Abstract
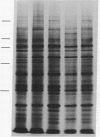
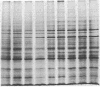
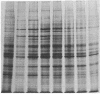
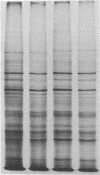
The glucose-regulated protein (GRP) system in mammalian cells is induced by glucose deprivation, anoxia, the calcium ionophore A23187, and 2-deoxyglucose. In Chinese hamster ovary cells the major GRPs are approximately equal to 76, 97, and 170 kDa. Removal of each of these four GRP-inducing stresses leads to the coordinate repression of GRPs and induction of the major heat shock proteins at 70 and 89 kDa. The application of each of these four GRP-inducing conditions leads to a significant induction of resistance to the drug doxorubicin. Removal of each GRP-inducing condition results in the rapid disappearance of this resistance in a manner that correlates with the repression of the GRPs. The retention of doxorubicin by GRP-induced cells does not explain the induced drug resistance. When the RIF in vitro/in vivo tumor system is probed with an antibody against the 76-kDa GRP, a significant increase in this GRP is observed in cells obtained from the central regions of tumors. Since hypoxia and/or nutrient deprivation can occur during tumor development, a GRP-induced state in the tumor may confer resistance to doxorubicin treatment.
Full text
PDF

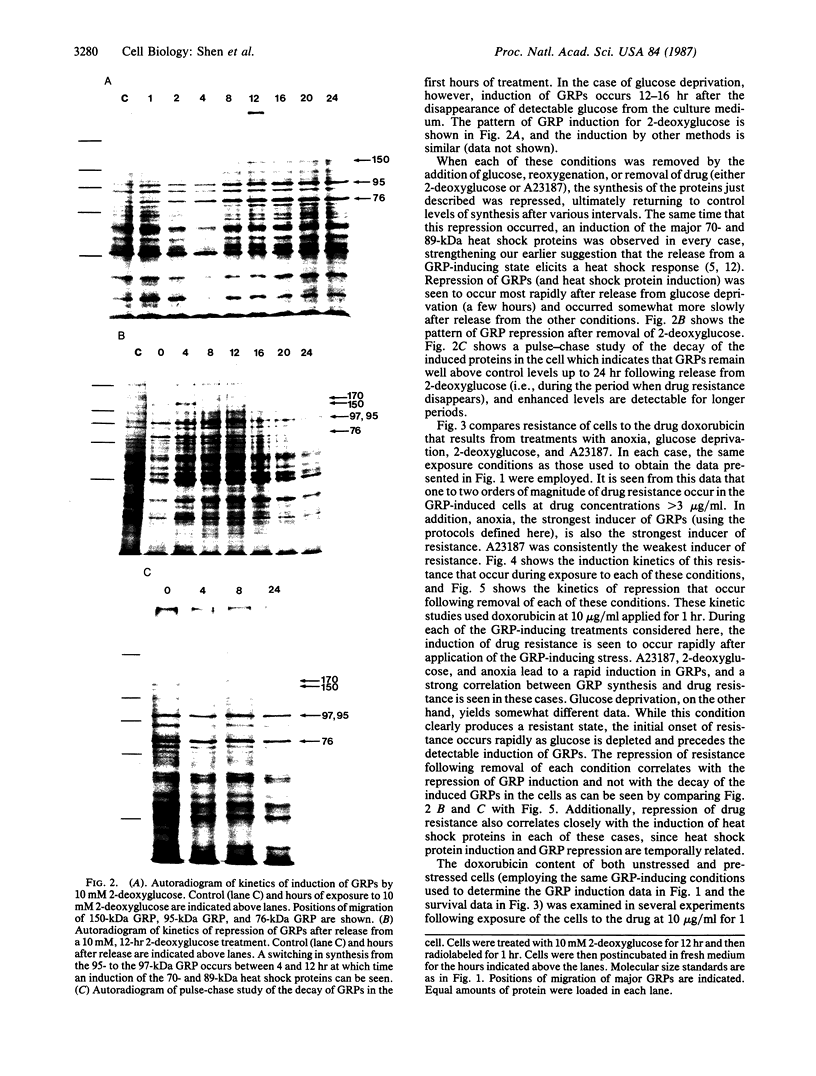
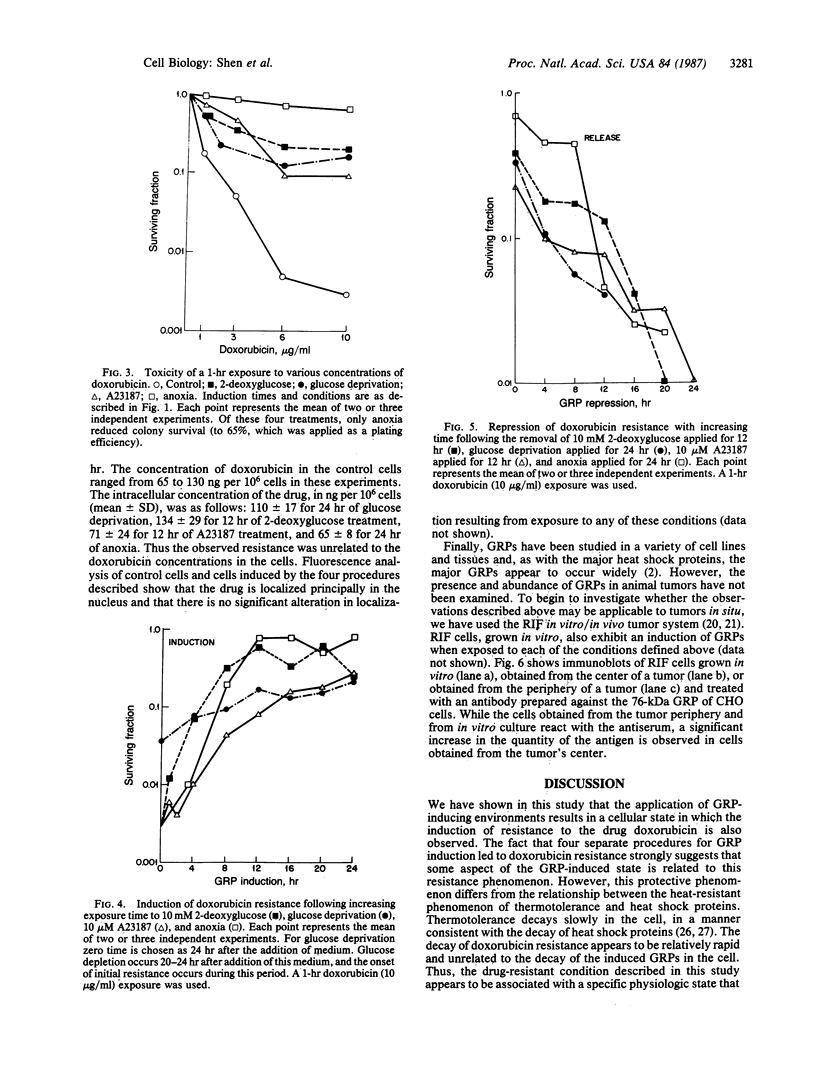

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolanowska W., Gessner T., Preisler H. A simplified method for determination of daunorubicin, adriamycin, and their chief fluorescent metabolites in human plasma by high-pressure liquid chromatography. Cancer Chemother Pharmacol. 1983;10(3):187–191. doi: 10.1007/BF00255759. [DOI] [PubMed] [Google Scholar]
- Born R., Eichholtz-Wirth H. Effect of different physiological conditions on the action of adriamycin on Chinese hamster cells in vitro. Br J Cancer. 1981 Aug;44(2):241–246. doi: 10.1038/bjc.1981.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. M., Twentyman P. R., Zamvil S. S. Response to the RIF-1 tumor in vitro and in C3H/Km mice to X-radiation (cell survival, regrowth delay, and tumor control), chemotherapeutic agents, and activated macrophages. J Natl Cancer Inst. 1980 Mar;64(3):605–611. [PubMed] [Google Scholar]
- Colofiore J. R., Ara G., Berry D., Belli J. A. Enhanced survival of adriamycin-treated Chinese hamster cells by 2-deoxy-D-glucose and 2,4-dinitrophenol. Cancer Res. 1982 Oct;42(10):3934–3940. [PubMed] [Google Scholar]
