Abstract
Behavioral management of risk, in which organisms must balance the requirements of obtaining food resources with the risk of predation, has been of considerable interest to ethologists for many years. Although numerous experiments have shown that animals alter their foraging behavior depending on the levels of perceived risk and demand for nutrients, few have considered the role of genetic variation in the trade-off between these variables. We performed a study of four zebrafish (Danio rerio (Hamilton, 1822)) strains to test for genetic variation in foraging behavior and whether this variation affected their response to both aversive stimuli and nutrient restriction. Zebrafish strains differed significantly in their latency to begin foraging from the surface of the water under standard laboratory conditions. Fish fed sooner when nutrients were restricted, although this was only significant in the absence of aversive stimuli. Aversive stimuli caused fish to delay feeding in a strain-specific manner. Strains varied in food intake and specific growth rate, and feeding latency was significantly correlated with food intake. Our results indicate significant genetic variation in foraging behavior and the perception of risk in zebrafish, with a pattern of strain variation consistent with behavioral adaptation to captivity.
Introduction
Behavioral management of risk, in which organisms must balance the requirements of obtaining food resources with the risk of predation, has been of considerable interest to ethologists for many years. Behavioral strategies to minimize predation risk include modifications to foraging behavior such as restricting foraging location (Pettersson and Bronmark 1993), latency to begin foraging (Morgan 1988; Angradi 1992; Cooper 2000), and alterations of foraging rate (Morgan 1988; Engstrom-Ost and Lehtiniemi 2004). Additionally, animals perform predator inspection (Walling et al. 2004), maintain group cohesion (Sogard and Olla 1997), use cover (Cooper 2000; Vehanen 2003), and lower activity levels (Horat and Semlitsch 1994; Vehanen 2003; Engstrom-Ost and Lehtiniemi 2004) to minimize risk of predation while foraging. Implementation of these behavioral strategies reflects a balance between two factors: the level of perceived risk of predation and the physiological demand for nutrients.
Elicitation of behavioral responses to elevated predation susceptibility is dependent on both experience and genetic factors. An animal’s perception of risk can be influenced by its susceptibility to predation based on body size (Roth and Johnson 2004; Urban 2007), as well as previous interactions with predators. Prior experience with predators has been shown to influence the perception of risk in a variety of taxa, including mammals (Owings et al. 2001), fish (Kelley and Magurran 2003; Walling et al. 2004), and lizards (Labra and Leonard 1999). Alternatively, O’Steen et al. (2002) showed that guppies (Poecilia reticulata Peters, 1859) descended from populations that experience differing levels of predation risk differed in survival rates under predation when reared and tested in laboratory experiments, indicating genetic variation resulting from local adaptation to different predation regimes.
Physiological demand for nutrients is also influenced by both environmental and genetic variations. Experience with food resources and temporal variation in food availability alters foraging behavior and risk management (Godin and Crossman 1994; Anholt and Werner 1995; Aubret et al. 2007). Genetic variation in physiological parameters such as growth rate (Abrahams and Sutterlin 1999) and feed conversion efficiency (Lister and Neff 2006) can also influence the drive to feed. For example, Abrahams and Sutterlin (1999) demonstrated that growth-enhanced transgenic Atlantic salmon (Salmo salar L., 1758) had increased consumption rates relative to control fish. Similarly, brown trout (Salmo trutta L., 1758) selected for increased body size (fork length) also showed higher feed intake relative to control lines (Sanchez et al. 2001; Mambrini et al. 2006).
Although the experiments discussed above indicate that animals alter their foraging behavior depending on the level of perceived risk and demand for nutrients, few studies have considered the role of genetic variation in the trade-off between these variables. In this study, we tested for the presence of population variation in behavioral management of risk during foraging among strains of zebrafish, Danio rerio (Hamilton, 1822). The zebrafish has historically been considered a model organism in developmental biology. However, there is growing interest in its use as a model for behavioral research (Gerlai 2003; Guo 2004; Robison and Rowland 2005; Sison et al. 2006). Recent work has demonstrated considerable interstrain variation in zebrafish behavior, including activity-related behaviors, aggression, and various measures of fear-like behavior such as place preference response to frightening stimuli and boldness towards a novel object (Wright et al. 2003; Robison and Rowland 2005; Moretz et al. 2007b). In addition, several studies of zebrafish foraging behavior have focused on resource defense (Grant and Kramer 1992; Hamilton and Dill 2002; Chapman and Kramer 1996) or shoaling cohesion (Miller and Gerlai 2007), but few have tested for variation among populations in foraging behavior (but see Moretz et al. 2007b). To our knowledge, no studies have tested whether zebrafish populations differentially modify foraging behavior in response to variation in risk and nutrient levels.
We therefore performed a study using four zebrafish strains to determine whether (i) strains of zebrafish differ in their foraging behavior (as measured by latency to feed), (ii) zebrafish alter foraging behavior in response to manipulations of nutrient availability and aversive stimuli, (iii) changes in foraging behavior in response to these manipulations vary among strains, and (iv) foraging behavior of individual strains is correlated with food intake and growth rate.
Materials and methods
Study populations
We used four strains of zebrafish that have been used in other studies examining interstrain behavioral variation in zebrafish (Robison and Rowland 2005; Moretz et al. 2007a, 2007b). The Gaighatta (G) strain was originally collected from its native habitat in India and brought to the University of Idaho zebrafish facility (Moscow, Idaho) in 2005. Fish from the Gaighatta strain used in this study were first generation laboratory-reared individuals from wild-born parents. The Nadia strain was originally collected from its native habitat in India and brought to the University of Oregon zebrafish facility (Eugene, Oregon) in 1999 (details available from http://zfin.org [accessed 9 September 2008]). Nadia fish used in this study were seven generations removed from wild-caught individuals. The Scientific Hatcheries (SH) strain is a commercially available strain that originated from a pet store and has since been maintained in a commercial system at high densities. According to its producer (Scientific Hatcheries, Huntington, California), this strain experienced selection for fast growth rate and high fecundity. It is difficult to precisely determine how long the SH strain has been reared in captivity, but we estimate this to be between 30 and 50 generations. The TM1 strain was established from pet store fish in 1986 and subsequently housed in the laboratory at the University of Miami for more than 24 generations (Gibbs and Schmale 2000). The strain was then obtained by our laboratory in 2001, and has subsequently undergone another 6 generations of laboratory breeding.
