Abstract
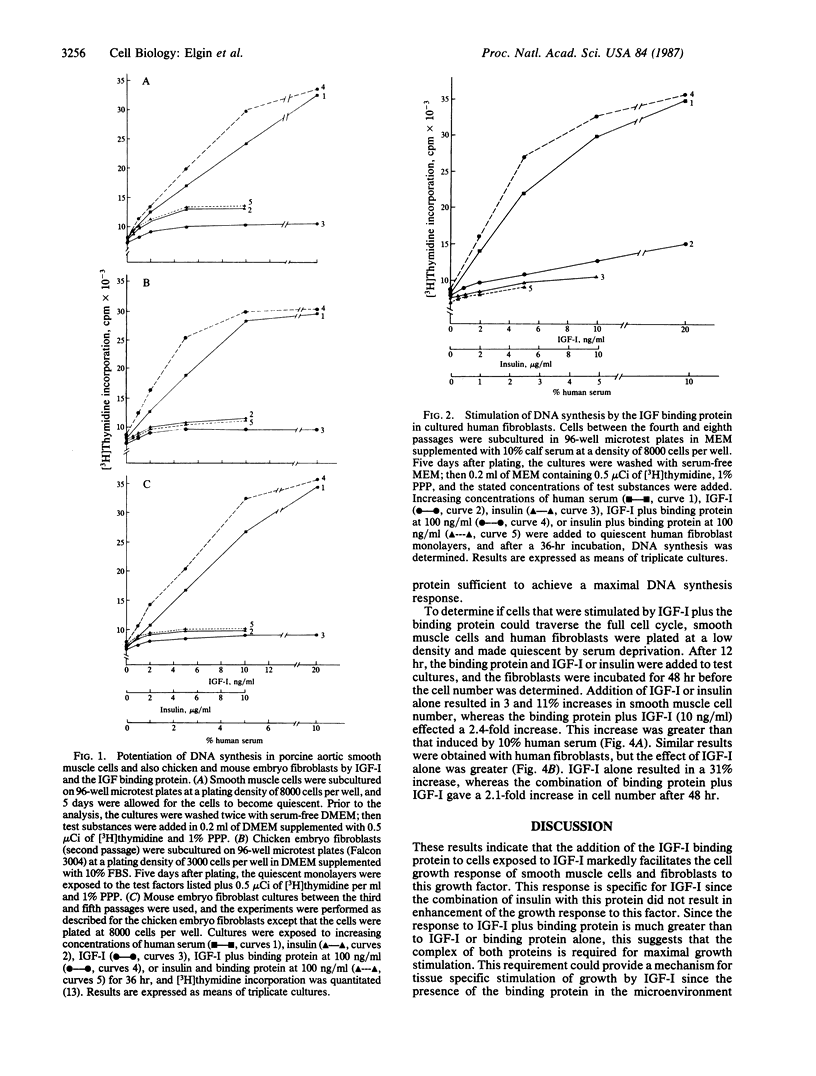
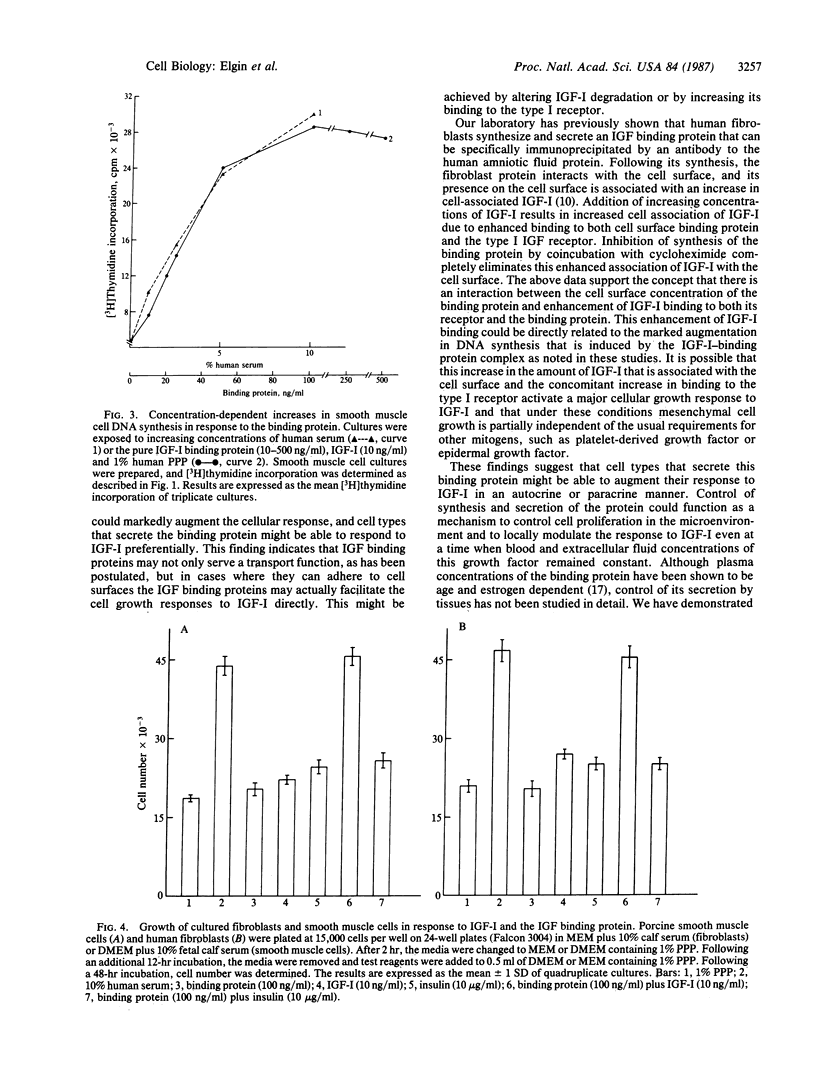
The insulin-like growth factors IGF-I and IGF-II circulate in blood bound to carrier proteins. The higher molecular mass IGF-binding protein complex (150 kDa) is composed of subunits, and one subunit that forms this complex is growth hormone dependent. In addition, many cell types and tissues secrete another form of IGF binding protein that is not growth hormone dependent. Both forms of the IGF binding protein are believed to inactivate the IGFs and to function as delivery systems to tissues. This conclusion was based on studies that determined the effects of impure preparations of these binding proteins or that examined the effect of these proteins only on the insulin-like actions of the IGFs. We report here that a pure preparation of the extracellular form of the IGF binding protein (purified from human amniotic fluid) markedly potentiated replication of several cell types in response to human IGF-I. Secondary cultures of human, mouse, and chicken embryo fibroblasts as well as porcine aortic smooth muscle cells showed marked enhancement of their DNA synthesis response (2.8- to 4.4-fold increases) to IGF-I in the presence of this protein. These responses were synergistic since the sum of the responses to either IGF-I or to the binding protein alone was between 8 and 17% of the increase obtained in cultures exposed to both peptides. The binding protein not only potentiated the DNA synthesis response but also enhanced the increase in cell number in response to IGF-I. This stimulation is specific for growth factors that bind to the binding protein since incubation with insulin, which binds to the type I IGF receptor but not to the binding protein, did not result in potentiation of this response. We conclude that a form of IGF binding protein that is present in extracellular fluids and is secreted by many types of cells can markedly potentiate the cellular response to IGF-I.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binoux M., Hardouin S., Lassarre C., Hossenlopp P. Evidence for production by the liver of two IGF binding proteins with similar molecular weights but different affinities for IGF I and IGF II. Their relations with serum and cerebrospinal fluid IGF binding proteins. J Clin Endocrinol Metab. 1982 Sep;55(3):600–602. doi: 10.1210/jcem-55-3-600. [DOI] [PubMed] [Google Scholar]
