Abstract
Symptom control in patients with moderate to severe persistent asthma is essential to reduce the significant morbidity associated with the disease. Poor adherence to controller medications has been identified as a major contributing factor to the high level of uncontrolled asthma. This review examines patient perspectives on, and preferences for, controller medications (inhaled corticosteroid and long-acting β2-agonist combinations [ICS/LABA]), and how this may affect adherence to therapy. Fluticasone/salmeterol and budesonide/formoterol, the currently available ICS/LABA combination products, have similar efficacy and tolerability based on a recent meta-analysis of asthma trials. Adherence is higher with the combination ICS/LABAs than when the components are administered separately. Investigations into patient preferences for desirable attributes of asthma medications indicate that an effective reliever with a fast onset and long duration of action is preferred and may lead to improved adherence. This rapid onset of effect was perceived and highly valued in patient surveys, and was associated with greater patient satisfaction. Thus, future research should be directed at therapy that offers both anti-inflammatory activity and a rapid onset of bronchodilator effect. To further improve patient adherence and treatment outcome, the effect of these characteristics as well as other factors on adherence should also be investigated.
Keywords: budesonide/formoterol, fluticasone/salmeterol, adherence, onset of effect, patient satisfaction
Clinician and patient perspectives on asthma control
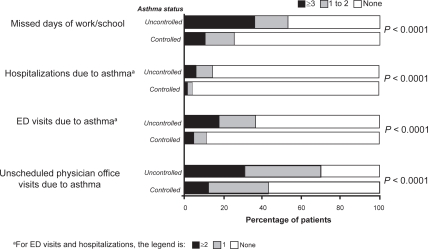
The overarching goal of asthma management is to achieve and maintain asthma control. Moderate to severe persistent asthma is associated with substantial morbidity if not adequately controlled.1 Despite the availability of evidence-based strategies for maximizing asthma control, the results of a national Web-based survey of 1812 adults with persistent asthma indicated that 55% had inadequate control of their asthma (Figure 1).2 While this shortcoming may be due in part to the limitations of current therapy, the results of the Gaining Optimal Asthma ControL (GOAL) study3 have shown that most patients can achieve and maintain guideline-defined control. Using a dose escalation strategy, patients with a wide range of asthma severity achieved control; at 1 year, 41% of patients had totally controlled asthma with inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) combination therapy versus 28% with ICS alone, while 71% maintained at least well-controlled asthma with ICS/LABA versus 59% with ICS alone.3 In addition, patients who achieved totally or well-controlled asthma for 8 weeks maintained a high level of asthma stability, whereas patients whose asthma was not well controlled had less stable levels of control and were more likely to make unscheduled use of healthcare resources.4 As the goal of treatment was to achieve total asthma control, most patients were receiving the maximum permitted dose of ICS or ICS/LABA by the end of the study. Although the study was not designed to establish a clinical model for the treatment of asthma, the results indicated that optimal control was most likely to occur with ICS/LABA combination therapy.
Figure 1.
Healthcare use and missed work/school in the past year in 809 patients with controlled asthma and 1003 patients with uncontrolled asthma. Drawn from data of Peters et al.2
Abbreviation: ED, emergency department.
Although it is recognized that asthma control cannot be obtained in all patients,3 poor adherence to prescribed controller medications is a significant contributor to the high level of uncontrolled asthma.5–7 Findings from a retrospective analysis of a large healthcare database showed that almost half of the patients with asthma failed to renew their prescription for controller medication in the first year of treatment.8 The results of another retrospective study of 405 patients with asthma in a Michigan-based health maintenance organization revealed that adherence to ICS therapy during the 2-year observation period was only 50%, and lack of adherence to ICS therapy was responsible for 60% of all asthma-related hospitalizations.7
Another reason for suboptimal asthma control may be healthcare providers’ failure to implement asthma treatment guidelines. Despite concerted efforts over the past decade to raise awareness of the NHLBI guidelines among primary care physicians, several urban-based studies have reported poor provider adherence to key asthma treatment recommendations, both in the pediatric-adolescent9,10 and adult11,12 outpatient settings. A recent (2003 to 2007) survey of 202 adult primary healthcare providers in inner-city New York assessed their adherence to 5 NHLBI guideline components.12 The survey results revealed suboptimal levels of implementation of peak flow monitoring, action plans, and referral for allergy testing, as reflected by adherence rates of 34%, 9%, and 10%, respectively.12 These shortcomings were attributed to the physicians’ lack of confidence in their ability to effectively implement the guideline recommendations and their low expectations of treatment outcome.12
Medication adherence is a complex issue, influenced by factors related to patients, healthcare providers, disease severity, and therapy. Improving adherence requires an approach that addresses these multiple factors.5,13 Findings from several studies have shown the need for patient education about the importance of daily use of controller medication even when symptoms are absent or not bothersome.14–19 Patients who have a “no symptoms, no asthma” belief have been shown to be significantly less likely to use ICS when they are asymptomatic and are less likely to engage in other self-management behaviors.20
Conversely, certain patient beliefs and perspectives may promote adherence to controller medication. For example, adherence is higher in those who believe that their controller medication is effective.18 Likewise, patients prefer treatments with immediate and noticeable effects, since this provides assurance that their medication is working.14 The purpose of this review is to examine how patient perspectives on, and preferences for, controller medication affect treatment adherence. The review focuses on the ICS/LABA combination products recommended by the NHLBI and GINA guidelines for the treatment of moderate to severe persistent asthma.21,22
Combination ICS/LABA controller medications
LABA is recommended in combination with ICS for long-term control and prevention of symptoms in patients aged 5 years or older who have moderate or severe persistent asthma or asthma that is inadequately controlled by ICS alone. ICS and LABA can be administered via separate inhalers (concurrent therapy) or via a single inhaler containing both drugs. Two single-inhaler ICS/LABA therapies are currently available and have been demonstrated to be effective and well tolerated: budesonide in combination with formoterol,23–26 and fluticasone in combination with salmeterol.27–31 Fluticasone/salmeterol (100/50 μg) is indicated for use in children aged 4 to 11 years, as well as in adolescents and adults.32 Currently, budesonide/formoterol pressurized metered-dose inhaler (pMDI) is indicated for use in individuals aged 12 years or older in the United States.33 Outside the United States, budesonide/formoterol dry powder inhaler (DPI) 80/4.5 μg and 160/4.5 μg doses are approved for patients aged 4 years or older, and the 320/9 μg dose is indicated for patients aged 12 years or older.
