Abstract
Iron is an essential but potentially hazardous biometal. Mammalian cells require sufficient amounts of iron to satisfy metabolic needs or to accomplish specialized functions. Iron is delivered to tissues by circulating transferrin, a transporter that captures iron released into the plasma mainly from intestinal enterocytes or reticuloendothelial macrophages. The binding of iron-laden transferrin to the cell-surface transferrin receptor 1 results in endocytosis and uptake of the metal cargo. Internalized iron is transported to mitochondria for the synthesis of haem or iron–sulfur clusters, which are integral parts of several metalloproteins, and excess iron is stored and detoxified in cytosolic ferritin. Iron metabolism is controlled at different levels and by diverse mechanisms. The present review summarizes basic concepts of iron transport, use and storage and focuses on the IRE (iron-responsive element)/IRP (iron-regulatory protein) system, a well known post-transcriptional regulatory circuit that not only maintains iron homoeostasis in various cell types, but also contributes to systemic iron balance.
Keywords: ferritin, ferroportin, iron-regulatory protein 1 (IRP1), iron-regulatory protein 2 (IRP2), iron–sulfur cluster (ISC), transferrin receptor (TfR)
Abbreviations: Abcb, ATP-binding cassette, subfamily B; ALA, 5-aminolevulinic acid; ALAS, ALA synthase; β-APP, β-amyloid precursor protein; BMP, bone morphogenetic protein; c-aconitase, cytosolic aconitase; C/EBPα, CCAAT/enhancer-binding protein α; Cfd1, cytosolic Fe–S cluster-deficient protein 1; Cul1, Cullin 1; Dcytb, duodenal cytochrome b; DHBA, dihydroxybenzoic acid; DMT1, divalent metal transporter 1; ER, endoplasmic reticulum; FBXL5, F-box and leucine-rich repeat protein 5; FLVCR, feline leukaemia virus, subgroup C, receptor; Grx, glutaredoxin; H, heavy; HIF, hypoxia-inducible factor; HO-1, haem oxygenase 1; Hpx, haemopexin; IOP1, iron-only hydrogenase-like protein 1; IRE, iron-responsive element; IRIDA, iron-refractory iron deficiency anaemia; IRP, iron-regulatory protein; ISC, iron–sulfur cluster; CIA, cytosolic ISC assembly; Isu, iron–sulfur cluster scaffold homologue; L, light; Lcn2, lipocalin 2; LIP, labile iron pool; m-aconitase, mitochondrial aconitase; MRCKα, myotonic dystrophy kinase-related Cdc42 (cell division cycle 42)-binding kinase α; Nar1, nuclear architecture-related protein 1; Nbp35, nucleotide-binding protein 35; Nfs, nitrogen fixation homologue; NTBI, non-transferrin-bound iron; PCBP1, poly(rC)-binding protein 1; Rbx1, Ring-box 1; ROS, reactive oxygen species; SDH, succinate dehydrogenase; Skp1, S-phase kinase-associated protein 1; SLC, solute carrier; STAT3, signal transducer and activator of transcription 3; Tf, transferrin; TfR, Tf receptor; UTR, untranslated region
BIOCHEMISTRY AND PHYSIOLOGY OF IRON
With minor exceptions, almost all cells employ iron as a cofactor for fundamental biochemical activities, such as oxygen transport, energy metabolism and DNA synthesis. This is due to the flexible coordination chemistry and redox reactivity of iron, which allow it to associate with proteins and bind to oxygen, transfer electrons or mediate catalytic reactions [1]. However, iron is also potentially toxic, because, under aerobic conditions, it catalyses the propagation of ROS (reactive oxygen species) and the generation of highly reactive radicals (such as the hydroxyl radical) through Fenton chemistry [2]. As iron readily shuttles between the reduced ferrous (Fe2+) and the oxidized ferric (Fe3+) forms, disruption of the cellular redox equilibrium requires only catalytic amounts of the metal. The ensuing oxidative stress is associated with damage of cellular macromolecules, tissue injury and disease [3,4]. It should also be noted that oxidized Fe3+ is poorly bioavailable, despite its high abundance, due to limited solubility. Thus the acquisition, usage and detoxification of iron pose a considerable challenge to cells and organisms, which have evolved sophisticated mechanisms to satisfy their metabolic needs and concomitantly minimize the risk of toxicity [5–7].
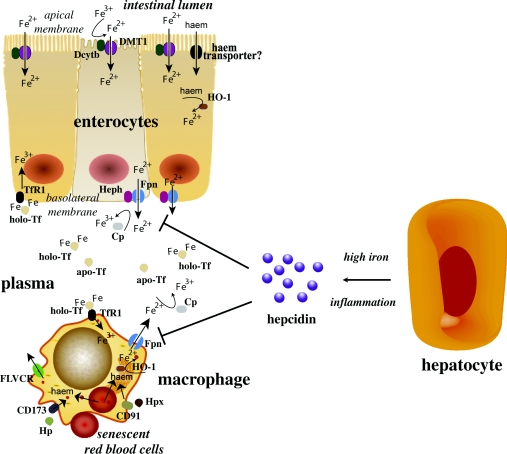
The vast majority of body iron (at least 2.1 g in humans) is distributed in the haemoglobin of red blood cells and developing erythroid cells and serves in oxygen transport. Significant amounts of iron are also present in macrophages (up to 600 mg) and in the myoglobin of muscles (~300 mg), whereas excess body iron (~ 1 g) is stored in the liver [8,9]. Other tissues contain lower, but not negligible, quantities of iron. Mammals lose iron from sloughing of mucosal and skin cells or during bleeding, but do not possess any regulated mechanism for iron excretion from the body. Therefore balance is maintained by the tight control of dietary-iron absorption in the duodenum (Figure 1). The uptake of nutritional iron involves reduction of Fe3+ in the intestinal lumen by ferric reductases [such as Dcytb (duodenal cytochrome b)] and the subsequent transport of Fe2+ across the apical membrane of enterocytes by DMT1 (divalent metal transporter 1), a member of the SLC (solute carrier) group of membrane transport proteins, also known as SLC11A2. Dietary haem can also be transported across the apical membrane by a yet unknown mechanism and subsequently metabolized in the enterocytes by HO-1 (haem oxygenase 1) to liberate Fe2+. Directly internalized or haem-derived Fe2+ is processed by the enterocytes and eventually exported across the basolateral membrane into the bloodstream via the solute carrier and Fe2+ transporter ferroportin (also known as SLC11A3). The ferroportin-mediated efflux of Fe2+ is coupled by its re-oxidation to Fe3+, catalysed by the membrane-bound ferroxidase hephaestin that physically interacts with ferroportin [10], and possibly also by its plasma homologue ceruloplasmin.
Figure 1. Hormonal regulation of iron efflux from duodenal enterocytes and reticuloendothelial macrophages by hepcidin.
Enterocytes absorb inorganic or haem iron from the diet and macrophages phagocytose iron-loaded senescent red blood cells, or acquire iron by other mechanisms (see the main text). Both cell types release Fe2+ into the plasma via ferroportin (Fpn), which is incorporated into apo-Tf following oxidation to Fe3+ via hephaestin (Heph) or ceruloplasmin (Cp). Hepatocytes generate the iron-regulatory hormone hepcidin in response to high iron or inflammatory signals, which inhibits the efflux of iron via ferroportin and promotes its retention within enterocytes and macrophages.
Exported iron is scavenged by Tf (transferrin), which maintains Fe3+ in a redox-inert state and delivers it into tissues. The total iron content of Tf (~3 mg) corresponds to less than 0.1% of body iron, but it is highly dynamic and undergoes more than ten times daily turnover to sustain erythropoiesis. The Tf iron pool is replenished mostly by iron recycled from effete red blood cells and, to a lesser extent, by newly absorbed dietary iron. Senescent red blood cells are cleared by reticuloendothelial macrophages, which metabolize haemoglobin and haem, and release iron into the bloodstream. By analogy to intestinal enterocytes, macrophages export Fe2+ from their plasma membrane via ferroportin, in a process coupled by re-oxidation of Fe2+ to Fe3+ by ceruloplasmin and followed by the loading of Fe3+ to Tf (Figure 1).
Ferroportin is expressed in many iron-exporting cells, including placental syncytiotrophoblasts, and plays a fundamental role in the release of iron from tissues into the bloodstream, but also in maternal iron transfer to the fetus. The complete disruption of mouse ferroportin is embryonic lethal, whereas its conditional inactivation leads to iron retention and accumulation in enterocytes, macrophages and hepatocytes [11].
REGULATION OF SYSTEMIC IRON TRAFFIC
The ferroportin-mediated efflux of Fe2+ from enterocytes and macrophages into the plasma is critical for systemic iron homoeostasis. This process is negatively regulated by hepcidin, a liver-derived peptide hormone that binds to ferroportin and promotes its phosphorylation, internalization and lysosomal degradation [6,12]. Hepcidin is primarily expressed in hepatocytes as a precursor pro-peptide. Pro-hepcidin undergoes proteolytic processing to yield a bioactive molecule of 25 amino acids that is secreted into the bloodstream. Hepcidin accumulates following iron intake and under inflammatory conditions, resulting in decreased dietary-iron absorption and iron retention in macrophages (Figure 1); during infection, this very probably serves to deprive invading bacteria from iron that is essential for growth. Conversely, hepcidin levels drop in iron deficiency, hypoxia or phlebotomy-induced anaemia, and this response promotes intestinal iron absorption and iron release from macrophages. The disruption of hepcidin is associated with systemic iron overload (haemochromatosis) [13], whereas pathological elevation of hepcidin levels contributes to the development of the anaemia of chronic disease [14] and accounts for the phenotype of hereditary IRIDA (iron-refractory iron deficiency anaemia) [15].
The expression of hepcidin is controlled transcriptionally. Basal hepcidin transcription requires C/EBPα (CCAAT/enhancer-binding protein α) [16]. Iron-dependent induction of hepcidin requires BMP (bone morphogenetic protein) signalling. Iron triggers the expression of BMP6 in the liver [17] and the intestine [18], which is thought to be secreted into the plasma for binding to a BMP receptor on the surface of hepatocytes. BMP6 signalling leads to phosphorylation of SMAD1/5/8 and translocation of SMAD4 to the nucleus, where it promotes hepcidin transcription upon binding to proximal and distal sites on its promoter. This model is supported by genetic data with BMP6−/− [19,20] and liver-specific SMAD4−/− [21] mice, which develop iron overload and express inappropriately low hepcidin levels. It has also been proposed that hepcidin responds to increased Tf saturation [22], possibly by a mechanism requiring a cross-talk between BMP and MAP (mitogen-activated protein kinase) signalling [23]. Further cofactors are required for iron-dependent activation of hepcidin, even though their exact mode of action is not yet clear. These include the haemochromatosis protein HFE, TfR2 (Tf receptor 2) and the BMP co-receptor HJV (haemojuvelin). Mutations in these proteins impair hepcidin expression and lead to hereditary haemochromatosis [13]. The membrane-bound serine protease matriptase 2 (TMPRSS6) appears to inhibit signalling to hepcidin by degrading HJV [24]. Mutations in matriptase 2 are associated with IRIDA, which is caused by hepcidin overexpression [15].
The pro-inflammatory cytokine IL-6 (interleukin-6) induces hepcidin transcription via STAT3 (signal transducer and activator of transcription 3) phosphorylation and translocation to the nucleus for binding to a proximal promoter element [25]. IL-1β activates hepcidin via the C/EBPα and BMP/SMAD pathways [26]. In addition, ER (endoplasmic reticulum) stress turns on hepcidin transcription via CREBH (cyclic AMP response element-binding protein H) [27] and/or CHOP (C/EBP homologous protein) [28]. Lipopolysaccharide promotes autocrine activation of hepcidin in macrophages via TLR4 (Toll-like receptor 4) signalling [29], whereas the pathogen Borrelia burgdorferi activates myeloid hepcidin via TLR2 [30].
Hepcidin transcription is suppressed during anaemia by a mechanism that requires erythropoietic activity [31]. In thalassaemic patients, hepcidin expression is blocked upon induction of GDF15 (growth differentiation factor 15) [32], a member of the TGF-β (transforming growth factor β) superfamily. Likewise, the erythroid regulator TWSG1 (twisted gastrulation homologue 1) was reported to contribute to hepcidin suppression in cell-culture experiments and in thalassaemic mice [33]. EPO (erythropoietin) signalling downregulates hepcidin following decreased binding of C/EBPα to its promoter [34]. Hepcidin transcription is also suppressed by hypoxia and oxidative stress. The role of HIFs (hypoxia-inducible factors) in the hypoxic pathway of hepcidin is debatable [35], whereas oxidative stress promotes histone deacetylation and decreased binding of C/EBPα and STAT3 to the hepcidin promoter [36].
There is no doubt that hormonal regulation of iron efflux from cells via the hepcidin/ferroportin axis is of paramount importance for systemic iron homoeostasis. However, it should be noted that the expression of ferroportin is also subjected to transcriptional [37] and post-transcriptional (see below) control. Recent results showed that hepcidin-dependent degradation of ferroportin does not suffice to limit dietary-iron absorption in mice lacking intestinal H-ferritin, adding further complexity to the mechanisms for regulation of systemic iron homoeostasis [38]. Along similar lines, dietary-iron absorption can be induced independently of the hepcidin pathway by transcriptional activation of DMT1 and Dcytb in duodenal enterocytes, a response orchestrated by HIF-2α [39,40].
MECHANISMS FOR CELLULAR IRON UPTAKE
The Tf cycle
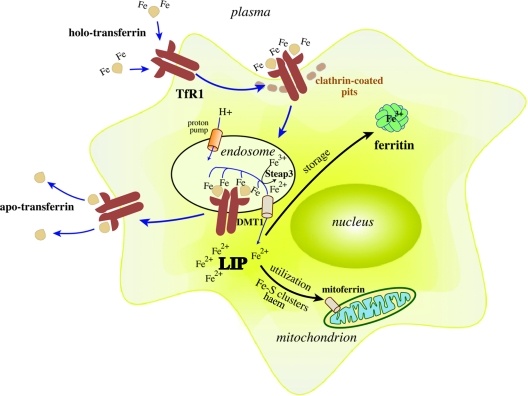
Developing erythroid cells, as well as most other cell types, acquire iron from plasma Tf. Iron-loaded holo-Tf binds with high affinity to TfR1 on the surface of cells [41], and the complex undergoes endocytosis via clathrin-coated pits (Figure 2). A proton pump promotes acidification of the endosome to pH 5.5, triggering the release of Fe3+ from Tf that remains bound to TfR1. The ferrireductase Steap3 reduces Fe3+ to Fe2+ [42], which is transported across the endosomal membrane by DMT1 to the cytosol or, possibly, directly to mitochondria in erythroid cells [43]. Following the release of iron, the affinity of Tf to TfR1 drops ~500-fold, resulting in its dissociation. In the final step of the cycle, apo-Tf is secreted into the bloodstream to recapture Fe3+.
