Abstract
Transforming growth factor-beta (TGFβ) is a pleiotropic multifunctional cytokine that regulates several essential cellular processes in many parts of the body including the cornea. Three isoforms of TGFβ are known in mammals and the human cornea expresses all of them. TGFβ1 has been shown to play a central role in scar formation in adult corneas whereas TGFβ2 and TGFβ3 have been implicated to play a critical role in corneal development and scarless wound healing during embryogenesis. The biological effects of TGFβ in the cornea have been shown to follow SMAD dependent as well as SMAD-independent signaling pathways depending upon cellular responses and microenvironment. Corneal TGFβ expression is necessary for maintaining corneal integrity and corneal wound healing. On the other hand, TGFβ is perhaps the most important cytokine in the pathogenesis of fibrotic disease in the cornea. Although the transformation of keratocytes to myofibroblasts induced by TGFβ is largely believed to cause corneal fibrosis or scarring, the precise molecular mechanism(s) involved in this process is still unknown. Currently no drugs are available to treat corneal scarring effectively without causing significant side effects. Many approaches to treat TGFβ-mediated corneal scarring are under investigation. These include blocking of TGFβ, TGFβ receptor, TGFβ function and/or TGFβ maturation. Other strategies such as modulating keratocyte proliferation, apoptosis, transcription and DNA condensation are also being investigated. The potential of gene therapy to neutralize the pathologic effects of TGFβ has also been demonstrated recently.
Keywords: Cornea, gene therapy, corneal scarring/haze, TGFβ
1. INTRODUCTION
Researchers discovered a new cytokine with the capability to transform fibroblasts in 1983 while studying epidermal and platelet derived growth factors in rat fibroblasts [1–2]. It has been almost three decades since transforming growth factor beta (TGFβ) was characterized as a distinct molecular entity after isolation from bovine kidney, human placenta, and human platelets. Originally called sarcoma growth factor, TGFβ was first isolated from neoplastic mouse tissue by Moloney sarcoma virus [3]. The TGFβ superfamily is made up of multifunctional cytokines that affect different cells and play a central role in wound healing and fibrosis of various organs including the cornea [4–5]. The TGFβ superfamily represents an extremely complex group of proteins in terms of structure, nomenclature, function, receptor types, sequence homology, and signaling pathways [1, 6]. TGFβ proteins are found in scores of multicellular organisms in nature highlighting the importance of this cytokine family [7, 8]. TGFβ elicits different responses depending on the context of action, concentration in different organs, and presence or absence of other growth factors influencing cellular and immunological activity.
The majority of cell types produce TGFβ. TGFβ is initially formed as precursor TGFβ containing the latency-associated protein and the latent TGF-binding protein. The extracellular activation of this complex is crucial in TGFβ-regulated biological activity. Upon activation, TGFβ transduces its signal through cooperative binding with two types of membrane bound TGFβ receptors and communicates the signals from the cell membrane to the nucleus via Smad-dependent or Smad-independent pathways [9]. The TGFβ superfamily includes three isoforms of TGFβ, five activins, the Mullerian inhibiting substance, and eight or more bone morphogenetic proteins (BMP) encoded by different genes. TGFβ cytokines are involved in wound repair and many other vital pre- and postnatal physiological processes such as cellular proliferation, migration, differentiation, apoptosis, and extracellular matrix (ECM) production in various tissues including the cornea [10–12]. TGFβ is linked to a number of human diseases and pathological processes of many organ systems including autoimmune disease, oncogenesis, atherosclerosis, fibrosis of the lungs, liver, kidneys, and skin, and corneal scarring and dystrophy [13–16]. In this review, we will first discuss TGFβ isoforms, biosynthesis, maturation, signal transduction, and biological function. Then, we will discuss TGFβ’s role in corneal homeostasis and wound healing followed by its clinical significance in the pathogenesis of corneal disease and treatment approaches to control pathologic effects of TGFβ.
2. TGFβ ISOFORMS
Five TGFβ isoforms have been identified in vertebrates. Three isoforms of TGFβ (β1, β2, and β3) have been identified in mammals while two additional isoforms have been reported, namely TGFβ4 and β5 in birds and amphibians respectively [6, 17–18]. All isoforms of mammalian TGFβ are 25 kDa homodimeric proteins and share an extensive similarity in their amino acid sequences although subtle differences exist [19–20]. For instance, TGFβ1 shows 75% similarity to TGFβ2, and about 80% to TGFβ3 whereas TGFβ2 has approximately 80% similarity to TGFβ3 [6]. Although these isoforms are homologues their amino acid sequences are encoded by three distinct genes suggesting that they may have different functions. First, the disulfide-linked functional protein, a monomer made up of two polypeptide chains, comes in either a homo- or heterodimer flavor. Second, the monomer is produced as the C-terminal region of a precursor molecule that also houses a propeptide and a petite N-terminal sequence required for secretion. Finally, all TGFβ superfamily members share a conserved C-terminal seven-cysteine domain as part of the monomer’s primary structure essential for dimerization and protein stability [6].
TGFβ is ubiquitously expressed by many cell types such as fibroblasts, endothelium, epithelium, smooth muscle cells, etc. Moreover, it is released by immune cells and detected in wound fluid especially during inflammation and tissue repair. Although all three TGFβ isoforms participate in wound healing TGFβ1 plays a dominant role in the wound repair process while TGFβ2 and TGFβ3 have been shown to play a key role in embryonic development and scarless wound healing [5, 21–25].
The cornea and many other anterior eye segment tissues such as limbus, conjunctiva, and tear fluid have been shown to express different TGFβ isoforms and their receptors [26–27]. This insinuates the importance of the TGFβ in the eye as will be described later.
3. TGFβ BIOSYNTHESIS AND MATURATION
The biosynthesis and maturation of TGFβ is essential for its biological function. TGFβ is released as a large inactive precursor lacking the ability to bind to its cell surface receptors [28]. All three TGFβ isoforms have differences in precursor nucleotide sequence, precursor amino acid, gene location, and gene expression site as shown in Table (1).
Table (1).
Comparison of TGF-β isoforms biosynthesis
| Isoforms | Precursor ATGC Sequence |
Glycosylation Site |
Precursor Amino Acid |
Cysteine Residues |
Gene Location (chromosome) |
Gene Expression Site |
|---|---|---|---|---|---|---|
| TGFβ1 | GC Rich | 3 | 390 | 3 | 19q13 | Endothelial, connective tissue and hematopoietic cells |
| TGFβ2 | AT Rich | 3 | 442/414 | 6 | 1q41 | Epithelial and CNS Cells |
| TGFβ3 | Mix AT/GC | 4 | 410 | 5 | 14q24 | Mesenchymal Cells |
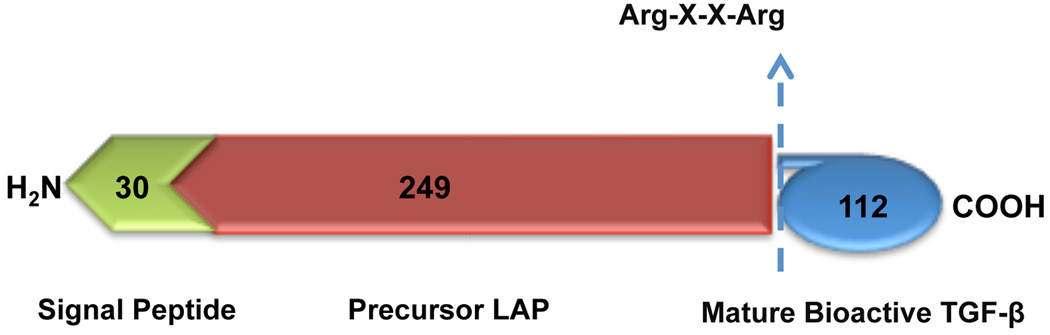
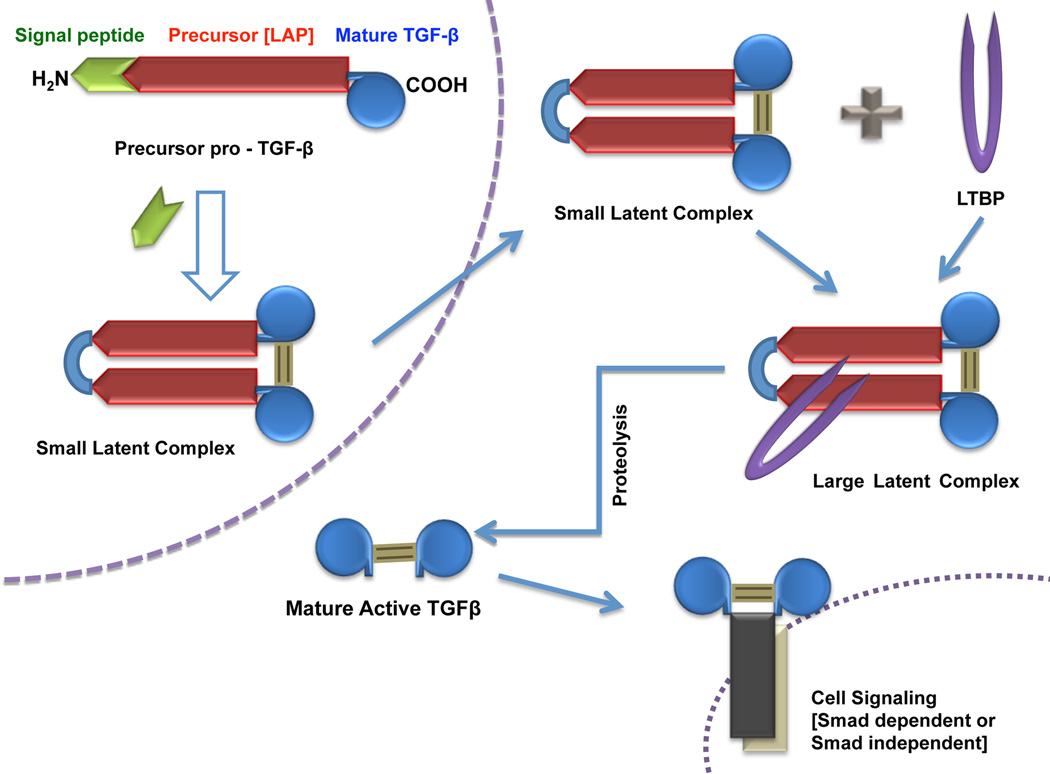
The inactive TGFβ precursor consists of 390–442 amino acids with three specialized regions made up of an N-terminal hydrophobic signal peptide region, the latency-associated peptide region of 249 amino acids, and the C-terminal region, a potentially bioactive TGFβ region consisting of 112 amino acids (Fig. (1)). The inactive TGFβ precursor is processed in the Golgi apparatus by endopeptidases, which cleave it at an Arg-X-X-Arg site (X=any amino acid) leading to the formation of a small latent complex. This complex, which contains a mature TGFβ homodimer noncovalently attached to two latency-associated peptides, is usually secreted from the cell with latent TGFβ binding protein as the large latent complex [29]. Latent TGFβ binding protein anchors inactive TGFβ to the ECM requiring proteolytic cleavage for conversion to mature TGFβ. Final activation of TGFβ involves the release of bioactive TGFβ from the latent complex via the interaction of latency-associated peptide with various proteins. Physical interactions with thrombospondin-1 or proteolytic cleavage brought about by plasmin, thrombin, endoglycosylase, or plasma transglutaminase causes a change in latency-associated peptide conformation liberating mature TGFβ [30] (Fig. (2)). In addition, recent studies evaluated the role of integrins in latent TGFβ activation and also showed both proteolytic and non-proteolytic (latent TGFβ conformational change via physical interaction) mechanisms [31].
