Abstract
Emerging evidence supporting chronic disease fighting properties of rice bran has advanced the development of stabilized rice bran for human use as a functional food and dietary supplement. A global and targeted metabolomic investigation of stabilized rice bran fermented with Saccharomyces boulardii was performed in three rice varieties. Metabolites from S. boulardii-fermented rice bran were detected by gas chromatography−mass spectrometry (GC−MS) and assessed for bioactivity compared to nonfermented rice bran in normal and malignant lymphocytes. Global metabolite profiling revealed significant differences in the metabolome that led to discovery of candidate compounds modulated by S. boulardii fermentation. Fermented rice bran extracts from three rice varieties reduced growth of human B lymphomas compared to each variety’s nonfermented control and revealed that fermentation differentially altered bioactive compounds. These data support that integration of global and targeted metabolite analysis can be utilized for assessing health properties of rice bran phytochemicals that are enhanced by yeast fermentation and that differ across rice varieties.
Keywords: rice bran, probiotics, metabolomics, Saccharomyces boulardii
Introduction
Stabilized rice bran is a unique whole food that naturally contains protein, vitamins, minerals, complex carbohydrates, phytonutrients, phospholipids, essential fatty acids, and more than 120 antioxidants.(1) Dietary rice bran intake and rice bran components have demonstrated chronic disease fighting activity, particularly for protection against cardiovascular disease and certain cancers.2−8 We and others have shown that rice varieties are not equal in content and composition of bioactive rice bran components.9,10 How these phytochemicals are altered by microbial fermentation and metabolism is an emerging area of research that merits scientific investigation when assessing bioactivity and health benefits. A few studies have evaluated rice bran as a dietary supplement or functional food ingredient;5,11−13 however, little is known about how chemical content changes with and without fermentation.
The yeast, Saccharomyces cerevisiae var. boulardii (S. boulardii), has probiotic activity and is widely used as a dietary supplement for intestinal disease prevention and treatment.14−16 The spectrum of biomedical activities and food processing applications reported with S. boulardii has significantly grown over the past decade and includes, but is not limited to, protection against enteric pathogens, modification of lymphocyte proliferation, and differential release of plant secondary metabolites from foods such as wine, sourdough and cheese.17−19Sacchromyces boulardii has been shown to be beneficial for modification of food components such as breakdown of dietary phytate and biofortification of folate to improve the nutritional value and health properties of food.20,21 The health benefit of S. boulardii both as a probiotic and for fermented foods was recently reviewed, and a meta-analysis of placebo-controlled treatment trials supports its safety and efficacy for protection against several types of diarrhea.16,22,23 Protection against specific enteric bacterial pathogens by S. boulardii may, in part, be due to anti-inflammatory actions and effects on immunity.15,19,23In vitro studies using mammalian cell cultures have shown that S. boulardii modifies host cell signaling pathways associated with proinflammatory responses, and that the mechanism may be based on blocking activation of nuclear factor-kappa B (NF-κB) and mitogen activated protein kinase (MAPK).24,25 Inhibition of these cell-signaling pathways is also an important mechanism for reducing cancer cell growth. Rice bran components have been reported to inhibit activation and promote apoptosis of malignant lymphocytes and to inhibit growth of intestinal cancers.3,11,26,27 In this report, we examined the effects of S. boulardii fermented rice bran across rice varieties on viability of normal human blood lymphocytes and B lymphoma in vitro. Rice bran chemical contents and the compounds altered by fermentation have not been previously assessed for effects on human B lymphomas, and were assessed using global and targeted metabolite profiling techniques.
A significant lack of knowledge exists regarding the ability of probiotics to alter the phytochemistry of rice bran for health benefits, and global metabolite profiling represents a novel approach to detect changes in rice bran phytochemical content due to fermentation without a bias toward certain chemical classes. A metabolite profiling approach based on gas chromatography−mass spectrometry (GC−MS) was recently used to investigate time-dependent metabolic changes during the germination of rice,(28) and more targeted studies have sought to identify bioactive and volatile compounds from rice bran oil or in bran polished from red and black rice varieties.9,29 Bran from three rice varieties was hypothesized herein to vary in bioactive chemical contents after fermentation with S. boulardii and to differentially inhibit human B lymphoma viability.
Materials and Methods
Reagents and Cell Culture
Caffeic acid, p-coumaric acid, ferulic acid, salicylic acid, β-sitosterol, and α-tocopherol standards were purchased from Sigma-Aldrich (St. Louis, MO). Saccharomyces boulardii was isolated from the commercial probiotic, Proboulardi (Metagenics Inc., San Clemente, CA), and confirmed by morphological tests. Cultures were maintained on yeast nitrogen base (YNB) amended with 0.5% (w/v) ammonium sulfate and 2% (w/v) dextrose. Raji B lymphomas were purchased from American Type Culture Collection. Whole blood from healthy volunteers was collected into 8 mL cell preparation tubes (CPT) with sodium citrate as an anticoagulent (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ). CPT tubes were centrifuged at 1500g for 30 min for separation and enrichment of normal human peripheral blood lymphocytes (PBL). PBL were washed two times with 1× phosphate buffered saline solution prior to resuspension in cell culture medium. Blood was obtained at Colorado State University according to Institutional Review Board approved protocols. Raji B lymphomas and freshly isolated normal PBL were cultured in RPMI medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 10 mg/mL penicillin, 10,000 IU/mL streptomycin, 25 mg/mL amphotericin, 1 mM sodium pyruvate, and 1× MEM nonessential amino acids.
Rice Bran Collection and Storage
Three rice varieties selected for investigation were Neptune, Wells and the near-isogenic line, Red Wells(30) (Table 1). Rice bran was provided as a generous gift from Dr. Anna McClung at the United States Department of Agriculture, Rice Research Center (Stuttgart, Arkansas). Bran was isolated by standard milling process, heat stabilized at 110 °C for 3 min, and stored at −20 °C.