Figure 2. Cellular iron uptake via the Tf cycle.
Iron-loaded holo-Tf binds to TfR1 on the cell surface and the complex undergoes endocytosis via clathrin-coated pits. A proton pump acidifies the endosome, resulting in the release of Fe3+, which is subsequently reduced to Fe2+ by Steap3 and transported across the endosomal membrane to the cytosol by DMT1. Internalized iron is directed to mitochondria via mitoferrin for metabolic utilization (such as synthesis of haem and ISCs), and excess iron is stored in ferritin. A cytosolic fraction of redox-active intracellular iron constitutes the LIP. The apo-Tf–TfR1 complex is recycled to the cell surface, where apo-Tf is released to capture plasma Fe3+.
The importance of the Tf cycle for iron delivery into erythroid cells is underscored by the embryonic lethal erythropoietic defects associated with the targeted disruption of mouse TfR1, and the microcytic hypochromic anaemia of TfR1+/− animals [44]. Moreover, hpx (hypotransferrinaemic) mice that fail to express appropriate Tf levels due to a splicing defect also develop microcytic hypochromic anaemia, which is associated with paradoxical hepatic iron overload [45]. These findings provide strong evidence that the Tf cycle is indispensable for iron delivery to erythroid cells, and Tf is the only physiological iron donor for erythropoiesis.
DMT1 does not only account for dietary-iron absorption in the intestine, but also constitutes a crucial component of the Tf cycle by mediating Fe2+ transport across the endosomal membrane. Thus DMT1−/− mice can neither absorb dietary iron, nor efficiently use iron for erythropoiesis and, consequently, develop severe and fatal microcytic hypochromic anaemia shortly after birth [46]. Animals with partially inactivated mutant DMT1 (mk/mk mice and Belgrade rats) exhibit severe anaemia [47,48].
Other mechanisms for iron uptake
Macrophages may use the Tf cycle for iron uptake, especially in culture. However, in vivo, resident macrophages acquire high amounts of iron by phagocytosis of effete red blood cells (Figure 1). Macrophages also have the capacity to clear haemoglobin and haem that is released in the circulation during intravascular hemolysis. Free haemoglobin is scavenged by Hp (haptoglobin), a liver-derived plasma protein, which in turn is internalized by macrophages upon binding to the receptor CD163 [49]. Likewise, free haem is scavenged by plasma Hpx (haemopexin) and undergoes endocytosis in macrophages upon binding to the receptor CD91 [50]. Directly internalized haem, or haem derived from the degradation of haemoglobin following direct haemoglobin uptake or erythrophagocytosis, is degraded by HO-1, yielding Fe2+, which is released into the plasma through ferroportin. Nramp1 (natural resistance-associated macrophage protein 1), a homologue of DMT1, contributes to efficient recycling of haemoglobin iron, especially during haemolytic anaemia [51].
Plasma Tf delivers iron to all tissues, except those that are separated from the blood by layers of endothelial cells forming a physical barrier (for example the brain, the testis or the eye). How iron crosses the blood–brain and other barriers, which do not allow the free passage of proteins and metabolites, is incompletely understood. It appears that the endothelial cells at the luminal site of blood capillaries express TfR1 and take up iron from plasma Tf [52,53]. The release of iron from endothelial cells (but also neurons or astrocytes) into the brain interstitium very probably involves ferroportin in conjunction with a ferroxidase activity, by analogy to the mechanism for basolateral iron transport in the intestinal epithelium. The ferroxidase activity can be provided by ferroportin-interacting proteins such as hephaestin, GPI (glycosylphosphatidylinositol)–ceruloplasmin (a membrane-associated ceruloplasmin isoform) [54] or β-APP (β-amyloid precursor protein) [55]. Inside the interstitial fluid, iron is captured by brain Tf that is secreted from oligodendrocytes.
Previously, plasma Tf was believed to be spared from substantial glomerular filtration in the kidney [56]. Later, it was established that polarized epithelial cells from the renal proximal tubules may acquire Tf-bound iron via the endocytic receptor cubilin, which operates in conjunction with its co-receptor megalin [57]. Cubilin (but not TfR1) is expressed on the apical (urine-facing) site of proximal tubule cells, which also contain high levels of endosomal DMT1, suggesting that at least a fraction of Tf is filtered in the kidney and retrieved from the glomerular filtrate upon binding to cubilin [56]. It is conceivable that the efflux of iron from the basolateral site of the proximal tubule cells to the bloodstream may involve ferroportin and hephaestin/ceruloplasmin.
Lcn2 (lipocalin 2), a mammalian protein that was identified by its capacity to interact with the bacterial siderophore enterobactin [58], is involved in iron-uptake pathways that operate during kidney development [59] and injury [60], and under inflammatory conditions [61]. Iron-loaded Lcn2 is internalized by the receptors 24p3R [61] and megalin [62]. Siderophores are low-molecular-mass iron-chelating metabolites, known to be synthesized by bacteria and fungi to acquire extracellular iron. It was recently demonstrated that mammals synthesize the siderophore 2,5-DHBA (dihydroxybenzoic acid), which is isomeric to 2,3-DHBA, the iron-binding component of enterobactin [63]. Importantly, depletion of 2,5-DHBA deregulated iron metabolism in mammalian cells and zebrafish embryos, highlighting the biological importance of Lcn2-dependent mechanisms. Lcn2−/− mice exhibit apparently normal iron metabolism; however, they fail to mount efficient innate immune responses against bacterial infection, suggesting that Lcn2 serves to deprive bacteria of nutritional iron [64].
Under physiological conditions, plasma Tf is hyposaturated (to ~30%) and displays a very high iron-binding capacity, to preclude the accumulation of unshielded NTBI (non-transferrin-bound iron). However, in hereditary haemochromatosis and other iron-overload disorders, the levels of plasma iron exceed the saturation capacity of Tf, and a pool of NTBI builds up that contributes significantly to hepatic iron loading [65]. The exact chemical nature of NTBI remains elusive, but its redox reactivity and toxicity is well established. It may consist of Fe3+ loosely chelated by albumin or by small organic molecules, such as citrate [66]. The mechanisms of NTBI uptake by cells are poorly understood, but a role for Lcn2 has been excluded [67]. The zinc transporter Zip14 (also known as SLC39A) appears capable of mediating NTBI uptake [68] as well as promoting the assimilation of Tf-bound iron [69].
Ferritin, the iron-storage protein (see below) has also been implicated in iron-transport pathways, which may predominate in pathological states, where iron-rich intracellular ferritin is released from damaged tissues. The membrane-bound protein TIM-2 (T-cell immunoglobulin-domain and mucin-domain 2) functions as a ferritin receptor in rodents [70]. More recently, it was shown that ferritin can be taken up by Scara 5 (scavenger receptor 5) and donate iron to the developing kidney [71], whereas H-ferritin can be internalized by cells upon specific binding to TfR1 [72].
CELLULAR IRON METABOLISM
Iron usage in mitochondria
Cells mostly use iron in mitochondria for the synthesis of haem and ISCs (iron–sulfur clusters). The mechanism for intracellular iron trafficking to mitochondria is incompletely understood. Kinetic evidence and microscopy studies in erythroid cells have provided support for the ‘kiss and run’ hypothesis, which postulates the direct delivery of Tf-derived iron to mitochondria through a transient contact with the endosome [43]. In other cell types, it is generally accepted that iron acquired during the Tf cycle is first released into the cytosol and then targeted to the mitochondria. The conserved cytosolic glutaredoxins Grx3 and Grx4 appear to play an essential role in intracellular iron sensing and trafficking, as their depletion in yeast leads to impaired iron transport to mitochondria and defects in iron-dependent pathways [73]. It is also well established that the entry of iron into mitochondria requires the SLC transporter mitoferrin (also known as SLC25A37), which is localized to the inner mitochondrial membrane [74]. Mitoferrin-1 is highly enriched in erythroid cells and is stabilized during differentiation and following interaction with the erythroid-specific ABC transporter Abcb10 (ATP-binding cassette, subfamily B, member 10) [75], whereas mitoferrin-2 is ubiquitously expressed and its half-life is not regulated [76]. Both mitoferrin isoforms are homologous; however, they exhibit limited functional redundancy, as overexpression of mitoferrin-2 cannot support mitochondrial iron assimilation in erythroid cells deficient of mitoferrin-1. The lack of functional mitoferrin-1 in the frs (frascati) zebrafish mutant is associated with severe defects in erythropoiesis, haem synthesis and ISC biogenesis [74]. Interestingly, depletion of the mammalian siderophore 2,5-DHBA also leads to defects in mitochondrial iron assimilation and haem synthesis [63], underscoring a role for the Lcn2 pathway in iron delivery to mitochondria.
All organisms synthesize the tetrapyrrol porphyrin ring of haem from the universal precursor ALA (5-aminolevulinic acid) by a conserved eight-step enzymatic pathway [77–79]. Most eukaryotes (except plants), generate ALA in mitochondria by the condensation of succinyl-CoA and glycine, which is catalysed by ALAS (ALA synthase). Mammals express a housekeeping (ALAS1) and an erythroid-specific (ALAS2) isoform of this enzyme. The universal precursor ALA is exported to the cytosol and converted into the intermediate metabolites porphobilinogen, hydroxymethylbilane, uroporphyrinogen III and coproporphyrinogen III. The latter is oxidized to protoporphyrinogen IX and imported into the mitochondria, where it is further oxidized to protoporphyrin IX. In the final reaction of the haem biosynthetic pathway, ferrochelatase catalyses the insertion of Fe2+ into protoporphyrin IX. Newly synthesized haem is exported to the cytosol for incorporation into haemoproteins. The transport of haem and its metabolic intermediates across the mitochondrial membranes may involve members of the ABC family of transporters [77] and the SLC transporter SLC25A39 [80]. In non-erythroid cells, the rate-limiting step of the haem biosynthetic pathway is the synthesis of ALA. By contrast, the synthesis of the porphyrin ring in erythroid cells depends on the iron supply, which is rate-limiting [79].
The assembly and repair of ISCs is mediated by complex pathways. The functions of several proteins of these pathways have been defined, yet new players continue to emerge [81–83]. The mitochondrial proteins Isu1/2 (also known as ISCU) and Isa1/2 (also known as ISCA1/2) provide a scaffold for the early steps of ISC biogenesis. The cysteine desulfurase Nfs1 (nitrogen fixation homologue 1, also known as ISCS), in complex with ISD11 (iron–sulfur protein biogenesis, desulfurase-interacting protein 11), generates elemental sulfur, whereas the iron-binding protein frataxin appears to serve as an iron donor. The mitochondrial proteins Grx5 and the transporter Abcb7 participate in the maturation of ISCs; however, their mode of action is not clear. The biogenesis of cytosolic ISC proteins requires mitochondria-derived ISC precursors, which are processed by a dedicated CIA (cytosolic ISC assembly) machinery [82]; to date, various CIA components have been identified, such as the P-loop NTPase Cfd1 (cytosolic Fe–S cluster-deficient protein 1), Nar1 (nuclear architecture-related protein 1)/IOP1 (iron-only hydrogenase-like protein 1), Cia1, Dre2 and Tah18. Cfd1 interacts with Nbp35 (nucleotide-binding protein 35) and the complex has been proposed to function as a scaffold for cytosolic ISC assembly [84]. An alternative model postulates that the cytosol is equipped with a complete machinery for de novo ISC assembly, consisting of cytosolic orthologues of mitochondrial ISC assembly factors such as Isu/ISCU and Nfs1/ISCS [83]. Importantly, defects in ISC biogenesis are associated with deregulation of cytosolic and cellular iron metabolism [43,83].
Handling of excess iron
Cells may eliminate excess intracellular iron by secretion of Fe2+ via ferroportin or by secretion of haem through the putative haem exporter FLVCR (feline leukaemia virus, subgroup C, receptor) [85]. Cells can also store and detoxify excess intracellular iron in the cytosol within ferritin, a conserved protein consisting of 24 H (heavy) and L (light) subunits, encoded by distinct genes [86]. Ferritin assembles into a shell-like structure with a cavity of ~80 Å (1 Å=0.1 nm) that provides storage space for up to 4500 Fe3+ ions in form of ferric oxy-hydroxide phosphate. Iron may enter ferritin with the aid of PCBP1 [poly(rC)-binding protein 1], which appears to function as an iron chaperone [87]. The incorporation of iron into holo-ferritin also requires the ferroxidase activity of H-ferritin, whereas L-ferritin chains provide a nucleation centre. The levels of H-ferritin and L-ferritin differ among various tissues; the former is enriched in the heart and the latter in the liver. Intracellular iron deposits may also be detected within haemosiderin, a structure consisting of ferritin degradation products and iron oxide clusters.
Iron stored within ferritin is considered to be bioavailable and may be mobilized for metabolic purposes during its lysosomal turnover [88] and, possibly, also following dynamic structural rearrangements of the ferritin subunits [89]. However, ferritin failed to donate iron for endogenous haem synthesis in experiments with cultured macrophages [90]. The induction of ferroportin promotes mobilization and export of ferritin-derived iron, followed by mono-ubiquitination and degradation of apo-ferritin by the proteasome [91]. Thus ferritin can be degraded by two different pathways, the lysosomal and the proteasomal pathways, which appears to require prior depletion of its iron [92]. The iron-storage function of ferritin is crucial for health. Thus the ablation of the gene encoding H-ferritin leads to early embryonic lethality [93], whereas the conditional disruption of this gene promotes liver damage due to oxidative stress [94]. Moreover, the intestinal disruption of H-ferritin misregulates dietary-iron absorption [38]. On the other hand, mutations in the gene encoding L-ferritin are associated with neuroferritinopathy, a rare hereditary movement disorder with an autosomal dominant transmission pattern, characterized by accumulation of redox-active iron in the brain [95].
Mitochondria contain a nuclear-encoded ferritin isoform [96]. Mitochondrial ferritin derives from an unusual intronless gene and is synthesized in the cytosol as a precursor polypeptide that is targeted to mitochondria by an N-terminal leader sequence. The mature protein possesses ferroxidase activity and assembles into functional ferritin nanocages. Mitochondrial ferritin is normally expressed at low levels and does not appear to have any major function in normal mitochondrial iron utilization. Its expression, however, is significantly induced in iron-loaded ring erythroblasts (sideroblasts) of sideroblastic anaemia patients and may serve as a sink for iron deposition [97].
A secreted glycosylated isoform of predominantly L-ferritin circulates in the bloodstream. It contains very low amounts of iron, suggesting that it does not play an essential role in iron storage or traffic, but it is used as a clinical marker for body iron stores. Experiments in mice suggested that serum ferritin derives from splenic macrophages and kidney proximal tubule cells [98].