Fig. (1).
Schematic representation of the protein structure related to TGFβ. An N-terminal hydrophobic signal peptide region (30 amino acids), the latent associated peptide (LAP) (249 amino acids) region and the C-terminal, a mature bioactive TGFβ region.
Fig. (2).
Schematic representation of TGFβ maturation. Precursor pro-TGFβ is cleaved by endopeptidase in Golgi apparatus to form small latent complex. This complex then covalently binds with latent TGFβ binding protein (LTBP) and forms the large latent complex that is finally released into the ECM as mature TGFβ.
Following the activation of bioactive TGFβ, a 25 kDa protein made up of two polypeptides of 12.5 kDa, signal transduction via different molecular signaling pathways may proceed.
4. TGFβ SIGNAL TRANSDUCTION
TGFβ functions as a regulatory cytokine and relays its commands from the cell surface to the nucleus by molecular signal transduction. Upon binding to cell-surface receptors, TGFβ ligands stimulate the assembly of serine/threonine kinase complexes which inaugurate signal transduction by cytoplasmic protein phosphorylation. This signal is then shuttled to the nucleus where it activates or suppresses target gene transcription leading to different cellular responses. The members of the TGFβ signal response incorporate Smad-dependent or Smad-independent signaling pathways depending on cell type.
4.1 SMAD DEPENDENT SIGNALING
Smad signaling pathway has been widely elucidated for the signaling of the TGFβ in regulating diverse cellular processes [5, 25, and 33]. TGFβ transduces its signal across the plasma membrane by way of specific cell surface receptors known as TGFβ receptors [34]. Based on their structure, function, and electrophoretic mobility, the TGFβ receptor family is divided into three subfamilies: type I, II, and III receptors [1, 6–7].
Type I receptors (TGFβR1) have been found to have a higher level of sequence similarity than type II receptors (TGFβR2), particularly in the kinase domain. TGFβR1 and TGFβR2 mainly affect the transduction of signals via serine-threonine membrane kinases that phosphorylate the hydroxyl site of serine-threonine on the target cell. Immediately upstream from its kinase domain, TGFβR1 has a conserved 30 amino acid region, the “juxtamembrane glycine-serine domain”, which play important role in TGFβR1 activation [35–36]. For phosphorylation, TGFβR1 requires activation by TGFβR2 to trigger its kinase function whereas TGFβR2 boasts intrinsic kinase activity. Furthermore, TGFβR1 requires TGFβR2 for ligand binding [37–38]. After phosphorylation, TGFβR1 phosphorylates and activates the highly-conserved cytoplasmic proteins of the Smad family. Besides TGFβR1 and TGFβR2, a third TGFβ receptor also exists. TGFβ receptor III (TGFβR3) is a transmembrane proteoglycan also known as betaglycan [39]. TGFβR3 binds to all three mammalian isoforms via its core protein and has a molecular weight ranging from 250–300 kDa. Betaglycan has been reported to be a mixture of heparan sulfate and chondroitin sulfate glycosaminoglycan [40, 41]. Along with endoglin, it is found to have the unique property of binding TGFβ with high affinity. There is also evidence that the highest level of sequence similarity between betaglycan and endoglin is found in the cytoplasmic and transmembrane domains [42].
Previously Cheiftz and collaborators evaluated the ligand binding property of betaglycan and its presence in serum and ECM [18]. Many studies have revealed that TGFβR3 is not directly involved in TGFβ signal transduction. However, after binding to various TGFβ superfamily members at the cell surface, it may act as a ligand reservoir for TGFβ receptors. Therefore, even though TGFβR3 is not believed to take part in signal transduction directly, it allows high affinity binding to TGFβR2. TGFβ1 and β3 bind TGFβR2 to initiate signal transduction whereas TGFβ2 requires TGFβR3 in order to bind TGFβR2 [43]. Upon binding to its corresponding receptor, TGFβ elicits its signals through Smad transducers (Fig. (3)).
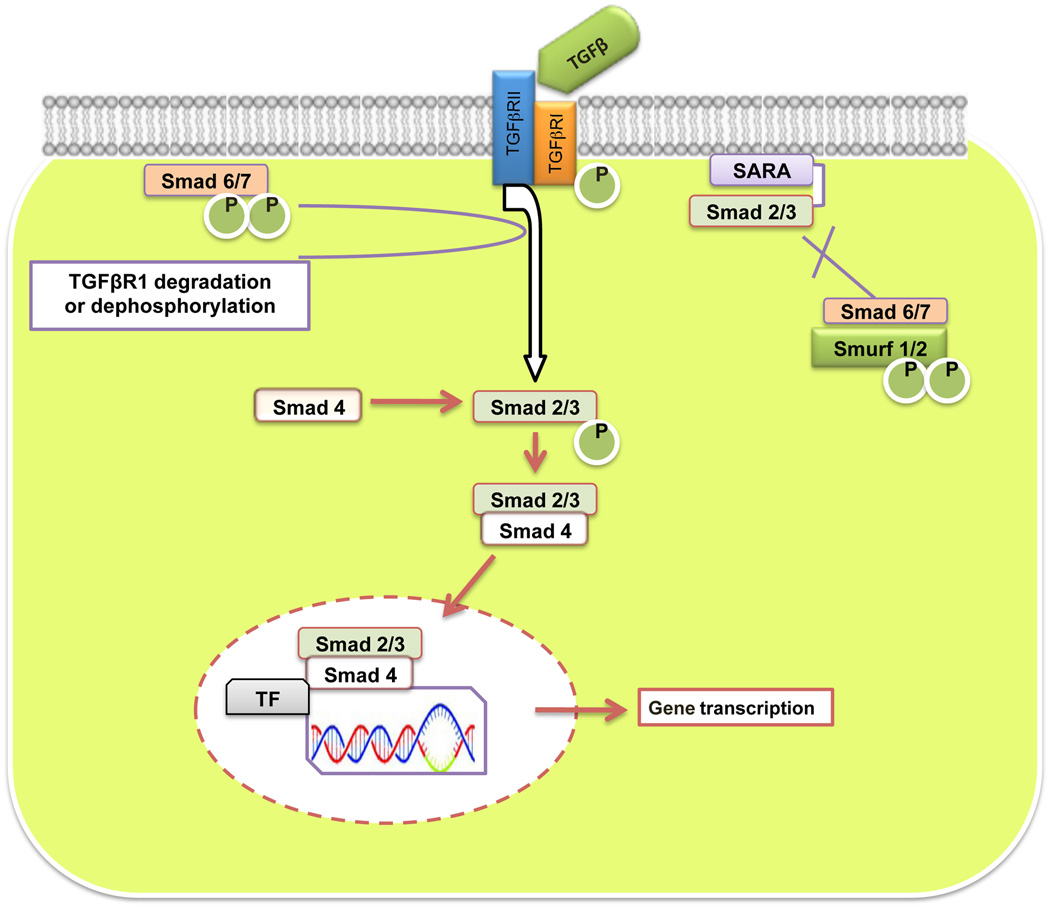
Fig. (3).
TGFβ Smad-dependent signaling pathway: TGFβ exerts its effect by binding and activating type II receptor (TGFβR2) and then type I receptor (TGFβR1). After phosphorylation, TGFβR2 phosphorylates TGFβR1, which subsequently phosphorylates and activates R-Smads (Smad2/3). Activated R-Smads are released from receptors then form a heterotrimeric complex with co-Smad (Smad4), which is translocated into the nucleus to regulate transcription of the target gene. I-Smads (Smad6 and Smad7) shows inhibitory Smad interaction and interfere with the activation of R-Smads while Smurf1/2 targets TGFβR-1 and TGFβR-2 along with various Smad family proteins for proteasome-mediated degradation.