Table 1. Rice Varieties Assessed for Bioactivity of the Bran.
| trait | Neptune | Wells | Red Wells |
|---|---|---|---|
| plant introduction ID | PI 655959 | PI 612439 | |
| yield | high | high | high |
| grain type | long | medium | medium |
| leaf type | erect | erect | erect |
| pericarp | brown | brown | red |
| sheath blight | moderately susceptible | moderately susceptible | moderately susceptible |
| rice blast | moderately resistant | moderately susceptible | moderately susceptible |
S. boulardii Fermentation and Metabolite Extraction
Rice bran, water and probiotic yeast fermentations were carried out using a modification of the methods described in refs (31) and (32). Briefly, 1.6 g of rice bran was added to 11.4 mL of sterilized water in the presence and absence of S. boulardii concentration of 6 × 105 cells mL−1, and samples were incubated at 37 °C for 24 h with gentle shaking (n = 3). Metabolites were extracted using two separate solvents: either (A) isopropanol:acetonitrile:water (3:2:2) for metabolite profiling or (B) methanol:water (80:20) for measuring bioactivity on lymphoma or peripheral blood lymphocytes in vitro. Solvent A was used for metabolite profiling and was previously shown to extract both lipids and organic acids.33,34 Solvent B was used to standardize cell culture treatments and conditions, as a similar single-phase aqueous-alcohol solvent was previously used to assess effects of rice bran compounds.26,27 After 24 h of fermentation in water, either isopropanol:acetonitrile or methanol was added to the culture for final 3:2:2 or 80:20 ratios, respectively. Samples were vortexed and incubated at room temperature for five minutes, and bran material and yeast cells were pelleted using centrifugation (1500g) for ten minutes followed by filtration. The supernatant was collected and stored at −80 °C until further chemical and biological analyses.
Gas Chromatography−Mass Spectrometry
Rice bran metabolites were detected by transferring 500 μL of extract to a new tube and dried using a vacuum centrifuge. The extract was derivatized by first adding 50 μL of a solution containing 20 mg/mL of methoxyamine hydrochloride in pyridine and incubating at 37 °C for two hours. Next, 50 μL of N-methyl-N-trimethylsilyltrifluoroacetamide with 1% trimethylchlorosilane (MSTFA + 1% TMCS) (Thermo Scientific) was added and the reaction was incubated at 37 °C for 60 min. Samples were centrifuged at 3000g for 5 min, and 80 μL of the supernatant was used for GC−MS analysis. Caffeic acid, coumaric acid, ferulic acid, salicylic acid, β-sitosterol, and α-tocopherol standards (Sigma-Aldrich, St. Louis, MO) were dissolved in an isopropanol/acetonitrile/water solution (3:2:2), evaporated, and derivatized under identical conditions.
The derivatized samples were equilibrated to room temperature, transferred to a 200 μL glass insert, and analyzed using a Trace GC Ultra coupled to a Thermo DSQ II scanning from m/z 50−650 at a rate of 5 scans/s in electron impact mode. Samples were injected at a 10:1 split ratio, and the inlet and transfer line were held at 280 °C. Separation was achieved on a 30 m TG-5MS column (Thermo Scientific, 0.25 mm i.d., 0.25 μm film thickness) using a temperature program of 80 °C for 0.5 min, then ramped at 15 °C per minute to 330 °C and held for 8 min, at a constant flow of 1.2 mL per minute. A single feature or known metabolite was defined by a given metabolite’s retention time and mass, and the peak area was used to determine the relative quantity of each feature or known metabolite.
Cell Viability
Raji B lymphomas and normal peripheral blood lymphocytes were plated to a density of 2.5 × 105 cells per mL. Rice bran extracts were dried in a vacuum centrifuge and resuspended in cell culture medium, and cells were incubated in the presence of rice bran extract for 24 h. Cells were centrifuged at 1500g for 5 min, resuspended in a solution consisting of cell culture medium and 1% resazurin sodium salt, and incubated at 37 °C for one hour. Fluorescence was measured at 765 nm, and viability was expressed as percent fluorescence relative to the vehicle control.35,36
S. boulardii Growth on Rice Bran
Saccharomyces boulardii cultures were maintained in YNB (MP Biomedicals, Solon, OH) with 0.5% ammonium sulfate and 2% dextrose at 37 °C. A liquid growth medium containing 5% rice bran and water was made with each rice variety. S. boulardii was added to the rice bran/water mixture at a final OD600 of 0.02 (approximately 6 × 105 cells mL−1). Cultures were incubated at 37 °C and sampled at 24, 48, and 72 h. Yeast cells were enumerated by drop plating serial dilutions on YNB plates to determine total colony forming units (CFUs).
Statistical Analysis
Chromatographic peaks between 2 and 25 min were detected by GC−MS and aligned using MarkerLynx software (Waters, Millford, MA, USA) with a retention time error window of 0.05 min. Masses used for analyses ranged between 50 and 650 m/z with a mass error tolerance of 0.4 m/z. Multivariate statistical analysis was performed using SIMCA P+ (v 12.0, Umetrics, Umeå, Sweden). Mean centering and pareto scaling were applied for all principal component, partial least-squares, and orthoganol projection to latent structures (OPLS) analyses. Each feature was analyzed independently in a linear mixed-effects model to determine the significance and percent variance attributed to fermentation (fixed effect), or variety and variety−fermentation interactions (random effects). Significance was determined with a P-value threshold of 0.05, and percent variation was determined using the sum of squares partitions of each random effect relative to the total sum of squares of the model. Fold changes due to fermentation were calculated for each feature using the peak areas of fermented divided by the nonfermented. Bioactive compounds were compared among varieties by one-way ANOVA (P < 0.05), and z-scores were calculated for metabolites from fermented varieties based on the mean and standard deviation of the nonfermented control. Effects on lymphoma viability and increased growth of S. boulardii on rice bran varieties were determined using a one-way ANOVA and Tukey’s HSD. Significant differences between treatments (rice bran varieties) and controls (YNB) were confirmed by a Dunnet’s 2-tailed comparison. These tests were performed using R software (v2.11.1), GraphPad Prism (v 5.0, GraphPad Software, Inc., La Jolla, CA) and XLStat-Pro (Addinsoft USA, New York, NY).