LIP (labile iron pool)
Various cell types contain a transient cytosolic pool of iron, presumably bound to low-molecular-mass intracellular chelates, such as citrate, various peptides, ATP, AMP or pyrophosphate. This LIP is redox-active and can be monitored in situ by using fluorescent sensors such as calcein or Phen Green SK [99,100]. Experiments with other fluorescent sensors that are selective for mitochondria also suggest the presence of chelatable iron in this organelle [101]. Ferritin and iron-chelating drugs protect cells against oxidative damage by reducing the cytosolic LIP in cultured cells [102] and in animals [103]. However, chronic overexpression of ferritin may lead to the opposite effect in mice, suggesting that iron sequestered in the ferritin shells may eventually become pro-oxidant [104]. The cytosolic LIP mirrors the cellular iron content and its fluctuations are considered to trigger homoeostatic adaptive responses.
THE IRE (IRON-RESPONSIVE ELEMENT)/IRP (IRON-REGULATORY PROTEIN 1) REGULATORY SYSTEM
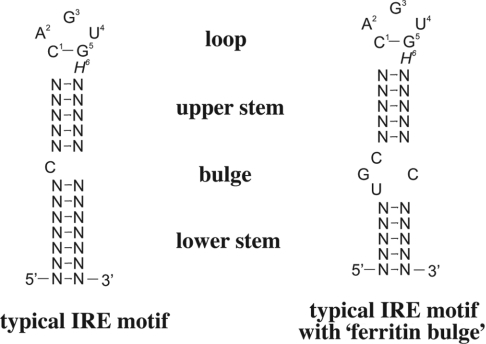
The expression of TfR1 and ferritin are co-ordinately regulated post-transcriptionally upon binding of IRP1 or IRP2 to IREs in the UTRs (untranslated regions) of their respective mRNAs [105–107]. IREs (Figure 3) are evolutionarily conserved hairpin structures of 25–30 nt [108]. A typical IRE stem consists of variable sequences that form base pairs of moderate stability (ΔG≈−7 kcal/mol), and folds into an α-helix that is slightly distorted by the presence of a small bulge in the middle (an unpaired C residue or an asymmetric UGC/C bulge/loop commonly found in the ferritin IRE). The loop contains a conserved 5′-CAGUGH-3′ sequence (H denotes A, C or U), where the underlined C and G residues form a base pair. TfR1 mRNA contains multiple IREs within its long 3′UTR, whereas the mRNAs encoding H- and L-ferritin contain a single IRE in their 5′UTRs.
Figure 3. Typical IRE motif.
A typical IRE motif consists of a hexanucleotide loop with the sequence 5′-CAGUGH-3′ (H could be A, C, or U) and a stem, interrupted by a bulge with an unpaired C residue (left) or an asymmetric tetranucleotide bulge (right); the latter is characteristic of ferritin IRE. Base-pairing between C1 and G5 of the loop is functionally important.
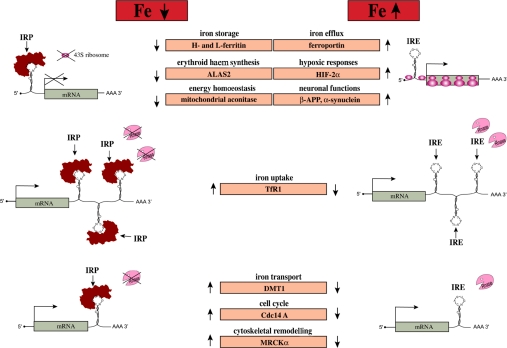
In iron-starved cells, IRPs bind with high affinity (Kd≈10−12 M) to cognate IREs. The IRE–IRP interactions stabilize TfR1 mRNA and, moreover, impose a steric blockade to ferritin mRNA translation (Figure 4). As a result, increased TfR1 levels stimulate acquisition of iron from plasma Tf to counteract iron deficiency. The inhibition of de novo ferritin synthesis leads to decreased abundance of this protein, as iron storage becomes obsolete under these conditions. Conversely, in cells with high iron content, both IRP1 and IRP2 become unavailable for IRE binding, allowing TfR1 mRNA degradation and ferritin mRNA translation. Thus when iron supply exceeds cellular needs, the IRE–IRP switch minimizes further iron uptake via TfR1, and favours the storage of excess iron in newly synthesized ferritin. The pathophysiological significance of the IRE–IRP system is illustrated in HHCS (hereditary hyperferritinaemia–cataract syndrome), an autosomal dominant disorder, characterized by supra-physiological serum ferritin levels (without iron overload) and early-onset cataract, that is caused by mutations in L-ferritin IRE that prevent IRP binding [109].
Figure 4. Post-transcriptional control of cellular pathways by the IRE–IRP regulatory system.
Translational-type IRE–IRP interactions in the 5′UTR modulate the expression of the mRNAs encoding H- and L-ferritin, ALAS2, m-aconitase, ferroportin, HIF-2α, β-APP and α-synuclein, which in turn control iron storage, erythroid iron utilization, energy homoeostasis, iron efflux, hypoxic responses and neurological pathways respectively. Conversely, IRE–IRP interactions in the 3′UTR stabilize the mRNAs encoding TfR1, DMT1, Cdc14A and MRCKα, which are involved in iron uptake, iron transport, the cell cycle and cytoskeletal remodelling respectively. Note that the regulation of DMT1, Cdc14A and MRCKα may require additional factors, and that the IREs in Cdc14A and MRCKα mRNAs are not phylogenetically conserved.
The IRE–IRP system was initially defined as a relatively simple and ubiquitous post-transcriptional regulatory circuit that maintains cellular iron homoeostasis in vertebrates by orchestrating co-ordinated iron uptake by TfR1 and storage in ferritin. The identification of additional IRE-containing mRNAs and the ongoing biochemical and physiological characterization of IRPs added considerable complexity and uncovered a functional significance for the IRE–IRP system that exceeds the narrow boundaries of cellular iron uptake and storage. Limitations of this system have also been reported, mostly in specialized cells that may override IRE–IRP-mediated control mechanisms for efficient iron handling. For example, TfR1 mRNA stability is uncoupled from iron supply and IRP regulation in erythroid progenitor cells, which take up extraordinary amounts of iron for haem synthesis and haemoglobinization [110]; under these conditions, TfR1 expression is regulated transcriptionally by an erythroid active element in its promoter [111]. IRE–IRP-independent regulation of TfR1 and ferritin at the transcriptional level is well established, and is reviewed elsewhere [112,113].
OTHER IRE-CONTAINING mRNAS
The biochemical characterization of IREs and the establishment of a canonical IRE motif prepared the way for the discovery of further IRE-containing mRNAs, some of them bearing atypical, yet functional, IREs [105–107]. An in silico screen of nucleotide databases led to the identification of a ‘translation-type’ IRE (an IRE that confers translational regulation) in the 5′UTR of ALAS2 mRNA. Considering that ALAS2 catalyses the initial reaction for haem biosynthesis in erythroid cells, the translational repression of its mRNA by IRPs associates the IRE–IRP system with systemic iron utilization and homoeostasis. This response may inhibit the accumulation of toxic protoporphyrin IX when iron is scarce. The severe hypochromic anaemia and early embryonic lethality of the sir (shiraz) zebrafish mutant is caused by a genetic defect in Grx5, which impairs the regulation of IRP1 (see below) and eventually leads to translational repression of ALAS2 [114]. A similar, but relatively milder, biochemical phenotype has been documented in a human patient with sideroblastic anaemia; interestingly, ALAS2 mRNA translation was suppressed by both IRPs because the deregulation of IRP1 was associated with cytosolic iron depletion and concomitant induction of IRP2 [115].
A single translation-type IRE was also found in the 5′UTR of the mRNAs encoding mammalian m-aconitases (mitochondrial aconitases) and the Ip (iron protein) subunit of Drosophila SDH (succinate dehydrogenase), which are both iron–sulfur enzymes of the citric acid cycle. The latter is not conserved in SDH transcripts of other insects [108]. Quantitatively, the degree of IRP-mediated translational repression of mammalian m-aconitases and Drosophila SDH is not profound (as compared with ferritin); however, it may suffice to co-ordinate the expression of these iron-containing proteins with iron availability and link the IRE–IRP system with energy metabolism.
The mRNAs encoding the iron transporters DMT1 and ferroportin are expressed in alternatively spliced isoforms, some of which are furnished with a translation-type IRE. Two out of four DMT1 transcripts contain a single IRE in their 3′UTR [116] that presumably operates as a stability control element and accounts for the high duodenal DMT1 expression in iron-deficient mice [117]. Nevertheless, the function of DMT1 IRE appears to be cell-specific and to require additional upstream regulatory elements in exon 1A [116]. Ferroportin mRNA is expressed in two alternatively spliced transcripts, one of which contains a single translation-type IRE in its 5′UTR [118] that is consistently associated with high ferroportin expression in the livers of iron-loaded mice [119]. Conversely, the lack of IRE in the alternative ferroportin transcript, which is enriched in duodenal enterocytes and erythroid precursor cells [118], allows the accumulation of ferroportin in these tissues during iron deficiency [119,120] by evading the translational blockade imposed by active IRPs. In an iron-deficient state, the bypass of the IRE–IRP system contributes to homoeostatic adaptation by (i) probably facilitating dietary-iron absorption in the duodenum, and (ii) possibly also permitting efflux of iron from erythroid cells in the bloodstream to restrict erythropoiesis and to make the metal available to iron-starved non-erythroid cells. The irradiation-induced deletion of 58 bp from mouse ferroportin IRE has been associated with diverse phenotypes, ranging from erythropoietin-dependent polycythaemia in heterozygous animals to microcytic hypochromic anaemia in homozygous counterparts [121]. These results may indicate further complexity in the post-transcriptional regulation of ferroportin by the IRE–IRP system.
More recently, a high-throughput biochemical screen revealed an atypical IRE in the 5′UTR of HIF-2α mRNA that functions as a translational control element [122]. In vitro, HIF-2α IRE interacts efficiently with recombinant IRP1 and IRP2 [122], but it appears to be a preferential target of IRP1 in cells [123,124]. Importantly, HIF-2α regulates hepatic erythropoietin production [125] and its ablation is associated with anaemia [126]. Thus translational inhibition of HIF-2α mRNA by IRP1 may lead to reduced erythropoietin expression, which can be viewed as a homoeostatic response to curtail erythropoiesis in iron deficiency. Since HIF-2α mediates the transcriptional activation of duodenal DMT1 and Dcytb in iron deficiency [39,40], its translational regulation by the IRE–IRP system is also predicted to fine-tune dietary-iron absorption. The development of animal models with disrupted HIF-2α IRE could provide a valuable tool to clarify the physiological role of IRPs in HIF-2α regulation.
Other experiments resulted in the identification of single IRE motifs in the 3′UTR of mRNA splice variants encoding MRCKα [myotonic dystrophy kinase-related Cdc42 (cell division cycle 42)-binding kinase α] [127] and human Cdc14A phosphatase [127,128]. Preliminary biochemical characterization suggests that these IRE motifs contribute to the regulation of mRNA stability, linking the IRE–IRP system with cytoskeletal remodelling and the cell cycle. Nevertheless, these IREs do not exhibit extensive phylogenetic conservation and appear to be restricted to primates and some mammals [108]. The mRNA encoding β-APP harbours a non-canonical IRE motif with a conserved 5′-CAGAG-3′ sequence (the underlined C and G residues form a base pair) as part of an extended loop in its 5′UTR, which preferentially interacts with IRP1 and functions as a translational control element [129]. Interestingly, α-synuclein mRNA also contains a predicted IRE-like motif [130] that awaits functional characterization. Aberrant expression of β-APP and α-synuclein is associated with Alzheimer's and Parkinson's diseases respectively; thus validation of the regulatory function of their IREs may couple the IRE–IRP system with human neurodegenerative conditions.
An extensive phylogenetic analysis confirmed that IRE-containing mRNAs are exclusively found in metazoans [108]. The ferritin IRE motif may represent the ancestral prototype, which was subsequently adopted during evolution by other genes in higher organisms. Notably, IRE-like sequences with iron-regulatory functions are present in some bacterial mRNAs [131–133]; however, their modes of action are only distally reminiscent of the metazoan IRE–IRP network.
Overall, as illustrated in Figure 4, functional IRE motifs have thus far been identified in mRNAs encoding proteins of iron uptake (TfR1), storage (H- and L-ferritin), erythroid utilization (ALAS2) and transport (DMT1 and ferroportin), as well as energy metabolism (m-aconitase and Drosophila SDH), hypoxic regulation (HIF-2α), cytoskeletal reorganization (MRCKα), cell-cycle control (Cdc14A) and neuronal function (β-APP and α-synuclein). It is also worth noting that the mRNAs encoding TfR2, mitochondrial ferritin and ALAS1 do not possess an IRE. The expanded list of IRE-containing mRNAs emphasizes the role of the IRE–IRP system as a master post-transcriptional iron-regulatory switch, but also implies further regulatory potential outside the context of iron metabolism in a strict sense.
IRPs: FUNCTIONAL AND STRUCTURAL FEATURES
IRP1 and IRP2 do not share sequence similarities with known RNA-binding proteins and do not contain any established RNA-binding motifs. They both belong to the family of ISC isomerases, which includes m-aconitase [105–107]. This enzyme catalyses the isomerization of citrate to iso-citrate via the intermediate cis-aconitate during the citric acid cycle, and contains a cubane [4Fe–4S]2+ ISC in its active site. Three of the iron atoms are attached to cysteine residues of the polypeptide, whereas the fourth iron (Fea) remains free and mediates catalytic chemistry.
IRP1 assembles an analogous to m-aconitase ISC that converts it to a c-aconitase (cytosolic aconitase). However, in contrast with m-aconitase, IRP1 only retains its ISC and its enzymatic function in iron-replete cells. In iron deficiency, holo-IRP1 is converted into apo-protein that possesses IRE-binding activity. Thus IRP1 is bifunctional and its mutually exclusive activities are reversibly regulated by an unusual ISC switch. IRP1 probably evolved independently of m-aconitase following an early duplication event that allowed it to acquire IRE-binding activity [134]. A second duplication event led to the evolution of IRP2 in higher eukaryotes.
IRP2 shares extensive homology with IRP1; however, it neither assembles an ISC nor retains aconitase active-site residues. Consequently, IRP2 only exhibits an IRE-binding activity and does not have any enzymatic function. A feature of IRP2 that distinguishes it from IRP1 is the presence of a conserved cysteine- and proline-rich stretch of 73 amino acids close to its N-terminus. This sequence is encoded by a separate exon and appears to be unstructured [135]. IRP2 is regulated in an irreversible manner, at the level of protein stability.