What are SMADS?
SMADs are evolutionarily conserved proteins that modulate the activity of the large family of proteins related to TGFβ. Smad proteins, the only substrates for type I receptor kinases, were first identified as the gene products of Drosophila melanogaster Mad and Caenorhabditis elegans Sma genes. The term Smad was thus derived from Sma and Mad (Mothers against decapentaplegic) gene homologues in C. elegans and D. melanogaster [44, 45]. The Smad family is classified according to function and consists of three main categories. These include receptor-regulated Smads (R-Smads), common mediator Smads (co-Smads), and inhibitory Smads (I-Smads). R-Smads (Smads1–3, Smad5, and Smad8) are ligand specific and become phosphorylated after directly interacting with activated TGFβR1. The receptor regulated Smads, Smad2 and Smad3, are mainly phosphorylated by TGFβ cytokines and activin receptors while Smad1, Smad5, and Smad8 are phosphorylated by BMP receptors [46]. Smad4, a co-Smad, is a common intermediary of all TGFβ superfamily members including TGFβ, activins, and BMP receptors for signal transduction from cell membrane to the nucleus. Furthermore Smad4 is essential for nucleus entry by associating with a receptor phosphorylated R-Smad. Finally, the third group comprises I-Smads also termed anti-Smads. I-Smads, Smad6 and Smad7, antagonize TGFβ signaling by interfering with the activation of R-Smads [47]. It has also been clearly noted that I-Smads demonstrate their action by associating with TGFβR1 and for the most part inhibit R-Smad recruitment by phosphorylation. In differentiating between the I-Smads, it has been reported that Smad7 mainly inhibits TGFβ and activin signaling while Smad6 inhibits BMP signaling in different cellular processes. Generally, Smads remain in their basal state in the cytoplasm to allow for their timely exposure to activated receptors. Besides this, type E3 ubiquitin ligases known as Smad ubiquitination regulatory factors-1/2 (Smurf-1/2), are also important in the regulation of different TGFβ signaling pathways [48]. By and large Smurf-1/2 targets TGFβR1, TGFβR2, and various Smads for proteasome-mediated degradation.
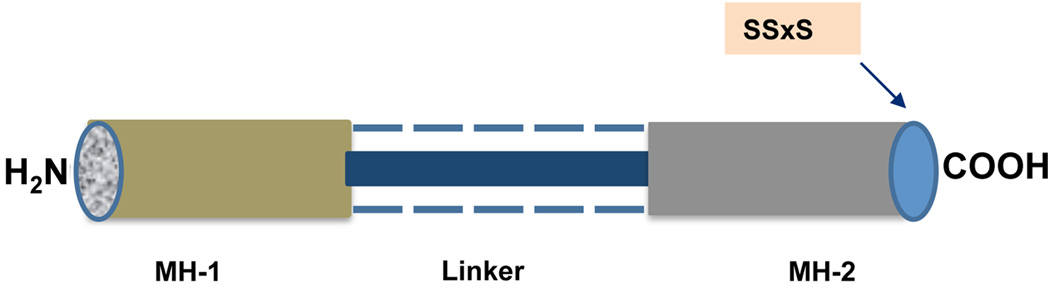
Structurally Smads are characterized by two highly conserved proline-rich globular domains, N-terminal Mad homology 1 (MH-1) and C-terminal Mad homology 2 (MH-2), that are held together by a nonconserved variable linker region. The MH1 domain recognizes the DNA sequence CAGAC whereas the MH2 domain mainly binds transcriptional coactivators.
The MH-1 and MH-2 domains of R-Smads and co-Smads show conserved regions as does the MH-2 domain of I-Smads. The MH-1 domain of I-Smads however, is not conserved and shows variation among species. Variability also exists in the functions of different MH-1 and MH-2 domains. For instance, the MH-1 domain of R-Smads binds DNA, while the MH-2 domain participates in intermolecular protein-protein interactions and is responsible for activating transcription. Moreover, studies have shown that the MH-1 domain of Smad3 and Smad4 possesses DNA binding ability whereas Smad2’s MH-1 domain does not directly bind to DNA efficiently [49–50]. Vasaliki and cohorts recently showed that amino acids glutamine 222 and proline 229 of the conserved variable linker region of Smad proteins plays principle roles in homo- as well as hetero-oligomerization and nuclear accumulation [51]. At the extreme C-terminus of the MH-2 domain, R-Smads contain a phosphorylation motif, SSxS, which is the site of Smad2 and Smad3 phosphorylation by TGFβR1 (Fig. (4)). Specifically, the last two Ser residues of the C-terminal SSxS motif are important and provide the site for the phosphorylation of R-Smads [52].
Fig. (4).
Schematic diagram of R-Smad containing MH-1 and MH-2 regions at amino (N) and carboxyl (C) terminal respectively linked together by variable linker region and specific phosphorylation motif, SSxS, at C-terminus.
The activated TGFβR1 then specifically recognizes and phosphorylates R-Smads, recruited by Smad anchor for receptor activation (SARA) that presents R-Smads as substrates to the activated TGFβ receptor complex. R-Smad phosphorylation decreases its affinity for SARA and leads to the formation of a heterocomplex with the unique co-Smad, Smad4. The activated R-Smads form a heterotrimeric complex with Smad4, and are then rapidly translocated to the nucleus. Smad4 translocates to the nucleus only when combined with R-Smads unlike R-Smads which may autonomously enter the nucleus from the cytoplasm without Smad4. When Smad4 is blocked or absent however, R-Smads may translocate but lack the ability to complete the signal to the nucleus via gene expression. This finding suggests that the main role of Smad4 is to regulate transcription rather than to transmit TGFβ signals from the cytoplasm to the nucleus. In many ways, the Smad heterotrimeric complex is viewed as a coactivator for various transcription factors. This is because it activates or suppresses transcription by way of its interactions with coactivators CBP/p300 [53–54]. After entrance into the nucleus, the complex interacts at the promoter site with transcription factors that contain sequence-specific DNA binding affinity to regulate gene expression. Here the heterotrimeric complexes essentially activate downstream responses and may act as transcriptional factors themselves controlled by a balance between transcriptional co-activators and co-repressors. In short, the heterotrimeric complex may recruit co-activators to stimulate transcription or recruit co-repressors to inhibit transcription leading to the expression of genes responsible for different cellular responses.
4.2 SMAD INDEPENDENT SIGNALING PATHWAY
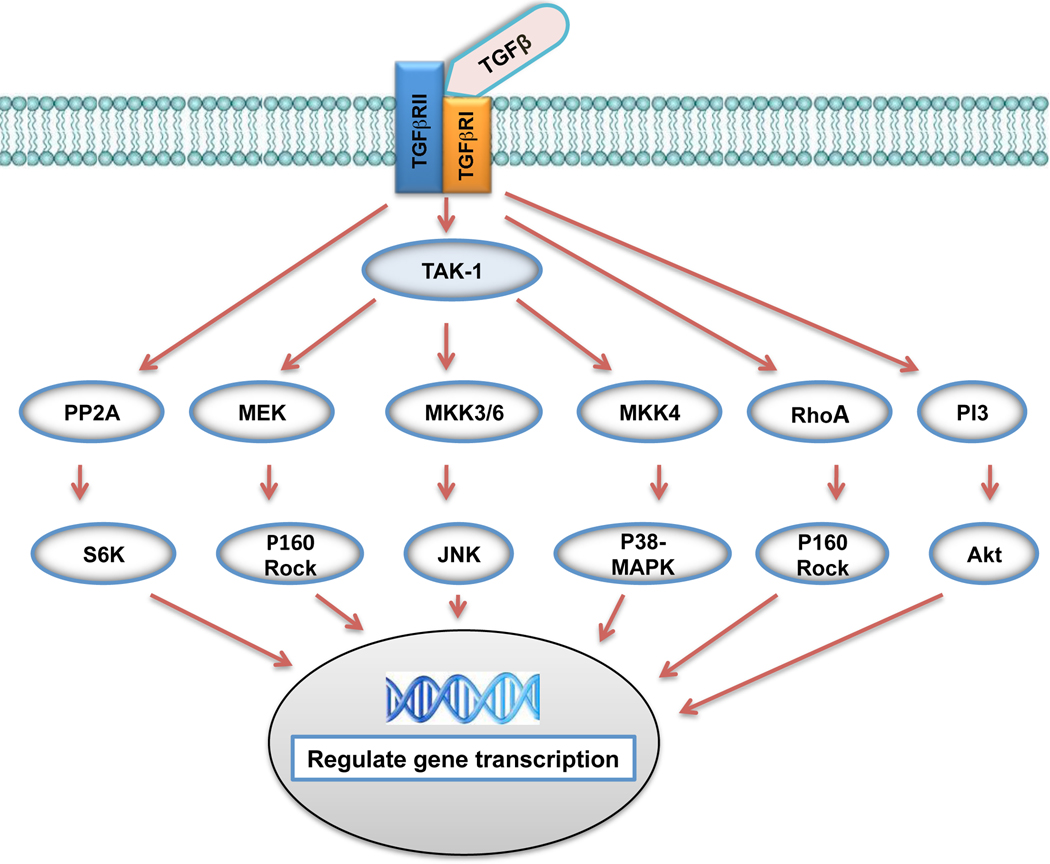
Accumulating evidence over the past few years indicates that TGFβ may signal through several pathways besides the Smad cascade for modulation of cell activity during both embryonic development and corneal wound healing. One such study conducted by the Saika group (2004) reported a lack of Smad3/4 localization in the nuclei of migrating corneal epithelium showing that the TGFβ/Smad pathway may not be directly involved in the regulation of cell proliferation. This is evident by the fact that TGFβ can activate several mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERKs), c-Jun N-terminal kinases (JNKs), and p38 kinases [55–56]. TGFβ also activates the phosphatidylinositol-3 kinase (PI-3) pathway, and in certain cells, signaling involving PP2Aphosphatase and RhoA (Fig. (5)). The MAPK signaling cassette is composed of the ERK, p38, and JNK/SAPK pathways which operate in parallel with one another. The MAPK pathways in mouse, rabbit, and human corneal epithelium mediate cell migration of corneal epithelium [57].
