Abstract
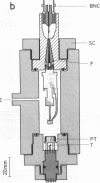
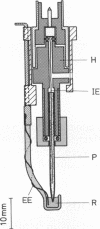
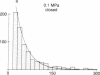
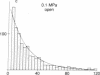
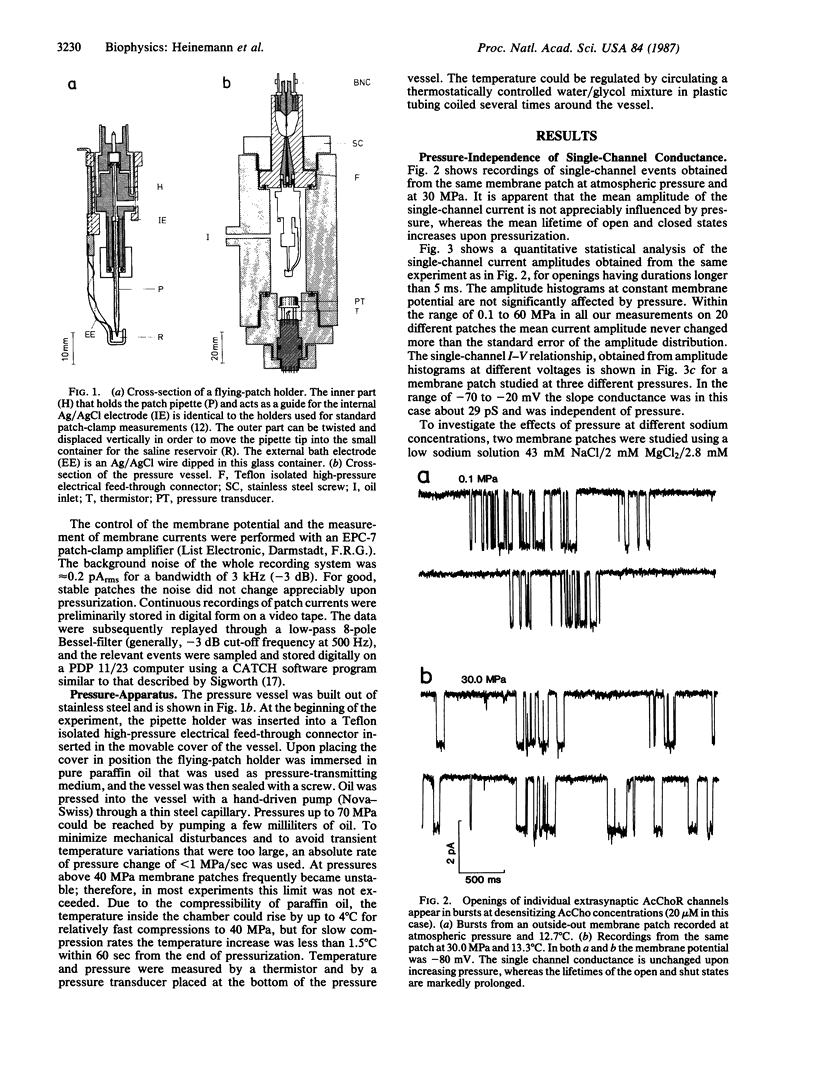
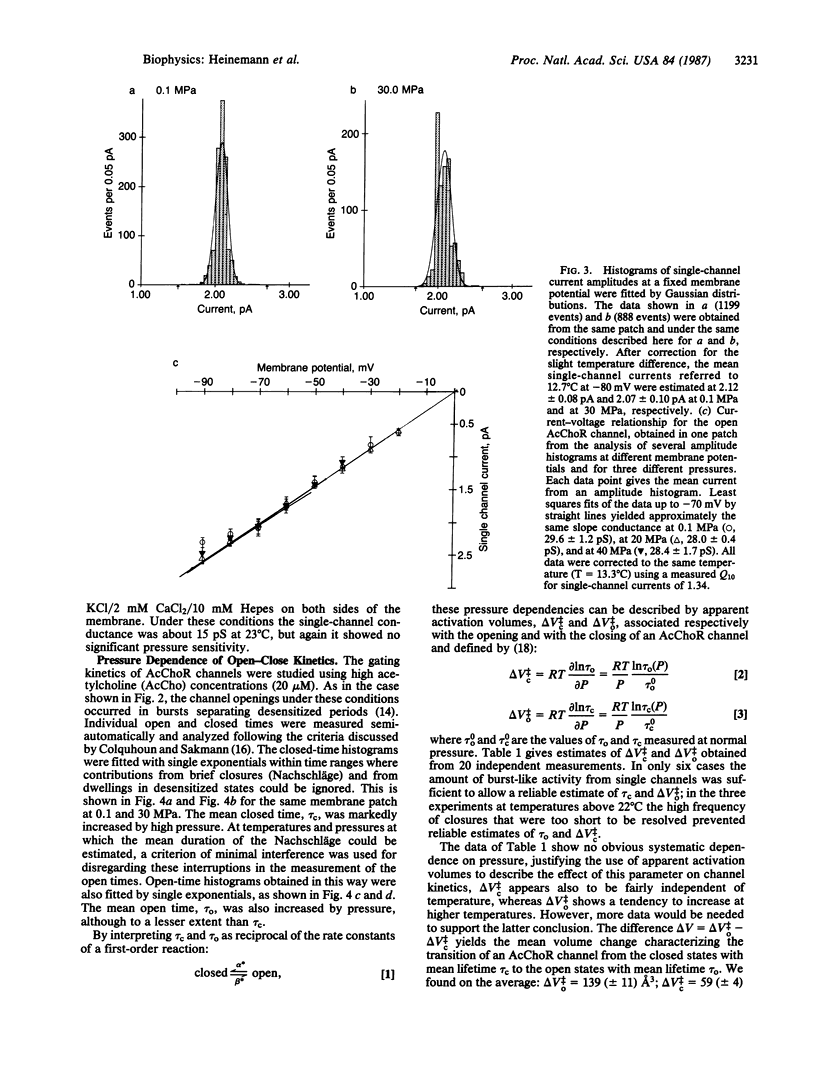
A technique for performing patch-clamp experiments under high hydrostatic (oil) pressure is described. The method allows the transfer of whole cell or membrane patches in a recording configuration into a pressure vessel, where pressure can be increased up to 60 MPa (approximately equal to 600 bar). We have studied in this way the pressure dependence of single acetylcholine receptor channels in excised "outside-out" membrane patches from cultured rat muscle cells. In the range of 0.1 to 60 MPa the open channel conductance in 140 mM NaCl solutions did not vary by more than 2%, which implies that the translocation of sodium ions through the channel pore does not involve steps with significant activation volumes. At high acetylcholine concentrations (20 microM) bursts of single-channel activity allowed measurements of the mean open and mean closed times of the channel. Pressurization to 40 MPa increased both mean open and mean closed times giving apparent activation volumes of about 59 and 139 A3, respectively. This implies a net volume increase of 80 A3, associated with the transition from the agonist-free state to the open state of the channel, which may be partially associated with the agonist-binding step. All the observed pressure effects were reversible. The activation volumes for the gating of acetylcholine receptor channels are comparable to those of sodium and potassium channels in the squid giant axon, suggesting that there is some basic common mechanism in the operation of ion-channel proteins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford M. L., MacDonald A. G., Wann K. T. The effects of hydrostatic pressure on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol. 1982 Dec;333:531–543. doi: 10.1113/jphysiol.1982.sp014467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner L. J., Hall J. E. Pressure effects on alamethicin conductance in bilayer membranes. Biophys J. 1983 Oct;44(1):39–47. doi: 10.1016/S0006-3495(83)84275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. J Physiol. 1985 Dec;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Fioravanti R., Segal J. R., Stühmer W. Pressure dependence of the potassium currents of squid giant axon. J Membr Biol. 1982;69(1):35–40. doi: 10.1007/BF01871239. [DOI] [PubMed] [Google Scholar]