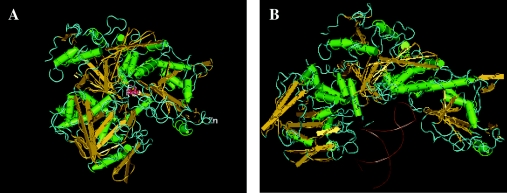
The crystal structure of IRP1 has been solved in both the c-aconitase-binding [136] and IRE-binding [137] forms (Figure 5), although the structure of IRP2 has not yet been determined. These results have validated earlier evidence that the site for catalysis and RNA-binding overlap, and the switch between the enzymatic and RNA-binding forms is associated with extensive conformational rearrangements. The folding of holo-IRP1 follows the pattern of m-aconitase [136], despite relatively limited sequence identity (22%), but consistently with the conservation of active-site residues. The protein is composed of four globular domains. Domains 1–3 are compact and join domain 4 through a surface linker. The ISC is located centrally at the interface of the four domains. The topology of the ISC and the surrounding environment are fairly conserved between c- and m-aconitases. Nevertheless, the overall structure of holo-IRP1, a protein of 889 amino acids, also shows differences to that of m-aconitase, which is smaller (780 amino acids). The short IRP1 fragments that do not superimpose with m-aconitase are exposed on the surface of the protein. As a result, the shapes and surface topologies of holo-IRP1 and m-aconitase diverge substantially, which may explain the fact that only the former can acquire IRE-binding activity.
Figure 5. Crystal structure of IRP1.
(A) c-Aconitase form (PDB code 2B3X); (B) IRE-binding form (PDB code 2IPY). A three-dimensional structure of this Figure is available at http://www.BiochemJ.org/bj/434/0365/bj4340365add.htm.
The structure of IRP1 in a complex with ferritin IRE (at 2.8 Å resolution) uncovered the details of the protein reorganization upon loss of its ISC [137]. The main features are a rotation of domain 4 by 32 °, but also an unpredicted extensive rearrangement of domain 3 by 52 ° that creates a hydrophilic cavity and allows access to the IRE. The RNA–protein interaction requires two crucial segments at the interface of domain 2 (residues 436–442) and domain 3 (residues 534–544). Thr438 and Asn439 make direct contacts with the IRE. The terminal residues of the IRE loop, A15, G16 and U17, interact with Ser371, Lys379 and Arg269 respectively within a cavity between domains 2 and 3. A second binding site is formed around the unpaired-C-bulge residue between the upper and lower stem, which occupies a pocket within domain 4, sandwiched between Arg713 and Arg780. The IRE–IRP1 complex is stabilized by additional bonds, ionic interactions and van der Waals contacts.
The structural studies described above offered detailed insights into the dual function of IRP1 as a c-aconitase and an IRE-binding protein. These findings also enabled the modelling of IRP2 structure and the design of site-directed mutagenesis experiments to investigate the role of single amino acid residues of IRP2 in IRE binding [138]. Nevertheless, the crystallization of IRP2 and the resolution of its structure, especially in a complex with IRE, will be necessary to precisely map the RNA–protein interaction and to understand the topology of the IRP2-specific 73 amino acid insert and its possible role in IRE binding.
IRON-DEPENDENT REGULATION OF IRP1
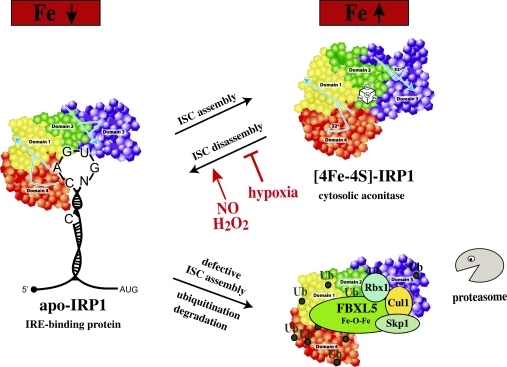
The ISC of IRP1 is the major site for its regulation (Figure 6). In vitro, holo-IRP1 can be easily reconstituted upon incubation of apo-IRP1 with ferrous salts, sulfide and reducing agents [139]. Within cells, the conversion of apo- to holo-IRP1 requires several cofactors, such as the mitochondrial proteins Nfs1 (ISCS) [140–142], frataxin [143,144], ISCU [145], Grx5 [114,115], ISD11 [146] or Abcb7 [147], as the silencing of these proteins favours expression of IRP1 in the IRE-binding form due to impaired ISC biogenesis. These results support an active role of mitochondria in the assembly of holo-IRP1. Moreover, mitochondrial ATP production is required for maintenance and repair of the IRP1 ISC [148], and ATP binds directly to IRP1 and promotes ISC stabilization [149]. Cytosolic homologues of Isu/ISCU and Nfs1/ISCS [145,150], as well as the cytosolic proteins Cfd1 [151], Nbp35 [152], Nar1/IOP1 [153], Cia1 [154], Dre2 [155] and, possibly, Tah18, which transfers electrons to Dre2 [156], are also implicated in the ISC assembly of IRP1.
Figure 6. Under physiological conditions, IRP1 is regulated by a reversible ISC switch.
Iron deficiency promotes ISC disassembly and a conformational rearrangement, resulting in the conversion of IRP1 from c-aconitase to an IRE-binding protein. The ISC is regenerated in iron-replete cells. Hypoxia favours maintenance of the ISC, whereas H2O2 or NO promote its disassembly. When the ISC-biogenesis pathway is not operational, iron leads to ubiquitination of apo-IRP1 by the FBXL5 E3 ligase complex (including Skp1, Cul1 and Rbx1), resulting in proteasomal degradation.
Iron starvation leads to conversion of holo-IRP1 into an IRE-binding apo-protein following depletion of its ISC. In cultured cells, this process is relatively lengthy (8–12 h) and does not require de novo protein synthesis. IRP1 is a fairly stable protein (half-life of ~24 h) and, under normal circumstances, its stability remains unaffected by iron levels. However, when ISC biogenesis is impaired by either inactivation of ISC assembly cofactors or phosphorylation of IRP1 at Ser138, iron leads to ubiquitination and slow degradation of apo-IRP1 by the proteasome [142,157,158]. This backup mechanism prevents accumulation of excessive apo-IRP1 in iron-loaded cells that may disrupt iron homoeostasis by its unregulated IRE-binding activity. Iron-chelating drugs promote considerably more efficient conversion of holo- to apo-IRP1 in typical cell-culture conditions with 21% oxygen, as compared with lower oxygen concentrations (3–6%) that are more relevant physiologically in tissues [159]. This is consistent with the predominance of holo-IRP1 in tissues of iron-deficient animals, and the conversion of only a small fraction to an IRE-binding apo-protein. The ISC of IRP1 exhibits sensitivity to oxidants (see below), whereas hypoxia favours its stabilization [123,160].
IRP1 can be phosphorylated by PKC (protein kinase C) at the conserved Ser138 and Ser711 residues [139,157,161]. Ser138 is located in proximity to the ISC and its phosphorylation appears to interfere with the ISC stability and alter the set-point for its disassembly in an oxygen-dependent manner [160]. The introduction of a phosphomimetic S138E mutation favours non-oxidative demetallation of [4Fe–4S]2+ to [3Fe–4S]0 by iron-chelating drugs in vitro [160] and sensitizes IRP1 to irondependent degradation in cells [157,158]. Phosphorylation of the C-terminal Ser711 may also regulate IRP1 functions. An S711E substitution inhibits the capacity of IRP1 to convert citrate into the intermediate cis-aconitate in vitro [139,161] and impairs its IRE-binding activity in cells [139]. PKC-mediated phosphorylation has been reported to promote the translocation of IRP1 from the cytosol to the ER and Golgi in iron-deficient cells, probably to facilitate its interaction with TfR1 IREs [162].
IRON-DEPENDENT REGULATION OF IRP2
IRP2 is synthesized de novo in response to low iron and remains stable under iron-starvation or hypoxia [105–107]. In iron-replete cells, however, IRP2 becomes destabilized and undergoes rapid ubiquitination and degradation by the proteasome. Earlier assumptions that the stability of IRP2 is regulated by its specific 73 amino acid insert have been experimentally rejected [163,164]. The analysis of a series of IRP2 deletion mutants provided evidence that its C-terminus contains sequences that are necessary, but not sufficient, for iron-dependent degradation [165]. These results suggested that the recognition of IRP2 by the proteasomal degradation machinery may require additional IRP structural elements. DMOG (dimethyl-oxalyl-glycine), an inhibitor of 2-oxoglutarate-dependent oxygenases, partially stabilizes IRP2 against iron, suggesting a possible involvement of this family of enzymes in tagging IRP2 for degradation [163,164]. Similar effects were observed within cells treated with succinylacetone, an inhibitor of haem synthesis, [166–168], highlighting a potential role of endogenous haem in the control of IRP2 stability.
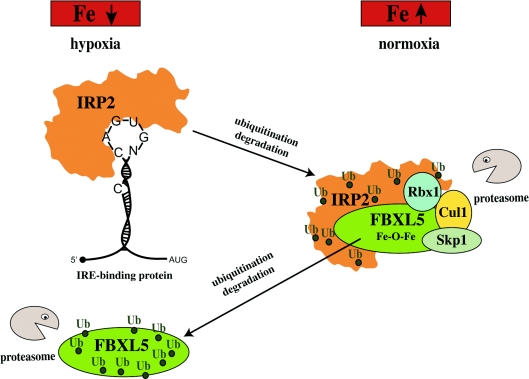
More recent results demonstrated that IRP2 (as well as apo-IRP1) are substrates of FBXL5 (F-box and leucine-rich repeat protein 5), a member of an E3 ubiquitin ligase complex that also includes Skp1 (S-phase kinase-associated protein 1), Cul1 (Cullin 1) and Rbx1 (Ring-box 1) [169,170]. FBXL5 contains an N-terminal haemerythrin domain with a characteristic Fe–O–Fe centre. Notably, FBXL5 is the first mammalian protein identified to harbour this ancient domain, which is used by oxygen-transport proteins in some bacteria and lower eukaryotes [171]. In iron-replete and oxygenated cells, FBXL5 accumulates and interacts with IRP2, mediating its ubiquitination and subsequent degradation (Figure 7). In contrast, in iron-deficient or hypoxic cells, FBXL5 itself undergoes proteasomal degradation by a yet unknown mechanism upon the loss of its Fe–O–Fe centre, which allows the stabilization of IRP2. Deletion of the haemerythrin domain or mutation of iron-binding ligands abolishes the regulatory function of FBXL5. Hence, FBXL5 senses iron and oxygen levels through the Fe–O–Fe centre of its haemerythrin domain and emerges as a novel regulator of cellular iron homoeostasis.
Figure 7. Iron and oxygen-dependent regulation of IRP2 stability by FBXL5.
IRP2 is stable in iron deficient and/or hypoxic cells; under these conditions, FBXL5 undergoes ubiquitination and proteasomal degradation. An increase in iron and oxygen levels stabilizes FBXL5 by formation of an Fe–O–Fe centre in its haemerythrin domain, triggering the assembly of an E3 ubiquitin ligase complex together with Skp1, Cul1 and Rbx1. This complex ubiquitinates (Ub) IRP2, leading to its recognition by the proteasome and its degradation.
IRP2 can be phosphorylated at Ser157 by Cdk1 (cyclin-dependent kinase 1)/cyclin B1 during the G2/M-phase of the cell cycle; this response decreases its IRE-binding activity and derepresses ferritin mRNA translation [172]. During mitotic exit, Ser157 undergoes dephosphorylation by the phosphatase Cdc14A. The regulation of Cdc14A during the cell cycle was found to be iron independent, despite the presence of the IRE motif in one of its mRNA splice variants. The stimulation of ferritin synthesis coupled to the inactivation of phosphorylated IRP2 is thought to protect DNA from iron-catalysed oxidative damage.
REDOX REGULATION OF IRON METABOLISM
Both IRP1 and IRP2 are sensitive to ROS and RNS (reactive nitrogen species) (reviewed in [173]). The redox regulation of IRP1 is mediated by its ISC. Exposure of cells to micromolar concentrations of H2O2 leads to removal of the ISC and induction of IRP1 for IRE binding via an incompletely characterized signalling mechanism. This response can be antagonized by myeloperoxidase-derived hypochlorite [174]. In vitro, ROS and RNS remove the Fea of the ISC and convert [4Fe–4S]2+-IRP to [3Fe–4S]2+-IRP1, which is non-functional. IRP1 also responds to NO, which likewise induces IRE binding at the expense of its aconitase activity, albeit by a diverse mechanism. NO may destroy the ISC of IRP1, but also promote iron efflux from cells [175], suggesting that the NO-mediated switch of IRP1 to its IRE-binding form could represent a homoeostatic response to iron starvation.
Consistent with this hypothesis, NO released from transfected fibroblasts was found to stabilize IRP2 in co-cultured cells [176]. The first hints for redox regulation of IRP2 were provided by experiments showing that antioxidants, such as ascorbate, α-tocopherol and N-acetylcysteine promote the proteasomal turnover of IRP2 [164]. More recently, it was shown that oxidants stabilize previously induced IRP2 against iron-dependent degradation [176a]. On the other hand, ROS may also oxidize Cys512 and Cys516, predicted to lie within the IRE-binding cleft of IRP2. Formation of a disulfide bridge between these two cysteine residues was shown to inhibit the IRE-binding activity of IRP2 and reduce TfR1 mRNA abundance [138].
The well documented induction of IRP1 by oxidative stress modulates cellular iron metabolism. Thus H2O2 leads to transient inhibition of ferritin synthesis, up-regulation of TfR1, increased uptake of 59Fe-labelled Tf and, notably, elevated storage of 59Fe into ferritin, despite the decrease in the ferritin content [177]. Oxidative stress may also regulate cellular iron metabolism independently of IRPs, as sustained non-toxic H2O2 concentrations (<5 μM), which mimic inflammatory conditions and do not affect IRE-binding activity, stimulate TfR1 mRNA translation [178]. Moreover, higher H2O2 doses promote proteasomal degradation of ferritin [179]. Importantly, ROS stabilize HIF-α subunits by oxidizing Fe2+ to Fe3+ and thereby depleting an essential cofactor of prolyl hydroxylases, which initiate their degradation in normoxia [180]. This has profound implications for iron metabolic pathways, considering that HIF transcriptionally regulates DMT1, Dcytb, Tf, TfR1, ceruloplasmin, HO-1 and ALAS2 [39,181,182]. The ROS-dependent repression of hepcidin via C/EBPα [36,183,184] further highlights the multitude of pathways for redox regulation of iron metabolism.
PHYSIOLOGICAL FUNCTIONS OF IRP1 AND IRP2
Targeted disruption of both IRP1 and IRP2 is incompatible with life and leads to embryonic lethality at the blastocyst stage [185], illustrating the importance of these proteins in early development. Mice with tissue-specific ablation of both IRP1 and IRP2 have been generated by Cre/loxP technology. In the intestine, the lack of IRPs is associated with growth defects, intestinal malabsorption, dehydration, weight loss and death within 4 weeks of birth [186]. The deregulated expression of TfR1, ferritin, ferroportin and DMT1 is consistent with a critical function of IRPs in intestinal iron transport and storage. The lack of IRPs in hepatocytes also leads to premature death due to liver failure, mitochondrial disfunction and alterations in haem and ISC biosynthetic pathways [187].