There are several potential advantages to single-inhaler combination therapy. Preclinical research has suggested that a potential synergy between corticosteroids and β2-agonists at the cellular and molecular level may translate into clinical benefits if they are deposited in the lungs simultaneously.34 Furthermore, ICS nonadherence is less likely with the combination product in a single inhaler, because it removes any confusion or inconvenience associated with the use of 2 separate inhalers.
Comparative safety and efficacy of fixed-dose regimens: budesonide/formoterol versus fluticasone/salmeterol combination therapy
The safety and efficacy of fixed doses of budesonide/formoterol and fluticasone/salmeterol have been directly compared in several large, randomized, controlled trials,35–39 which formed the basis of a recently published Cochrane meta-analysis.40 Drawing on 5 randomized studies including data from 5537 adolescent and adult patients with asthma (Table 1), the meta-analysis found that budesonide/formoterol and fluticasone/salmeterol were equally effective and well tolerated, with no significant differences between treatments on primary efficacy outcomes (exacerbations requiring oral steroids or hospitalization; serious adverse events [including asthma-related deaths and intubation]) (Table 2) or secondary efficacy outcomes (exacerbations leading to emergency department visit/hospital admission, morning and evening peak flow, clinical spirometry measures, symptoms, reliever medication use, and tolerability).40
Table 1.
Randomized, controlled studies of fixed-dose budesonide/formoterol versus fluticasone/salmeterol combination therapy in asthma
| Study | Design | Patients | Treatments (FD arms) | Primary efficacy outcome | Safety outcomes |
|---|---|---|---|---|---|
| Aalbers et al36 | 4-week, randomized, double-blind, parallel-group 6-month, open-label, extension phase | Symptomatic patients aged ≥12 years with asthma ≥6 months Mean symptom score 1.5 Mean ICS 735 μg/day Mean FEV1 84% predicted |
BUD/FM 160/4.5 μg × 2 inhalations bid (n = 215) FP/SM: 250/50 μg × 1 inhalation bid (n = 224) |
Similar odds of achieving a well-controlled asthma week for FP/SM and BUD/FM (OR: 1.29; 95% CI: 0.98, 1.69) | Similar AE incidence Most mild to moderate in severity |
| Dahl et al38 | 24-week, randomized, double-blind, parallel group | Patients ≥18 years with asthma ≥6 months Mean FEV1 79% predicted Previous ICS use (BDP 1000–2000 μg/day or equivalent) |
BUD/FM 200/6 μg × 2 inhalations bid (n = 697) FP/SM: 250/50 μg × 1 inhalation bid (n = 694) |
Similar rates of exacerbations: FP/SM, 2.69; BUD/FM, 2.79 (OR: 0.96; 95% CI: 0.84, 1.10) |
Similar AE incidence Both drugs similarly well tolerated |
| Kuna et al39 | 6-month, randomized, double-blind, parallel-group | Symptomatic patients ≥12 years with asthma ≥6 months Mean FEV1 73% predicted Mean ICS 744–750 μg/day |
BUD/FM 320/9 μg × 1 inhalation bid (n = 1105) FP/SM: 125/25 μg × 2 inhalations bid (n = 1123) |
Similar rates of severe exacerbations for BUD/FMvs FP/SM (HR: 0.91; 95% CI: 0.72,1.16) | Similar AE incidence and severity Both drugs well tolerated |
| Busse et al37 | 7-month, randomized, open-label | Patients ≥12 years with moderate to severe persistent asthma ≥6 months Mean FEV1 ∼78% predicted Mean ICS ∼550 μg/day |
BUD/FM 160/4.5 μg × 2 inhalations bid (n = 427) FP/SM: 250/50 μg × 1 inhalation bid (n = 406) |
Similar times to first asthma exacerbation for BUD/FM vs FP/SM (HR: 0.99; 95% CI: 0.79, 1.25) | Similar AE pattern; mostly mild or moderate severity Both drugs well tolerated |
| SAM4004835 | 12-week, randomized, double-blind, parallel group | Symptomatic patients >18 years with moderate asthma Mean symptom score ≥1 Mean FEV1 65% predicted Previous ICS use (BDP 1000 μg/day or equivalent) |
BUD/FM 200/6 μg × 1 inhalation bid (n = 127) FP/SM: 250/50 μg × 1 inhalation bid (n = 121) |
Similar changes in Fev1 % predicted after 12 weeks, mean (SD): BUD/FM, 10.9 (13.3); FP/SM, 14.0 (16.4) | Similar AE incidence |
Abbreviations: AE, adverse event; BDP, beclomethasone dipropionate; BUD, budesonide; CI, confidence interval; FD, fixed dose; FEV1, forced expiratory volume in 1 second; FM, formoterol; FP, fluticasone propionate; HR, hazard ratio; ICS, inhaled corticosteroid; OR, odds ratio; SD, standard deviation; SM, salmeterol.