- Binoux M., Hossenlopp P., Lassarre C., Hardouin N. Production of insulin-like growth factors and their carrier by rat pituitary gland and brain explants in culture. FEBS Lett. 1981 Feb 23;124(2):178–184. doi: 10.1016/0014-5793(81)80131-7. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Elgin R. G., Han V. K., Casella S. J., D'Ercole A. J., Van Wyk J. J. Cultured fibroblast monolayers secrete a protein that alters the cellular binding of somatomedin-C/insulinlike growth factor I. J Clin Invest. 1986 May;77(5):1548–1556. doi: 10.1172/JCI112470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R. Interaction of circulating cell-derived and plasma growth factors in stimulating cultured smooth muscle cell replication. J Cell Physiol. 1984 Nov;121(2):425–430. doi: 10.1002/jcp.1041210222. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J., Pledger W. J. Sequential addition of platelet factor and plasma to BALB/c 3T3 fibroblast cultures stimulates somatomedin-C binding early in cell cycle. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6644–6648. doi: 10.1073/pnas.77.11.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drop S. L., Kortleve D. J., Guyda H. J., Posner B. I. Immunoassay of a somatomedin-binding protein from human amniotic fluid: levels in fetal, neonatal, and adult sera. J Clin Endocrinol Metab. 1984 Nov;59(5):908–915. doi: 10.1210/jcem-59-5-908. [DOI] [PubMed] [Google Scholar]
- Drop S. L., Valiquette G., Guyda H. J., Corvol M. T., Posner B. I. Partial purification and characterization of a binding protein for insulin-like activity (ILAs) in human amniotic fluid: a possible inhibitor of insulin-like activity. Acta Endocrinol (Copenh) 1979 Mar;90(3):505–518. doi: 10.1530/acta.0.0900505. [DOI] [PubMed] [Google Scholar]
- Knauer D. J., Smith G. L. Inhibition of biological activity of multiplication-stimulating activity by binding to its carrier protein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7252–7256. doi: 10.1073/pnas.77.12.7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Proper J. A., Goustin A. S., Shipley G. D., DiCorleto P. E., Moses H. L. Induction of c-sis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta: a proposed model for indirect mitogenesis involving autocrine activity. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2453–2457. doi: 10.1073/pnas.83.8.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Baxter R. C. Insulin-like growth factor-binding protein from human plasma. Purification and characterization. J Biol Chem. 1986 Jul 5;261(19):8754–8760. [PubMed] [Google Scholar]