Results
Rice Bran Metabolome Differences among Rice Varieties before and after Fermentation with S. boulardii
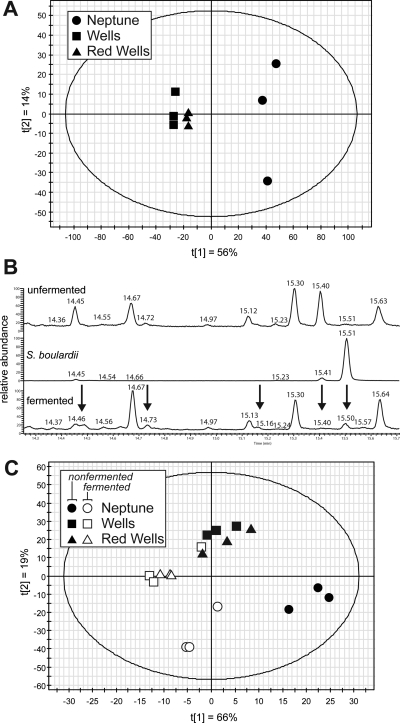
Bran from three rice varieties (Table 1) was extracted for metabolite profiling and analyzed by gas chromatography coupled to mass spectrometry (GC−MS). The rice varieties Neptune and Wells are U.S. semidwarf varieties, and Red Wells is isogenic to Wells apart from a single deleted base pair in the proanthocyanidin gene Rc. This mutation results in the production of red pigment in the bran layer of the seed.(30) Principal component analysis was used to elucidate varietal differences in the metabolome of nonfermented rice bran (Figure 1A). Varietal differences were largely explained by the first component (56%), and the second component differentiated between biological replicates (14%). Rice bran was then incubated in the presence and absence of S. boulardii and metabolites were extracted and detected by GC−MS. The GC−MS chromatograms of nonfermented rice bran, S. boulardii fermented rice bran, and S. boulardii extracts showed unique differences among the treatments (Figure 1B). Partial least squares discriminant analysis (PLS-DA) was used to detect differences in the metabolome among all three varieties and with or without fermentation with S. boulardii (Figure 1C). The first two components of the PLS-DA model explained 66% and 19% of the variation, respectively.
Figure 1.
Metabolite profiling of rice bran from three varieties with and without fermentation by S. boulardii. (A) Principal component analysis (PCA) of bran extracts from three rice varieties (Neptune, Wells and Red Wells) show diversity in metabolite profiles. The first principal component separated the three varieties, and the second component was mostly composed of variation among replicates within a single variety. (B) A representative portion of a GC−MS chromatograph showed change in metabolites in fermented rice bran. Neptune rice variety alone (top), S. boulardii extract alone (middle) and bran from Neptune variety fermented with S. boulardii (bottom). Some peaks are present in only one sample, and others are present in both but vary in quantity, as indicated by arrows above differential peaks. (C) PLS-DA model of three varieties nonfermented (black shading) or fermented with S. boulardii (white). The first component separated each variety from its fermented counterpart, and the second component separated Neptune from Wells and Red Wells.
This model demonstrates the ability to apply metabolite-profiling techniques to differentiate chemical contents of fermented rice bran from nonfermented rice bran, irrespective of the rice variety tested.
Rice Bran Metabolites Modulated by S. boulardii Fermentation
S. boulardii fermentation induced changes in metabolite content for all three varieties were determined by quantitative analysis of peak areas from 10,260 GC−MS derived features. For Neptune, Wells, and Red Wells, 448, 127, and 311 features varied due to fermentation, respectively (Student’s t test, P < 0.05). The mean percent variance explained by the linear mixed model for all features was 14.8%. Genotype and genotype−fermentation effects explained a mean of 10% and 4.8% of the total variation, respectively.
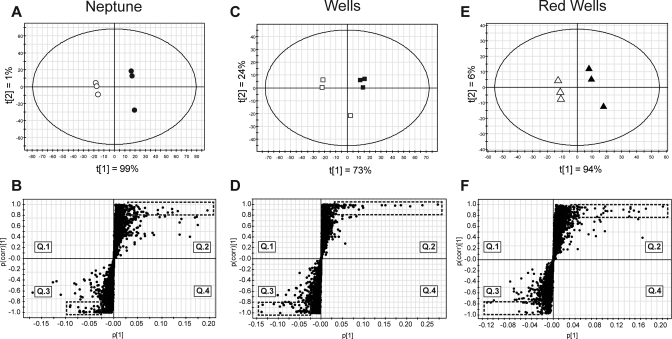
A series of PLS-DA and OPLS models were applied to each rice variety to determine metabolites with the most significant changes due to fermentation (Figure 2). The effect of fermentation on each variety’s metabolome was explained by the first component in each PLS-DA model. OPLS analyses were conducted to determine metabolites quantitatively altered by yeast probiotic fermentation. Metabolites of interest contained p(1) and p(corr) values greater than 0.02 and 0.8, respectively, and were analyzed for quantitative differences between samples by fold-change due to fermentation and by Student’s t test (P < 0.05) (Table 2). Mass spectra of significant peaks were screened in the National Institute of Technology Standards metabolite database for probable matches. Rice varieties differed in candidate metabolites altered by S. boulardii fermentation and by chemical classes. For the three varieties, there was wide variation in both the relative quantities of metabolites increased and the types of predicted metabolites. S. boulardii fermentation of rice bran differs with regard to variety and was next evaluated for impact on anticancer properties of rice bran.
Figure 2.
PLS-DA and OPLS models to determine metabolite variation induced by fermentation with S. boulardii. Each variety was independently analyzed for metabolite differences induced by S. boulardii fermentation. (A) Neptune PLS-DA showed the metabolome differs between unfermented (black) and fermented (white) samples. (B) The Neptune OPLS analysis showed metabolites that highly differ based on fermentation, indicated by the dashed box for quadrant 2 (nonfermented) and quadrant 3 (fermented). p(corr) values correspond to deviation across replicates, and p(1) values are proportional to the quantity of metabolite. Wells (C and D) and Red Wells (E and F) also showed altered metabolite content induced by S. boulardii.