Table 2.
Primary outcomes of meta-analysis comparing the efficacy and safety of fluticasone/salmeterol versus budesonide/formoterol combination therapy40
| Outcome | Study | Fluticasone/salmeterol n/N (%) | Budesonide/formoterol n/N (%) | Odds ratio (95% CI) |
|---|---|---|---|---|
| Exacerbations requiring oral steroid treatment | Busse et al37 | 37/404 (9.2) | 37/422 (9.2) | 1.05 (0.65, 1.69) |
| Kuna et al39 | 109/1199 (9.1) | 108/1099 (9.8) | 0.92 (0.69, 1.21) | |
| Dahl et al38 | 63/694 (9.1) | 79/697 (11.3) | 0.78 (0.55, 1.11) | |
| Total | 209/2297 (9.1) | 224/2218 (10.1) | 0.89 (0.73, 1.09) | |
| Exacerbations requiring hospitalization | Aalbers et al36 | 0/219 (0.0) | 1/215 (0.5) | 0.33 (0.01, 8.04) |
| Busse et al37 | 2/404 (0.5) | 1/422 (0.2) | 2.09 (0.19, 23.19) | |
| Kuna et al39 | 15/1123 (1.2) | 13/1105 (1.2) | 1.14 (0.54, 2.40) | |
| Dahl et al38 | 4/694 (0.6) | 1/697 (0.1) | 4.03 (0.45, 36.19) | |
| Total | 21/2440 (0.8) | 16/2439 (0.7) | 1.29 (0.68, 2.47) | |
| Asthma-related serious AEs | Aalbers et al36 | 0/224 (0.0) | 1/215 (0.5) | 0.32 (0.01, 7.86) |
| Kuna et al39 | 15/1119 (1.3) | 12/1099 (1.1) | 1.23 (0.57, 2.64) | |
| Dahl et al38 | 6/697 (0.9) | 1/700 (0.1) | 6.07 (0.73, 50.55) | |
| Total | 21/2040 (1.0) | 14/2014 (0.7) | 1.47 (0.75, 2.86) |
Notes: Odds ratio represents the event risk for fluticasone/salmeterol relative to budesonide/formoterol.
Abbreviations: AEs, adverse events; CI, confidence interval.
Onset of bronchodilator action of fixed-dose regimens: budesonide/formoterol versus fluticasone/salmeterol combination therapy
One characteristic that differs between budesonide/formoterol and fluticasone/salmeterol with the potential to influence patient preference is the onset of effect. Comparative trials have shown that formoterol has a rapid onset of effect, similar to that of albuterol, and significantly more rapid than that of salmeterol.41,42 Similar results have been found in randomized, crossover studies with the combination inhalers budesonide/formoterol and fluticasone/salmeterol. When both combinations were delivered by DPI, budesonide/formoterol (160/4.5 μg and 320/9 μg) showed a significantly faster onset of bronchodilatory action than fluticasone/salmeterol (250/50 μg), as measured by mean forced expiratory volume in 1 second (FEV1) at 3 minutes after inhalation.43,44 Likewise, a comparison of the bronchodilatory effects of budesonide/formoterol pMDI (160/9 μg), fluticasone/salmeterol DPI (250/50 μg), and albuterol pMDI (180 μg) showed that the improvement in FEV1 observed at 3 minutes after dosing was significantly greater with budesonide/formoterol pMDI than with fluticasone/salmeterol DPI, and similar to that with albuterol pMDI.45
ICS/LABA combination therapy: patient adherence, satisfaction, and preferences
Adherence to ICS/LABA combination therapy
Use of combination ICS/LABA therapy may help with patient nonadherence. Administration of the ICS/LABA combination in a single inhaler simplifies the treatment regimen and improves patient adherence, as shown by analyses of medical and pharmacy claims from a large managed care organization and Medicaid patients.46,47 In both analyses, patient adherence was significantly greater with fluticasone/salmeterol administered in a single inhaler than with the individual drug components administered separately. In 1 analysis, short-acting β2-agonist (SABA) use was significantly lower when combination fluticasone/salmeterol therapy was administered in a single inhaler than when administered separately;47 however, this finding was not seen in a study by Stempel and colleagues.46 Retrospective analysis of matched cohort data from a Canadian provincial healthcare and public drug insurance plan database revealed that combination ICS/LABA therapy was associated with significantly greater persistence, better treatment adherence, fewer asthma exacerbations, and lower use of SABAs than concurrent inhaler therapy.8 These studies are retrospective and based on pharmacy claims data and not direct measures of adherence.
