: structure of carboxyl-terminal domains, sites of gene expression, and chromosomal localization. Science. 1985 Oct 4;230(4721):37–43. doi: 10.1126/science.2994225. [DOI] [PubMed] [Google Scholar]
- Költringer P., Jürgens G. A dominant role of lipoprotein(a) in the investigation and evaluation of parameters indicating the development of cervical atherosclerosis. Atherosclerosis. 1985 Dec;58(1-3):187–198. doi: 10.1016/0021-9150(85)90065-6. [DOI] [PubMed] [Google Scholar]
- Lerch P. G., Rickli E. E. Studies onthe chemical nature of lysine-binding sites and on their localization in human plasminogen. Biochim Biophys Acta. 1980 Oct 21;625(2):374–378. doi: 10.1016/0005-2795(80)90302-5. [DOI] [PubMed] [Google Scholar]
- Maartmann-Moe K., Berg K. Lp(a) lipoprotein enters cultured fibroblasts independently of the plasma membrane low density lipoprotein receptor. Clin Genet. 1981 Nov;20(5):352–362. doi: 10.1111/j.1399-0004.1981.tb01047.x. [DOI] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Summaria L., Hsieh B., Shah R. J. The peptide chains of human plasmin. Mechanism of activation of human plasminogen to plasmin. J Biol Chem. 1967 May 25;242(10):2333–2342. [PubMed] [Google Scholar]
- Seman L. J., Breckenridge W. C. Isolation and partial characterization of apolipoprotein (a) from human lipoprotein (a). Biochem Cell Biol. 1986 Oct;64(10):999–1009. doi: 10.1139/o86-133. [DOI] [PubMed] [Google Scholar]
- Sodetz J. M., Brockway W. J., Castellino F. J. Multiplicity of rabbit plasminogen. Physical characterization. Biochemistry. 1972 Nov 21;11(24):4451–4458. doi: 10.1021/bi00774a005. [DOI] [PubMed] [Google Scholar]
- Utermann G., Weber W. Protein composition of Lp(a) lipoprotein from human plasma. FEBS Lett. 1983 Apr 18;154(2):357–361. doi: 10.1016/0014-5793(83)80182-3. [DOI] [PubMed] [Google Scholar]
- Wiman B., Lijnen H. R., Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochim Biophys Acta. 1979 Jul 25;579(1):142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]