Fig. (5).
TGFβ Smad-independent Signaling Pathways: TGFβ exerts its effect by binding and activating type II receptor (TGFβR2) and then type I receptor (TGFβR1). Smad independent pathways are then activated and include various branches of MAP kinase (MAPK) pathways, Rho-like GTPase signaling pathways, phosphatidylinositol-3-kinase (PI3K)/AKT pathways and protein phosphatase 2A (PP2A) pathway. These pathways then regulate gene transcription in the nucleus. The exact mechanism of activation of these various Smad-independent signaling pathways remains to be elaborated.
5. TGFβ BIOLOGICAL FUNCTION
TGFβ affects a vast range of biological processes. It regulates cell replication, proliferation, differentiation, and migration [32, 58, and 59]. TGFβ also controls cell cycle progression, programmed cell death, ECM production, immune tolerance, inflammation, angiogenesis, hematopoiesis, bone formation, wound healing, and fibrosis [60–62]. In addition, TGFβ stimulates collagen synthesis and the production of several key proteins such as tenascins, fibronectin, thrombospondin, proteoglycans, tissue inhibitor of metalloprotease-1 (TIMP-1), and plasminogen activator inhibitor-1 (PAI-1) [10, 63]. Furthermore, TGFβ is known to act in an isoform-dependent way to protect neurons. In many instances TGFβ has two-way functional capabilities allowing it to inhibit various cellular processes in one context while stimulating the same processes in another. For example, TGFβ functions as both a stimulator and inhibitor of cellular replication thus controlling ECM production [64]. As is evident, TGFβ has various effects in different cells in diverse settings. It is essential for the maintenance of life (tissue homeostasis) as it mediates tissue growth and development as well as tissue wound healing following injury. Overexpression of this vital growth factor however, may lead to disease and in some instances, death.
5.1 TGFβ’S ROLE IN FIBROSIS AND OTHER DISEASES
Following an injury, the body expends a great deal of energy in an effort to repair itself and restore tissue function. This leads to healing via a fibroproliferative response which entails TGFβ-mediated deposition of excessive collagen along with ECM. This type of wound healing involves inflammation, angiogenesis, migration and proliferation of fibroblasts, and connective tissue remodeling that leads to scarring. Previous studies have identified TGFβ1 and TGFβ2 as potent stimulators of fibrosis in the skin [65–66]. After sustaining an injury, TGFβ regulates damage repair and endeavors to restore previous tissue function and morphology. This maintenance of tissue homeostasis involves delicate interactions among TGFβ, the ECM, and different cells. This restores vital barrier function, integrity, and tensile strength of the tissue [67–69]. Unlike adult tissue, fetal tissue exhibits the ability to swiftly heal skin wounds and render them scar free. This is thought to be due to several mechanistic differences that exist between fetal and adult wound healing as well as TGFβ levels [66, 70]. For instance, adult tissue is contaminated and desiccated while fetal tissue is surrounded by amniotic fluid and is housed in a germ-free environment. Additionally, adult tissue is fully differentiated and fibroblast migration along with reepithelialization occurs at a much slower rate than in fetal tissue. Furthermore, in adult wounds there is a massive inflammatory response compared with fetal wounds. Potent platelet degranulation of active cytokines, especially TGFβ, is also visible in adult wounds while a reduced amount is seen in fetal wounds [71]. In regards to specific TGFβ isoforms, TGFβ3 has been shown to predominate in fetal wound healing where TGFβ action is modulated by fibromodulin. However, this is not the case in adults as TGFβ1 is the chief isoform and is modulated by proteoglycans including decorin. This suggests that scarless wound healing may be influenced by the relative ratio of different TGFβ isoforms in particular TGFβ1 and β3 [72]. Past studies have also found that the addition of exogenous TGFβ to fetal wounds switches the scarless tissue regeneration healing process to adult-like wound healing where scarring results [30, 73]. This also reemphasizes the importance of various isoforms of TGFβ in homeostasis, wound repair, and fibrosis.
The disturbance of TGFβ homeostasis may result in various pathological entities including fibrosis. TGFβ deficiency has been linked with defective wound repair, as well as autoimmune diseases, inflammation, atherosclerosis, and oncogenesis. Excessive TGFβ levels have been associated with hypertension, angiogenesis, and cancer [74].
TGFβ has bidirectional capabilities that are very much context specific. In various physiologic and disease states, it may function as an inhibitor of various cellular processes in one instance but promote the same processes in another. In the immune system for example, TGFβ regulates leukocyte activation along with chemotaxis. By so doing, it protects the body from the development of autoimmune diseases while allowing the immune system to combat foreign pathogens. If TGFβ is overexpressed however, immunopathologies such as cancer may result. Early in oncogenesis, TGFβ operates as an anti-mitogenic factor and a tumor suppressor [75]. Later on as the cancer progresses however, the once-protective cytokine promotes angiogenesis as well as myofibroblast transformation furthering tumor expansion, advancement, and metastasis [76]. In other systems, an increase or decrease in TGFβ may lead to disease in both cases. This is seen in chronic obstructive pulmonary disease where overactive TGFβ signaling leads to small airway disease while decreased TGFβ signaling triggers parenchymal tissue obliteration in emphysema [77]. We, along with others, speculate that assorted gene transcription regulation is thought to be the avenue by which TGFβ exerts contradictory effects.
As discussed earlier, TGFβ also directs ECM production as it is a stimulator and inhibitor of cell replication [64]. TGFβ stimulates the synthesis of collagens, fibronectin, proteoglycans, thrombospondin, PAI-1 and TIMP-1. The increased secretion of PAI-1 and MMP-9 brought about by TGFβ2 has been shown to play a role in prostate cancer where TGFβ mainly induces apoptosis of the epithelium. In the developing embryo TGFβ has been reported to be a powerful inhibitor of hematopoietic stem cell proliferation. Nevertheless, its role in adult hematopoiesis has yet to be elucidated. The role of TGFβ superfamily in myocardial infarction including infarct healing, cardiac repair, and remodeling has also been reported although the molecular mechanism is still unknown [78].
5.2 TGFβ EXPRESSION AND LOCALIZATION IN THE CORNEA
TGFβ is expressed in the cornea reflecting its complex role in maintaining corneal integrity and promoting corneal wound healing [79–80]. The cornea, a transparent avascular tissue that covers and protects the front surface of the eye, is made up of three cell layers and two membranes from anterior to posterior: epithelium, epithelial basement membrane (Bowman’s layer), stroma, Descemet’s membrane, and endothelium. Although constitutively expressed by the uninjured cornea, TGFβ has been reported in varying quantities in different layers of the cornea depending on the condition of the cornea [80]. For example, TGFβ is normally restricted to healthy intact corneal epithelium but is secreted into the stroma during wound healing [81]. In the healthy cornea TGFβ1 is detected inside epithelial cells while the isoforms TGFβ2 and β3 are present in the extracellular environment [15]. In the wounded cornea TGFβ1 is the minor isoform observed while TGFβ2, the major mediator of the corneal fibrotic response, is detected in large amounts.
Previous studies report that both TGFβ1 and β2 are seen within the corneal epithelium and stroma in the injured cornea while TGFβ3 is not found in the anterior eye at all [12, 82–83]. Recently however, Huh and colleagues reported unique differences in isoform expression including TGFβ3 following corneal wounding in chick cornea via immunohistochemistry. TGFβ3 was found in basal cells of regenerating areas as well as uninjured regions of the cornea after corneal injury while TGFβ1 levels were increased in Bowman’s layer and TGFβ2 demonstrated strong expression in migrating and proliferating epithelial cells, fibroblasts, Descemet’s membrane, and the endothelium [80]. Other investigators also hold that all three isoforms occur after corneal injury and are present in the stroma. Variation in TGFβ corneal expression also exists among species. For example, TGFβ1 was observed in normal chick cornea but was absent in normal and wounded mice corneal epithelium. This could be due to differences in corneal composition as mice lack Bowman’s layer in the cornea [84].