All animals were bred and housed in our recirculating zebrafish facility built by Aquaneering Inc. (San Diego, California). Fish were fed twice daily with flake food and live brine shrimp and maintained at 28.5 °C on a 14 h light : 10 h dark photoperiod. All animals were cared for in accordance with guidelines of the University of Idaho Animal Care and Use Committee.
Experimental design
Twenty-five adult (3 months old) fish from each strain (total n = 100 fish) were each randomly assigned to an individual 1 L tank (25 cm × 5 cm × 15 cm; Aquaneering, San Diego, California) in our zebrafish facility. All tanks were assigned a numeric identifier allowing the observer to record data without knowledge of the strain of each fish. White paper barriers were placed between tanks to eliminate the potential for individuals in adjoining tanks to influence each other’s foraging behavior through visual cues. Fish were allowed to acclimate for 4 days to the experimental tanks before data collection began and were fed 8 ± 2 mg of flake food twice per day (0900 and 1300). This amount of food was chosen because it was sufficient to satiate all fish in the experiment. The advantage of performing behavioral assays on individuals is that it increases statistical power and avoids the problem of tank effects. However, the zebrafish is a shoaling species. Although foraging behavior in individuals may differ slightly from groups, it has been shown that zebrafish shoal cohesion decreases while for aging (Miller and Gerlai 2007), indicating independent individual behavior.
Behavioral assay
We chose to measure latency to begin feeding from the water surface as a proxy measure of foraging behavior. Previous studies have utilized latency to begin feeding after a stimulus as an indicator of food motivation in fish (reviewed by Lima and Dill 1990) and as a proxy for antipredator behavior (Whitham and Mathis 2000; Yamamoto and Reinhardt 2003), as well as stress recovery (Moretz et al. 2007b). Our delivery methods were consistent with the normal feeding procedure used in the fish facility, where flake food is delivered by hand through a 1.5 cm hole in the lid of the tank. The observer recorded by stopwatch the amount of time between delivery of flake food (Tetramin flake food; 8 ± 2 mg per feeding event) and commencement of the feeding behavior at the surface of the water. Timing of feeding latency stopped at 180 s and any fish that had not obtained food from the surface by that time was assigned a value of 180 s. Feeding latency data were collected once per day at approximately 0900. It is important to note that the collection of data in real time by the person responsible for feeding and performing the manipulations described below introduces the possibility of observer bias in tests of stimulus effects. However, because the fish were randomly assigned to tanks and marked only with a numeric identifier, this limitation does not extend to tests of the effect of strain.
We chose to measure feeding behavior that occurred at the water surface for two reasons. First, we considered surface feeding to be more objective, as it can be difficult to accurately and precisely determine when small fish consume minute food particles suspended in the water column. Second, fish often perceive the surface of the water as riskier habitat; other studies have linked the surface of the water with predation risk in zebrafish (Hamilton and Dill 2002) and other fish species (Reinhardt 2001; Weber and Fausch 2003; Biro et al. 2004; Huntingford 2004). Aversive stimuli such as movement of the tank lid (described below) would likely be perceived as elevating risk and therefore potentially influence timing of feeding at this location. It should be noted, however, that in rare instances food penetrated into the water column before an individual began feeding from the surface. We recorded if the fish under observation began feeding from within the water column, but waited until feeding occurred at the surface to record the feeding latency score. This behavior occurred too infrequently for statistical analysis.
Treatments
Below, we describe the six treatment combinations in which we manipulated levels of nutrient availability and risk in a repeated-measures design. Each treatment combination lasted for 4 days, during which behavioral data were collected once daily. Performing these treatments in a repeated-measures design has the advantage of accounting for potential variations in the stimulus across days. The treatment periods were performed in series, starting with the control conditions for both nutrient intake and risk and ending with an alarm cue, restricted diet combination (Table 1). Fish were given a recovery period of 4 days in between each treatment during which feeding levels were returned to control levels and no risk-cue manipulations were conducted to reduce any carry over effects on behavior.
Table 1.
Summary of nutrient availability and risk-cue combinations to assess variation in feeding latency among zebrafish (Danio rerio) strains
| Risk | Nutrient | Abbreviation |
|---|---|---|
| Control* | Control† | CC |
| Control* | Restricted§ | CR |
| Surface‡ | Control† | SC |
| Surface‡ | Restricted§ | SR |
| Control* | Control† | CC2 |
| Alarm∥ | Control† | AC |
| Alarm∥ | Restricted§ | AR |
No risk cues were provided.
Fish were fed twice daily.
Fish were fed once per day.
Tank was raised and lowered prior to feeding.
An injection of zebrafish alarm substance was injected into the tank prior to feeding.
Control conditions
We first collected feeding latency data under standard laboratory conditions to establish a baseline of behavior. After the 4 day acclimation, we began conducting the assays of feeding latency as described above. In addition to the food provided during the surface feeding latency assay, fish were fed an equal amount of food in the afternoon at the normal feeding time in the facility. As this treatment period did not differ from our standard laboratory conditions, we termed this treatment the “control” period. Collection of feeding latency data during this period allowed us to test whether there was variation in feeding behavior among zebrafish strains under normal laboratory conditions.
Alteration of nutrient availability
We established two nutrient availability levels in our experiment. First, fish were maintained at a control level, where individuals were fed in the afternoon in addition to the morning feeding that was used to determine feeding latency. The second nutrient availability level (restricted) was a restriction to half-rations, during which fish were not fed in the afternoon.
Alteration of risk cues
We established three levels of risk during the feeding latency assay using potentially aversive stimuli. The control treatment was consistent with the standard laboratory environment, as described above. The next risk level was a disturbance at the surface of the water (“surface”). We raised the front of the tank lid to a height of 3 cm and immediately dropped it back in place 2 s before providing food for the feeding latency assay. This stimulus provided both a mechanical cue (the impact of the lid) and a visual cue (change in light conditions) of a disturbance at the point of food delivery.