Table 2. Varietal Differences in Candidate Compounds Altered by S. boulardii Fermentation.
| compound | class | fold change after fermentation | p-value |
|---|---|---|---|
| Neptune | |||
| galactose | sugar | −10.32 | 0.03 |
| palmitic acid | fatty acid | −1.2 | 0.04 |
| α-linoleic acid | fatty acid | −1.23 | 0.04 |
| unknown disaccharide | sugar | 26.44 | 0.001 |
| xylitol | sugar-alcohol | 14.79 | <0.001 |
| glucitol | sugar-alcohol | a | <0.001 |
| alanine | amino acid | 4.63 | 0.02 |
| phosphoric acid | mineral | 2 | <0.001 |
| 1,2,3-propanetricarboxylic acid | organic acid | 4.34 | 0.02 |
| Wells | |||
| d-fructose | sugar | −6.38 | 0.005 |
| ribitol | sugar-alcohol | b | <0.001 |
| linoleic acid methyl ester | fatty acid | 1.4 | 0.04 |
| Red Wells | |||
| palmitic acid | fatty acid | a | 0.03 |
| unknown disaccharide | sugar | a | <0.001 |
Metabolite only present in fermented extracts.
Metabolite only present in nonfermented extracts.
S. boulardii Fermented Rice Bran Extracts Differentially Inhibit Lymphoma Viability
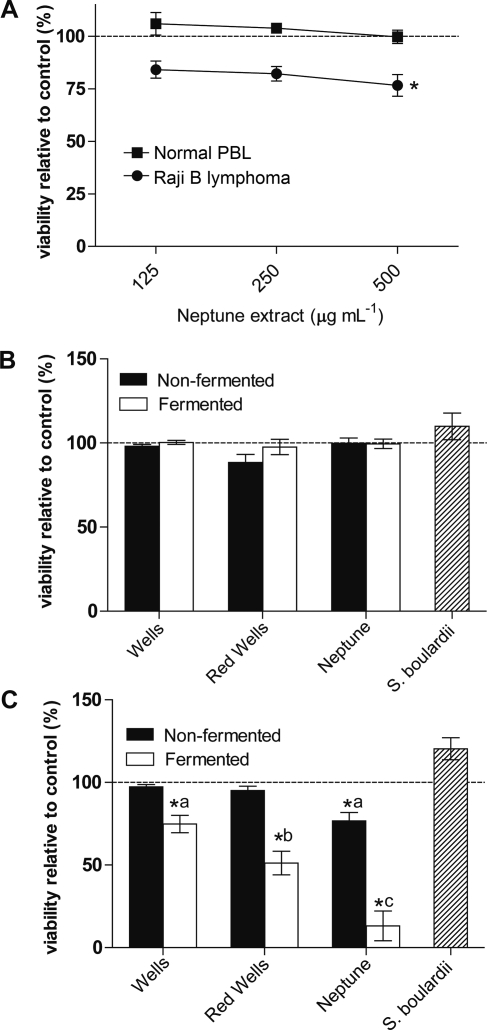
Polar rice bran extracts were previously shown to inhibit tumor promotion of lymphoblastoid B cells, and rice bran agglutinin inhibited growth of monoblastic leukemia U937 cells.(26) Methanol-soluble metabolites from Neptune rice bran were screened for dose dependent effects on viability of normal human peripheral blood lymphocytes (PBL) and malignant human B-cell lymphoma (Figure 3A). Viability was measured by resazurin stain after 24 h of incubation with fermented and nonfermented rice bran extracts. The rice bran extracts did not affect the viability of normal PBL (Figure 3A). A significant reduction in lymphoma viability was demonstrated at the 500 μg mL−1 dose of Neptune rice bran extract, while the 125 and 250 μg mL−1 were not significantly reduced from vehicle control (Figure 3A). The 500 μg mL−1 dose of rice bran extract was next used to examine effects of both nonfermented and fermented rice bran extracts across varieties on normal PBL and lymphoma. None of the rice bran extracts altered the viability of normal PBL (Figure 3B). The S. boulardii-fermented rice bran significantly inhibited lymphoma viability compared to vehicle controls for all varieties tested (Figure 3C). The nonfermented Neptune rice bran extracts showed a 23% reduction in viability, and S. boulardii-fermented Neptune rice bran extracts reduced viability by 85% compared to control. At 500 μg mL−1, unfermented extracts of Wells and Red Wells had no effect on lymphoma viability relative to the control, however fermented extracts inhibited viability by 75% and 51%, respectively (Figure 3C). The percent reduction in viability differed among varieties of the three fermented extracts (ANOVA, Tukey post hoc, P < 0.05). The differential reduction in viability by fermented rice bran among rice varieties supports that variation in metabolite contents as detected in Figure 1 may be important for bioactivity. The isopropanol:acetonitrile:water (3:2:2) solvent used for metabolite profiling of fermented bran extracts (Figure 2) was also examined for effects on lymphoma viability, however this solvent demonstrated suboptimal background activity as a vehicle control and was therefore not utilized to compare effects across rice varieties (data not shown).
Figure 3.
S. boulardii fermented rice bran inhibits lymphoma viability. (A) Different doses of nonfermented methanolic rice bran extracts (Neptune) were added to normal human peripheral blood lymphocytes (PBL) and Raji B lymphoma cultures for 24 h. Values are expressed as the mean percent viable cells relative to the vehicle control ± SEM. Extracts of nonfermented Neptune reduced lymphoma viability at 500 μg mL−1 (Student’s t test, P < 0.05). (B) Fermented and nonfermented extracts of all three varieties at 500 μg mL−1 had no effect on viability of normal PBL. (C) Fermented and nonfermented extracts of all three varieties at 500 μg mL−1 differentially affected lymphoma viability, as measured by cell fluorescence after the addition of resaruzin (ANOVA, Tukey post hoc, P < 0.05). Significance from vehicle control is represented by an asterisk, and statistical groupings are denoted by the letters a, b, and c.
