Abstract
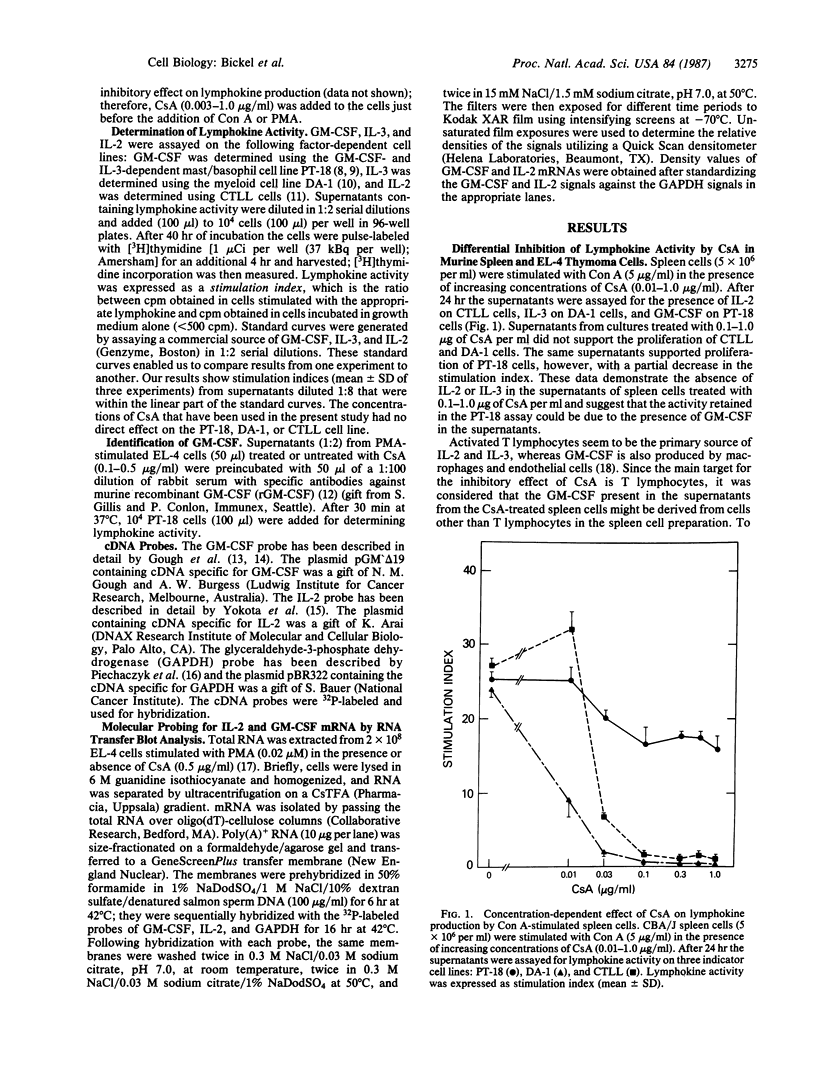
Stimulation of T lymphocytes with mitogens or antigens is followed by proliferation and lymphokine production. Although cyclosporin A (CsA), an immunosuppressive drug, has been shown to inhibit the production of certain lymphokines, including interleukin 2 (IL-2), interleukin 3 (IL-3), and gamma-interferon, its effect on the production of granulocyte/macrophage colony-stimulating factor (GM-CSF) has not been evaluated. In the current study, concanavalin A (Con A)-stimulated murine spleen cells secreted GM-CSF, IL-3, and IL-2, and in the presence of CsA (0.1-1.0 micrograms/ml), IL-2 and IL-3 activities were inhibited. In contrast, significant activity was detected when the CsA-treated culture supernatants were assayed on a cell line that is dependent on GM-CSF and/or IL-3. Similar CsA-resistant activity was observed when the EL-4 thymoma cells were stimulated with a phorbol ester [phorbol 12-myristate 13-acetate (PMA)] in the presence of CsA. The activity resistant to CsA was identified as GM-CSF by the ability of specific antibodies against murine recombinant GM-CSF to neutralize its activity. These findings indicate that GM-CSF, in contrast to IL-2 and IL-3, was not inhibited by CsA. In additional experiments, transfer blot of poly(A)+ RNA isolated from PMA-induced EL-4 cells in the presence or the absence of CsA was hybridized with GM-CSF and IL-2 cDNA probes. Expression of the GM-CSF gene in EL-4 cells was detected independent of CsA, whereas CsA inhibited the expression of the IL-2 gene. The present data show that production of IL-2 and IL-3, but not that of GM-CSF, is inhibited by CsA and suggest a differential control mechanism for lymphokine synthesis in T lymphocytes.