Patient satisfaction with ICS/LABA combination therapy
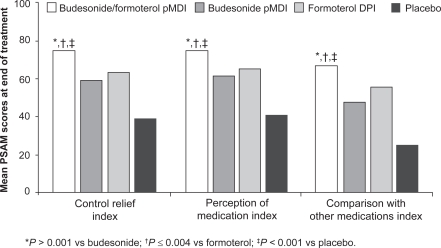
Satisfaction with treatment is an important factor in a patient’s resolve to continue daily asthma medication. A search of the medical literature on patient preference or satisfaction with ICS/LABA therapy was conducted using PubMed, EMBASE, and ISI Web of Knowledge (Thomson Reuters), as well as a search of clinicaltrials.gov for ongoing trials, revealing overall limited information. Patient satisfaction with ICS/LABA combination therapy administered from a single inhaler was investigated in 2 randomized, double-blind, placebo-controlled, 12-week studies of budesonide/formoterol pMDI.48,49 In the first study, conducted in 405 patients with mild to moderate persistent asthma, budesonide/formoterol pMDI 160/9 μg bid was compared with budesonide pMDI 160 μg bid and formoterol DPI 9 μg bid.49 Patient satisfaction was assessed using the Patient Satisfaction with Asthma Medication (PSAM) questionnaire,50 a validated, 23-item, asthma-specific instrument covering 4 domains: perception of medication index, control relief index, comparison with other medications index, and inhaler index (this last domain was not assessed, because all patients used both types of inhalers [pMDI and DPI]). The perception of medication index assesses satisfaction with medical benefits received, overall perception, influence on sense of well being, and whether a patient would recommend the treatment to others. The control relief index rates the onset, degree, and duration of symptom relief, and the patient’s willingness to continue treatment. The comparison with other medications index compares the current treatment versus all other inhaled medications for onset, degree, and duration of relief. Scores range from 0 to 100, with 0 representing the lowest level of satisfaction and 100 representing the highest.50
After 12 weeks of treatment, mean PSAM scores were significantly higher with budesonide/formoterol pMDI than with budesonide pMDI or formoterol DPI on all 3 assessed indices (Figure 2).49 A greater proportion of patients also reported higher satisfaction with budesonide/formoterol pMDI than with the other treatments on the individual items within each of the 3 assessed PSAM indices. These results suggest not only that treatment with budesonide/formoterol pMDI leads to greater satisfaction with asthma medication, but also that both components contribute to this improvement.49
Figure 2.
Mean Patient Satisfaction with Asthma Medication (PSAM) index scores at the end of 12 weeks of treatment with budesonide/formoterol, budesonide alone, formoterol alone, or placebo. Drawn from data of Murphy et al.49
Abbreviations: DPI, dry powder inhaler; pMDI, pressurized metered-dose inhaler.
In the second study, patient-reported outcomes were compared in 553 adults with moderate to severe persistent asthma who were randomized to treatment with budesonide/formoterol pMDI 320/9 μg bid (administered via a single inhaler), budesonide pMDI 320 μg bid plus formoterol DPI 9 μg bid (administered consecutively via 2 separate inhalers), budesonide pMDI 320 μg bid, formoterol DPI 9 μg bid, or placebo.48 Patient health-related quality of life was assessed using the standardized Asthma Quality of Life Questionnaire (AQLQ[S]),51 the Medical Outcomes Survey (MOS) Sleep Scale,52 and the PSAM questionnaire. No significant differences in patient-related outcomes were noted between budesonide/formoterol and budesonide plus formoterol (ie, between combination therapy administered from a single inhaler versus 2 separate inhalers); however, end-of-treatment improvements in AQLQ(S) overall and domain scores and in PSAM scores for control relief and perception of medication were significantly greater with budesonide/formoterol pMDI than with the individual components.
Patient preferences for attributes of asthma therapy
Conjoint analysis is a technique used in market research to investigate the relative importance of groups of attributes of consumer products, and it can also be used to analyze patient preferences for various treatment options.53 It provides a method for comparing the value to the patient of desirable attributes (eg, efficacy) versus undesirable ones (eg, high cost) and for identifying which factors influence patient preference for one regimen over another. Patient preference for attributes of asthma medication was investigated using conjoint analysis in a Swedish study involving 298 adult patients with asthma receiving ICS plus SABA, ICS plus LABA, or ICS/LABA.54 Different combinations of 6 attributes of asthma treatment were rated by questionnaire with predefined levels for each attribute. These included: (1) type of maintenance treatment (ICS alone, LABA + ICS, or ICS/LABA in a single inhaler); (2) need for additional inhaler for acute symptom relief (yes/no); (3) time to onset of action of reliever (≤3 minutes, 10 to 15 minutes); (4) duration of action of reliever (3 to 6 hours, ≥12 hours); (5) number of symptom-free days per month (<10, 10 to 14, 15 to 20, >20); and (6) out-of-pocket costs per month (Sk100, 240, 380, 520). Conjoint analysis showed that patients focused primarily on the effectiveness of treatment. While the most important aspect of asthma maintenance treatment was the number of symptom-free days, this was found only when the number of symptom-free days increased from <10 to >20 per month. The highest-ranked preferences were treatment with a reliever with fast onset and long duration of action (preferred by 78% of patients) and treatment with an ICS/LABA combination inhaler rather than separate inhalers (preferred by 50% of patients). The preferred asthma regimen was an ICS/LABA combination that could be used for both controller and reliever therapy. Overall, 85% of patients preferred an alternative treatment to their current regimen and were willing to pay more (on average about US$36) for the alternative.54
More recently, a US nationwide telephone survey of 200 randomly selected adults with asthma was conducted to establish the factors that influence patients’ adherence to asthma controller medication.55 When questioned about treatment-related factors that might improve their adherence, the most frequent responses were: (1) “If it controlled my symptoms better”; (2) “If it had long-lasting control of my symptoms”; (3) “If it meant I would need rescue medication less often”; (4) “If it meant I would need less additional medication to control my asthma overall”; (5) “If I knew I would feel better and could be more active without having asthma symptoms”; and (6) “If I could feel it helping my asthma soon after taking it.” Nonadherence was primarily the consequence of an active decision to use medication only as needed. A subgroup of patients who reported poor adherence to their controller medication (n = 75) listed different factors that might improve their adherence: (1) “If I could do more things I normally can’t do now”; (2) “If I could feel it helping my asthma soon after taking it”; (3) “If it had long-lasting control of my symptoms”; (4) “If it controlled my symptoms better”; (5) If it meant I would need rescue medication less often”; and (6) “If I could be certain that it was safe.” Many of the 200 patients strongly preferred a medication that worked quickly; this preference was especially apparent in the nonadherent group.55
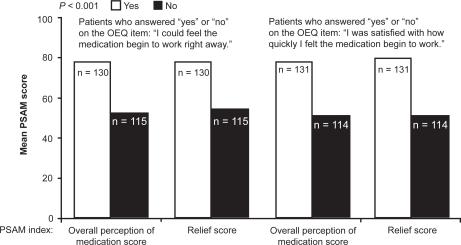
Patient preference for a rapid bronchodilator effect has been investigated recently using a newly developed measure, the Onset of Effect Questionnaire (OEQ).56 The OEQ is a validated weekly diary that elicits ratings for 5 items: during the past week (1) you could tell that your study medication was working; (2) you could feel your study medication begin to work right away; (3) you felt physical sensations shortly after taking your medication that reassured you that it was working; (4) your study medication worked as quickly as your albuterol; and (5) you were satisfied with how quickly you felt your study medication begin to work.56 Perception of, and satisfaction with, the feeling that controller therapy is working right away as measured by the OEQ items 2 and 5 has been shown to be a significant predictor of overall satisfaction with medication and satisfaction with the onset and duration of relief of asthma symptoms, as assessed by the PSAM (Figure 3).56 This suggests that patients who perceive that their medication is acting rapidly will not only receive positive reinforcement from use of the medication, but will also be more satisfied with treatment, possibly resulting in better treatment adherence.56 In 2 double-blind, randomized, placebo-controlled clinical trials employing the OEQ in asthma, significantly more budesonide/formoterol-treated patients than budesonide- or placebo-treated patients reported that they could feel their study medication beginning to work right away.57
Figure 3.