S. boulardii Modulation of Bioactive Rice Bran Compounds
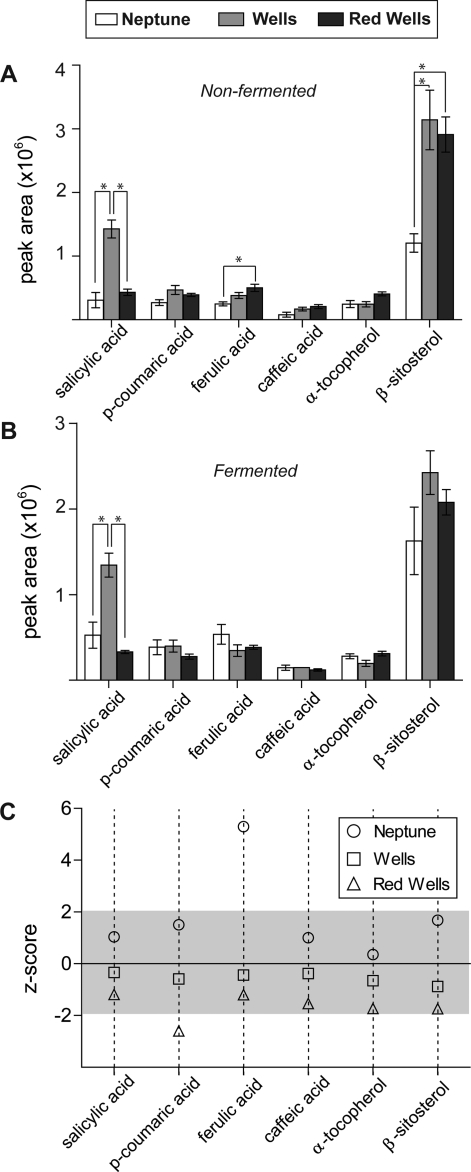
Given the varietal differences in anticancer activity of S. boulardii fermented rice bran extracts, a number of bioactive rice bran compounds were selected for relative quantification. Rice bran contains a number of metabolites with reported anticancer effects, notably phenolics and phytosterols.37−39 Salicylic, p-coumaric, ferulic, and caffeic acid, and also α-tocopherol and β-sitosterol were detected in nonfermented and fermented rice bran from each of the three varieties by comparing the initial GC−MS chromatograms to purchased standards (Figure 4). Nonfermented extracts from Wells contained a greater quantity of salicylic acid than both Red Wells and Neptune (Figure 4A). Red Wells contained higher amounts of ferulic acid than Neptune, and Neptune contained significantly less β-sitosterol than both Wells and Red Wells (ANOVA, Tukey post hoc, P < 0.05). A z-score analysis was conducted to determine significant changes in metabolite quantity due to fermentation with S. boulardii, using nonfermented rice bran as a control. The data shown in Figure 4C supports that S. boulardii fermentation reduced the quantity of p-coumaric acid in Red Wells, and increased ferulic acid in Neptune (Figure 4C).
Figure 4.
Bioactive food components in rice bran with and without S. boulardii fermentation. Relative quantification of metabolites without fermentation (A) and with fermentation (B) was based on the area of the GC−MS chromatograph. Metabolites showing a significant difference by relative quantity and between two varieties were indicated by an asterisk (ANOVA, Tukey post hoc, P < 0.05). Values are expressed as mean peak area ± SEM. (C) z-score for metabolites from fermented extracts using the nonfermented as a control. Significant changes in metabolite quantity are indicated by z-score values outside of the shaded region. Increased ferulic acid was detected in S. boulardii fermented Neptune rice bran, and decreased p-coumaric acid was detected from fermented Red Wells compared to nonfermented.
Rice Bran As Sole Carbon Source for S. boulardii
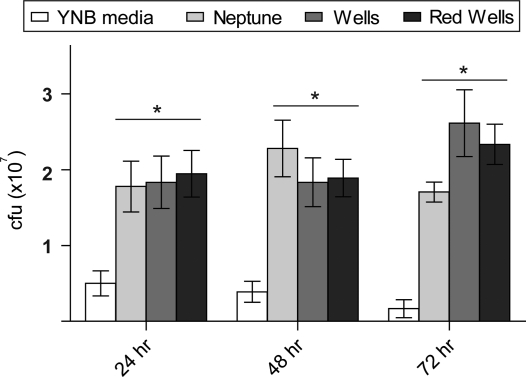
The ability of S. boulardii to utilize and quantitatively alter chemical components of rice bran was confirmed by measuring its growth on rice bran as a sole carbon source. Overnight cultures of S. boulardii inoculated into medium containing 5% rice bran from each of the varieties grew significantly better than cultures inoculated into YNB broth with dextrose as the primary carbon source (Figure 5). In addition, S. boulardii cultures maintained viability and cell numbers for 3 days on rice bran medium, while the number of cells in the YNB cultures steadily declined. No significant differences in the growth of S. boulardii were detected among the three rice varieties (ANOVA, Tukey post hoc, P < 0.05).
Figure 5.
Rice bran enhances growth of S. boulardii. Rice bran from all three rice varieties significantly increased the growth of S. boulardii compared to YNB medium alone after 24, 48, and 72 h. An asterisk indicates difference in the quantity of yeast colonies compared to the YNB control (one way ANOVA, Tukey post hoc, P < 0.05).
Discussion
This study demonstrates the utility of integrating global and targeted metabolite profiling for analysis of rice bran phytochemicals in the presence and absence of S. boulardii fermentation, and has advanced our knowledge about how probiotic fermentation of rice bran can enhance the bioactivity of extracts. Metabolomics is one strategy used to measure the wide array of phytochemicals that are typically evaluated in the “free” forms from food extracts, as these small molecules dissolve quickly and are immediately absorbed into the bloodstream. This high throughput, yet sensitive, approach is also useful to assess the “bound” forms of rice molecules, which are attached to the plant cell walls and must be released by microbes during digestion before they can be absorbed. These findings set the stage for developing metabolomics as a tool for investigating rice bran phytochemical diversity and digestion by probiotics.