- Merimee T. J., Zapf J., Froesch E. R. Dwarfism in the pygmy. An isolated deficiency of insulin-like growth factor I. N Engl J Med. 1981 Oct 22;305(17):965–968. doi: 10.1056/NEJM198110223051701. [DOI] [PubMed] [Google Scholar]
- Moses A. C., Nissley S. P., Passamani J., White R. M. Further characterization of growth hormone-dependent somatomedin-binding proteins in rat serum and demonstration of somatomedin-binding proteins produced by rat liver cells in culture. Endocrinology. 1979 Feb;104(2):536–546. doi: 10.1210/endo-104-2-536. [DOI] [PubMed] [Google Scholar]
- Mottola C., MacDonald R. G., Brackett J. L., Mole J. E., Anderson J. K., Czech M. P. Purification and amino-terminal sequence of an insulin-like growth factor-binding protein secreted by rat liver BRL-3A cells. J Biol Chem. 1986 Aug 25;261(24):11180–11188. [PubMed] [Google Scholar]
- Pantazis N. J., Blanchard M. H., Arnason B. G., Young M. Molecular properties of the nerve growth factor secreted by L cells. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1492–1496. doi: 10.1073/pnas.74.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Póvoa G., Enberg G., Jörnvall H., Hall K. Isolation and characterization of a somatomedin-binding protein from mid-term human amniotic fluid. Eur J Biochem. 1984 Oct 15;144(2):199–204. doi: 10.1111/j.1432-1033.1984.tb08449.x. [DOI] [PubMed] [Google Scholar]
- Rutanen E. M., Koistinen R., Sjöberg J., Julkunen M., Wahlström T., Bohn H., Seppälä M. Synthesis of placental protein 12 by human endometrium. Endocrinology. 1986 Mar;118(3):1067–1071. doi: 10.1210/endo-118-3-1067. [DOI] [PubMed] [Google Scholar]
- Scott C. D., Martin J. L., Baxter R. C. Rat hepatocyte insulin-like growth factor I and binding protein: effect of growth hormone in vitro and in vivo. Endocrinology. 1985 Mar;116(3):1102–1107. doi: 10.1210/endo-116-3-1102. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Mitchell W. M., Cohen S. Characterization of the high molecular weight form of epidermal growth factor. J Biol Chem. 1974 May 25;249(10):3198–3203. [PubMed] [Google Scholar]
- Waziri M., Patil S. R., Hanson J. W., Bartley J. A. Abnormality of chromosome 11 in patients with features of Beckwith-Wiedemann syndrome. J Pediatr. 1983 Jun;102(6):873–876. doi: 10.1016/s0022-3476(83)80014-6. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schoenle E., Jagars G., Sand I., Grunwald J., Froesch E. R. Inhibition of the action of nonsuppressible insulin-like activity on isolated rat fat cells by binding to its carrier protein. J Clin Invest. 1979 May;63(5):1077–1084. doi: 10.1172/JCI109377. [DOI] [PMC free article] [PubMed] [Google Scholar]