The other stimulus (“alarm”) was an injection of 0.07 mL of chemical alarm substance (Schreckstoff) into the tank water 3 s before providing food for the feeding latency assay. Previous studies have shown that contact with alarm substance from conspecifics solicits an unlearned alarm response in zebrafish (Suboski et al. 1990). Because it is not known if populations differ in the production of alarm substance, we chose to create a zebrafish alarm substance from two size-matched adult fish from each of the four strains, utilizing skin from the entire fish (approximately 2 cm2 from each fish). Zebrafish skin was homogenized in 300 mL of deionized water and filtered through glass wool to make the alarm substance, which was aliquoted and frozen at −20 °C until use. Preliminary experiments (data not shown) confirmed that 0.07 mL of alarm substance was sufficient to elicit an immediate (within 3 s) alarm reaction in zebrafish (characterized by rapid zig-zag swimming and (or) freezing in place). Alarm substance was delivered into the tanks from a syringe via each tank’s water delivery tubes. Efferent water containing the alarm substance was treated as part of the facility’s recirculation system. Although the stability of alarm substance is unknown, we assumed that a combination of large-scale dilution (7 mL per day of alarm substance introduced into a system-wide volume of more than 12 000 L) combined with physical (5 μm filters) and chemical (Fluidized sand bed) filtrations prevented any transient behavioral effects.
An alternative design for this experiment would have been to use fish assigned only to a single treatment group rather than our repeated-measures design in which each fish received each treatment. However, logistical constraints such as tank space (>500 tanks would have been required) made implementation of this alternative impossible. As there was potential for experimental time, rather than treatments, to influence results in our design, we performed an additional data collection period under control conditions between the surface disturbance and the alarm substance treatments. During this time point, the fish were given the standard 4 day recovery period, and behavioral data collection then began under control conditions of risk and diet. This control condition period (control 2) allowed us to test our assumption that the 4 day recovery period was sufficient to allow fish to return to baseline levels of foraging behavior before the next manipulation, as well as whether any observed changes in behavior from the surface disturbance to alarm substance treatments were related to time (or a carry-over from the previous treatment) rather than alarm treatment.
Food intake and growth rate
Our next step was to determine whether interstrain variation in feeding latency behavior was associated with food intake and growth rate, as variation in foraging behavior can result from variation in food demand (Abrahams and Sutterlin 1999). Thirty days after the feeding latency trials were complete, the fish were weighed and measured for standard length. Fish were also visually sexed at this time using secondary sexual characters (Westerfield 1993). Individuals from the same strain were then randomly combined into tanks of five fish each (n = 5 tanks per strain). Fish were pooled because the very small mass of food consumed by individual fish made it difficult to monitor food intake on a per fish basis. Fish were fed to apparent satiety (food remaining at the water surface 5 min after delivery and no apparent feeding activity; Halver and Hardy 2002) twice per day for 5 days to acclimate them to group living and foraging. We assumed that the 35 days between the two experiments was sufficient recovery time to limit potential behavioral carry-over from the alarm substance treatments. At the end of the acclimation period, the food intake and growth rate trial was begun, where each tank was fed to apparent satiety twice per day for the next 30 days. The mass of food provided per tank from tank-specific vials was recorded each day. Although there was unconsumed food remaining at the water’s surface included in the calculation of food mass, this amount was negligible relative to the amount consumed and was consistent across tanks. After 30 days, fish were again weighed and measured for standard length. Food intake was quantified as a percentage of biomass, where the amount of food provided to each tank per day was divided by the beginning biomass in the tank. We also estimated specific growth rate (SGR), where the natural log of the change in each tank’s biomass was divided by the number of experimental days (i.e., 30). Unlike the feeding latency assays, where the experimental unit was an individual fish, tank was used as the experimental unit for growth and feed intake analyses.
Statistical analyses
Surface feeding latency data from all treatment periods were analyzed using repeated measures mixed model ANOVA within the SAS version 9.1.3 (SAS Institute Inc. 2007) Mixed procedure. The full mixed model included a fully crossed factorial design of strain, nutrient level, aversive stimulus, and sex. As behavior was measured daily for 4 days within each treatment combination, the day of each behavioral assay was included in the model as a block effect nested within nutrient and risk levels and as a repeated measure with each fish as the subject. Significant deviation from normality of model residuals was rectified by rank-transforming the data; the model was too complex for standard nonparametric techniques (Conover 1999). Significance of variation among strains and across treatments was tested using the Tukey–Kramer’s test on least square means calculated from the full model.
For the food intake and growth experiment, the GLM procedure within SAS was used to test for interstrain variation in both food intake and SGR, with tank as the experimental unit. We also tested for correlations between feeding latency, SGR, and food intake using Proc Corr within SAS.
Results
Strain variation in feeding latency
Using the full mixed model, we detected significant variation in feeding latency among strains (Table 2). We also detected a significant sex × strain interaction across all treatments (Table 2), and present least square means of ranked feeding latency from the control (CC) period for both males and females within each strain in Fig. 1. The general pattern during these control conditions was for Nadia and Gaighatta to have higher feeding latencies (i.e., they took more time to commence feeding) than TM1 and SH, with females having a lower feeding latency than males in all strains.
Table 2.
Results of the repeated measures mixed model ANOVA using rank-transformed feeding latency in four strains of zebrafish (Danio rerio)
| Effect | df | F | P |
|---|---|---|---|
| Day | 4, 344 | 8.42 | <0.0001 |
| Nutrient | 1, 91 | 8.28 | 0.005 |
| Risk | 2, 181 | 641.94 | <0.0001 |
| Sex | 1, 91 | 54.55 | <0.0001 |
| Strain | 3, 91 | 126.54 | <0.0001 |
| Nutrient × risk | 2, 179 | 11.27 | <0.0001 |
| Nutrient × sex | 1, 91 | 0.3 | 0.5824 |
| Nutrient × strain | 3, 91 | 0.92 | 0.4352 |
| Risk × sex | 2, 181 | 0.01 | 0.9892 |
| Risk × strain | 6, 181 | 8.7 | <0.0001 |
| Strain × sex | 3, 91 | 4.96 | 0.0031 |
| Nutrient × risk × sex | 2, 179 | 0.58 | 0.5596 |
| Nutrient × risk × strain | 6, 179 | 1.6 | 0.1503 |
| Nutrient × strain × sex | 3, 91 | 0.34 | 0.7988 |
| Risk × strain × sex | 6, 181 | 2.07 | 0.0593 |
| Nutrient × risk × strain × sex | 6, 179 | 0.85 | 0.5299 |
Fig. 1.