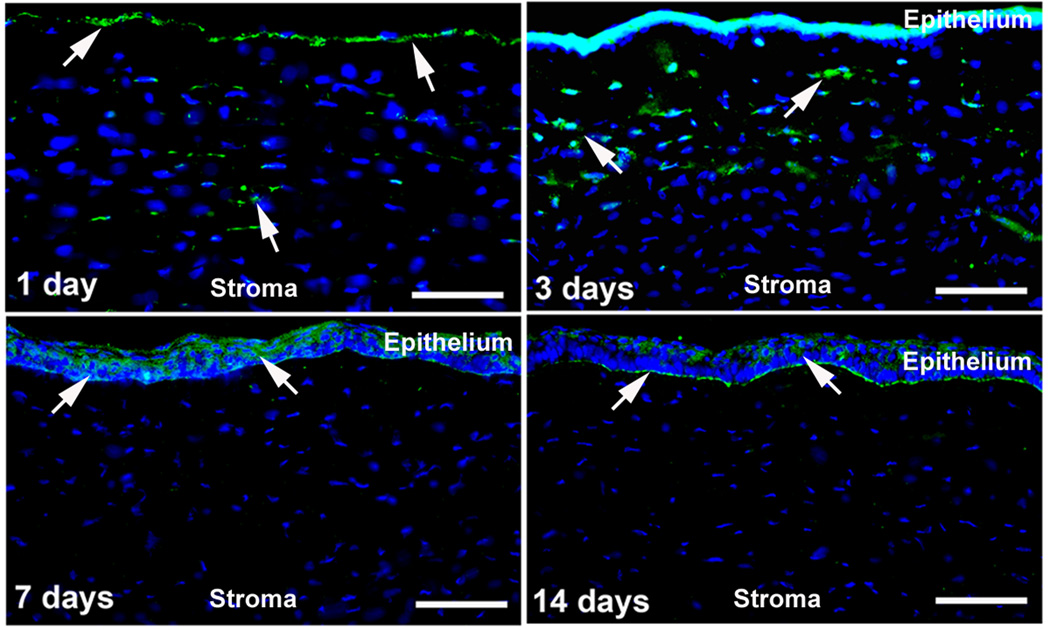
From clinical observation and extensive experimentation, we and other researchers have postulated that the integrity of Bowman’s layer may play a key part in TGFβ release from the corneal stroma in corneal wound healing. Upon debriding the corneal epithelium from Bowman’s layer, cells of the corneal stroma undergo programmed cell death and the stroma is regenerated via mitosis. This occurs without the hypercellularity and excess ECM deposition seen in fibrosis as will be discussed in following sections. The expression of fibrotic markers is observed only if Bowman’s layer is penetrated and the stroma is ablated after debridement of the corneal epithelium as seen in photorefractive keratectomy (PRK). Laser-assisted in situ keratomileusis (LASIK) takes advantage of this fact as Bowman’s layer is only pierced around the borders of a flap. This allows for replacement of dead keratocytes surrounding the ablated area without stimulating the fibrotic response leading to subsequent corneal haze [81, 85]. A recent study conducted by our lab sought to observe systematic and time-dependent monitoring of TGFβ following injury using a PRK rabbit model. Fig. (6) shows time-dependent localization and levels of TGFβ1 in −9.0 D PRK-treated rabbit cornea. PRK caused a significant increase in TGFβ1 in the stroma from day-1 to day-3. The increase in TGFβ1 expression at day 1 and day 3 in the stroma of post-PRK cornea was found statistically significant (p<0.001 or <0.01) compared to naïve corneas. Nonetheless, a decline in an elevated level of TGFβ1 was observed after these two time points as extremely low or no TGFβ1 expression was detected at day 7 or day 14 in the stroma. As expected TGFβ1 expression was observed in the regenerated epithelium and basement membrane, which was removed prior to performing PRK. The levels of TGFβ2 expression in the rabbit stroma after PRK injury was markedly different from the TGFβ1, however, the pattern of change in expression level was similar but not identical as evident from Fig. (7). Like TGFβ1, the TGFβ2 levels in the anterior stroma at day 1 and day 3 after PRK was significantly (p<0.01) higher than the naïve controls. This strongly suggests that TGFβ is essential in initiating the corneal wound healing response and once activated, the response may prove self-perpetuating.
Fig. (6).
Representative immunofluorescence images showing time-dependent expression and localization of TGFβ1 in −9.0 D PRK-treated rabbit cornea. PRK caused a significant increase in TGFβ1 in the stroma at day 1 and day 3 (p<0.001 or <0.01) compared to naïve corneas. The elevated level of TGFβ1 showed progressive decline as extremely low or no expression was detected at day 7 or day 14 in the stroma. The naïve corneas did not show any TGFβ1 expression (data not shown). Scale bar denotes 50 µm.
Fig. (7).
Representative immunofluorescence images showing time-dependent expression and localization of TGFβ2 in −9.0 D PRK-treated rabbit cornea. PRK induced TGFβ2 expression in the stroma at an early time points (day 1 and day 3) as no expression of TGFβ2 was detected in naïve corneas (data not shown). Scale bar denotes 50 µm.
All three isoforms are found in different regions of the cornea reflecting their different roles. Jester and colleagues previously showed the role of TGFβ1 following refractive surgery in keratocyte activation, myofibroblast transformation and proliferation, and wound healing [86]. TGFβ2 plays a key role in corneal development (epithelial-mesenchymal interactions) as well as homeostasis and repair [87]. Following corneal wounding for example, TGFβ2 is detectable in the central corneal wound region where it is responsible for myofibroblast transformation, proliferation and stratification that leads to regeneration of corneal epithelial cells. The mechanism by which this occurs includes migration of activated keratocytes at the wound edge into the fibrin clot which is largely replaced by fibroblasts that become TGFβ2-producing myofibroblasts. Along with TGFβ1 and TGFβ2, TGFβ3 also stimulates epithelial cell proliferation in the cornea especially in unwounded regions [80].
5.3 TGFβ’S ROLE IN CORNEAL WOUND HEALING AND SCARRING
Wound healing plays an integral part in the maintenance of corneal transparency and is thus crucial to normal visual function [25]. Corneal injury has been shown to trigger an unfettered wound healing response that all but induces scarring leading to visual loss. Corneal scarring is an important ocular pathology and is the third leading cause of blindness worldwide [88]. Numerous growth factors and cytokines have been found to regulate corneal wound healing and include TGFβ, connective tissue growth factor, ECM, distinct cell types, etc. TGFβ is arguably the most important ligand in the pathogenesis of fibrotic disease in the cornea as well as in the eye as a whole. Examples range from corneal scarring to lens capsule fibrosis following cataract surgery to tractional retinal detachment in proliferative diabetic retinopathy [25]. In addition to being a sequela of corneal infection, TGFβ-mediated corneal haze development is also apparent following surgical procedures such as PRK, a common laser eye surgery used globally to correct refractive errors in humans [4, 89]. As shown in Fig (6) and (7), TGFβ1 and TGFβ2 detected immediately following PRK injury affect keratocyte, fibroblast, and myofibroblast population in the corneal stroma. This strongly suggests that TGFβ is essential in initiating the corneal wound healing response and once activated, the response may prove self-perpetuating.
It has been reported that following corneal injury, TGFβ overexpression leads to corneal turbidity. This is because increased TGFβ levels augment JNK 1/2-mediated connective tissue growth factor gene expression. Proliferation and migration of ECM proteins collagen and fibronectin by corneal fibroblasts ensues leading to corneal haze [90]. Specifically, the release of TGFβ from injured corneal epithelium induces transformation of quiescent keratocytes into corneal fibroblasts and myofibroblasts, a molecular mechanism known to cause haze. Myofibroblasts are metabolically active and highly contractile cells with reduced transparency attributable to decreased intracellular crystallin production [89].
To date, numerous efforts have been made to identify the mechanism for the effect of TGFβ on improving healing wounds and epithelialization. Following corneal injury, TGFβ leads to an increase in pro-fibrotic molecules and cytokines that control the immune response as well as inflammation. In particular, TGFβ affects chemotaxis of pro-inflammatory cells such as monocytes, lymphocytes, neutrophils, and fibroblasts as well as the production and remodeling of ECM, mitosis induction in certain fibroblasts, and angiogenesis [76, 91]. Once fibroblasts commence the proliferation process, collagen production is increased. The amplified production of collagen coupled with decreased ECM degradation leads to an increase in scar formation [92].
TGFβ1 is responsible for myofibroblastic differentiation, the transdifferentiation of quiescent keratocytes to fibroblasts and myofibroblasts seen in corneal fibrotic disease alluded to earlier. Myofibroblast transformation leads to wound contraction and subsequent aberrant tissue function. Myofibroblasts, which originate from mesenchymal cells or epithelial cells via epithelial-mesenchymal transition, can be distinguished by the expression of α-smooth muscle actin (α-SMA) and alter ECM production in corneal wound healing. Biochemically and morphologically an intermediate between smooth muscle cells and fibroblasts, myofibroblasts generate a powerful contractile force, essential to wound closure. Myofibroblastic differentiation brought about by TGFβ1 is often accompanied by gene expression augmentations of essential wound healing proteins. Examples include cytoskeletal proteins (α-SMA), ECM proteins (fibronectin, proteoglycans, and collagen), and inhibitory proteins that deter ECM degradation. Additionally, myofibroblastic transformation leads to the loss of cell-cell contact between fibroblasts in cell cultures [93].
TGFβ in other areas of the eye in close proximity to the cornea affects corneal scarring. A recent study showed that increased expression of TGFβ1 in the anterior chamber leads to myofibroblast transformation in all corneal layers. The loss of corneal homeostasis due to endothelial damage following injury or surgery deep to the cornea may also play a role in corneal fibrosis and subsequent corneal opacification. In addition to the aqueous humor in the anterior chamber, the cornea is also in direct contact with the tear film suggesting that TGFβ and other cytokines and growth factors present in this layer also influence corneal wound healing [23].
6. CURRENT AND FUTURE TREATMENTS TARGETING TGFβ
TGFβ superfamily members have been implicated in various biological and pathological processes. Thus corneal haze is a result of TGFβ-modulated wound healing following ocular infection or trauma including injury and surgery. Current conventional small molecule drug treatments do not effectively treat corneal scarring and angiogenesis, require repeated drug application and are associated with side effects. On the horizon, other novel approaches such as gene therapy are proving to have potential in corneal haze treatment. It is also thought that the timing of therapy is critical as early treatment is imperative to prevent corneal scarring. Ongoing experiments in our laboratory are focused on the suppressor functions of TGFβ1, a potential strategy to treat corneal scarring, and aim to elucidate the unique molecular mechanisms underlying the role of this vital cytokine in wound healing for corneal eye disease.