Single IRP1−/− or IRP2−/− mice are viable, indicating a functional redundancy of the ubiquitously expressed IRP1 and IRP2. Nonetheless, it appears that IRE-containing mRNAs are primarily regulated by IRP2 in vivo, as IRP1 is predominantly expressed as c-aconitase within tissues [105,188]. Hence, it is not surprising that IRP1−/− mice do not exhibit any overt pathological phenotype under standard laboratory conditions, and merely misregulate TfR1 and ferritin expression in the kidney and brown fat [189]. IRP1−/− mice also mount an efficient inflammatory signalling response to turpentine [190] and do not exhibit differential sensitivity to anthracycline-induced cardiotoxicity compared with wild-type animals [191]. The lack of any discernible pathology in IRP1−/− mice may raise questions on the physiological significance of IRP1 as c-aconitase. Considering the important roles of citrate in metabolism [192], it will be important to examine IRP1−/− animals for potential metabolic defects.
IRP2−/− mice develop microcytic hypochromic anaemia, manifest excessive iron deposition in the duodenum and the liver and have relative iron deficiency in the spleen [193,194]. Erythroid precursor cells of IRP2−/− animals exhibit reduced TfR1 expression, which may account for the decreased iron levels in the bone marrow. These cells also contain high levels of protoporphyrin IX, apparently due to unrestricted expression of ALAS2, which is reminiscent of erythropoietic protoporphyria. The characterization of mice with tissue-specific disruption of IRP2 in either enterocytes, hepatocytes or macrophages showed that the deregulation of tissue iron levels during ubiquitous IRP2 deficiency is largely explained by cell-autonomous functions of IRP2 [195]. Thus selective ablation of IRP2 in enterocytes, hepatocytes or macrophages impairs iron homoeostasis in these cells, but does not alter haematological or plasma iron parameters, suggesting that the microcytosis of IRP2−/− mice is caused by an intrinsic defect in haematopoiesis.
The disruption of IRP2 has also been associated with neuropathology. Aging IRP2−/− mice accumulate excessive amounts of iron in specific areas of the brain and develop a progressive neurodegenerative disorder [196,197]. This clinical phenotype is further aggravated in a genetic background of IRP1 haploinsufficiency (IRP2−/− IRP1+/− mice) [198]. Treatment of IRP2−/− mice with the ISC-disrupting nitroxide Tempol partially rescued neuropathy in these animals, due to activation of endogenous IRP1 for IRE binding [199]. However, Tempol failed to correct microcytosis, possibly due to lower expression of IRP1 in erythroblasts compared with the forebrain. Interestingly, isogenic IRP2−/− mice generated by a different targeting strategy do not manifest severe neurodegeneration, but perform relatively poorly in neurobehavioural tests [200]. The role of IRP2 in neuronal physiology is unclear. It has been hypothesized that disruption of iron homoeostasis in neurons of IRP2−/− animals (which show suppression of TfR1 and unrestrained expression of ferritin and, possibly, also ferroportin) may cause a state of functional iron deficiency [105]. In addition, the lack of IRP2 may trigger a decrease in brain copper levels by derepressing translation of the IRE-containing β-APP mRNA, which is also implicated in copper efflux [201].
Taken together, the results obtained from the analysis of currently available IRP knockout mice demonstrate an essential role of the IRE–IRP system in body iron homoeostasis. Further characterization of these animals, as well as the generation of new models, e.g. bearing spatial and/or temporal disruption of IRPs, or engineered for tissue-specific expression of constitutive mutants, is expected to improve our understanding on the function of the IRE–IRP network in health and disease.
IRPs AND CANCER
There is evidence for possible involvement of IRPs in cancer biology. The overexpression of wild-type IRP1 or IRP1C437S, a mutant with constitutive IRE-binding activity, in human H1299 lung cancer cells did not affect their proliferation in culture, but significantly impaired the growth of tumour xenografts formed upon injection of these cells into nude mice [202]. In contrast, in a similar setting, the overexpression of IRP2 strongly accelerated tumour xenograft growth, and this apparent pro-oncogenic activity was abolished by deletion of the IRP2-specific domain of 73 amino acids [203]. Interestingly, these contrasting phenotypes were not associated with differential expression of known IRP targets. In fact, ferritin evaded the suppressor activity of both IRP1 and IRP2 transgenes in the tumour xenografts, as previously shown in dense cultures of IRP1C437S-overexpressing cells [204]. IRP1- and IRP2-overexpressing tumours exhibited distinct gene-expression profiles, suggesting that IRPs may differentially modulate cancer growth by mechanisms independent of their function as IRE-binding proteins. The pro-oncogenic activity of IRP2 correlated with increased ERK1/2 (extracellular-signal-regulated kinase 1/2) phosphorylation and high levels of c-myc, which is particularly intriguing considering that this proto-oncogene transcriptionally activates IRP2 [205]. Further work is required to understand the molecular basis underlying the apparent tumour suppressor activity of IRP1 and to elucidate whether IRP2 functions as a bona fide oncogene and, possibly, participates in a feedback regulatory loop with c-myc to control tumour growth.
PERSPECTIVES
Until the mid-1980s, the bio-iron field was restricted to the study of Tf, TfR1 and ferritin. The subsequent discovery of the IRE–IRP system prepared the way for understanding the co-ordinate regulation of cellular iron metabolism. In the new millennium, the identification of hepcidin as a master hormonal switch of systemic iron homoeostasis has provided a framework to comprehend the regulation of physiological mechanisms for iron traffic in the body and to decipher the molecular basis underlying various iron-related disorders. Today, an in-depth characterization of the signalling pathways and cross-talk activities culminating in the activation of hepcidin continues to pose a major challenge. This includes elucidation of the early responses to systemic iron perturbations or alterations in erythropoietic activity that translate into hepcidin regulation. It should also be noted that the profound biomedical implications associated with the modulation of hepcidin pathways render the hepcidin–ferroportin axis an attractive target for drug development.
It will be important to further characterize and better understand the regulatory links between systemic and cellular iron metabolism. For example, the post-transcriptional regulation of ferroportin and HIF-2α by IRPs is of particular interest, considering that the former is the target of hepcidin, whereas the latter is an upstream regulator of erythropoiesis and intestinal iron absorption. Moreover, TfR1, a target of IRPs, is crucial for iron-dependent regulation of hepcidin. Future work is also expected to clarify the role of the IRE–IRP system in neuronal biology, in light of the biochemically characterized non-canonical IRE and the predicted IRE in the mRNAs encoding β-APP and α-synuclein respectively. It is also conceivable that additional IRE-containing mRNAs, especially with atypical IRE motifs, remain to be discovered. Although studies of IRP knockout animals have provided crucial insights into the role of the IRE–IRP regulatory system in vivo, the physiological significance of an IRE motif in the regulation of a given mRNA could be better appreciated by generating animals with specific disruption of this motif, to uncouple mRNA expression from the control of IRPs.
Several outstanding questions on cellular iron metabolism, primarily related to the movement of iron across organelles, await further investigation. Future work will probably shed light on the roles of the siderophore 2,5-DHBA, Grx3 and Grx4, PCBP1 and the E3 ubiquitin ligase FBXL5 in intracellular iron sensing and trafficking, and on the roles of ABC and SLC transporters and of FLVCR in the transport of haem and its precursors. The rapidly growing field of ISC biogenesis is expected to provide novel links between cellular iron metabolism and utilization, and the identification of iron-regulatory pathways involving microRNAs may also be anticipated. Finally, characterization of the roles of IRP1 and IRP2 in cancer biology may uncover novel functions for these proteins, not necessarily related to iron metabolism.
FUNDING
K.P. is funded by the Canadian Institutes for Health Research (CIHR) [grant number MOP-86514] and holds a Chercheur National career award from the Fonds de la Recherche en Santé du Quebéc (FRSQ).
References
- 1.Aisen P., Enns C., Wessling-Resnick M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001;33:940–959. doi: 10.1016/s1357-2725(01)00063-2. [DOI] [PubMed] [Google Scholar]
- 2.Koppenol W. H. The centennial of the Fenton reaction. Free Radical Biol. Med. 1993;15:645–651. doi: 10.1016/0891-5849(93)90168-t. [DOI] [PubMed] [Google Scholar]
- 3.Galaris D., Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit. Rev. Clin. Lab. Sci. 2008;45:1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- 4.Kell D. B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genomics. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Domenico I., McVey Ward D., Kaplan J. Regulation of iron acquisition and storage: consequences for iron-linked disorders. Nat. Rev. Mol. Cell Biol. 2008;9:72–81. doi: 10.1038/nrm2295. [DOI] [PubMed] [Google Scholar]
- 6.Hentze M. W., Muckenthaler M. U., Galy B., Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Andrews N. C. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews N. C. Disorders of iron metabolism. N. Engl. J. Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 9.Olsson K. S., Norrby A. Comment to: Hepcidin: from discovery to differential diagnosis. Haematologica 2008; 93:90–7. Haematologica. 2008;93:e51. doi: 10.3324/haematol.12814. [DOI] [PubMed] [Google Scholar]
- 10.Yeh K. Y., Yeh M., Mims L., Glass J. Iron feeding induces ferroportin 1 and hephaestin migration and interaction in rat duodenal epithelium. Am. J. Physiol. Gastrointest. Liver Physiol. 2009;296:G55–G65. doi: 10.1152/ajpgi.90298.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan A., Lima C. A., Pinkus J. L., Pinkus G. S., Zon L. I., Robine S., Andrews N. C. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1:191–200. doi: 10.1016/j.cmet.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth E., Ganz T. The role of hepcidin in iron metabolism. Acta Haematol. 2009;122:78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P. L., Beutler E. Regulation of hepcidin and iron-overload disease. Annu. Rev. Pathol. 2009;4:489–515. doi: 10.1146/annurev.pathol.4.110807.092205. [DOI] [PubMed] [Google Scholar]
- 14.Weiss G., Goodnough L. T. Anemia of chronic disease. N. Engl. J. Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 15.Finberg K. E. Iron-refractory iron deficiency anemia. Semin. Hematol. 2009;46:378–386. doi: 10.1053/j.seminhematol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Courselaud B., Pigeon C., Inoue Y., Inoue J., Gonzalez F. J., Leroyer P., Gilot D., Boudjema K., Guguen-Guillouzo C., Brissot P., et al. C/EBPα regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J. Biol. Chem. 2002;277:41163–41170. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 17.Kautz L., Meynard D., Monnier A., Darnaud V., Bouvet R., Wang R. H., Deng C., Vaulont S., Mosser J., Coppin H., Roth M. P. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112:1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 18.Arndt S., Maegdefrau U., Dorn C., Schardt K., Hellerbrand C., Bosserhoff A. K. Iron-induced expression of bone morphogenic protein 6 in intestinal cells is the main regulator of hepatic hepcidin expression in vivo. Gastroenterology. 2010;138:372–382. doi: 10.1053/j.gastro.2009.09.048. [DOI] [PubMed] [Google Scholar]
- 19.Meynard D., Kautz L., Darnaud V., Canonne-Hergaux F., Coppin H., Roth M. P. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat. Genet. 2009;41:478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 20.Andriopoulos B., Jr, Corradini E., Xia Y., Faasse S. A., Chen S., Grgurevic L., Knutson M. D., Pietrangelo A., Vukicevic S., Lin H. Y., Babitt J. L. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat. Genet. 2009;41:482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang R. H., Li C., Xu X., Zheng Y., Xiao C., Zerfas P., Cooperman S., Eckhaus M., Rouault T., Mishra L., Deng C. X. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Gao J., Chen J., Kramer M., Tsukamoto H., Zhang A. S., Enns C. A. Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab. 2009;9:217–227. doi: 10.1016/j.cmet.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramey G., Deschemin J. C., Vaulont S. Cross-talk between the mitogen activated protein kinase and bone morphogenetic protein/hemojuvelin pathways is required for the induction of hepcidin by holotransferrin in primary mouse hepatocytes. Haematologica. 2009;94:765–772. doi: 10.3324/haematol.2008.003541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvestri L., Pagani A., Nai A., De Domenico I., Kaplan J., Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8:502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleming R. E. Iron and inflammation: cross-talk between pathways regulating hepcidin. J. Mol. Med. 2008;86:491–494. doi: 10.1007/s00109-008-0349-8. [DOI] [PubMed] [Google Scholar]