Full text
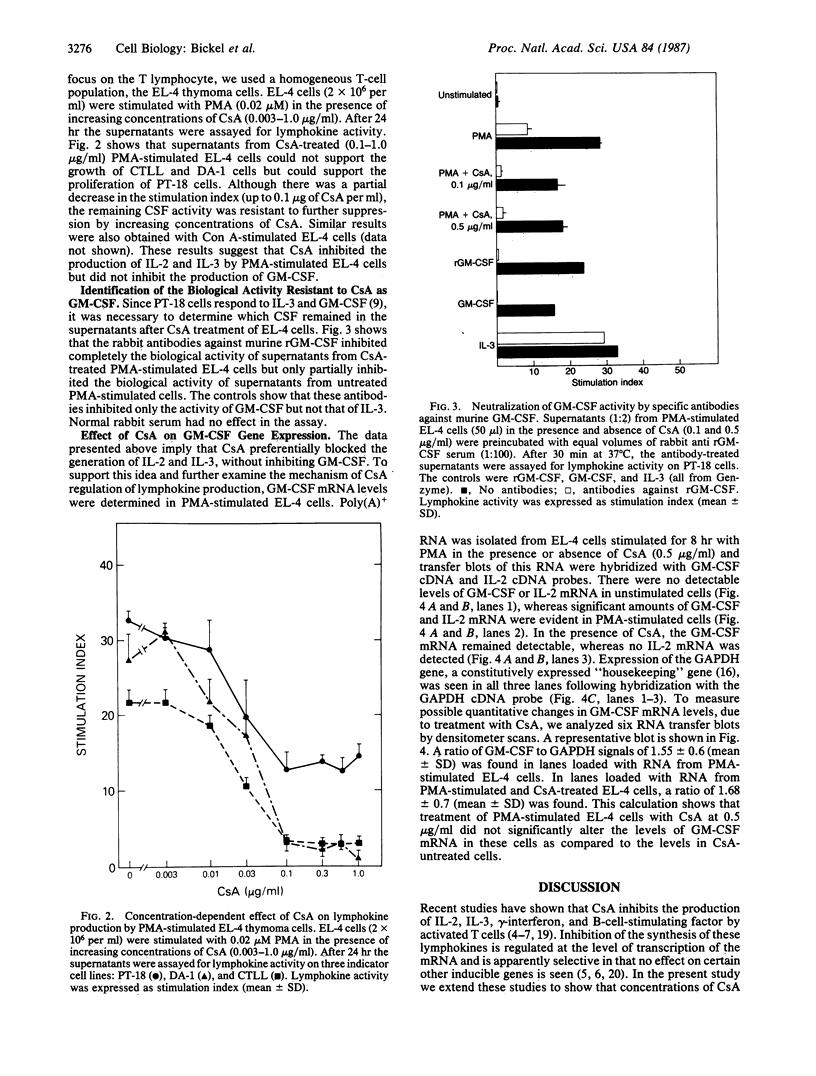
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. E., Gillis S., Smith K. A. Monoclonal cytolytic T-cell lines. J Exp Med. 1979 Jan 1;149(1):273–278. doi: 10.1084/jem.149.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Elliott J. F., Lin Y., Mizel S. B., Bleackley R. C., Harnish D. G., Paetkau V. Induction of interleukin 2 messenger RNA inhibited by cyclosporin A. Science. 1984 Dec 21;226(4681):1439–1441. doi: 10.1126/science.6334364. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Metcalf D., Gough J., Grail D., Dunn A. R. Structure and expression of the mRNA for murine granulocyte-macrophage colony stimulating factor. EMBO J. 1985 Mar;4(3):645–653. doi: 10.1002/j.1460-2075.1985.tb03678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Andrus L., Steinman R. M. Lymphokine and nonlymphokine mRNA levels in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1986 Apr 1;163(4):922–937. doi: 10.1084/jem.163.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli-Piperno A., Inaba K., Steinman R. M. Stimulation of lymphokine release from T lymphoblasts. Requirement for mRNA synthesis and inhibition by cyclosporin A. J Exp Med. 1984 Dec 1;160(6):1792–1802. doi: 10.1084/jem.160.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L. Secretion of interleukin 2, macrophage-activating factor, interferon, and colony-stimulating factor by alloreactive T lymphocyte clones. J Immunol. 1984 Jun;132(6):2924–2931. [PubMed] [Google Scholar]
- Kelso A., Metcalf D., Gough N. M. Independent regulation of granulocyte-macrophage colony-stimulating factor and multi-lineage colony-stimulating factor production in T lymphocyte clones. J Immunol. 1986 Mar 1;136(5):1718–1725. [PubMed] [Google Scholar]
- Krönke M., Leonard W. J., Depper J. M., Arya S. K., Wong-Staal F., Gallo R. C., Waldmann T. A., Greene W. C. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Park L. S., Urdal D. L. Development and characterization of antiserum to murine granulocyte-macrophage colony-stimulating factor. J Immunol. 1986 May 15;136(10):3706–3709. [PubMed] [Google Scholar]
- Orosz C. G., Roopenian D. C., Widmer M. B., Bach F. H. Analysis of cloned T cell function. II. Differential blockade of various cloned T cell functions by cyclosporine. Transplantation. 1983 Dec;36(6):706–711. doi: 10.1097/00007890-198336060-00024. [DOI] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Todd I., Brown M., Rittenberg M. B. Immunologic memory to phosphorylcholine. VI. Heterogeneity in light chain gene expression. Eur J Immunol. 1985 Feb;15(2):177–183. doi: 10.1002/eji.1830150213. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Biology and biochemistry of T cell-derived lymphokines. I. The coordinate synthesis of interleukin 2 and colony-stimulating factors in a murine T cell lymphoma. J Immunol. 1983 Jul;131(1):293–297. [PubMed] [Google Scholar]
- Yokota T., Arai N., Lee F., Rennick D., Mosmann T., Arai K. Use of a cDNA expression vector for isolation of mouse interleukin 2 cDNA clones: expression of T-cell growth-factor activity after transfection of monkey cells. Proc Natl Acad Sci U S A. 1985 Jan;82(1):68–72. doi: 10.1073/pnas.82.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ythier A. A., Abbud-Filho M., Williams J. M., Loertscher R., Schuster M. W., Nowill A., Hansen J. A., Maltezos D., Strom T. B. Interleukin 2-dependent release of interleukin 3 activity by T4+ human T-cell clones. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7020–7024. doi: 10.1073/pnas.82.20.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]