Presently no effective remedy exists to eliminate corneal scarring without significant adverse effects. Although topical corticosteroids are frequently utilized in the treatment of corneal scarring, they are only beneficial short-term, cause many side effects, and seldom prove efficacious in reducing corneal scarring [94]. Numerous corneal surgeons apply topical mitomycin C (MMC) prior to PRK to avert laser-induced corneal haze. MMC, an alkylating chemotherapeutic agent, attempts to limit corneal haze by blocking proliferation brought about by TGFβ. However, MMC causes several complications such as corneal endothelial damage, abnormal wound healing, limbal and scleral necrosis, and loss of keratocytes [95–96]. Netto and colleagues recently demonstrated that topical application of MMC also inhibits repopulation of keratocytes and establishes an acellular zone in rabbit corneas up to six months post application [97].
Several other agents such as synthetic inhibitors of metalloproteinase, cyclosporine-A, vitamin E, diclofenac, etc. have shown some benefit in animal studies. However, their high toxicity combined with other deleterious side effects limit their use in humans [98–100]. Nerve growth factor and amniotic membrane have also been reported to control corneal scar and corneal ulcer formation. Even so, clinical trials with nerve growth factor failed to show significant benefits compared to conventional treatment (tarsorrhaphy or bandage contact lens) [101–102]. Phototherapeutic keratectomy, a laser surgical technique, has also been used to treat corneal scarring resulting from gunpowder explosion but leads to undesirable side effects including hyperopic shift and astigmatism among others. The complications and, in some cases, toxicity of current practices in the management of corneal scarring illustrates the inherent risks of these treatments and advocates for the development of safer, improved pharmacologic agents in reducing corneal haze without serious side effects [98–100].
We recently tested our hypothesis that agents having inactivation as well as neutralization properties of TGFβ can inhibit haze progression in the cornea in vivo. We reported that trichostatin A (TSA), a histone deacetylase inhibitor that suppresses TGFβ-induced fibrogenesis in many nonocular tissues, reduces corneal haze [103]. Although not currently in clinical use, TSA may be incorporated into treatment of ophthalmology patients to reduce corneal haze in the near future. In addition to TSA, our laboratory also studied vorinostat, an FDA-approved derivative of TSA presently in clinical use for the treatment of progressive, persistent, or recurrent cutaneous T-cell lymphoma. In preliminary experiments, vorinostat showed similar potential to TSA in impeding corneal haze development [a]. Besides vorinostat, moxifloxacin has been shown to inhibit myofibroblast development by blocking TGFβRI [b].
The feasibility of treating corneal scarring via gene therapy, an innovative treatment modality approach, has also been demonstrated [104]. Gene therapy in the cornea seeks to employ appropriate vectors (viral or non-viral) to transfer genes to the cornea in order to increase or decrease the expression of a particular protein, cytokine, or growth factor that will in turn modulate corneal disease. In the case of corneal scarring, the cytokine of concern is none other than TGFβ. We conjecture that genes blocking TGFβ activity will prevent myofibroblast transformation and thus scarring in the cornea in vivo. We recently demonstrated inhibition of TGFβ-mediated transdifferentiation of corneal keratocytes to myofibroblasts with decorin, a natural modulator of TGFβ, using an in vitro model. In our study we demonstrated the presence of decorin mRNA and protein in rabbit cornea. Studies in non-ocular tissues have demonstrated that collagen-bound decorin does not efficiently neutralize TGFβ. Since decorin in the cornea is bound to collagen, it may not be available to neutralize PRK-induced increased TGFβ levels in the corneal stroma. Therefore, PRK-induced corneal haze may represent an imbalance between TGFβ and its endogenous inhibitor decorin [105]. Another approach that has been tested in preventing corneal scarring is the blockade of cyclins and cyclin-dependent kinases, both of which play an active role in controlling cell division. Further studies are needed to delineate the utility of decorin in treating corneal scarring.
Other avenues in gene therapy endeavor to block certain targets in the TGFβ signaling pathway. In this manner, beneficial effects of TGFβ such as JNK-dependent fibronectin-mediated epithelialization of wounds are preserved while detrimental effects of TGFβ such as Smad3-induced collagen overproduction are inhibited. Examples include blocking specific R-Smads, such as Smad3. In wound healing experiments, it was found that the overexpression of I-Smads, like Smad7, had similar effects to blocking Smad3 in reducing TGFβ1 gene expression. In addition to its effects in the cytoplasm, Smad7 may block transcription factors in the nucleus. Major advantages of gene therapy include lower dose for effect, lower cost, increased efficiency, and a longer period of gene expression eliminating the need for frequent repeated injections or applications which can further hamper wound healing. A concern with gene therapy however, is possible harmful side effects. Although gene transfer with viral vectors may result in a secondary inflammatory reaction, this can be circumvented with the utilization of non-viral vectors like plasmid DNA [92].
Another approach in gene therapy for corneal scarring is the use of soluble TGFβR2. Soluble TGFβR2 blocks TGFβ activity by binding TGFβ thus rendering it unable to bind its cell surface receptor. In corneal injury model, a single dose of recombinant adenovirus encoding for soluble TGFβR2 decreased corneal opacification as well as inflammation, and neovascularization [104]. Unfortunately, like other gene therapy methods, this approach is unregulated and untargeted leading to numerous adverse effects. Although these examples show the potential of gene therapy in treating corneal scarring, they also point out major obstacles, like safety profile, that need to be addressed if gene therapy is to make the transition from the laboratory to clinical practice in patients. Our lab is working to optimize tissue-selective targeted gene delivery into keratocytes employing AAV vectors that are non-pathogenic to humans. With the utilization of a specific mutant vector and specialized surgical techniques, we have demonstrated successful transduction of genes into the corneal endothelium.
On the protein level, TGFβ antagonists such as monoclonal antibodies are also showing promise in treating corneal scarring. Earlier, Shah and collaborators reported results from a ground-breaking study where anti-TGFβ1 and β2 antibodies were shown to inhibit formation of cutaneous scars in rodent wounds. Not only did the antibody-treated wounds demonstrate decreased TGFβ1, collagen, and fibronectin levels, they also showed an increase in normal cutaneous structure, and equal tensile strength as compared to other wounds. A follow up study where TGFβ3 was added to the wounds also showed decreased cutaneous scarring [106]. Furthermore, it was established by the same group that early treatment is paramount in decreasing scar formation as the first dose of TGFβ stimulates TGFβ overproduction. This comes about by way of chemotaxis and autoinduction which in turn induces collagen overproduction and fibroblast proliferation while simultaneously inhibiting ECM breakdown [92].
Carrington and cohorts found that in the cornea TGFβ1 delays re-epithelialization, accelerates keratocyte proliferation, increases repopulation of cells around the wound, and encourages the transformation of keratocytes to myofibroblasts [107]. In contrast, they also found that the anti-TGFβ1 monoclonal antibody CAT-192 enhances re-epithelialization of the cornea while reducing stromal repopulation. Comparable results to CAT-192 were also reported with low doses of IL-10 and exogenous TGFβ3, although TGFβ3 elicited little effect on corneal re-epithelialization. This recognizes IL-10 and TGFβ3 as potential agents in treating and preventing fibrosis in the cornea [107]. Although monoclonal antibodies may seem to be an optimum treatment choice for corneal scarring, a major problem accompanying their use is their stability at room temperature. In addition to monoclonal antibodies, treatment for corneal scarring on the protein level includes biglycan as well as soluble TGFβ receptors and decorin as explained above [96].
Another approach to thwart the effects of TGFβ may be to block its maturation from latent TGFβ. Zieske and cohorts demonstrated in a mouse model that αVβ6 integrin, a key protein involved in latent TGFβ1 activation by latent-associated peptide cleavage, is increased during epithelial wound healing. A significant reduction in laminin, α-SMA, and mature TGFβ1 was seen as well as an increase in large latent TGFβ1 in β6-null mice following keratectomy. This is another strategy where TGFβ modulation can be achieved [c].
Corneal wound healing, mediated by various cytokines such as TGFβ, necessitates functioning stem cells from the adjacent corneoscleral junction also known as the limbus. The loss of these vital stem cells consequently leads to wound healing difficulties. Although not well characterized, several signaling pathways including TGFβ control corneal stem cells. Limbal stem cells have successfully been used in human autograft transplantation in the past. For example, one study demonstrated that limbal explants containing limbal epithelial stem cells obtained from the healthy eye of an individual could be transplanted into the contralateral diseased eye of the patient leading to corneal re-epithelialization along with regression of both angiogenesis and persistent epithelial defects. This has subsequently led to the development and utilization of limbal stem cells grown on amniotic membrane as well as autologous mucosal epithelial cell grafts [108].
Previously, so called ‘‘wandering cells’’ were thought to play a part in tissue repair and wound healing. Recent stem cell research has revived this idea as reports point to similarities in the cornea. There is an increasing body of evidence suggesting that wandering cells are actually stem cells originating from the bone marrow, and can assist in tissue repair in various tissues including the cornea [81]. Recently a pioneer study demonstrated the migration of GFP-labeled BM-derived cells into the corneal stroma of mice [109]. It was shown immunohistochemically that some of the migrating cells were BM-derived dendritic cells and macrophages [110]. The role of bone marrow–derived stem cells in corneal wound repair is an area with scant information that will benefit from thorough exploration.