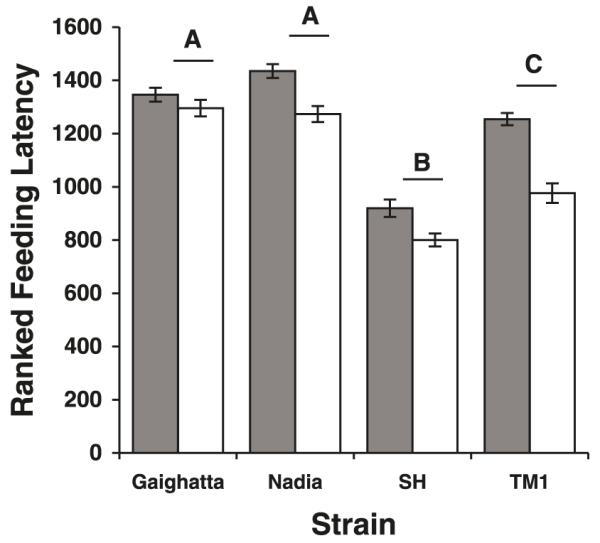
Feeding latency for male (shaded bars) and female (open bars) zebrafish (Danio rerio) from four strains during control conditions (CC). Bars represent least square means (±SE) of rank-transformed data. Strains with different letters are significantly different (p < 0.05). Higher values of ranked feeding latency indicate a longer time to commence feeding.
Treatment effects on feeding latency
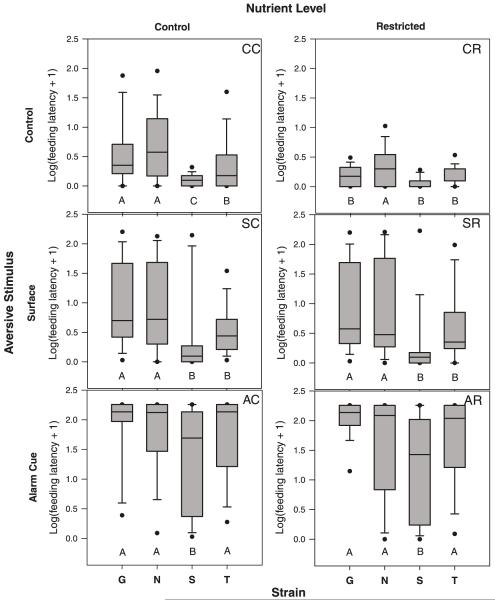
Manipulations of nutrient availability and risk both affected foraging behavior, as indicated by significant main effects for both variables (Table 2). The restricted diet tended to reduce feeding latency and individual variation, while increased risk manipulations increased feeding latency and individual variation (Fig. 2). However, we also observed a significant interaction between diet and risk, primarily because restricting diets by half reduced feeding latency only at control levels of risk. During both the surface disturbance and the alarm-cue treatments, the restricted diet had no effect on foraging behavior.
Fig. 2.
Boxplots of feeding latency during nutrient and risk-cue manipulation in four zebrafish (Danio rerio) strains (G, Gaighatta; N, Nadia; S, Scientific Hatcheries; T, TM1). Log-transformed data are shown to facilitate comparisons of the alarm-cue treatments with other treatments. Different letters along the x axis within each plot denote significant differences (p < 0.05) among strains. Letters in the upper right-hand corner of each plot represent the abbreviated treatment names from Table 1.
When conditions were returned to control levels between the surface disturbance and the alarm-cue treatments (CC2), feeding latency was not significantly different from that observed in the initial control period (Fig. 3; F[1,90] = 2.94, p = 0.091).
Fig. 3.
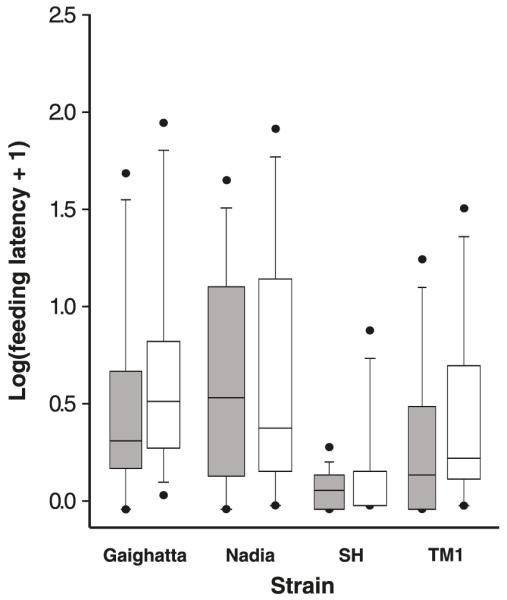
Boxplots comparing feeding latency of zebrafish (Danio rerio) in the initial control period (CC; shaded bars) with feeding latency during the second control period (CC2; open bars). While there was a significant strain effect during each period, there was no significant effect of time on feeding latency within strains.
There was a significant main effect of day in the model (Table 2); however, there was no consistent trend for ranked feeding latency to increase or decrease with time. In addition, the only treatment for which day was significant was the alarm cue – control diet combination (F[4,371] = 11.80, p < 0.001). In this treatment, ranked feeding latency increased with day.