Relationship between perception of, and satisfaction with, the feeling that medication is working right away on the Onset of effect Questionnaire (OEQ) and ratings from the Patient Satisfaction with Asthma Medication (PSAM) index on satisfaction with medication and asthma symptom relief scores. Drawn from data of Murphy et al.49
Clinicians’ perspectives regarding the value to the patient of a perceived rapid onset of treatment effect have also been evaluated.58 A 12-member consensus panel of community-based healthcare professionals who reviewed the above-mentioned OEQ findings with budesonide/formoterol pMDI, but were blinded to the drug name,56 unanimously concluded that the fact that patients could feel their medication working right away was clinically relevant and meaningful to decision making, and that this attribute might improve patient adherence.58
Patient satisfaction with ICS/LABA: budesonide/formoterol versus fluticasone/salmeterol
Patient satisfaction with the budesonide/formoterol pMDI (adjustable-dose and fixed-dose) compared with the fluticasone/salmeterol DPI has been assessed using the Asthma Treatment Satisfaction Measure (ATSM)59 in a large, randomized, open-label, multicenter, 7-month study of adult patients with moderate to severe persistent asthma.60 The ATSM is a newly developed, validated instrument that incorporates the domains of patient expectations, treatment preferences, self-reported treatment outcomes, and overall treatment satisfaction.59 As measured by the ATSM, patient satisfaction with treatment was greater with budesonide/formoterol (adjustable dose or fixed dose) than with fixed-dose fluticasone/salmeterol. Patients receiving adjustable-dose budesonide/formoterol reported significantly greater satisfaction on the ATSM overall score and scores for feel medication working, dosing management, and timely relief of symptoms compared with those receiving fixed-dose fluticasone/salmeterol. Patients receiving fixed-dose budesonide/formoterol reported significantly greater satisfaction for timely relief of symptoms and feel medication working scores than those receiving fixed-dose fluticasone/salmeterol.60
In summary, assessment of patient satisfaction and preference for treatment provides insight into those features of combination controller medication that are important to patients and may lead to greater treatment adherence. These assessments showed that the rapid onset of bronchodilator effect associated with budesonide/formoterol was highly valued by patients and was associated with greater patient satisfaction than was fluticasone/salmeterol. However, it is important to note that patients’ stated preference for rapid onset of effect does not necessarily mean improved adherence to therapy will be a direct result. Further studies are needed to evaluate the impact of this characteristic of an ICS/LABA combination on adherence.
Implications for future clinical research and development
Use of combination therapy for both controller and reliever therapy
Research suggests that patients would prefer an ICS/LABA combination that could be used as both controller and reliever medication.54 The strategy of using a single ICS/LABA for both controller and reliever therapy, known by the acronym SMART (Symbicort® [budesonide/formoterol] Maintenance and Reliever Therapy), is currently approved outside the United States. Symbicort SMART is based on the premise that for patients who are already receiving a daily maintenance dose of budesonide/formoterol, use of as-needed budesonide/formoterol would allow more rapid adjustment of anti-inflammatory therapy in conjunction with rapid relief of symptoms. More rapid ICS adjustment, in turn, is associated with improved asthma control. At the same time, the LABA component would provide rapid symptom relief. This premise has been confirmed by findings from numerous studies;39,61–65 however, further research is needed to determine if it leads to improved adherence to therapy.
The rapid onset of effect of formoterol is advantageous not only because it allows the combination with budesonide to be used as both controller and reliever therapy, but also because it contributes to patient satisfaction with treatment.56 Pharmaceutical research and development should be directed toward combination therapies with rapid-acting LABAs, because of the importance to patients of rapid onset of action.54,56 Promising new ICS/rapid-acting LABA combinations include ciclesonide/formoterol, mometasone/formoterol, fluticasone/formoterol, and mometasone/indacaterol. Investigation into patient preference and the impact on adherence with these medications will be essential as we continue to advance asthma care.
Conclusions
Advances in the management of asthma are aimed at providing therapies that are not only safe and effective, but also have the potential for overcoming insufficient medication adherence, which is a critical barrier to successful treatment. Treatment with ICS/LABA is highly effective and recommended for patients whose asthma is not controlled on ICS alone. Combination treatment with a single inhaler is preferred by patients and is associated with improved adherence compared with ICS alone or ICS and LABA in separate inhalers.