In conclusion, TGFβ is a remarkable cytokine that plays a vital role in the homeostasis of all tissues in the human body including the cornea. It is essential for growth and development before birth and throughout life but can also lead to tissue failure and premature death if unregulated. Its different isoforms, biosynthesis, maturation, signal transduction involving the SMAD pathway, and biological function including its role in fibrosis and wound healing have been well documented. However, other signaling pathways have yet to be completely elucidated as it has become accepted that cross-talk is evident between pathways. In addition to regulating numerous other growth factors and cytokines, TGFβ is controlled not only by itself in a negative feedback manner, but also by other factors. Many of these factors, such as connective tissue growth factor, have been studied extensively but others have yet to be afforded the same attention. Importantly, TGFβ may hold the key to scarless tissue repair thus warranting further investigation. This is especially important in the cornea as TGFβ-mediated corneal scarring is a leading cause of blindness. Effective treatment of fibrosis and scarring in the cornea is still under investigation as current modalities offer little resolution and are plagued with serious side effects. However, with continued research and the implementation of novel approaches such as targeted gene therapy and stem cell transplantation, the future seems bright in the field of corneal wound healing.
ACKNOWLEDGEMENTS
The work was supported by the RO1EY17294 (RRM) grant from the National Eye Institute, National Institutes of Health, Bethesda, MD and an unrestricted grant from the Research to Prevent Blindness, New York, NY.
Footnotes
Commercial Relationship: A. Tandon, None; J.C.K. Tovey, None; A. Sharma, None; R. Gupta, None; R. R. Mohan, None.
Lopez, V., Tovey, J., Sharma, A., Waggoner, M., Cowden, J.W. and Mohan, R.R. Vorinostat Effectively Reduces TGFβ-Mediated Myofibroblast Formation in the Cornea. Program Summary of ARVO 2010 Meeting, Fort Lauderdale, Florida, May 2–l6, 2010; p. 57.
Chang, S.W., Chen, T.C., Wang, T.Y., and Chou, S.F. Moxifloxacin Suppresses Myofibroblast Differentiation via Blocking TGF-betaRI on Corneal Fibroblasts. Program Summary of ARVO 2010 Meeting, Fort Lauderdale, Florida, May 2–l6, 2010; p 57.
Blanco, T., Hutcheon, A.E.K., and Zieske, J.D. Up-Regulation of TGF-β1 by αVβ6 Integrin Enhances Corneal Wound Healing in Mice. Program Summary of ARVO 2010 Meeting, Fort Lauderdale, Florida, May 2–l6, 2010; p 172
REFRENCES
- 1.Chin D, Boyle GM, Parsons PG, Coman WB. Br J Plast Surg. 2004;57:215–221. doi: 10.1016/j.bjps.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. J. Biol. Chem. 1983;258:7155–7160. [PubMed] [Google Scholar]
- 3.De Larco JE, Todaro GJ. Proc. Nati Acad. Sci. 1978;75:4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jester JV, Barry Lane PA, Petroll WM, Olsen DR, Cavanagh HD. Cornea. 1997;16:177–187. [PubMed] [Google Scholar]
- 5.Saika S. Cornea. 2004;23:25–30. doi: 10.1097/01.ico.0000136668.41000.73. [DOI] [PubMed] [Google Scholar]
- 6.Cordeiro MF. Prog Retin Eye Res. 2002;21:75–89. doi: 10.1016/s1350-9462(01)00021-0. Review. [DOI] [PubMed] [Google Scholar]
- 7.Attisano L, Wrana JL. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 8.Shi Y, Massague J. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 9.Annes JP, Munger JS, Rifkin DB. J. Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 10.Roberts AB. Miner Electrolyte Metab. 1998;24:111–119. doi: 10.1159/000057358. [DOI] [PubMed] [Google Scholar]
- 11.Wilson SE, Chen L, Mohan RR, Liang Q, Liu J. Exp Eye Res. 1999;68:377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 12.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Prog Retin Eye Res. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 13.Blobe GC, Schiemann WP, Lodish HF. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 14.Sharma GD, He J, Bazan HE. J Biol Chem. 2003;278:21989–21997. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 15.Saika S. Lab Invest. 2006;86:106–115. doi: 10.1038/labinvest.3700375. [DOI] [PubMed] [Google Scholar]
- 16.Vij N, Sharma A, Thakkar M, Sinha S, Mohan RR. Mol Vis. 2008;30:1020–1027. [PMC free article] [PubMed] [Google Scholar]
- 17.Kondaiah P, Sands MJ, Smith JM, Fields A, Roberts AB, Sporn MB, Melton DA. J Biol Chem. 1990;265:1089–1093. [PubMed] [Google Scholar]
- 18.Cheifetz S, Hernandez H, Laiho M, ten Dijke P, Iwata KK, Massague J. J Biol Chem. 1990;265:20533–20538. [PubMed] [Google Scholar]
- 19.Tucker RF, Volkenant ME, Branum EL, Moses HL. Cancer Res. 1983;43:1581–1586. [PubMed] [Google Scholar]
- 20.Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV. Nature (London) 1985;316:701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- 21.Miyazono K. Blood. 2008;112:3533–3544. doi: 10.1182/blood-2008-07-165696. [DOI] [PubMed] [Google Scholar]
- 22.Lu L, Reinach PS, Kao WW. Exp Biol Med (Maywood) 2001;226:653–664. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 23.Reneker LW, Bloch A, Xie L, Overbeek PA, Ash JD. Brain Res Bull. 2009 doi: 10.1016/j.brainresbull.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karamichos D, Guo XQ, Hutcheon AE, Zieske JD. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.09-3860. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saika S, Yamanaka O, Okada Y, Tanaka S, Miyamoto T, Sumioka T, Kitano A, Shirai K, Ikeda K. Front Biosci. (Schol Ed) 2009;1:376–390. doi: 10.2741/S32. [DOI] [PubMed] [Google Scholar]
- 26.Long Q, Chu R, Zhou X, Dai J, Chen C, Rao SK, Lam DS. J Refract Surg. 2006;22:708–712. doi: 10.3928/1081-597X-20060901-13. [DOI] [PubMed] [Google Scholar]
- 27.Kruse FE, Tseng SC. Ophthalmologe. 1994;91:617–623. [PubMed] [Google Scholar]
- 28.Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Microsc. Res. Tech. 2001;52:354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Javelaud D, Mauviel A. The International Journal of Biochemistry & Cell Biology. 2004;36:1161–1165. doi: 10.1016/S1357-2725(03)00255-3. [DOI] [PubMed] [Google Scholar]
- 30.Houghton PE, Keefer KA, Krummel TM. Wound Repair Regen. 1995;3:229–236. doi: 10.1046/j.1524-475X.1995.30215.x. [DOI] [PubMed] [Google Scholar]
- 31.Wipff PJ, Hinz B. Eur J Cell Biol. 2008;87:601–615. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Massague J, Cheifetz S, Laiho M, Ralph DA, Weis FM, Zentella A. Cancer Surv. 1992;12:81–103. [PubMed] [Google Scholar]
- 33.Feng XH, Derynck R. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. Review. [DOI] [PubMed] [Google Scholar]
- 34.Derynck R, Feng XH. Biochim Biophys Acta. 1997;1333:F105–F150. doi: 10.1016/s0304-419x(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 35.Wieser R, Wrana JL, Massague J. EMBO J. 1995;15:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Franzen P, Heldin CH, Miyazono K. Biochem Biophys Res Commun. 1995;15:682–689. doi: 10.1006/bbrc.1995.1241. [DOI] [PubMed] [Google Scholar]
- 37.Attisano L, Wrana JL, Cheifetz S, Massague J. Cell. 1992;68:97–108. doi: 10.1016/0092-8674(92)90209-u. [DOI] [PubMed] [Google Scholar]
- 38.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massague J. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 39.Segarini PR, Seyedin SM. J. Biol. Chem. 1988;263:8366–8370. [PubMed] [Google Scholar]
- 40.Lopez-Casillas F, Cheifetz S, Doody J, Andres JL, Lane WS, Massague J. Cell. 1991;67:785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Casillas F, Payne HM, Andres JL, Massague J. J. Cell Biol. 1994;124:557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. J. Biol. Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 43.Laverty HG, Wakefield LM, Occleston NL, O'Kane S, Ferguson MW. 2009;20:305–317. doi: 10.1016/j.cytogfr.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, Tamaki K, Hanai J, Heldin CH, Miyazono K, Dijke P. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohei Miyazono. Journal of Bone and Mineral Metabolism. 1998;16:133–138. [Google Scholar]