Strain variation in treatment response
There was no significant interaction between nutrient intake level and strain on feeding latency (Table 2), indicating that all strains responded similarly to the dietary manipulation. There was, however, a significant interaction between risk level and strain. Least square means using the risk × strain interaction term indicated that each strain responded to the surface disturbance and alarm-cue manipulations by significantly increasing feeding latency (p < 0.0001 for all strains). However, strains varied in the magnitude of their response to each aversive stimulus so that the pattern of interstrain variation changed with each of the three treatments (CC, SC, and AC in Fig. 2). At control risk levels, Nadia had the longest feeding latency, SH the shortest, and Gaighatta and TM1 were intermediate. During the surface disturbance, Nadia and Gaighatta were most similar to each other, while SH and TM1 group together at a shorter latency to feed. The pattern of strain variation also changed in the alarm-cue treatment, where the SH had the lowest latency to feed relative to the other three strains. The SH were also notable for their marked increase in variation among individuals in response to the aversive stimuli (Fig. 2).
Food intake and growth rate
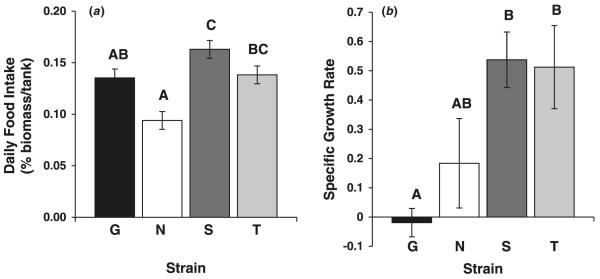
There was a significant effect of strain on SGR (F[3,19] = 5.27, p = 0.0102) and food intake (F[3,19] = 11.02, p = 0.0004; Fig. 4). Gaighatta and Nadia fish had lower SGR and food intake per unit body mass than TM1 and SH fish. Mean feeding latency (calculated as the mean value of ranked feeding latency of all individuals within the tank) was negatively correlated with food intake (r = −0.60, p = 0.0055). Thus, fish that delayed surface feeding in the control treatment of the feeding latency assays also consumed less food in the group tanks. Food intake and SGR were also significantly correlated (r = 0.48, p = 0.032).
Fig. 4.
(a) Mean (±SE) food intake and (b) mean specific growth rate of four zebrafish (Danio rerio) strains (G, Gaighatta; N, Nadia; S, Scientific Hatcheries; T, TM1). Letters indicate significant differences (p < 0.05) among the strains.
Discussion
Our study revealed significant interstrain variation in zebrafish surface feeding latency, and this variation was dependent on conditions of nutrient availability and manipulations of aversive stimuli. Feeding latency was reduced when nutrient availability was restricted, but manipulations of perceived risk using aversive stimuli overrode nutrient restriction and increased latency to feed. Although the response to dietary restriction was consistent across strains, the degree of response to the aversive stimuli was strain-dependent. Food intake was negatively correlated with latency to feed and there was significant strain variation in both food intake and SGR. Collectively, these data indicate that zebrafish strains vary in the management of the trade-off between the perceived risk and the physiological drive to acquire nutrients.
There are several potential explanations for strain variation in the management of risk during foraging. If we consider that the fish need to balance their demand for food against the risk of predation, it may be that the four strains vary in their perception of risk, which would allow some fish to begin foraging in situations that others considered to be too risky. Interstrain variation in the response to the risk treatments suggests that this is a plausible explanation, as the pattern of variation among strains in feeding behavior changed depending on the risk treatment. For example, although Gaighatta showed the greatest behavioral change in response to the surface disturbance treatments, both Gaighatta and Nadia had significantly higher latencies to feed during these treatments than SH and TM1. This may indicate that SH and TM1 perceived the surface disturbances as less risky than the other two strains. Variation in the perception of risk has been shown in other species of fish. Most notably, risk-related phenotypes in the guppy such as schooling behavior, predator inspection behavior, response to overhead threats, habitat selection, foraging behavior, and sexual characters respond to variations in local predation pressure (reviewed in Magurran et al. 1993).
Another potential explanation for interstrain variation in foraging behavior is that the strains differ in their physiological demand for nutrients. As all of our fish experienced the same physical conditions in their rearing environment since fertilization, the phenotypic variation in our study likely has a significant genetic component. In fish, increases in growth hormone levels (Abrahams and Sutterlin 1999) and selection for increased growth rates (Mambrini et al. 2006) both increase food intake. Genetic variation in these growth-related traits also influences feeding behavior; individuals with the highest growth rates require the most food and therefore take the greatest risk to obtain it. For example, Abrahams and Sutterlin (1999) demonstrated that transgenic Atlantic salmon with increased levels of growth hormone not only exhibited increased growth, but also increased consumption rates and spent more time feeding in the presence of predators than control fish. Alternatively, Lister and Neff (2006) showed that food conversion efficiency could influence foraging behavior, with individuals of lower efficiency taking greater risks while foraging; progeny of bluegill (Lepomis macrochirus Rafinesque, 1819) from males with different reproductive tactics differed in the amount of time spent foraging in risky habitat (Lister and Neff 2006) and in food conversion efficiency (Neff 2004). In our study, the fish that consumed the most food (as estimated by the amount of food provided) were also the fastest growing and were the quickest to begin feeding even in the presence of aversive stimuli. However, it is important to note that feeding behavior and growth rate were measured in different contexts (i.e., measurements on individuals versus groups). Our results are similar to those observed by Abrahams and Sutterlin (1999), in which the fish with the highest growth rates engaged in more risky behavior.
Ultimately, the relative effects of perception of risk and demand for food on foraging behavior are difficult to disentangle. We therefore suggest that the interstrain variation in foraging behavior we observed may be a product of both an increased demand for food and a reduced perception of disturbances at the water surface as “risky” in the SH and TM1 strains relative to Gaighatta and Nadia. However, there are several ways in which future studies may disentangle the relative effects of risk perception and demand for food. In particular, the rapid generation time of the zebrafish allows construction of segregating F2 populations for QTL (quantitative trait locus) analysis (for example see Wright et al. 2006). Experiments of this sort could determine whether interstrain variation in food demand and perception of risk are genetically correlated or segregating as independent traits. Alternatively, more detailed experiments using serially increasing levels of risk (e.g., different concentrations of alarm substance) and food availability could reveal whether populations varied in the critical level of risk beyond which feeding is completely inhibited.