- 26.Matak P., Chaston T. B., Chung B., Srai S. K., McKie A. T., Sharp P. A. Activated macrophages induce hepcidin expression in HuH7 hepatoma cells. Haematologica. 2009;94:773–780. doi: 10.3324/haematol.2008.003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vecchi C., Montosi G., Zhang K., Lamberti I., Duncan S. A., Kaufman R. J., Pietrangelo A. ER stress controls iron metabolism through induction of hepcidin. Science. 2009;325:877–880. doi: 10.1126/science.1176639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oliveira S. J., Pinto J. P., Picarote G., Costa V. M., Carvalho F., Rangel M., de Sousa M., de Almeida S. F. ER stress-inducible factor CHOP affects the expression of hepcidin by modulating C/EBPα activity. PLoS ONE. 2009;4:e6618. doi: 10.1371/journal.pone.0006618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peyssonnaux C., Zinkernagel A. S., Datta V., Lauth X., Johnson R. S., Nizet V. TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood. 2006;107:3727–3732. doi: 10.1182/blood-2005-06-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koening C. L., Miller J. C., Nelson J. M., Ward D. M., Kushner J. P., Bockenstedt L. K., Weis J. J., Kaplan J., De Domenico I. Toll-like receptors mediate induction of hepcidin in mice infected with Borrelia burgdorferi. Blood. 2009;114:1913–1918. doi: 10.1182/blood-2009-03-209577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pak M., Lopez M. A., Gabayan V., Ganz T., Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanno T., Bhanu N. V., Oneal P. A., Goh S. H., Staker P., Lee Y. T., Moroney J. W., Reed C. H., Luban N. L., Wang R. H., et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat. Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 33.Tanno T., Porayette P., Sripichai O., Noh S. J., Byrnes C., Bhupatiraju A., Lee Y. T., Goodnough J. B., Harandi O., Ganz T., et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114:181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto J. P., Ribeiro S., Pontes H., Thowfeequ S., Tosh D., Carvalho F., Porto G. Erythropoietin mediates hepcidin expression in hepatocytes through EPOR signaling and regulation of C/EBPα. Blood. 2008;111:5727–5733. doi: 10.1182/blood-2007-08-106195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volke M., Gale D. P., Maegdefrau U., Schley G., Klanke B., Bosserhoff A. K., Maxwell P. H., Eckardt K. U., Warnecke C. Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors. PLoS ONE. 2009;4:e7875. doi: 10.1371/journal.pone.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miura K., Taura K., Kodama Y., Schnabl B., Brenner D. A. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48:1420–1429. doi: 10.1002/hep.22486. [DOI] [PubMed] [Google Scholar]
- 37.Ludwiczek S., Aigner E., Theurl I., Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 38.Vanoaica L., Darshan D., Richman L., Schumann K., Kuhn L. C. Intestinal ferritin H is required for an accurate control of iron absorption. Cell Metab. 2010;12:273–282. doi: 10.1016/j.cmet.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Shah Y. M., Matsubara T., Ito S., Yim S. H., Gonzalez F. J. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009;9:152–164. doi: 10.1016/j.cmet.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mastrogiannaki M., Matak P., Keith B., Simon M. C., Vaulont S., Peyssonnaux C. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Invest. 2009;119:1159–1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponka P., Beaumont C., Richardson D. R. Function and regulation of transferrin and ferritin. Semin. Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 42.Ohgami R. S., Campagna D. R., Greer E. L., Antiochos B., McDonald A., Chen J., Sharp J. J., Fujiwara Y., Barker J. E., Fleming M. D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005;37:1264–1269. doi: 10.1038/ng1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richardson D. R., Lane D. J., Becker E. M., Huang M. L., Whitnall M., Rahmanto Y. S., Sheftel A. D., Ponka P. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc. Natl. Acad. Sci. U.S.A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levy J. E., Jin O., Fujiwara Y., Kuo F., Andrews N. C. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat. Genet. 1999;21:396–399. doi: 10.1038/7727. [DOI] [PubMed] [Google Scholar]
- 45.Trenor C. C., Campagna D. R., Sellers V. M., Andrews N. C., Fleming M. D. The molecular defect in hypotransferrinemic mice. Blood. 2000;96:1113–1118. [PubMed] [Google Scholar]
- 46.Gunshin H., Fujiwara Y., Custodio A. O., Direnzo C., Robine S., Andrews N. C. Slc11a2 is required for intestinal iron absorption and erythropoiesis but dispensable in placenta and liver. J. Clin. Invest. 2005;115:1258–1266. doi: 10.1172/JCI24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleming M. D., Trenor C. C. I., Su M. A., Foernzler D., Beier D. R., Dietrich W. F., Andrews N. C. Microcytic anaemia mice have a mutation in Nramp2, a candidate iron transporter gene. Nat. Genet. 1997;16:383–386. doi: 10.1038/ng0897-383. [DOI] [PubMed] [Google Scholar]
- 48.Fleming M. D., Romano M. A., Su M. A., Garrick L. M., Garrick M. D., Andrews N. C. Nramp2 is mutated in the anemic Belgrade (b) rat: evidence of a role for Nramp2 in endosomal iron transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1148–1153. doi: 10.1073/pnas.95.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kristiansen M., Graversen J. H., Jacobsen C., Sonne O., Hoffman H. J., Law S. K., Moestrup S. K. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 50.Hvidberg V., Maniecki M. B., Jacobsen C., Hojrup P., Moller H. J., Moestrup S. K. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- 51.Soe-Lin S., Apte S. S., Andriopoulos B., Jr., Andrews M. C., Schranzhofer M., Kahawita T., Garcia-Santos D., Ponka P. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc. Natl. Acad. Sci. U.S.A. 2009;106:5960–5965. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zecca L., Youdim M. B., Riederer P., Connor J. R., Crichton R. R. Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 2004;5:863–873. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- 53.Rouault T. A., Zhang D. L., Jeong S. Y. Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab. Brain Dis. 2009;24:673–684. doi: 10.1007/s11011-009-9169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jeong S. Y., David S. Glycosylphosphatidylinositol-anchored ceruloplasmin is required for iron efflux from cells in the central nervous system. J. Biol. Chem. 2003;278:27144–27148. doi: 10.1074/jbc.M301988200. [DOI] [PubMed] [Google Scholar]
- 55.Duce J. A., Tsatsanis A., Cater M. A., James S. A., Robb E., Wikhe K., Leong S. L., Perez K., Johanssen T., Greenough M. A., et al. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Smith C. P., Thevenod F. Iron transport and the kidney. Biochim. Biophys. Acta. 2009;1790:724–730. doi: 10.1016/j.bbagen.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Kozyraki R., Fyfe J., Verroust P. J., Jacobsen C., Dautry-Varsat A., Gburek J., Willnow T. E., Christensen E. I., Moestrup S. K. Megalin-dependent cubilin-mediated endocytosis is a major pathway for the apical uptake of transferrin in polarized epithelia. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12491–12496. doi: 10.1073/pnas.211291398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goetz D. H., Holmes M. A., Borregaard N., Bluhm M. E., Raymond K. N., Strong R. K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 59.Yang J., Goetz D., Li J. Y., Wang W., Mori K., Setlik D., Du T., Erdjument-Bromage H., Tempst P., Strong R., Barasch J. An iron delivery pathway mediated by a lipocalin. Mol. Cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 60.Mori K., Lee H. T., Rapoport D., Drexler I. R., Foster K., Yang J., Schmidt-Ott K. M., Chen X., Li J. Y., Weiss S., et al. Endocytic delivery of lipocalin–siderophore–iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Invest. 2005;115:610–621. doi: 10.1172/JCI23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Devireddy L. R., Gazin C., Zhu X., Green M. R. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell. 2005;123:1293–1305. doi: 10.1016/j.cell.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 62.Hvidberg V., Jacobsen C., Strong R. K., Cowland J. B., Moestrup S. K., Borregaard N. The endocytic receptor megalin binds the iron transporting neutrophil-gelatinase-associated lipocalin with high affinity and mediates its cellular uptake. FEBS Lett. 2005;579:773–777. doi: 10.1016/j.febslet.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 63.Devireddy L. R., Hart D. O., Goetz D. H., Green M. R. A mammalian siderophore synthesized by an enzyme with a bacterial homolog involved in enterobactin production. Cell. 2010;141:1006–1017. doi: 10.1016/j.cell.2010.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flo T. H., Smith K. D., Sato S., Rodriguez D. J., Holmes M. A., Strong R. K., Akira S., Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 65.Breuer W., Hershko C., Cabantchik Z. I. The importance of non-transferrin bound iron in disorders of iron metabolism. Transfus. Sci. 2000;23:185–192. doi: 10.1016/s0955-3886(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 66.Hider R. C. Nature of nontransferrin-bound iron. Eur. J. Clin. Invest. 2002;32(Suppl. 1):50–54. doi: 10.1046/j.1365-2362.2002.0320s1050.x. [DOI] [PubMed] [Google Scholar]
- 67.Huang H., Akira S., Santos M. M. Is the iron donor lipocalin 2 implicated in the pathophysiology of hereditary hemochromatosis? Hepatology. 2009;49:1012–1016. doi: 10.1002/hep.22699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liuzzi J. P., Aydemir F., Nam H., Knutson M. D., Cousins R. J. Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc. Natl. Acad. Sci. U.S.A. 2006;103:13612–13617. doi: 10.1073/pnas.0606424103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao N., Gao J., Enns C. A., Knutson M. D. ZRT/IRT-like protein 14 (ZIP14) promotes the cellular assimilation of iron from transferrin. J. Biol. Chem. 2010;285:32141–32150. doi: 10.1074/jbc.M110.143248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen T. T., Li L., Chung D. H., Allen C. D., Torti S. V., Torti F. M., Cyster J. G., Chen C. Y., Brodsky F. M., Niemi E. C., et al. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J. Exp. Med. 2005;202:955–965. doi: 10.1084/jem.20042433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J. Y., Paragas N., Ned R. M., Qiu A., Viltard M., Leete T., Drexler I. R., Chen X., Sanna-Cherchi S., Mohammed F., et al. Scara5 is a ferritin receptor mediating non-transferrin iron delivery. Dev. Cell. 2009;16:35–46. doi: 10.1016/j.devcel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li L., Fang C. J., Ryan J. C., Niemi E. C., Lebron J. A., Bjorkman P. J., Arase H., Torti F. M., Torti S. V., Nakamura M. C., Seaman W. E. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3505–3510. doi: 10.1073/pnas.0913192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muhlenhoff U., Molik S., Godoy J. R., Uzarska M. A., Richter N., Seubert A., Zhang Y., Stubbe J., Pierrel F., Herrero E., et al. Cytosolic monothiol glutaredoxins function in intracellular iron sensing and trafficking via their bound iron–sulfur cluster. Cell Metab. 2010;12:373–385. doi: 10.1016/j.cmet.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw G. C., Cope J. J., Li L., Corson K., Hersey C., Ackermann G. E., Gwynn B., Lambert A. J., Wingert R. A., Traver D., et al. Mitoferrin is essential for erythroid iron assimilation. Nature. 2006;440:96–100. doi: 10.1038/nature04512. [DOI] [PubMed] [Google Scholar]
- 75.Chen W., Paradkar P. N., Li L., Pierce E. L., Langer N. B., Takahashi-Makise N., Hyde B. B., Shirihai O. S., Ward D. M., Kaplan J., Paw B. H. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc. Natl. Acad. Sci. U.S.A. 2009;106:16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paradkar P. N., Zumbrennen K. B., Paw B. H., Ward D. M., Kaplan J. Regulation of mitochondrial iron import through differential turnover of mitoferrin 1 and mitoferrin 2. Mol. Cell. Biol. 2009;29:1007–1016. doi: 10.1128/MCB.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Severance S., Hamza I. Trafficking of heme and porphyrins in metazoa. Chem. Rev. 2009;109:4596–4616. doi: 10.1021/cr9001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryter S. W., Tyrrell R. M. The heme synthesis and degradation pathways: role in oxidant sensitivity. Heme oxygenase has both pro- and antioxidant properties. Free Radical Biol. Med. 2000;28:289–309. doi: 10.1016/s0891-5849(99)00223-3. [DOI] [PubMed] [Google Scholar]
- 79.Ponka P. Tissue-specific regulation of iron metabolism and heme synthesis: distinct control mechanisms in erythroid cells. Blood. 1997;89:1–25. [PubMed] [Google Scholar]
- 80.Nilsson R., Schultz I. J., Pierce E. L., Soltis K. A., Naranuntarat A., Ward D. M., Baughman J. M., Paradkar P. N., Kingsley P. D., Culotta V. C., et al. Discovery of genes essential for heme biosynthesis through large-scale gene expression analysis. Cell Metab. 2009;10:119–130. doi: 10.1016/j.cmet.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. Structure, function, and formation of biological iron–sulfur clusters. Annu. Rev. Biochem. 2005;74:247–281. doi: 10.1146/annurev.biochem.74.082803.133518. [DOI] [PubMed] [Google Scholar]
- 82.Lill R. Function and biogenesis of iron–sulphur proteins. Nature. 2009;460:831–838. doi: 10.1038/nature08301. [DOI] [PubMed] [Google Scholar]
- 83.Ye H., Rouault T. A. Human iron–sulfur cluster assembly, cellular iron homeostasis, and disease. Biochemistry. 2010;49:4945–4956. doi: 10.1021/bi1004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Netz D. J., Pierik A. J., Stumpfig M., Muhlenhoff U., Lill R. The Cfd1–Nbp35 complex acts as a scaffold for iron–sulfur protein assembly in the yeast cytosol. Nat. Chem. Biol. 2007;3:278–286. doi: 10.1038/nchembio872. [DOI] [PubMed] [Google Scholar]
- 85.Keel S. B., Doty R. T., Yang Z., Quigley J. G., Chen J., Knoblaugh S., Kingsley P. D., De Domenico I., Vaughn M. B., Kaplan J. A heme export protein is required for red blood cell differentiation and iron homeostasis. Science. 2008;319:825–828. doi: 10.1126/science.1151133. [DOI] [PubMed] [Google Scholar]
- 86.Arosio P., Ingrassia R., Cavadini P. Ferritins: a family of molecules for iron storage, antioxidation and more. Biochim. Biophys. Acta. 2009;1790:589–599. doi: 10.1016/j.bbagen.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 87.Shi H., Bencze K. Z., Stemmler T. L., Philpott C. C. A cytosolic iron chaperone that delivers iron to ferritin. Science. 2008;320:1207–1210. doi: 10.1126/science.1157643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Y., Mikhael M., Xu D., Li Y., Soe-Lin S., Ning B., Li W., Nie G., Zhao Y., Ponka P. Lysosomal proteolysis is the primary degradation pathway for cytosolic ferritin and cytosolic ferritin degradation is necessary for iron exit. Antioxid. Redox Signaling. 2010;13:999–1009. doi: 10.1089/ars.2010.3129. [DOI] [PubMed] [Google Scholar]
- 89.Takagi H., Shi D., Ha Y., Allewell N. M., Theil E. C. Localized unfolding at the junction of three ferritin subunits. A mechanism for iron release? J. Biol. Chem. 1998;273:18685–18688. doi: 10.1074/jbc.273.30.18685. [DOI] [PubMed] [Google Scholar]
- 90.Mikhael M., Sheftel A. D., Ponka P. Ferritin does not donate its iron for haem synthesis in macrophages. Biochem. J. 2010;429:463–471. doi: 10.1042/BJ20100346. [DOI] [PubMed] [Google Scholar]
- 91.De Domenico I., Vaughn M. B., Li L., Bagley D., Musci G., Ward D. M., Kaplan J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006;25:5396–5404. doi: 10.1038/sj.emboj.7601409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Domenico I., Ward D. M., Kaplan J. Specific iron chelators determine the route of ferritin degradation. Blood. 2009;114:4546–4551. doi: 10.1182/blood-2009-05-224188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ferreira C., Bucchini D., Martin M. E., Levi S., Arosio P., Grandchamp B., Beaumont C. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 2000;275:3021–3024. doi: 10.1074/jbc.275.5.3021. [DOI] [PubMed] [Google Scholar]