In addition to the convenience of a single inhaler, patients prefer medications with a rapid onset of effect. Patients who exhibit poor adherence believed that a rapid onset of effect would improve their adherence to treatment. Currently available data based on patients’ perspectives on ICS/LABA therapy suggests that patients receiving rapidly acting combination therapy perceive immediate benefit from their medication and experience high levels of satisfaction with treatment. Research is needed to evaluate if patient preference for a drug with rapid onset of effect results in improved adherence to therapy and outcome. Thus, future research should be directed at combination therapy that offers not only anti-inflammatory activity, but a rapid onset of bronchodilator effect.
Acknowledgments
Susan Sutch, PharmD, and Andrew Fitton, PhD, from Evidence Scientific Solutions, provided medical writing support funded by AstraZeneca LP.
Footnotes
Disclosures
Dr Murphy has received honoraria for consulting from AstraZeneca LP, Schering-Plough, Merck, Dey, and Sepracor, and research support from AstraZeneca LP, GlaxoSmithKline, Merck, Schering-Plough, and Novartis. Dr Bender has received honoraria for consulting from AstraZeneca LP, Genentech, Kyorin, and Merck, served on the speaker bureau for GlaxoSmithKline and Merck, and received research support from AstraZeneca LP and Sepracor.
References
- 1.Fuhlbrigge AL, Adams RJ, Guilbert TW, et al. The burden of asthma in the United States: level and distribution are dependent on interpretation of the national asthma education and prevention program guidelines. Am J Respir Crit Care Med. 2002;166(8):1044–1049. doi: 10.1164/rccm.2107057. [DOI] [PubMed] [Google Scholar]
- 2.Peters SP, Jones CA, Haselkorn T, Mink DR, Valacer DJ, Weiss ST. Real-world Evaluation of Asthma Control and Treatment (REACT): findings from a national Web-based survey. J Allergy Clin Immunol. 2007;119(6):1454–1461. doi: 10.1016/j.jaci.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 4.Bateman ED, Bousquet J, Busse WW, et al. Stability of asthma control with regular treatment: an analysis of the Gaining Optimal Asthma controL (GOAL) study. Allergy. 2008;63(7):932–938. doi: 10.1111/j.1398-9995.2008.01724.x. [DOI] [PubMed] [Google Scholar]
- 5.Bender BG, Rand C. Medication non-adherence and asthma treatment cost. Curr Opin Allergy Clin Immunol. 2004;4(3):191–195. doi: 10.1097/00130832-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Lasmar L, Camargos P, Champs NS, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy. 2009;64(5):784–789. doi: 10.1111/j.1398-9995.2008.01877.x. [DOI] [PubMed] [Google Scholar]
- 7.Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Marceau C, Lemière C, Berbiche D, Perreault S, Blais L. Persistence, adherence, and effectiveness of combination therapy among adult patients with asthma. J Allergy Clin Immunol. 2006;118(3):574–581. doi: 10.1016/j.jaci.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Cabana MD, Rand CS, Becher OJ, Rubin HR. Reasons for pediatrician nonadherence to asthma guidelines. Arch Pediatr Adolesc Med. 2001;155(9):1057–1062. doi: 10.1001/archpedi.155.9.1057. [DOI] [PubMed] [Google Scholar]
- 10.Legorreta AP, Christian-Herman J, O’Connor RD, Hasan MM, Evans R, Leung KM. Compliance with national asthma management guidelines and specialty care: a health maintenance organization experience. Arch Intern Med. 1998;158(5):457–464. doi: 10.1001/archinte.158.5.457. [DOI] [PubMed] [Google Scholar]
- 11.Halm EA, Wisnivesky JP, Leventhal H. Quality and access to care among a cohort of inner-city adults with asthma: who gets guideline concordant care? Chest. 2005;128(4):1943–1950. doi: 10.1378/chest.128.4.1943. [DOI] [PubMed] [Google Scholar]
- 12.Wisnivesky JP, Lorenzo J, Lyn-Cook R, et al. Barriers to adherence to asthma management guidelines among inner-city primary care providers. Ann Allergy Asthma Immunol. 2008;101(3):264–270. doi: 10.1016/S1081-1206(10)60491-7. [DOI] [PubMed] [Google Scholar]
- 13.Baiardini I, Braido F, Giardini A, et al. Adherence to treatment: assessment of an unmet need in asthma. J Investig Allergol Clin Immunol. 2006;16(4):218–223. [PubMed] [Google Scholar]
- 14.Bender BG. Overcoming barriers to nonadherence in asthma treatment. J Allergy Clin Immunol. 2002;109(6 Suppl):S554–S559. doi: 10.1067/mai.2002.124570. [DOI] [PubMed] [Google Scholar]
- 15.Bender BG, Bender SE. Patient-identified barriers to asthma treatment adherence: responses to interviews, focus groups, and questionnaires. Immunol Allergy Clin North Am. 2005;25(1):107–130. doi: 10.1016/j.iac.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130(1 Suppl):65S–72S. doi: 10.1378/chest.130.1_suppl.65S. [DOI] [PubMed] [Google Scholar]
- 17.Markson LE, Vollmer WM, Fitterman L, et al. Insight into patient dissatisfaction with asthma treatment. Arch Intern Med. 2001;161(3):379–384. doi: 10.1001/archinte.161.3.379. [DOI] [PubMed] [Google Scholar]
- 18.Ulrik CS, Backer V, Søes-Petersen U, Lange P, Harving H, Plaschke PP. The patient’s perspective: adherence or non-adherence to asthma controller therapy? J Asthma. 2006;43(9):701–704. doi: 10.1080/02770900600925569. [DOI] [PubMed] [Google Scholar]
- 19.Van Ganse E, Mörk AC, Osman LM, et al. Factors affecting adherence to asthma treatment: patient and physician perspectives. Prim Care Respir J. 2003;12(2):46–51. doi: 10.1038/pcrj.2003.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halm EA, Mora P, Leventhal H. No symptoms, no asthma: the acute episodic disease belief is associated with poor self-management among inner-city adults with persistent asthma. Chest. 2006;129(3):573–580. doi: 10.1378/chest.129.3.573. [DOI] [PubMed] [Google Scholar]
- 21.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention. www.ginasthma.org. Updated December 2008. Accessed January 12, 2009.