The pattern of interstrain variation we observed indicates a difference between strains recently derived from wild populations (Gaighatta and Nadia) and those maintained in the laboratory for 30 or more generations (SH and TM1). There are two potential explanations for this result. First, this variation may represent naturally occurring variation among zebrafish strains. Previous work using wild zebrafish populations has demonstrated variation in boldness towards novel objects, but not shoaling tendencies, indicating that at least some behaviors are variable among populations recently collected in the wild (Wright et al. 2003). Variation in foraging behavior among the zebrafish strains in our study may therefore reflect phylogenetic relatedness or local adaptation of source populations to similar habitat features, such as variation in predation risk (O’Steen et al. 2002).
Alternatively, the patterns of intraspecific variation in for-aging behavior observed in our study may be a result of adaptation to captivity, which in fish can result in numerous phenotypic and genetic changes. These include changes in place preference, reductions in antipredator behavior, increases in boldness and risk taking, and increases in food intake and growth rate (reviewed by Huntingford 2004). Hatchery salmonids and trout emerge from cover more quickly after a disturbance, take greater risks while foraging, and display longer feeding latencies than wild fish (Johnsson and Abrahams 1991; Einum and Fleming 1997; Abrahams and Sutterlin 1999; Yamamoto and Reinhardt 2003; Biro et al. 2004). Hatchery rearing can also result in fish with higher growth rates than wild fish (Johnsson et al. 1996; Reinhardt 2001; Mambrini et al. 2006), as well as increased food intake (Abrahams and Sutterlin 1999; Sanchez et al. 2001; Yamamoto and Reinhardt 2003; Mambrini et al. 2006). Similar to our study, Yamamoto and Reinhardt (2003) showed that hatchery-raised masu salmon (Oncorhynchus masou (Brevoort, 1856)) fed more and were more willing to leave cover and feed under chemically simulated predation risk than wild fish.
The pattern of strain variation in our study matches that described in other fish species undergoing adaptation to captivity. The two strains most recently derived from wild populations (Gaighatta and Nadia) had longer latencies to feed even during control conditions, had a greater response to surface disturbances, consumed less food, and had lower growth rates relative to the SH and TM1 fish that have been in the laboratory for more than 30 generations. Like hatchery fish, laboratory zebrafish are not under selection pressure from predators, nor are they constrained by limited or patchy resources. Rather, they are subjected to artificial and indirect selection during strain propagation. The fish that mature quickly and have the greatest fecundity are those most often selected to produce offspring. This may impose selection on growth rates and reproductive traits, as well as related behaviors such as food intake, feeding latency, boldness, and competitive abilities. In the case of the SH strain, the supplier has explicitly stated that their fish have undergone selection for increased growth rate. We therefore consider behavioral adaptation to captivity to be the most likely explanation for the patterns of behavioral variation that we observed.
The only other study to show variation among zebrafish strains in foraging behavior also examined latency to feed (Moretz et al. 2007a, 2007b). Similar to our study, these authors observed that the Nadia strain had a higher latency to feed than the TM1 strain. However, in their study the SH fish were not significantly different from Nadia. Although methodological differences make direct comparisons of our results with these two studies difficult, all three demonstrate that foraging behavior can vary among strains. Taken together, these studies also indicate that zebrafish foraging behavior is influenced by both genetic and environmental factors that may be specific to a given experiment.
Predictably, both restricting food availability and increasing levels of risk influenced foraging behavior in our study, indicating that both factors are important in zebrafish foraging decisions. However, we found that restricted diet only reduced feeding latency when risk cues were at control levels. Similarly, Angradi (1992) found that simulated avian predation increased feeding latency in rainbow trout (Oncorhynchus mykiss (Walbaum, 1792)), but restricting food had no significant effect. Our results are also similar to experiments in bluntnose minnows (Pimephales notatus (Rafinesque, 1820)), which increased latency to feed in the presence of predators, but decreased feeding latency with food deprivation (Morgan 1988). Further experiments are required to determine whether reductions in feeding latency during aversive stimuli can be overcome by a more severe level of dietary restriction.
Although we observed significant effects of our manipulations on surface feeding latency, the tests of treatment effects require careful consideration. During the study, the observer remained blind relative to the strain identity of the fish, but by necessity was aware of the manipulations of nutrient availability and aversive stimuli. This feature of the design introduces the possibility of observer bias in tests of treatment effects. Similarly, the order in which the manipulations were performed was chosen to minimize the potential carry-over effects of alarm substance on behavior. We assumed that a 4 day rest period between manipulations was sufficient to reduce or eliminate carry-over effects of previous treatments on subsequent behavior. This assumption is partially supported by the fact that the feeding latency of all strains returned to control levels after the 4 day rest period midway through the experiment (Fig. 3). Although we cannot rule out these potential sources of bias, they do not affect our conclusions regarding significant differences among strains within treatments.
Our results support the prediction that foraging behavior changes in response to elevated perceived risk; however, the behavioral response to this elevated risk can vary among populations. Our study suggests that the observed intraspecific variation in foraging behavior has a genetic basis and that future behavioral research in zebrafish should carefully consider the possibility that behavioral responses to manipulations may be strain-specific. This study also highlights the potential utility of the zebrafish in studies of the genetic basis of variation in foraging behavior.
Acknowledgements
We thank Erin Churchill for her valuable technical assistance, Katie Schmidt for her aid in data collection, Josh Boyce-Derricott for donating alarm substance, and Robert Drew for his statistical assistance. Robert Drew and Gordon Murdoch also provided valuable feedback on earlier versions of the manuscript. This research was supported by National Science Foundation (NSF) Idaho EPSCoR Program and NSF under EPS-0447689 and IOS-0818904, and the zebrafish facility was constructed with funding from the National Institutes of Health grant no. P20 RR016448 from the COBRE Program of the National Center for Research Resources.