- 46.Miyazono K, Kamiya Y, Morikawa M. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- 47.Heldin CH, Miyazono K, ten Dijke P. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 48.Datto M, Wang XF. Cell. 2005;8:2–4. doi: 10.1016/j.cell.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Yingling JM, Datto MB, Wong C, Frederick JP, Liberati NT, Wang XF. Mol Cell Biol. 1997;17:7019–7028. doi: 10.1128/mcb.17.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chai J, Wu JW, Yan N, Massagué J, Pavletich NP, Shi Y. J Biol Chem. 2003;30:20327–20331. doi: 10.1074/jbc.C300134200. [DOI] [PubMed] [Google Scholar]
- 51.Vasilaki E, Siderakis M, Papakosta P, Skourti-Stathaki K, Mavridou S, Kardassis D. Biochemistry. 2009;8:8366–8378. doi: 10.1021/bi9005489. [DOI] [PubMed] [Google Scholar]
- 52.Itoh F, Divecha N, Brocks L, Oomen L, Janssen H, Calafat J, Itoh S, Dijke Pt P. Genes Cells. 2002;7:321–331. doi: 10.1046/j.1365-2443.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 53.Shi W, Chen H, Sun J, Chen C, Zhao J, Wang YL, Anderson KD, Warburton D. Am J Physiol Lung Cell Mol Physiol. 2004;286:293–300. doi: 10.1152/ajplung.00228.2003. [DOI] [PubMed] [Google Scholar]
- 54.Derynck R, Zhang YE. Nature. 2003;425:577–584. doi: 10.1038/nature02006. Review. [DOI] [PubMed] [Google Scholar]
- 55.Bazan HE, Varner L. Curr Eye Res. 1997;16:372–379. doi: 10.1076/ceyr.16.4.372.10699. [DOI] [PubMed] [Google Scholar]
- 56.Li DQ, Luo L, Chen Z, Kim HS, Song XJ, Pflugfelder SC. Exp Eye Res. 2006;82:588–596. doi: 10.1016/j.exer.2005.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kretzschmar M, Doody J, Timokhina I, Massague J. Genes Dev. 1999;13:804–816. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saarma M. Eur J Biochem. 2000;267:6968–6971. doi: 10.1046/j.1432-1327.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- 59.Anumanthan G, Halder SK, Osada H, Takahashi T, Massion PP, Carbone DP, Datta PK. Br J Cancer. 2005;14:1157–1167. doi: 10.1038/sj.bjc.6602831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bikfalvi A. Eur J Cancer. 1995;31:1101–1104. doi: 10.1016/0959-8049(95)00169-j. [DOI] [PubMed] [Google Scholar]
- 61.Taipale J, Saharinen J, Keski-Oja J. Adv Cancer Res. 1998;75:87–134. doi: 10.1016/s0065-230x(08)60740-x. [DOI] [PubMed] [Google Scholar]
- 62.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 63.Laiho M, Saksela O, Keski-Oja J. J Biol Chem. 1987;262:17467–17474. [PubMed] [Google Scholar]
- 64.Streuli CH, Schmidhauser C, Kobrin M, Bissell MJ, Derynck R. J Cell Biol. 1993;120:253–260. doi: 10.1083/jcb.120.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah RM, Friedman AC, Ostrum BJ, Sexauer W, Fiel SB. Crit Rev Diagn Imaging. 1995;36:441–477. Review. [PubMed] [Google Scholar]
- 66.Longaker MT, Adzick NS. Plast Reconstr Surg. 1991;87:788–798. doi: 10.1097/00006534-199104000-00032. [DOI] [PubMed] [Google Scholar]
- 67.Werner S, Grose R. Physiol Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 68.Singer AJ, Thode HCJ, McClain SA. Acad Emerg Med. 2000;7:1083–1088. doi: 10.1111/j.1553-2712.2000.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 69.Martin P, Parkhurst SM. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- 70.Whitby DJ, Ferguson MW. Dev Biol. 1991;147:207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- 71.Bullard KM, Longaker MT, Lorenz HP. World J. Surg. 2003;27:54–61. doi: 10.1007/s00268-002-6737-2. [DOI] [PubMed] [Google Scholar]
- 72.O'Kane S, Ferguson MW. Int J Biochem Cell Biol. 1997;29:63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- 73.Krummel TM, Michna BA, Thomas BL, Sporn MB, Nelson JM, Salzberg AM, Cohen IK, Diegelmann RF. J Pediatr Surg. 1988;23:647–652. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- 74.August P, Suthanthiran M. N Engl J Med. 2006;354:2721–2723. doi: 10.1056/NEJMcibr062143. [DOI] [PubMed] [Google Scholar]
- 75.Reiss M. Microbes Infect. 1999;1:1327–1347. doi: 10.1016/s1286-4579(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 76.Wrzesinski SH, Wan YY, Flavell RA. Clin Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 77.Konigshoff M, Kneidinger N, Eickelberg O. Swiss Med Wkly. 2009;139:554–563. doi: 10.4414/smw.2009.12528. [DOI] [PubMed] [Google Scholar]
- 78.Bujak M, Frangogiannis NG. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Exp Eye Res. 1994;59:63–71. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]
- 80.Huh MI, Chang Y, Jung JC. Histol Histopathol. 2009;24:1405–1416. doi: 10.14670/HH-24.1405. [DOI] [PubMed] [Google Scholar]
- 81.Fini ME, Stramer BM. Cornea. 2005;24:S2–S11. doi: 10.1097/01.ico.0000178743.06340.2c. [DOI] [PubMed] [Google Scholar]
- 82.Nishida K, Kinoshita S, Yokoi N, Kaneda M, Hashimoto K, Yamamoto S. Invest Ophthalmol Vis Sci. 1994;35:3289–3294. [PubMed] [Google Scholar]
- 83.Pasquale LR, Dorman-Pease ME, Lutty GA, Quigley HA, Jampel HD. Invest Ophthalmol Vis Sci. 1993;34:23–30. [PubMed] [Google Scholar]
- 84.Stramer BM, Zieske JD, Jung JC, Austin JS, Fini ME. Invest Ophthalmol Vis Sci. 2003;44:4237–4246. doi: 10.1167/iovs.02-1188. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura K, Kurosaka D, Bissen-Miyajima H, Tsubota K. Br J Ophthalmol. 2001;85:209–213. doi: 10.1136/bjo.85.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Cornea. 1996;15:505–516. [PubMed] [Google Scholar]
- 87.Strissel KJ, Rinehart WB, Fini ME. Invest Ophthalmol Vis Sci. 1995;36:151–162. [PubMed] [Google Scholar]
- 88.Foster A, Resnikoff S. Eye. 2005;19:1133–1135. doi: 10.1038/sj.eye.6701973. [DOI] [PubMed] [Google Scholar]
- 89.Jester JV, Petroll WM, Cavanagh HD. Prog Retin Eye Res. 1999;18:311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- 90.Chang Y, Wu XY. J Int Med Res. 2009;37:727–736. doi: 10.1177/147323000903700316. [DOI] [PubMed] [Google Scholar]
- 91.Padua D, Massague J. Cell Research. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 92.Liu W, Wang DR, Cao YL. Curr Gene Ther. 2004;4:123–136. doi: 10.2174/1566523044578004. [DOI] [PubMed] [Google Scholar]
- 93.Masur SK, Dewal HS, Dinh TT, Erenburg I, Petridou S. Proc Natl Acad Sci. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Price FW, Jr, Willes L, Price M, Lyng A, Ries J. Ophthalmology. 2001;108:1236–1245. doi: 10.1016/s0161-6420(01)00624-8. [DOI] [PubMed] [Google Scholar]
- 95.Nassaralla BA, McLeod SD, Jr, Nassaralla JJ. J Refract Surg. 2007;23:226–232. doi: 10.3928/1081-597X-20070301-04. [DOI] [PubMed] [Google Scholar]
- 96.Nassiri N, Farahangiz S, Rahmani L, Nassiri N. J Cataract Refract Surg. 2008;34:902–908. doi: 10.1016/j.jcrs.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 97.Netto MV, Mohan RR, Sinha S, Sharma A, Gupta PC, Wilson SE. J. Refractive Surg. 2006;22:562–574. doi: 10.3928/1081-597x-20060601-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang JH, Kook MC, Lee JH, Chung H, Wee WR. Exp Eye Res. 1998;66:389–396. doi: 10.1006/exer.1997.0415. [DOI] [PubMed] [Google Scholar]
- 99.Bilgihan K, Ozdek S, Ozogul C, Gurelik G, Bilgihan A, Hasanreisoglu B. Eye. 2000;14:231–237. doi: 10.1038/eye.2000.60. [DOI] [PubMed] [Google Scholar]
- 100.Nassaralla BA, Szerenyi K, Wang XW, Reaves T, McDonnell PJ. Opthalmol. 1995;102:469–474. doi: 10.1016/s0161-6420(95)30998-0. [DOI] [PubMed] [Google Scholar]
- 101.Cellini M, Bendo E, Bravetti GO, Campos EC. Ophthalmic Res. 2006;38:177–181. doi: 10.1159/000092626. [DOI] [PubMed] [Google Scholar]
- 102.Arora R, Mehta D, Jain V. Eye. 2005;19:273–278. doi: 10.1038/sj.eye.6701490. [DOI] [PubMed] [Google Scholar]
- 103.Sharma A, Mehan MM, Cowden JW, Mohan RR. Invest. Ophthalmol Vis Sci. 2009;50:2695–2701. doi: 10.1167/iovs.08-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Prog Retin Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 105.Mohan RR, Gupta R, Mehan MM, Cowden JW, Wilson SE, Sinha S. Exp Eye Res. 2010 doi: 10.1016/j.exer.2010.05.013. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shah M, Foreman DM, Ferguson MW. Lancet. 1992;25:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 107.Carrington LM, Albon J, Anderson I, Kamma C, Boulton M. Invest Ophthalmol Vis Sci. 2006;47:1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- 108.Secker GA, Daniels JT. Stem Cell Rev. 2008;4:159–168. doi: 10.1007/s12015-008-9029-x. [DOI] [PubMed] [Google Scholar]
- 109.Wilson SE, Mohan RR, Netto M, Perez V, Possin D, Huang J, Kwon R, Alekseev A, Rodriguez-Perez JP. Invest Ophthalmol Vis Sci. 2004;45:2201–2211. doi: 10.1167/iovs.03-1162. [DOI] [PubMed] [Google Scholar]
- 110.Nakamura T, Ishikawa F, Sonoda KH, Hisatomi T, Qiao H, Yamada J, Fukata M, Ishibashi T, Harada M, Kinoshita S. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]