- Freyer J. P., Sutherland R. M. Proliferative and clonogenic heterogeneity of cells from EMT6/Ro multicellular spheroids induced by the glucose and oxygen supply. Cancer Res. 1986 Jul;46(7):3513–3520. [PubMed] [Google Scholar]
- Gerweck L. E., Epstein L. F. Cell proliferation, protein turnover, and the decay of thermotolerance in CHO cells. Radiat Res. 1986 Jun;106(3):311–320. [PubMed] [Google Scholar]
- Kessel D. Enhanced glycosylation induced by adriamycin. Mol Pharmacol. 1979 Jul;16(1):306–312. [PubMed] [Google Scholar]
- Krishan A., Sauerteig A., Wellham L. L. Flow cytometric studies on modulation of cellular adriamycin retention by phenothiazines. Cancer Res. 1985 Mar;45(3):1046–1051. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Landry J., Chrétien P., Bernier D., Nicole L. M., Marceau N., Tanguay R. M. Thermotolerance and heat shock proteins induced by hyperthermia in rat liver cells. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):59–62. doi: 10.1016/0360-3016(82)90385-6. [DOI] [PubMed] [Google Scholar]
- Laszlo A., Li G. C. Heat-resistant variants of Chinese hamster fibroblasts altered in expression of heat shock protein. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8029–8033. doi: 10.1073/pnas.82.23.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Petersen N. S., Mitchell H. K. Induced thermal tolerance and heat shock protein synthesis in Chinese hamster ovary cells. Int J Radiat Oncol Biol Phys. 1982 Jan;8(1):63–67. doi: 10.1016/0360-3016(82)90386-8. [DOI] [PubMed] [Google Scholar]
- Ling V., Gerlach J., Kartner N. Multidrug resistance. Breast Cancer Res Treat. 1984;4(2):89–94. doi: 10.1007/BF01806390. [DOI] [PubMed] [Google Scholar]
- Martin W. M., McNally N. J. Cytotoxicity of adriamycin to tumour cells in vivo and in vitro. Br J Cancer. 1980 Dec;42(6):881–889. doi: 10.1038/bjc.1980.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Infection with paramyxoviruses stimulates synthesis of cellular polypeptides that are also stimulated in cells transformed by Rous sarcoma virus or deprived of glucose. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6120–6124. doi: 10.1073/pnas.75.12.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps D. S. Electrophoretic transfer of proteins from fixed and stained gels. Anal Biochem. 1984 Sep;141(2):409–412. doi: 10.1016/0003-2697(84)90062-9. [DOI] [PubMed] [Google Scholar]
- Pouysségur J., Shiu R. P., Pastan I. Induction of two transformation-sensitive membrane polypeptides in normal fibroblasts by a block in glycoprotein synthesis or glucose deprivation. Cell. 1977 Aug;11(4):941–947. doi: 10.1016/0092-8674(77)90305-1. [DOI] [PubMed] [Google Scholar]