- 94.Darshan D., Vanoaica L., Richman L., Beermann F., Kuhn L. C. Conditional deletion of ferritin H in mice induces loss of iron storage and liver damage. Hepatology. 2009;50:852–860. doi: 10.1002/hep.23058. [DOI] [PubMed] [Google Scholar]
- 95.Levi S., Cozzi A., Arosio P. Neuroferritinopathy: a neurodegenerative disorder associated with L-ferritin mutation. Best Pract. Res. Clin. Haematol. 2005;18:265–276. doi: 10.1016/j.beha.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 96.Levi S., Arosio P. Mitochondrial ferritin. Int. J. Biochem. Cell Biol. 2004;36:1887–1889. doi: 10.1016/j.biocel.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 97.Cazzola M., Invernizzi R., Bergamaschi G., Levi S., Corsi B., Travaglino E., Rolandi V., Biasiotto G., Drysdale J., Arosio P. Mitochondrial ferritin expression in erythroid cells from patients with sideroblastic anemia. Blood. 2003;101:1996–2000. doi: 10.1182/blood-2002-07-2006. [DOI] [PubMed] [Google Scholar]
- 98.Cohen L. A., Gutierrez L., Weiss A., Leichtmann-Bardoogo Y., Zhang D. L., Crooks D. R., Sougrat R., Morgenstern A., Galy B., Hentze M. W., et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116:1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 99.Breuer W., Shvartsman M., Cabantchik Z. I. Intracellular labile iron. Int. J. Biochem. Cell Biol. 2008;40:350–354. doi: 10.1016/j.biocel.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 100.Petrat F., de Groot H., Sustmann R., Rauen U. The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 2002;383:489–502. doi: 10.1515/BC.2002.051. [DOI] [PubMed] [Google Scholar]
- 101.Rauen U., Springer A., Weisheit D., Petrat F., Korth H. G., de Groot H., Sustmann R. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. ChemBioChem. 2007;8:341–352. doi: 10.1002/cbic.200600311. [DOI] [PubMed] [Google Scholar]
- 102.Epsztejn S., Glickstein H., Picard V., Slotki I. N., Breuer W., Beaumont C., Cabantchik Z. I. H-Ferritin subunit overexpression in erythroid cells reduces the oxidative stress response and induces multidrug resistance properties. Blood. 1999;94:3593–3603. [PubMed] [Google Scholar]
- 103.Kaur D., Yantiri F., Rajagopalan S., Kumar J., Mo J. Q., Boonplueang R., Viswanath V., Jacobs R., Yang L., Beal M. F., et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 104.Kaur D., Rajagopalan S., Andersen J. K. Chronic expression of H-ferritin in dopaminergic midbrain neurons results in an age-related expansion of the labile iron pool and subsequent neurodegeneration: implications for Parkinson's disease. Brain Res. 2009;1297:17–22. doi: 10.1016/j.brainres.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rouault T. A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- 106.Recalcati S., Minotti G., Cairo G. Iron regulatory proteins: from molecular mechanisms to drug development. Antioxid. Redox Signaling. 2010;13:1593–1616. doi: 10.1089/ars.2009.2983. [DOI] [PubMed] [Google Scholar]
- 107.Wallander M. L., Leibold E. A., Eisenstein R. S. Molecular control of vertebrate iron homeostasis by iron regulatory proteins. Biochim. Biophys. Acta. 2006;1763:668–689. doi: 10.1016/j.bbamcr.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Piccinelli P., Samuelsson T. Evolution of the iron-responsive element. RNA. 2007;13:952–966. doi: 10.1261/rna.464807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roetto A., Bosio S., Gramaglia E., Barilaro M. R., Zecchina G., Camaschella C. Pathogenesis of hyperferritinemia cataract syndrome. Blood Cells Mol. Dis. 2002;29:532–535. doi: 10.1006/bcmd.2002.0590. [DOI] [PubMed] [Google Scholar]
- 110.Schranzhofer M., Schifrer M., Cabrera J. A., Kopp S., Chiba P., Beug H., Mullner E. W. Remodeling the regulation of iron metabolism during erythroid differentiation to ensure efficient heme biosynthesis. Blood. 2006;107:4159–4167. doi: 10.1182/blood-2005-05-1809. [DOI] [PubMed] [Google Scholar]
- 111.Lok C. N., Ponka P. Identification of an erythroid active element in the transferrin receptor gene. J. Biol. Chem. 2000;275:24185–24190. doi: 10.1074/jbc.M000944200. [DOI] [PubMed] [Google Scholar]
- 112.Torti F. M., Torti S. V. Regulation of ferritin genes and protein. Blood. 2002;99:3505–3516. doi: 10.1182/blood.v99.10.3505. [DOI] [PubMed] [Google Scholar]
- 113.Ponka P., Lok C. N. The transferrin receptor: role in health and disease. Int. J. Biochem. Cell Biol. 1999;31:1111–1137. doi: 10.1016/s1357-2725(99)00070-9. [DOI] [PubMed] [Google Scholar]
- 114.Wingert R. A., Galloway J. L., Barut B., Foott H., Fraenkel P., Axe J. L., Weber G. J., Dooley K., Davidson A. J., Schmidt B., et al. Deficiency of glutaredoxin 5 reveals Fe–S clusters are required for vertebrate haem synthesis. Nature. 2005;436:1035–1039. doi: 10.1038/nature03887. [DOI] [PubMed] [Google Scholar]
- 115.Ye H., Jeong S. Y., Ghosh M. C., Kovtunovych G., Silvestri L., Ortillo D., Uchida N., Tisdale J., Camaschella C., Rouault T. A. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J. Clin. Invest. 2010;120:1749–1761. doi: 10.1172/JCI40372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hubert N., Hentze M. W. Previously uncharacterized isoforms of divalent metal transporter (DMT)- 1: implications for regulation and cellular function. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12345–12350. doi: 10.1073/pnas.192423399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gunshin H., Mackenzie B., Berger U. V., Gunshin Y., Romero M. F., Boron W. F., Nussberger S., Gollan J. L., Hediger M. A. Cloning and characterization of a mammalian protein-coupled metal-ion transporter. Nature. 1997;388:482–488. doi: 10.1038/41343. [DOI] [PubMed] [Google Scholar]
- 118.Zhang D. L., Hughes R. M., Ollivierre-Wilson H., Ghosh M. C., Rouault T. A. A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab. 2009;9:461–473. doi: 10.1016/j.cmet.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abboud S., Haile D. J. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 2000;275:19906–19912. doi: 10.1074/jbc.M000713200. [DOI] [PubMed] [Google Scholar]
- 120.McKie A. T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T. J., Farzaneh F., et al. A novel duodenal iron-regulated transporter IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell. 2000;5:299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 121.Mok H., Jelinek J., Pai S., Cattanach B. M., Prchal J. T., Youssoufian H., Schumacher A. Disruption of ferroportin 1 regulation causes dynamic alterations in iron homeostasis and erythropoiesis in polycythaemia mice. Development. 2004;131:1859–1868. doi: 10.1242/dev.01081. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez M., Galy B., Muckenthaler M. U., Hentze M. W. Iron-regulatory proteins limit hypoxia-inducible factor-2α expression in iron deficiency. Nat. Struct. Mol. Biol. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- 123.Zimmer M., Ebert B. L., Neil C., Brenner K., Papaioannou I., Melas A., Tolliday N., Lamb J., Pantopoulos K., Golub T., Iliopoulos O. Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Mol. Cell. 2008;32:838–848. doi: 10.1016/j.molcel.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zimmer M., Lamb J., Ebert B. L., Lynch M., Neil C., Schmidt E., Golub T. R., Iliopoulos O. The connectivity map links iron regulatory protein-1-mediated inhibition of hypoxia-inducible factor-2a translation to the anti-inflammatory 15-deoxy-Δ12,14-prostaglandin J2. Cancer Res. 2010;70:3071–3079. doi: 10.1158/0008-5472.CAN-09-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rankin E. B., Biju M. P., Liu Q., Unger T. L., Rha J., Johnson R. S., Simon M. C., Keith B., Haase V. H. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J. Clin. Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gruber M., Hu C. J., Johnson R. S., Brown E. J., Keith B., Simon M. C. Acute postnatal ablation of Hif-2α results in anemia. Proc. Natl. Acad. Sci. U.S.A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cmejla R., Petrak J., Cmejlova J. A novel iron responsive element in the 3′UTR of human MRCKα. Biochem. Biophys. Res. Commun. 2006;341:158–166. doi: 10.1016/j.bbrc.2005.12.155. [DOI] [PubMed] [Google Scholar]
- 128.Sanchez M., Galy B., Dandekar T., Bengert P., Vainshtein Y., Stolte J., Muckenthaler M. U., Hentze M. W. Iron regulation and the cell cycle: identification of an iron-responsive element in the 3′-untranslated region of human cell division cycle 14A mRNA by a refined microarray-based screening strategy. J. Biol. Chem. 2006;281:22865–22874. doi: 10.1074/jbc.M603876200. [DOI] [PubMed] [Google Scholar]
- 129.Cho H. H., Cahill C. M., Vanderburg C. R., Scherzer C. R., Wang B., Huang X., Rogers J. T. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J. Biol. Chem. 2010;285:31217–31232. doi: 10.1074/jbc.M110.149161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Friedlich A. L., Tanzi R. E., Rogers J. T. The 5′-untranslated region of Parkinson's disease α-synuclein messenger RNA contains a predicted iron responsive element. Mol. Psychiatry. 2007;12:222–223. doi: 10.1038/sj.mp.4001937. [DOI] [PubMed] [Google Scholar]
- 131.Tang Y., Guest J. R. Direct evidence for mRNA binding and post-transcriptional regulation by Escherichia coli aconitases. Microbiology. 1999;145:3069–3079. doi: 10.1099/00221287-145-11-3069. [DOI] [PubMed] [Google Scholar]
- 132.Alen C., Sonenshein A. L. Bacillus subtilis aconitase is an RNA-binding protein. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10412–10417. doi: 10.1073/pnas.96.18.10412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dandekar T., Beyer K., Bork P., Kenealy M. -R., Pantopoulos K., Hentze M. W., Sonntag-Buck V., Flouriot G., Gannon F., Keller W., Schreiber S. Systematic genomic screening and analysis of mRNA in untranslated regions and mRNA precursors: combining experimental and computational approaches. Bioinformatics. 1998;14:271–278. doi: 10.1093/bioinformatics/14.3.271. [DOI] [PubMed] [Google Scholar]
- 134.Gruer M. J., Artymiuk P. J., Guest J. R. The aconitase family: three structural variations on a common theme. Trends Biochem. Sci. 1997;22:3–6. doi: 10.1016/s0968-0004(96)10069-4. [DOI] [PubMed] [Google Scholar]
- 135.Dycke C., Bougault C., Gaillard J., Andrieu J. P., Pantopoulos K., Moulis J. M. Human iron regulatory protein 2 is easily cleaved in its specific domain: consequences for the heme binding properties of the protein. Biochem. J. 2007;408:429–439. doi: 10.1042/BJ20070983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dupuy J., Volbeda A., Carpentier P., Darnault C., Moulis J. M., Fontecilla-Camps J. C. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006;14:129–139. doi: 10.1016/j.str.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 137.Walden W. E., Selezneva A. I., Dupuy J., Volbeda A., Fontecilla-Camps J. C., Theil E. C., Volz K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. doi: 10.1126/science.1133116. [DOI] [PubMed] [Google Scholar]
- 138.Zumbrennen K. B., Wallander M. L., Romney S. J., Leibold E. A. Cysteine oxidation regulates the RNA-binding activity of iron regulatory protein 2. Mol. Cell. Biol. 2009;29:2219–2229. doi: 10.1128/MCB.00004-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fillebeen C., Caltagirone A., Martelli A., Moulis J. M., Pantopoulos K. IRP1 Ser-711 is a phosphorylation site, critical for regulation of RNA-binding and aconitase activities. Biochem. J. 2005;388:143–150. doi: 10.1042/BJ20041623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Biederbick A., Stehling O., Rösser R., Niggemeyer B., Nakai Y., Elsässer H. P., Lill R. Role of human mitochondrial Nfs1 in cytosolic iron-sulfur protein biogenesis and iron regulation. Mol. Cell. Biol. 2006;26:5675–5687. doi: 10.1128/MCB.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Fosset C., Chauveau M. J., Guillon B., Canal F., Drapier J. C., Bouton C. RNA silencing of mitochondrial m-Nfs1 reduces Fe-S enzyme activity both in mitochondria and cytosol of mammalian cells. J. Biol. Chem. 2006;281:25398–25406. doi: 10.1074/jbc.M602979200. [DOI] [PubMed] [Google Scholar]
- 142.Wang J., Fillebeen C., Chen G., Biederbick A., Lill R., Pantopoulos K. Iron-dependent degradation of apo-IRP1 by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 2007;27:2423–2430. doi: 10.1128/MCB.01111-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stehling O., Elsasser H. P., Bruckel B., Muhlenhoff U., Lill R. Iron–sulfur protein maturation in human cells: evidence for a function of frataxin. Hum. Mol. Genet. 2004;13:3007–3015. doi: 10.1093/hmg/ddh324. [DOI] [PubMed] [Google Scholar]
- 144.Seznec H., Simon D., Bouton C., Reutenauer L., Hertzog A., Golik P., Procaccio V., Patel M., Drapier J. C., Koenig M., Puccio H. Friedreich ataxia: the oxidative stress paradox. Hum. Mol. Genet. 2005;14:463–474. doi: 10.1093/hmg/ddi042. [DOI] [PubMed] [Google Scholar]
- 145.Tong W. H., Rouault T. A. Functions of mitochondrial ISCU and cytosolic ISCU in mammalian iron–sulfur cluster biogenesis and iron homeostasis. Cell Metab. 2006;3:199–210. doi: 10.1016/j.cmet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 146.Shi Y., Ghosh M., Tong W. H., Rouault T. A. Human ISD11 is essential for both iron–sulfur cluster assembly and maintenance of normal cellular iron homeostasis. Hum. Mol. Genet. 2009;18:3014–3025. doi: 10.1093/hmg/ddp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pondarre C., Antiochos B. B., Campagna D. R., Clarke S. L., Greer E. L., Deck K. M., McDonald A., Han A. P., Medlock A., Kutok J. L., et al. The mitochondrial ATP-binding cassette transporter Abcb7 is essential in mice and participates in cytosolic iron–sulfur cluster biogenesis. Hum. Mol. Genet. 2006;15:953–964. doi: 10.1093/hmg/ddl012. [DOI] [PubMed] [Google Scholar]
- 148.Bouton C., Chauveau M. J., Lazereg S., Drapier J. C. Recycling of RNA binding iron regulatory protein 1 into an aconitase after nitric oxide removal depends on mitochondrial ATP. J. Biol. Chem. 2002;277:31220–31227. doi: 10.1074/jbc.M203276200. [DOI] [PubMed] [Google Scholar]
- 149.Popovic Z., Templeton D. M. Interaction of iron regulatory protein-1 (IRP-1) with ATP/ADP maintains a non-IRE-binding state. Biochem. J. 2010;430:315–324. doi: 10.1042/BJ20100111. [DOI] [PubMed] [Google Scholar]
- 150.Li K., Tong W. H., Hughes R. M., Rouault T. A. Roles of the mammalian cytosolic cysteine desulfurase, ISCS, and scaffold protein, ISCU, in iron–sulfur cluster assembly. J. Biol. Chem. 2006;281:12344–12351. doi: 10.1074/jbc.M600582200. [DOI] [PubMed] [Google Scholar]