References
- Abrahams MV, Sutterlin A. The foraging and antipredator behaviour of growth-enhanced transgenic Atlantic salmon. Anim. Behav. 1999;58:933–942. doi: 10.1006/anbe.1999.1229. doi:10.1006/anbe.1999.1229. PMID: 10564595. [DOI] [PubMed] [Google Scholar]
- Angradi TR. Effects of predation risk on foraging behavior of juvenile rainbow trout (Oncorhynchus mykiss) Can. J. Zool. 1992;70:355–360. doi:10.1139/z92-053. [Google Scholar]
- Anholt BR, Werner EE. Interaction between food availability and predation mortality mediated by adaptive behavior. Ecology. 1995;76:2230–2234. doi:10.2307/1941696. [Google Scholar]
- Aubret F, Bonnet X, Bradshaw D. Food versus risk: foraging decision in young Tiger snakes, Notechis scutatus. Amphib.-Reptilia. 2007;28:304–308. doi:10.1163/156853807780202396. [Google Scholar]
- Biro PA, Abrahams MV, Post JR, Parkinson EA. Predators select against high growth rates and risk-taking behaviour in domestic trout populations. Proc. R. Soc. Lond. B Biol. Sci. 2004;271:2233–2237. doi: 10.1098/rspb.2004.2861. doi:10.1098/rspb.2004.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman MR, Kramer DL. Guarded resources: the effect of intruder number on the tactics and success of defenders and intruders. Anim. Behav. 1996;52:83–94. doi:10.1006/anbe.1996.0154. [Google Scholar]
- Conover WJ. Practical nonparametric statistics. John Wiley and Sons; New York: 1999. [Google Scholar]
- Cooper WE., Jr. Tradeoffs between predation risk and feeding in a lizard, the broad-headed skink (Eumeces laticeps) Behaviour. 2000;137:1175–1189. doi:10.1163/156853900502583. [Google Scholar]
- Einum S, Fleming IA. Genetic divergence and interactions in the wild among native, farmed and hybrid Atlantic salmon. J. Fish Biol. 1997;50:634–651. doi:10.1111/j.1095-8649.1997.tb01955.x. [Google Scholar]
- Engstrom-Ost J, Lehtiniemi M. Threat-sensitive predator avoidance by pike larvae. J. Fish Biol. 2004;65:251–261. doi:10.1111/j.0022-1112.2004.00448.x. [Google Scholar]
- Gerlai R. Zebrafish: an uncharted behavior genetic model. Behav. Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. doi:10.1023/A:1025762314250. PMID:14574124. [DOI] [PubMed] [Google Scholar]
- Gibbs PDL, Schmale MC. GFP as a genetic marker scorable throughout the life cycle of transgenic zebra fish. Mar. Biotechnol. 2000;2:107–125. doi: 10.1007/s101269900014. PMID:10811950. [DOI] [PubMed] [Google Scholar]
- Godin JGJ, Crossman SL. Hunger-dependent predator inspection and foraging behaviors in the threespine stickleback (Gasterosteus aculeatus) under predation risk. Behav. Ecol. Sociobiol. 1994;34:359–366. doi:10.1007/BF00197006. [Google Scholar]
- Grant JWA, Kramer DL. Temporal clumping of food arrival reduces its monopolization and defence by zebrafish, Brachydanio rerio. Anim. Behav. 1992;44:101–110. doi:10.1016/S0003-3472(05)80759-6. [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. doi:10.1046/j.1601-183X.2003.00053.x. PMID:15005714. [DOI] [PubMed] [Google Scholar]
- Halver JE, Hardy RW. Fish nutrition. Academic Press; San Diego: 2002. [Google Scholar]
- Hamilton IM, Dill LM. Monopolization of food by zebrafish (Danio rerio) increases in risky habitats. Can. J. Zool. 2002;80:2164–2169. doi:10.1139/z02-199. [Google Scholar]
- Horat P, Semlitsch RD. Effects of predation risk and hunger on the behavior of two species of tadpoles. Behav. Ecol. Sociobiol. 1994;34:393–401. doi:10.1007/BF00167330. [Google Scholar]
- Huntingford FA. Implications of domestication and rearing conditions for the behaviour of cultivated fishes. J. Fish Biol. 2004;65:122–142. doi:10.1111/j.0022-1112.2004.00562.x. [Google Scholar]
- Johnsson JI, Abrahams MV. Interbreeding with domestic strain increases foraging under threat of predation in juvenile steelhead trout (Oncorhynchus mykiss) — an experimental study. Can. J. Fish. Aquat. Sci. 1991;48:243–247. doi:10.1139/f91-033. [Google Scholar]
- Johnsson JI, Petersson E, Jonsson E, Bjornsson BT, Jarvi T. Domestication and growth hormone alter antipredator behaviour and growth patterns in juvenile brown trout, Salmo trutta. Can. J. Fish. Aquat. Sci. 1996;53:1546–1554. doi:10.1139/cjfas-53-7-1546. [Google Scholar]
- Kelley JL, Magurran AE. Learned predator recognition and antipredator responses in fishes. Fish Fish. 2003;4:216–226. [Google Scholar]
- Labra A, Leonard R. Intraspecific variation in antipredator responses of three species of lizards (Liolaemus): possible effects of human presence. J. Herpetol. 1999;33:441–448. doi:10.2307/1565641. [Google Scholar]
- Lima SL, Dill LM. Behavioral decisions made under the risk of predation — a review and prospectus. Can. J. Zool. 1990;68:619–640. doi:10.1139/z90-092. [Google Scholar]
- Lister JS, Neff BD. Paternal genetic effects on foraging decision-making under the risk of predation. Ethology. 2006;112:963–970. doi:10.1111/j.1439-0310.2006.01248.x. [Google Scholar]
- Magurran AE, Seghers BH, Carvalho GR, Shaw PW. Evolution of adaptive variation in antipredator behavior. Mar. Behav. Physiol. 1993;23:29–44. [Google Scholar]
- Mambrini M, Labbe L, Randriamanantsoa F, Boujard T. Response of growth-selected brown trout (Salmo trutta) to challenging feeding conditions. Aquaculture. 2006;252:429–440. doi:10.1016/j.aquaculture.2005.07.001. [Google Scholar]
- Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav. Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. doi:10.1016/j.bbr.2007.07.007. PMID:17707522. [DOI] [PubMed] [Google Scholar]