- 22.National Heart Lung and Blood Institute . National Heart Lung and Blood Institute; Expert Panel Report 3: Guidelines for the diagnosis and management of asthma. www.nhlbi.nih.gov/guidelines/asthma/index.htm. Updated August 5, 2008. Accessed November 5, 2008. [Google Scholar]
- 23.Corren J, Korenblat PE, Miller CJ, O’Brien CD, Mezzanotte WS. Twelve-week, randomized, placebo-controlled, multicenter study of the efficacy and tolerability of budesonide and formoterol in one metered-dose inhaler compared with budesonide alone and formoterol alone in adolescents and adults with asthma. Clin Ther. 2007;29(5):823–843. doi: 10.1016/j.clinthera.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 24.Lalloo UG, Malolepszy J, Kozma D, et al. Budesonide and formoterol in a single inhaler improves asthma control compared with increasing the dose of corticosteroid in adults with mild-to-moderate asthma. Chest. 2003;123(5):1480–1487. doi: 10.1378/chest.123.5.1480. [DOI] [PubMed] [Google Scholar]
- 25.Noonan M, Rosenwasser LJ, Martin P, O’Brien CD, O’Dowd L. Efficacy and safety of budesonide and formoterol in one pressurised metered-dose inhaler in adults and adolescents with moderate to severe asthma: a randomised clinical trial. Drugs. 2006;66(17):2235–2254. doi: 10.2165/00003495-200666170-00006. [DOI] [PubMed] [Google Scholar]
- 26.Lyseng-Williamson KA, Simpson D. Budesonide/formoterol pressurized metered-dose inhaler. Drugs. 2008;68(13):1855–1864. doi: 10.2165/00003495-200868130-00005. [DOI] [PubMed] [Google Scholar]
- 27.Aubier M, Pieters WR, Schlösser NJ, Steinmetz KO. Salmeterol/fluticasone propionate (50/500 microg) in combination in a Diskus inhaler (Seretide) is effective and safe in the treatment of steroid-dependent asthma. Respir Med. 1999;93(12):876–884. doi: 10.1016/s0954-6111(99)90053-7. [DOI] [PubMed] [Google Scholar]
- 28.Bateman ED, Britton M, Carrillo J, Almeida J, Wixon C. Salmeterol/fluticasone combination inhaler: a new, effective and well tolerated treatment for asthma. Clin Drug Investig. 1998;16(3):193–201. doi: 10.2165/00044011-199816030-00003. [DOI] [PubMed] [Google Scholar]
- 29.Chapman KR, Ringdal N, Backer V, Palmqvist M, Saarelainen S, Briggs M. Salmeterol and fluticasone propionate (50/250 microg) administered via combination Diskus inhaler: as effective as when given via separate Diskus inhalers. Can Respir J. 1999;6(1):45–51. doi: 10.1155/1999/894803. [DOI] [PubMed] [Google Scholar]
- 30.Kavuru M, Melamed J, Gross G, et al. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2000;105(6 Pt 1):1108–1116. doi: 10.1067/mai.2000.105711. [DOI] [PubMed] [Google Scholar]
- 31.Nelson HS, Busse WW, Kerwin E, et al. Fluticasone propionate/salmeterol combination provides more effective asthma control than low-dose inhaled corticosteroid plus montelukast. J Allergy Clin Immunol. 2000;106(6):1088–1095. doi: 10.1067/mai.2000.110920. [DOI] [PubMed] [Google Scholar]
- 32.Advair [package insert] Research Triangle Park, NC: GlaxoSmithKline; 2008. [Google Scholar]
- 33.Symbicort [package insert] Wilmington, DE: AstraZeneca; 2009. [Google Scholar]
- 34.Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting β2-agonists and corticosteroids. Eur Respir J. 2002;19(1):182–191. doi: 10.1183/09031936.02.00283202. [DOI] [PubMed] [Google Scholar]
- 35.Randomised, double-blind, parallel group study on the efficacy and tolerability of the salmeterol 50 mcg/fluticasone 250 mcg combination Diskus compared to the formoterol 6 mcg/budesonide 200 mcg combination Turbohaler administered twice daily in patients with moderate bronchial asthma. Study No. SAM40048 GlaxoSmithKline Clinical Study Register. http://gsk-clinicalstudyregister.com/files/pdf/997.pdf. Updated November 14, 2005. Accessed November 15, 2008.