- 151.Roy A., Solodovnikova N., Nicholson T., Antholine W., Walden W. E. A novel eukaryotic factor for cytosolic Fe–S cluster assembly. EMBO J. 2003;22:4826–4835. doi: 10.1093/emboj/cdg455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stehling O., Netz D. J., Niggemeyer B., Rosser R., Eisenstein R. S., Puccio H., Pierik A. J., Lill R. Human Nbp35 is essential for both cytosolic iron–sulfur protein assembly and iron homeostasis. Mol. Cell. Biol. 2008;28:5517–5528. doi: 10.1128/MCB.00545-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Song D., Lee F. S. A role for IOP1 in mammalian cytosolic iron–sulfur protein biogenesis. J. Biol. Chem. 2008;283:9231–9238. doi: 10.1074/jbc.M708077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Balk J., Aguilar Netz D. J., Tepper K., Pierik A. J., Lill R. The essential WD40 protein Cia1 is involved in a late step of cytosolic and nuclear iron–sulfur protein assembly. Mol. Cell. Biol. 2005;25:10833–10841. doi: 10.1128/MCB.25.24.10833-10841.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y., Lyver E. R., Nakamaru-Ogiso E., Yoon H., Amutha B., Lee D. W., Bi E., Ohnishi T., Daldal F., Pain D., Dancis A. Dre2, a conserved eukaryotic Fe/S cluster protein, functions in cytosolic Fe/S protein biogenesis. Mol. Cell. Biol. 2008;28:5569–5582. doi: 10.1128/MCB.00642-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Netz D. J., Stumpfig M., Dore C., Muhlenhoff U., Pierik A. J., Lill R. Tah18 transfers electrons to Dre2 in cytosolic iron–sulfur protein biogenesis. Nat. Chem. Biol. 2010;6:758–765. doi: 10.1038/nchembio.432. [DOI] [PubMed] [Google Scholar]
- 157.Clarke S. L., Vasanthakumar A., Anderson S. A., Pondarre C., Koh C. M., Deck K. M., Pitula J. S., Epstein C. J., Fleming M. D., Eisenstein R. S. Iron-responsive degradation of iron-regulatory protein 1 does not require the Fe–S cluster. EMBO J. 2006;25:544–553. doi: 10.1038/sj.emboj.7600954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fillebeen C., Chahine D., Caltagirone A., Segal P., Pantopoulos K. A phosphomimetic mutation at Ser-138 renders iron regulatory protein 1 sensitive to iron-dependent degradation. Mol. Cell. Biol. 2003;23:6973–6981. doi: 10.1128/MCB.23.19.6973-6981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Meyron-Holtz E. G., Ghosh M. C., Rouault T. A. Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science. 2004;306:2087–2090. doi: 10.1126/science.1103786. [DOI] [PubMed] [Google Scholar]
- 160.Deck K. M., Vasanthakumar A., Anderson S. A., Goforth J. B., Kennedy M. C., Antholine W. E., Eisenstein R. S. Evidence that phosphorylation of iron regulatory protein 1 at serine 138 destabilizes the [4Fe–4S] cluster in cytosolic aconitase by enhancing 4Fe–3Fe cycling. J. Biol. Chem. 2009;284:12701–12709. doi: 10.1074/jbc.M807717200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pitula J. S., Deck K. M., Clarke S. L., Anderson S. A., Vasanthakumar A., Eisenstein R. S. Selective inhibition of the citrate-to-isocitrate reaction of cytosolic aconitase by phosphomimetic mutation of serine-711. Proc. Natl. Acad. Sci. U.S.A. 2004;101:10907–10912. doi: 10.1073/pnas.0404308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Patton S. M., Pinero D. J., Surguladze N., Beard J., Connor J. R. Subcellular localization of iron regulatory proteins to Golgi and ER membranes. J. Cell Sci. 2005;118:4365–4373. doi: 10.1242/jcs.02570. [DOI] [PubMed] [Google Scholar]
- 163.Hanson E. S., Rawlins M. L., Leibold E. A. Oxygen and iron regulation of iron regulatory protein 2. J. Biol. Chem. 2003;278:40337–40342. doi: 10.1074/jbc.M302798200. [DOI] [PubMed] [Google Scholar]
- 164.Wang J., Chen G., Muckenthaler M., Galy B., Hentze M. W., Pantopoulos K. Iron-mediated degradation of IRP2: an unexpected pathway involving a 2-oxoglutarate-dependent oxygenase activity. Mol. Cell. Biol. 2004;24:954–965. doi: 10.1128/MCB.24.3.954-965.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang J., Chen G., Lee J., Pantopoulos K. Iron-dependent degradation of IRP2 requires its C-terminal region and IRP structural integrity. BMC Mol. Biol. 2008;9:15. doi: 10.1186/1471-2199-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bourdon E., Kang D. K., Ghosh M. C., Drake S. K., Wey J., Levine R. L., Rouault T. A. The role of endogenous heme synthesis and degradation domain cysteines in cellular iron-dependent degradation of IRP2. Blood Cells Mol. Dis. 2003;31:247–255. doi: 10.1016/s1079-9796(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 167.Wang J., Fillebeen C., Chen G., Andriopoulos B., Pantopoulos K. Sodium nitroprusside promotes IRP2 degradation via an increase in intracellular iron and in the absence of S-nitrosylation at C178. Mol. Cell. Biol. 2006;26:1948–1954. doi: 10.1128/MCB.26.5.1948-1954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kim S., Wing S. S., Ponka P. S-nitrosylation of IRP2 regulates its stability via the ubiquitin–proteasome pathway. Mol. Cell. Biol. 2004;24:330–337. doi: 10.1128/MCB.24.1.330-337.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vashisht A. A., Zumbrennen K. B., Huang X., Powers D. N., Durazo A., Sun D., Bhaskaran N., Persson A., Uhlen M., Sangfelt O., et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science. 2009;326:718–721. doi: 10.1126/science.1176333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Salahudeen A. A., Thompson J. W., Ruiz J. C., Ma H. W., Kinch L. N., Li Q., Grishin N. V., Bruick R. K. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science. 2009;326:722–726. doi: 10.1126/science.1176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.French C. E., Bell J. M., Ward F. B. Diversity and distribution of hemerythrin-like proteins in prokaryotes. FEMS Microbiol. Lett. 2008;279:131–145. doi: 10.1111/j.1574-6968.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- 172.Wallander M. L., Zumbrennen K. B., Rodansky E. S., Romney S. J., Leibold E. A. Iron-independent phosphorylation of iron regulatory protein 2 regulates ferritin during the cell cycle. J. Biol. Chem. 2008;283:23589–23598. doi: 10.1074/jbc.M803005200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fillebeen C., Pantopoulos K. Redox control of iron regulatory proteins. Redox Rep. 2002;7:15–22. doi: 10.1179/135100002125000136. [DOI] [PubMed] [Google Scholar]
- 174.Mütze S., Hebling U., Stremmel W., Wang J., Arnhold J., Pantopoulos K., Mueller S. Myeloperoxidase-derived hypochlorous acid antagonizes the oxidative stress-mediated activation of iron regulatory protein 1. J. Biol. Chem. 2003;278:40542–40549. doi: 10.1074/jbc.M307159200. [DOI] [PubMed] [Google Scholar]
- 175.Watts R. N., Hawkins C., Ponka P., Richardson D. R. Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc. Natl. Acad. Sci. U.S.A. 2006;103:7670–7675. doi: 10.1073/pnas.0602515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang J., Chen G., Pantopoulos K. Nitric oxide inhibits the degradation of IRP2. Mol. Cell. Biol. 2005;25:1347–1353. doi: 10.1128/MCB.25.4.1347-1353.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176a.Hausmann A., Lee J., Pantopoulos K. Redox control of iron regulatory protein 2 stability. FEBS Lett. 2011 doi: 10.1016/j.febslet.2011.01.036. doi:101016/j.febslet.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 177.Caltagirone A., Weiss G., Pantopoulos K. Modulation of cellular iron metabolism by hydrogen peroxide. Effects of H2O2 on the expression and function of iron-responsive element-containing mRNAs in B6 fibroblasts. J. Biol. Chem. 2001;276:19738–19745. doi: 10.1074/jbc.M100245200. [DOI] [PubMed] [Google Scholar]
- 178.Andriopoulos B., Hegedusch S., Mangin J., Riedel H. D., Hebling U., Wang J., Pantopoulos K., Mueller S. Sustained hydrogen peroxide induces iron uptake by transferrin receptor-1 independent of the IRP/IRE network. J. Biol. Chem. 2007;282:20301–20308. doi: 10.1074/jbc.M702463200. [DOI] [PubMed] [Google Scholar]
- 179.Mehlhase J., Sandig G., Pantopoulos K., Grune T. Oxidation-induced ferritin turnover in microglial cells: role of proteasome. Free Radical Biol. Med. 2005;38:276–285. doi: 10.1016/j.freeradbiomed.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 180.Gerald D., Berra E., Frapart Y. M., Chan D. A., Giaccia A. J., Mansuy D., Pouyssegur J., Yaniv M., Mechta-Grigoriou F. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 181.Salahudeen A. A., Bruick R. K. Maintaining Mammalian iron and oxygen homeostasis: sensors, regulation, and cross-talk. Ann. N. Y. Acad. Sci. 2009;1177:30–38. doi: 10.1111/j.1749-6632.2009.05038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hofer T., Wenger R. H., Kramer M. F., Ferreira G. C., Gassmann M. Hypoxic up-regulation of erythroid 5-aminolevulinate synthase. Blood. 2003;101:348–350. doi: 10.1182/blood-2002-03-0773. [DOI] [PubMed] [Google Scholar]
- 183.Choi S. O., Cho Y. S., Kim H. L., Park J. W. ROS mediate the hypoxic repression of the hepcidin gene by inhibiting C/EBPα and STAT-3. Biochem. Biophys. Res. Commun. 2007;356:312–317. doi: 10.1016/j.bbrc.2007.02.137. [DOI] [PubMed] [Google Scholar]
- 184.Harrison-Findik D. D., Schafer D., Klein E., Timchenko N. A., Kulaksiz H., Clemens D., Fein E., Andriopoulos B., Pantopoulos K., Gollan J. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006;281:22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 185.Smith S. R., Ghosh M. C., Ollivierre-Wilson H., Hang Tong W., Rouault T. A. Complete loss of iron regulatory proteins 1 and 2 prevents viability of murine zygotes beyond the blastocyst stage of embryonic development. Blood Cells Mol. Dis. 2006;36:283–287. doi: 10.1016/j.bcmd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 186.Galy B., Ferring-Appel D., Kaden S., Grone H. J., Hentze M. W. Iron regulatory proteins are essential for intestinal function and control key iron absorption molecules in the duodenum. Cell Metab. 2008;7:79–85. doi: 10.1016/j.cmet.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 187.Galy B., Ferring-Appel D., Sauer S. W., Kaden S., Lyoumi S., Puy H., Kolker S., Grone H. J., Hentze M. W. Iron regulatory proteins secure mitochondrial iron sufficiency and function. Cell Metab. 2010;12:194–201. doi: 10.1016/j.cmet.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 188.Muckenthaler M. U., Galy B., Hentze M. W. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 189.Meyron-Holtz E. G., Ghosh M. C., Iwai K., LaVaute T., Brazzolotto X., Berger U. V., Land W., Ollivierre-Wilson H., Grinberg A., Love P., Rouault T. A. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. EMBO J. 2004;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Viatte L., Grone H. J., Hentze M. W., Galy B. In vivo role(s) of the iron regulatory proteins (IRP) 1 and 2 in aseptic local inflammation. J. Mol. Med. 2009;87:913–921. doi: 10.1007/s00109-009-0494-8. [DOI] [PubMed] [Google Scholar]
- 191.Corna G., Galy B., Hentze M. W., Cairo G. IRP1-independent alterations of cardiac iron metabolism in doxorubicin-treated mice. J. Mol. Med. 2006;84:551–560. doi: 10.1007/s00109-006-0068-y. [DOI] [PubMed] [Google Scholar]
- 192.Tong W. H., Rouault T. A. Metabolic regulation of citrate and iron by aconitases: role of iron–sulfur cluster biogenesis. Biometals. 2007;20:549–564. doi: 10.1007/s10534-006-9047-6. [DOI] [PubMed] [Google Scholar]
- 193.Cooperman S. S., Meyron-Holtz E. G., Olivierre-Wilson H., Ghosh M. C., McConnell J. P., Rouault T. A. Microcytic anemia, erythropoietic protoporphyria and neurodegeneration in mice with targeted deletion of iron regulatory protein 2. Blood. 2005;106:1084–1091. doi: 10.1182/blood-2004-12-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Galy B., Ferring D., Minana B., Bell O., Janser H. G., Muckenthaler M., Schumann K., Hentze M. W. Altered body iron distribution and microcytosis in mice deficient for iron regulatory protein 2 (IRP2) Blood. 2005;106:2580–2589. doi: 10.1182/blood-2005-04-1365. [DOI] [PubMed] [Google Scholar]
- 195.Ferring-Appel D., Hentze M. W., Galy B. Cell-autonomous and systemic context-dependent functions of iron regulatory protein 2 in mammalian iron metabolism. Blood. 2009;113:679–687. doi: 10.1182/blood-2008-05-155093. [DOI] [PubMed] [Google Scholar]
- 196.LaVaute T., Smith S., Cooperman S., Iwai K., Land W., Meyron-Holtz E., Drake S. K., Miller G., Abu-Asab M., Tsokos M., et al. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nat. Genet. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- 197.Ghosh M. C., Ollivierre-Wilson H., Cooperman S., Rouault T. A. Reply to Iron homeostasis in the brain: complete iron regulatory protein 2 deficiency without symptomatic neurodegeneration in the mouse. Nat. Genet. 2006;38:969–970. doi: 10.1038/ng0906-967. [DOI] [PubMed] [Google Scholar]
- 198.Smith S. R., Cooperman S., Lavaute T., Tresser N., Ghosh M., Meyron-Holtz E., Land W., Ollivierre H., Jortner B., Switzer R., III, et al. Severity of neurodegeneration correlates with compromise of iron metabolism in mice with iron regulatory protein deficiencies. Ann. N. Y. Acad. Sci. 2004;1012:65–83. doi: 10.1196/annals.1306.006. [DOI] [PubMed] [Google Scholar]
- 199.Ghosh M. C., Tong W. H., Zhang D., Ollivierre-Wilson H., Singh A., Krishna M. C., Mitchell J. B., Rouault T. A. Tempol-mediated activation of latent iron regulatory protein activity prevents symptoms of neurodegenerative disease in IRP2 knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12028–12033. doi: 10.1073/pnas.0805361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Galy B., Hölter S. M., Klopstock T., Ferring D., Becker L., Kaden S., Wurst W., Gröne H.-J., Hentze M. W. Iron homeostasis in the brain: complete iron regulatory protein 2 deficiency without symptomatic neurodegeneration in the mouse. Nat. Genet. 2006;38:967–969. doi: 10.1038/ng0906-967. [DOI] [PubMed] [Google Scholar]
- 201.Mueller C., Magaki S., Schrag M., Ghosh M. C., Kirsch W. M. Iron regulatory protein 2 is involved in brain copper homeostasis. J. Alzheimers Dis. 2009;18:201–210. doi: 10.3233/JAD-2009-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chen G., Fillebeen C., Wang J., Pantopoulos K. Overexpression of iron regulatory protein 1 suppresses growth of tumor xenografts. Carcinogenesis. 2007;28:785–791. doi: 10.1093/carcin/bgl210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maffettone C., Chen G., Drozdov I., Ouzounis C., Pantopoulos K. Tumorigenic properties of iron regulatory protein 2 (IRP2) mediated by its specific 73-amino acids insert. PLoS ONE. 2010;5:e10163. doi: 10.1371/journal.pone.0010163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang J., Pantopoulos K. Conditional de-repression of ferritin synthesis in cells expressing a constitutive IRP1 mutant. Mol. Cell. Biol. 2002;22:4638–4651. doi: 10.1128/MCB.22.13.4638-4651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wu K. J., Polack A., Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science. 1999;283:676–679. doi: 10.1126/science.283.5402.676. [DOI] [PubMed] [Google Scholar]