Metabolite profiles in this study showed variation among the three U.S. rice varieties Neptune, Wells, and Red Wells (Figure 1A, Table 1). The Neptune metabolite profile separated from both Wells and Red Wells. This cluster was expected given the near-isogenic state of the “Wells” varieties and provided strong rationale for investigating differential bioactive properties. Rice bran fermentation with the S. boulardii probiotic enhanced metabolite diversity (Figure 1C), and showed rice varietal differences in bran extract-mediated reduction of lymphoma growth (Figure 3B). No apparent toxicity was demonstrated for S. boulardii fermented rice bran on normal peripheral blood lymphocytes. Candidate metabolites that were significantly increased postfermentation also differed among the three varieties (Table 2). The ability of S. boulardii to utilize rice bran as a sole carbon source substrate for cellular metabolism and growth (Figure 5) suggests that rice bran contains unique prebiotic characteristics. Although no differences were detected among the Neptune, Wells and Red Wells rice varieties to increase probiotic growth, these findings warrant further investigation of rice bran prebiotic components and the synergistic effects of prebiotic/probiotic combinations on human health. To our knowledge, only two studies have examined distinct rice bran varieties for differential anticarcinogenic activity.27,28 Data from these studies demonstrate that rice varieties with pigmented seed coat also exhibit differential activity when compared to nonpigmented. The findings presented in this report suggest that some of the inconsistent results of past rice bran investigations on cancer cell growth may be due to the rice variety tested and not just those chemicals responsible for pigment.
Another plausible explanation for inconclusive data on rice bran is not only differences in metabolite content among varieties but also the influence of probiotics altering the bioavailability of cancer-protective compounds in select tissues. The ability of phenols, particularly ferulic, salicylic, caffeic, and p-coumaric acids and α-tocopherol (a lipid-soluble antioxidant) found in rice bran, to scavenge free radicals, alter enzymes, affect biochemical pathways, and interfere with gene expression has attracted the attention of researchers in search of cancer-fighting agents.21,29,30 The efficacy of ferulic acid, which remains in the bloodstream longer than other known antioxidants and therefore may provide more protection, is dependent on its bioavailability and dosage.(31) However, plant phenols are often found in a biologically unavailable form due to an ester-bond to cell wall polysaccharides. Therefore, the optimal dose of rice bran required to achieve cancer-fighting levels of ferulic acid is unknown. Humans and rats have been shown to release diferulic acid from bran fiber using gastrointestinal esterases found in the large and small intestines, thus enhancing the bioavailability of this compound.(32) The data shown in Figure 4C supports that the Neptune rice variety may exhibit higher probiotic-induced ferulic acid release and bioavailability than the other two varieties, and that consuming the whole food postfermentation with S. boulardii may be a viable alternative for achieving enhanced levels of this compound without losing the benefits of the others. Yeast cells typically only maintain viability for several hours after they have reached stationary phase and depleted their carbon source. Our results show that rice bran medium allows S. boulardii cells to maintain viability over several days, suggesting that secondary fermentation by the yeast may be occurring and may further alter the phytochemical content of the rice bran (Figure 5). Thus, it will be necessary to optimize fermentation times to advance our understanding of the kinetics of rice bran phytochemical metabolism and release by S. boulardii.
Emerging evidence supports additive and/or synergistic effects of rice bran components for protection against certain cancers,5,11,40,41 however few studies have examined differences in phytochemical contents in commercially available rice varieties. Our data support that many rice bran components were fermented by the yeast probiotic (Figure 2) and these components work together to enhance probiotic growth (Figure 5). One study examined a yeast fermentation of rice bran for changes in the stability, palatability, and nutritional status (carbohydrate, methionine, calcium, and ash content) of the bran, but did not address the alteration of potentially bioactive phytochemicals.(42) Given the evidence for cancer fighting activities of rice bran phytochemicals, the data presented herein support that S. boulardii-fermented rice bran should be next tested for bioavailability of bioactive components and for reducing lymphoma viability in vivo. Chemopreventive single agent compounds found in rice bran include, but are not limited to, tocopherols, polyphenols, inositol hexaphosphate (IP6), nonstarchy polysaccharides, γ-oryzanol and phytosterols.2,4,43−45 Our metabolite profile analysis of fermented rice bran revealed extensive rice bran chemical diversity, and can be used to further the identities of novel combinations of bioactive compounds that display phytochemical teamwork.46,47 Whole rice bran consumption is undoubtedly recognized as important for providing more comprehensive protection against cancer cells when compared to supplementation with isolated ingredients, and the metabolite profiling techniques and chemical analyses presented herein support further interrogation of rice bran effects on intestinal microbe interactions as well as probiotic growth and metabolism. The metabolomics strategy applied herein has advanced our understanding of the health importance of rice bran phytochemical diversity in the presence and absence of fermentation and for disease fighting activity. Single agent nutritional “magic bullets” too often fail to achieve the health benefits indicated by cell-based assays. Available methodologies have also limited the scientific investigations of rice bran to these reductive approaches. By utilizing global metabolomic profiling, we can now more holistically approach complex mixtures of small molecules in rice bran and improve studies linking bioactive food components and human health.
Acknowledgments
We would like to thank Dr. Ming-Hsuan Chen and Dr. Anna McClung from the USDA-ARS Rice Research Unit for supplying the bran from Neptune, Wells and Red Wells rice varieties.
Glossary
Abbreviations Used
- GC−MS
gas chromatography−mass spectrometry
- U.S.
United States.
The Shipley Foundation and NIH 5R03CA150070-2 supported this work.