- Moretz JA, Martins EP, Robison BD. The effects of early and adult social environment on zebrafish (Danio rerio) behavior. Environ. Biol. Fishes. 2007a;80:91–101. doi:10.1007/s10641-006-9122-4. [Google Scholar]
- Moretz JA, Martins EP, Robison BD. Behavioral syndromes and the evolution of correlated behavior in zebrafish. Behav. Ecol. 2007b;18:556–562. doi:10.1093/beheco/arm011. [Google Scholar]
- Morgan MJ. The influence of hunger, shoal size and predator presence on foraging in bluntnose minnows. Anim. Behav. 1988;36:1317–1322. doi:10.1016/S0003-3472(88)80200-8. [Google Scholar]
- Neff BD. Increased performance of offspring sired by parasitic males in bluegill sunfish. Behav. Ecol. 2004;15:327–331. doi:10.1093/beheco/arh016. [Google Scholar]
- O’Steen S, Cullum AJ, Bennett AF. Rapid evolution of escape ability in Trinidadian guppies (Poecilia reticulata) Evolution. 2002;56:776–784. doi: 10.1111/j.0014-3820.2002.tb01388.x. PMID:12038535. [DOI] [PubMed] [Google Scholar]
- Owings DH, Coss RG, McKernon D, Rowe MP, Arrowood PC. Snake-directed antipredator behavior of rock squirrels (Spermophilus variegatus): population differences and snake-species discrimination. Behaviour. 2001;138:575–595. doi:10.1163/156853901316924485. [Google Scholar]
- Pettersson LB, Bronmark C. Trading off dafety against food: state dependent habitat choice and foraging in crucian carp. Oecologia (Berl.) 1993;95:353–357. doi: 10.1007/BF00320988. doi:10.1007/BF00320988. [DOI] [PubMed] [Google Scholar]
- Reinhardt UG. Selection for surface feeding in farmed and sea-ranched masu salmon juveniles. Trans. Am. Fish. Soc. 2001;130:155–158. doi:10.1577/1548-8659(2001)130<0155:SFSFIF>2.0.CO;2. [Google Scholar]
- Robison BD, Rowland W. A potential model systemfor studying the genetics of domestication: behavioral variation among wild and domesticated strains of zebra danio (Danio rerio) Can. J. Fish. Aquat. Sci. 2005;62:2046–2054. doi:10.1139/f05-118. [Google Scholar]
- Roth ED, Johnson JA. Size-based variation in antipredator behavior within a snake (Agkistrodon piscivorus) population. Behav. Ecol. 2004;15:365–370. doi:10.1093/beheco/arh024. [Google Scholar]
- Sanchez MP, Chevassus B, Labbe L, Quillet E, Mambrini M. Selection for growth of brown trout (Salmo trutta) affects feed intake but not feed efficiency. Aquat. Living Resour. 2001;14:41–48. doi:10.1016/S0990-7440(00)01103-7. [Google Scholar]
- SAS Institute Inc. SAS. Version 9.1.3 [computer program] SAS Institute Inc.; Cary, N.C.: 2007. [Google Scholar]
- Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes influencing vertebrate behavior: zebrafish making headway. Lab Anim. 2006;35:33–39. doi: 10.1038/laban0506-33. doi:10.1038/laban0506-33. PMID:16645614. [DOI] [PubMed] [Google Scholar]
- Sogard SM, Olla BL. The influence of hunger and predation risk on group cohesion in a pelagic fish, walleye pollock Theragra chalcogramma. Environ. Biol. Fishes. 1997;50:405–413. doi:10.1023/A:1007393307007. [Google Scholar]
- Suboski MD, Bain S, Carty AE, McQuoid LM, Seelen MI, Seifert M. Alarm reaction in acquisition and social transmission of simulated-predator recognition by zebra danio fish (Brachydanio rerio) J. Comp. Psychol. 1990;104:101–112. doi:10.1037/0735-7036.104.1.101. [Google Scholar]
- Urban MC. Risky prey behavior evolves in risky habitats. Proc. Natl. Acad. Sci. U.S.A. 2007;104:14377–14382. doi: 10.1073/pnas.0704645104. doi:10.1073/pnas.0704645104. PMID:17724339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehanen T. Adaptive flexibility in the behaviour of juvenile Atlantic salmon: short-term responses to food availability and threat from predation. J. Fish Biol. 2003;63:1034–1045. doi:10.1046/j.1095-8649.2003.00228.x. [Google Scholar]
- Walling CA, Dawnay N, Kazem AJN, Wright J. Predator inspection behaviour in three-spined sticklebacks (Gasterosteus aculeatus): body size, local predation pressure and cooperation. Behav. Ecol. Sociobiol. 2004;56:164–170. doi:10.1007/s00265-004-0763-z. [Google Scholar]
- Weber ED, Fausch KD. Interactions between hatchery and wild salmonids in streams: differences in biology and evidence for competition. Can. J. Fish. Aquat. Sci. 2003;60:1018–1036. doi:10.1139/f03-087. [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio) University of Oregon Press; Eugene: 1993. [Google Scholar]
- Whitham J, Mathis A. Effects of hunger and predation risk on foraging behavior of graybelly salamanders, Eurycea multiplicata. J. Chem. Ecol. 2000;26:1659–1665. doi:10.1023/A:1005590913680. [Google Scholar]
- Wright D, Rimmer LB, Pritchard VL, Krause J, Butlin RK. Inter and intra-population variation in shoaling and boldness in the zebrafish (Danio rerio) Naturwissenschaften. 2003;90:374–377. doi: 10.1007/s00114-003-0443-2. doi:10.1007/s00114-003-0443-2. PMID:12955228. [DOI] [PubMed] [Google Scholar]
- Wright D, Nakamichi R, Krause J, Butlin RK. QTL analysis of behavioral and morphological differentiation between wild and laboratory zebrafish (Danio rerio) Behav. Genet. 2006;36:271–284. doi: 10.1007/s10519-005-9029-4. doi:10.1007/s10519-005-9029-4. PMID:16408248. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Reinhardt UG. Dominance and predator avoidance in domesticated and wild masu salmon Oncorhynchus masou. Fish. Sci. 2003;69:88–94. doi:10.1046/j.1444-2906.2003.00591.x. [Google Scholar]