- Conti F., Fioravanti R., Segal J. R., Stühmer W. Pressure dependence of the sodium currents of squid giant axon. J Membr Biol. 1982;69(1):23–34. doi: 10.1007/BF01871238. [DOI] [PubMed] [Google Scholar]
- Conti F., Inoue I., Kukita F., Stühmer W. Pressure dependence of sodium gating currents in the squid giant axon. Eur Biophys J. 1984;11(2):137–147. doi: 10.1007/BF00276629. [DOI] [PubMed] [Google Scholar]
- Dwyer T. M., Farley J. M. Permeability properties of chick myotube acetylcholine-activated channels. Biophys J. 1984 Mar;45(3):529–539. doi: 10.1016/S0006-3495(84)84190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harper A. A., Macdonald A. G., Wann K. T. The action of high hydrostatic pressure on the membrane currents of Helix neurones. J Physiol. 1981 Feb;311:325–339. doi: 10.1113/jphysiol.1981.sp013587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J. V., Jr, Gilbert D. L. Slowing of ionic currents in the voltage-clamped squid axon by helium pressure. Nature. 1975 Nov 27;258(5533):351–352. doi: 10.1038/258351a0. [DOI] [PubMed] [Google Scholar]
- Heremans K. High pressure effects on proteins and other biomolecules. Annu Rev Biophys Bioeng. 1982;11:1–21. doi: 10.1146/annurev.bb.11.060182.000245. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Macdonald A. G. The effects of pressure on the molecular structure and physiological functions of cell membranes. Philos Trans R Soc Lond B Biol Sci. 1984 Jan 7;304(1118):47–68. doi: 10.1098/rstb.1984.0008. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B., Steinbach J. H. The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978 Jul 18;375(2):219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- Parmentier J. L., Shrivastav B. B., Bennett P. B. Hydrostatic pressure reduces synaptic efficiency by inhibiting transmitter release. Undersea Biomed Res. 1981 Sep;8(3):175–183. [PubMed] [Google Scholar]
- Sakmann B., Bormann J., Hamill O. P. Ion transport by single receptor channels. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):247–257. doi: 10.1101/sqb.1983.048.01.027. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Patlak J., Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature. 1980 Jul 3;286(5768):71–73. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Open channel noise. I. Noise in acetylcholine receptor currents suggests conformational fluctuations. Biophys J. 1985 May;47(5):709–720. doi: 10.1016/S0006-3495(85)83968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]