- Rubin P., Casarett G. Microcirculation of tumors. I. Anatomy, function, and necrosis. Clin Radiol. 1966 Jul;17(3):220–229. doi: 10.1016/s0009-9260(66)80027-2. [DOI] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R., Hughes C. S. Induction of glucose-regulated proteins during anaerobic exposure and of heat-shock proteins after reoxygenation. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4843–4847. doi: 10.1073/pnas.81.15.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciandra J. J., Subjeck J. R. The effects of glucose on protein synthesis and thermosensitivity in Chinese hamster ovary cells. J Biol Chem. 1983 Oct 25;258(20):12091–12093. [PubMed] [Google Scholar]
- Scotto K. W., Biedler J. L., Melera P. W. Amplification and expression of genes associated with multidrug resistance in mammalian cells. Science. 1986 May 9;232(4751):751–755. doi: 10.1126/science.2421411. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Pouyssegur J., Pastan I. Glucose depletion accounts for the induction of two transformation-sensitive membrane proteinsin Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3840–3844. doi: 10.1073/pnas.74.9.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirotnak F. M., Yang C. H., Mines L. S., Oribé E., Biedler J. L. Markedly altered membrane transport and intracellular binding of vincristine in multidrug-resistant Chinese hamster cells selected for resistance to vinca alkaloids. J Cell Physiol. 1986 Feb;126(2):266–274. doi: 10.1002/jcp.1041260217. [DOI] [PubMed] [Google Scholar]
- Smith E., Stratford I. J., Adams G. E. Cytotoxicity of adriamycin on aerobic and hypoxic chinese hamster V79 cells in vitro. Br J Cancer. 1980 Oct;42(4):568–573. doi: 10.1038/bjc.1980.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subjeck J. R., Sciandra J. J., Johnson R. J. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982 Aug;55(656):579–584. doi: 10.1259/0007-1285-55-656-579. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T., Shen J., Johnson R. J. Association between the mammalian 110,000-dalton heat-shock protein and nucleoli. J Cell Biol. 1983 Nov;97(5 Pt 1):1389–1395. doi: 10.1083/jcb.97.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasovic S. P., Steck P. A., Heitzman D. Heat-stress proteins and thermal resistance in rat mammary tumor cells. Radiat Res. 1983 Aug;95(2):399–413. [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Welch W. J., Garrels J. I., Thomas G. P., Lin J. J., Feramisco J. R. Biochemical characterization of the mammalian stress proteins and identification of two stress proteins as glucose- and Ca2+-ionophore-regulated proteins. J Biol Chem. 1983 Jun 10;258(11):7102–7111. [PubMed] [Google Scholar]