Funding Statement
National Institutes of Health, United States
References
- Kahlon T. S.Rice Bran: Production, Composition, Functionality and Food Applications, Physiological Benefits. In Fiber Ingredients: Food Applications and Health Benefits; Cho S. S., Samuel P., Eds.; Taylor and Francis Group, LLC: Boca Raton, 2009; pp 305−21. [Google Scholar]
- Cai H.; Al-Fayez M.; Tunstall R. G.; Platton S.; Greaves P.; Steward W. P.; Gescher A. J. The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in ApcMin mice. Mol. Cancer Ther. 2005, 4 (9), 1287–92. [DOI] [PubMed] [Google Scholar]
- Kannan A.; Hettiarachchy N.; Johnson M. G.; Nannapaneni R. Human colon and liver cancer cell proliferation inhibition by peptide hydrolysates derived from heat-stabilized defatted rice bran. J. Agric. Food Chem. 2008, 56 (24), 11643–7. [DOI] [PubMed] [Google Scholar]
- Kong C. K.; Lam W. S.; Chiu L. C.; Ooi V. E.; Sun S. S.; Wong Y. S. A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem. Pharmacol. 2009, 77 (9), 1487–96. [DOI] [PubMed] [Google Scholar]
- Phutthaphadoong S.; Yamada Y.; Hirata A.; Tomita H.; Taguchi A.; Hara A.; Limtrakul P. N.; Iwasaki T.; Kobayashi H.; Mori H. Chemopreventive effects of fermented brown rice and rice bran against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in female A/J mice. Oncol. Rep. 2009, 21 (2), 321–7. [PubMed] [Google Scholar]
- Kestin M.; Moss R.; Clifton P. M.; Nestel P. J. Comparative effects of three cereal brans on plasma lipids, blood pressure, and glucose metabolism in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 1990, 52 (4), 661–6. [DOI] [PubMed] [Google Scholar]
- Kuriyan R.; Gopinath N.; Vaz M.; Kurpad A. V. Use of rice bran oil in patients with hyperlipidaemia. Natl. Med. J. India 2005, 18 (6), 292–6. [PubMed] [Google Scholar]
- Wilson T. A.; Nicolosi R. J.; Woolfrey B.; Kritchevsky D. Rice bran oil and oryzanol reduce plasma lipid and lipoprotein cholesterol concentrations and aortic cholesterol ester accumulation to a greater extent than ferulic acid in hypercholesterolemic hamsters. J. Nutr. Biochem. 2007, 18 (2), 105–12. [DOI] [PubMed] [Google Scholar]
- Sukhonthara S.; Theerakulkait C.; Miyazawa M. Characterization of Volatile Aroma Compounds from Red and Black Rice Bran. J. Oleo Sci. 2009, 58 (3), 155–160. [DOI] [PubMed] [Google Scholar]
- Heuberger A. L.; L. M.; Chen M. H.; Brick M. A.; Leach J. E.; Ryan E. P. Metabolomic and Functional Genomic Analyses Reveal Varietal Differences in Bioactive Compounds of Cooked Rice. PLOS One 2010, 5 (9), e12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyama M.; Yoshimi N.; Yamada Y.; Sakata K.; Kuno T.; Yoshida K.; Qiao Z.; Vihn P. Q.; Iwasaki T.; Kobayashi H.; Mori H. Preventive effect of fermented brown rice and rice bran against colon carcinogenesis in male F344 rats. Oncol. Rep. 2002, 9 (4), 817–22. [PubMed] [Google Scholar]
- Koh J. H.; Suh H. J. Biological Activities of Thermo-tolerant Microbes from Fermented Rice Bran as an Alternative Microbial Feed Additive. Appl. Biochem. Biotechnol. 2009, 157 (3), 420–430. [DOI] [PubMed] [Google Scholar]
- Koh J. H.; Yu K. W.; Suh H. J. Biological activities of Saccharomyces cerevisiae and fermented rice bran as feed additives. Lett. Appl. Microbiol. 2002, 35 (1), 47–51. [DOI] [PubMed] [Google Scholar]
- Zanello G.; Meurens F.; Berri M.; Salmon H. Saccharomyces boulardii effects on gastrointestinal diseases. Curr. Issues Mol. Biol. 2009, 11 (1), 47–58. [PubMed] [Google Scholar]
- Czerucka D.; Piche T.; Rampal P. Review article: yeast as probiotics -- Saccharomyces boulardii. Aliment. Pharmacol. Ther. 2007, 26 (6), 767–78. [DOI] [PubMed] [Google Scholar]
- Yan F.; Polk D. B. Probiotics as functional food in the treatment of diarrhea. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9 (6), 717–21. [DOI] [PubMed] [Google Scholar]
- Fleet G. H. Yeasts in foods and beverages: impact on product quality and safety. Curr. Opin. Biotechnol. 2007, 18 (2), 170–175. [DOI] [PubMed] [Google Scholar]
- Jespersen L. Occurrence and taxonomic characteristics of strains of Saccharomyces cerevisiae predominant in African indigenous fermented foods and beverages. FEMS Yeast Res. 2003, 3 (2), 191–200. [DOI] [PubMed] [Google Scholar]
- Martins F. S.; Nardi R. M.; Arantes R. M.; Rosa C. A.; Neves M. J.; Nicoli J. R. Screening of yeasts as probiotic based on capacities to colonize the gastrointestinal tract and to protect against enteropathogen challenge in mice. J. Gen. Appl. Microbiol. 2005, 51 (2), 83–92. [DOI] [PubMed] [Google Scholar]
- Rekha C. R.; Vijayalakshmi G., Bioconversion of isoflavone glycosides to aglycones, mineral bioavailability and vitamin B complex in fermented soymilk by probiotic bacteria and yeast. J. Appl. Microbiol. [DOI] [PubMed] [Google Scholar]
- Sindhu S. C.; Khetarpaul N. Effect of probiotic fermentation on antinutrients and in vitro protein and starch digestibilities of indigenously developed RWGT food mixture. Nutr. Health 2002, 16 (3), 173–81. [DOI] [PubMed] [Google Scholar]
- Skovgaard N. New trends in emerging pathogens. Int. J. Food Microbiol. 2007, 120 (3), 217–24. [DOI] [PubMed] [Google Scholar]
- de Vrese M.; Marteau P. R. Probiotics and prebiotics: effects on diarrhea. J. Nutr. 2007, 137 (3 Suppl. 2), 803S–11S. [DOI] [PubMed] [Google Scholar]
- Dalmasso G.; Cottrez F.; Imbert V.; Lagadec P.; Peyron J. F.; Rampal P.; Czerucka D.; Groux H.; Foussat A.; Brun V. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology 2006, 131 (6), 1812–25. [DOI] [PubMed] [Google Scholar]
- Chen X.; Kokkotou E. G.; Mustafa N.; Bhaskar K. R.; Sougioultzis S.; O’Brien M.; Pothoulakis C.; Kelly C. P. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo and protects against Clostridium difficile toxin A-induced enteritis. J. Biol. Chem. 2006, 281 (34), 24449–54. [DOI] [PubMed] [Google Scholar]
- Nam S. H.; Choi S. P.; Kang M. Y.; Koh H. J.; Kozukue N.; Friedman M. Bran extracts from pigmented rice seeds inhibit tumor promotion in lymphoblastoid B cells by phorbol ester. Food Chem. Toxicol. 2005, 43 (5), 741–745. [DOI] [PubMed] [Google Scholar]
- Miyoshi N.; Koyama Y.; Katsuno Y.; Hayakawa S.; Mita T.; Ohta T.; Kaji K.; Isemura M. Apoptosis Induction Associated with Cell Cycle Dysregulation by Rice Bran Agglutinin. J. Biochem. 2001, 130, 799–805. [DOI] [PubMed] [Google Scholar]
- Shu X. L.; Frank T.; Shu Q. Y.; Engel K. H. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56 (24), 11612–20. [DOI] [PubMed] [Google Scholar]
- Goffman F. D.; Pinson S.; Bergman C. Genetic diversity for lipid content and fatty acid profile in rice bran. J. Am. Oil Chem. Soc. 2003, 80 (5), 485–90. [Google Scholar]
- Brooks S. A.; Yan W.; Jackson A. K.; Deren C. W. A natural mutation in rc reverts white-rice-pericarp to red and results in a new, dominant, wild-type allele: Rc-g. Theor. Appl. Genet. 2008, 117, 575–80. [DOI] [PubMed] [Google Scholar]
- Sindhu S. C.; Khetarpaul N. Effect of probiotic fermentation on antinutrients and in vitro protein and starch digestibilities of indigenously developed RWGT food mixture. Nutr. Health 2002, 16 (3), 173–181. [DOI] [PubMed] [Google Scholar]
- SINDHU S. C. a. N. K. Fermentation with one step single and sequential cultures of yeast and lactobacilli: Effect on antinutrients and digestibilities (in vitro) of starch and protein in an indigenously developed food mixture. Plant Foods Hum. Nutr. 2003, 58, 1–10.12859008 [Google Scholar]
- Palazoglu M.; Fiehn O. Metabolite identification in blood plasma using GC/MS and the Agilent Fiehn GC/MS metabolomics RTL library. Agilent Application Note 2009, 5990–3638EN. [Google Scholar]
- Skogerson K.; Harrigan G. G.; Reynolds T. L.; Halls S. C.; Ruebelt M.; Iandolino A.; Pandravada A.; Glenn K. C.; Fiehn O. Impact of Genetics and Environment on the Metabolite Composition of Maize Grain. J. Agric. Food Chem. 2010, 58 (6), 3600–10. [DOI] [PubMed] [Google Scholar]
- Xiao J.; Zhang Y.; Wang J.; Yu W.; Wang W.; Ma X., Monitoring of Cell Viability and Proliferation in Hydrogel-Encapsulated System by Resazurin Assay. Appl. Biochem. Biotechnol. [DOI] [PubMed] [Google Scholar]
- Duarte M.; Giordani R. B.; De Carli G. A.; Zuanazzi J. A.; Macedo A. J.; Tasca T. A quantitative resazurin assay to determinate the viability of Trichomonas vaginalis and the cytotoxicity of organic solvents and surfactant agents. Exp. Parasitol. 2009, 123 (2), 195–8. [DOI] [PubMed] [Google Scholar]
- Taniguchi H.; Hosoda A.; Tsuno T.; Maruta Y.; Nomura E. Preparation of ferulic acid and its application for the synthesis of cancer chemopreventive agents. Anticancer Res. 1999, 19 (5A), 3757–61. [PubMed] [Google Scholar]
- Bouic P. J. D.; Etsebeth S.; Liebenberg R. W.; Albrecht C. F.; Pegel K.; Van Jaarsveld P. P. Beta-sitosterol and beta-sitosterol glucoside stimulate human peripheral blood lymphocyte proliferation: Implications for their use as an immunomodulatory vitamin combination. Int. J. Immunopharmacol. 1996, 18 (12), 693–700. [DOI] [PubMed] [Google Scholar]
- Piironen V.; Lindsay D. G.; Miettinen T. A.; Toivo J.; Lampi A.-M. Plant sterols: biosynthesis, biological function and their importance to human nutrition. J. Sci. Food Agric. 2000, 80 (7), 939–66. [Google Scholar]
- Mori H.; Kawabata K.; Yoshimi N.; Tanaka T.; Murakami T.; Okada T.; Murai H. Chemopreventive effects of ferulic acid on oral and rice germ on large bowel carcinogenesis. Anticancer Res. 1999, 19 (5A), 3775–8. [PubMed] [Google Scholar]
- Hudson E. A.; Dinh P. A.; Kokubun T.; Simmonds M. S. J.; Gescher A. Characterization of Potentially Chemopreventive Phenols in Extracts of Brown Rice That Inhibit the Growth of Human Breast and Colon Cancer Cells. Cancer Epidemiol., Biomarkers Prev. 2000, 9, 1163–70. [PubMed] [Google Scholar]
- Feddern V.; Furlong E. B.; Soares L. A. d. S. Effects of fermentation on the physicochemical and nutritional properties of rice bran. Ciênc. Tecnol. Aliment., Campinas 2007, 27 (4), 800–4. [Google Scholar]
- Takeshita M.; Nakamura S.; Makita F.; Ohwada S.; Miyamoto Y.; Morishita Y. Antitumor effect of RBS (rice bran saccharide) on ENNG-induced carcinogenesis. Biotherapy 1992, 4 (2), 139–45. [DOI] [PubMed] [Google Scholar]
- Aoe S.; Oda T.; Tojima T.; Tanaka M.; Tatsumi K.; Mizutani T. Effects of rice bran hemicellulose on 1,2-dimethylhydrazine-induced intestinal carcinogenesis in Fischer 344 rats. Nutr. Cancer 1993, 20 (1), 41–9. [DOI] [PubMed] [Google Scholar]
- Norazalina S.; Norhaizan M. E.; Hairuszah I.; Norashareena M. S. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp. Toxicol. Pathol 2010, 62 (3), 259–68. [DOI] [PubMed] [Google Scholar]
- Adom K. K.; Sorrells M. E.; Liu R. H. Phytochemical Profiles and Antioxidant Activity of Wheat Varieties. J. Agric. Food Chem. 2003, 51 (26), 7825–34. [DOI] [PubMed] [Google Scholar]
- Lila M. A. From beans to berries and beyond: teamwork between plant chemicals for protection of optimal human health. Ann. N.Y. Acad. Sci. 2007, 1114, 372–80. [DOI] [PubMed] [Google Scholar]