- 36.Aalbers R, Backer V, Kava TT, et al. Adjustable maintenance dosing with budesonide/formoterol compared with fixed-dose salmeterol/fluticasone in moderate to severe asthma. Curr Med Res Opin. 2004;20(2):225–240. doi: 10.1185/030079903125002928. [DOI] [PubMed] [Google Scholar]
- 37.Busse WW, Shah SR, Somerville L, Parasuraman B, Martin P, Goldman M. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. J Allergy Clin Immunol. 2008;121(6):1407–1414. doi: 10.1016/j.jaci.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 38.Dahl R, Chuchalin A, Gor D, Yoxall S, Sharma R. EXCEL: a randomised trial comparing salmeterol/fluticasone propionate and formoterol/budesonide combinations in adults with persistent asthma. Respir Med. 2006;100(7):1152–1162. doi: 10.1016/j.rmed.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Kuna P, Peters MJ, Manjra AI, et al. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract. 2007;61(5):725–736. doi: 10.1111/j.1742-1241.2007.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lasserson TJ, Cates CJ, Ferrara G, Casali L. Combination fluticasone and salmeterol versus fixed dose combination budesonide and formoterol for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008;(3):CD004106. doi: 10.1002/14651858.CD004106.pub3. [DOI] [PubMed] [Google Scholar]
- 41.Ankerst J, Lötvall J, Cassidy S, Byrne N. Comparison of the bronchodilating effects of formoterol and albuterol delivered by hydrofluoroalkane pressurized metered-dose inhaler. Treat Respir Med. 2005;4(2):123–127. doi: 10.2165/00151829-200504020-00006. [DOI] [PubMed] [Google Scholar]
- 42.Grembiale RD, Pelaia G, Naty S, Vatrella A, Tranfa CM, Marsico SA. Comparison of the bronchodilating effects of inhaled formoterol, salmeterol and salbutamol in asthmatic patients. Pulm Pharmacol Ther. 2002;15(5):463–466. doi: 10.1006/pupt.2002.0387. [DOI] [PubMed] [Google Scholar]
- 43.Palmqvist M, Arvidsson P, Beckman O, Peterson S, Lötvall J. Onset of bronchodilation of budesonide/formoterol vs. salmeterol/fluticasone in single inhalers. Pulm Pharmacol Ther. 2001;14(1):29–34. doi: 10.1006/pupt.2000.0260. [DOI] [PubMed] [Google Scholar]
- 44.van der Woude HJ, Boorsma M, Bergqvist PB, Winter TH, Aalbers R. Budesonide/formoterol in a single inhaler rapidly relieves methacholine-induced moderate-to-severe bronchoconstriction. Pulm Pharmacol Ther. 2004;17(2):89–95. doi: 10.1016/j.pupt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Hampel FC, Martin P, Mezzanotte WS. Early bronchodilatory effects of budesonide/formoterol pMDI compared with fluticasone/salmeterol DPI and albuterol pMDI: 2 randomized controlled trials in adults with persistent asthma previously treated with inhaled corticosteroids. J Asthma. 2008;45(4):265–272. doi: 10.1080/02770900801890505. [DOI] [PubMed] [Google Scholar]
- 46.Stempel DA, Stoloff SW, Carranza Rosenzweig JR, Stanford RH, Ryskina KL, Legorreta AP. Adherence to asthma controller medication regimens. Respir Med. 2005;99(10):1263–1267. doi: 10.1016/j.rmed.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Stoloff SW, Stempel DA, Meyer J, Stanford RH, Carranza Rosenzweig JR. Improved refill persistence with fluticasone propionate and salmeterol in a single inhaler compared with other controller therapies. J Allergy Clin Immunol. 2004;113(2):245–251. doi: 10.1016/j.jaci.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Chervinsky P, Baker J, Bensch G, et al. Patient-reported outcomes in adults with moderate to severe asthma after use of budesonide and formoterol administered via 1 pressurized metered-dose inhaler. Ann Allergy Asthma Immunol. 2008;101(5):463–473. doi: 10.1016/S1081-1206(10)60284-0. [DOI] [PubMed] [Google Scholar]
- 49.Murphy K, Nelson H, Parasuraman B, Boggs R, Miller C, O’Dowd L. The effect of budesonide and formoterol in one pressurized metered-dose inhaler on patient-reported outcomes in adults with mild-to-moderate persistent asthma. Curr Med Res Opin. 2008;24(3):879–894. doi: 10.1185/030079908X273354. [DOI] [PubMed] [Google Scholar]
- 50.Mathias SD, Warren EH, Colwell HH, Sung JC. A new treatment satisfaction measure for asthmatics: a validation study. Qual Life Res. 2000;9(7):873–882. doi: 10.1023/a:1008913209828. [DOI] [PubMed] [Google Scholar]
- 51.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47(2):76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hays RD, Stewart AL. Sleep measures: definitions and issues. In: Stewart AL, Ware JE, editors. Measuring Function and Well-Being: The Medical Outcomes Study Approach. Durham, NC: Duke University Press; 1991. pp. 235–259. [Google Scholar]
- 53.Szeinbach SL, Murawski MM, McGhan WF, Corey R, Barnes JH. Using conjoint analysis to evaluate health state preferences. Drug Inf J. 1999;33(3):849–858. [Google Scholar]
- 54.Johansson G, Ställberg B, Tornling G, et al. Asthma treatment preference study: a conjoint analysis of preferred drug treatments. Chest. 2004;125(3):916–923. doi: 10.1378/chest.125.3.916. [DOI] [PubMed] [Google Scholar]
- 55.Bender BG, Long A, Parasuraman B, Tran ZV. Factors influencing patient decisions about the use of asthma controller medication. Ann Allergy Asthma Immunol. 2007;98(4):322–328. doi: 10.1016/S1081-1206(10)60877-0. [DOI] [PubMed] [Google Scholar]
- 56.Leidy NK, Matthias SD, Parasuraman BM, Patrick DL, Pathak D. Development and validation of an onset of effect questionnaire for patients with asthma. Allergy Asthma Proc. 2008;29(6):590–599. doi: 10.2500/aap.2008.29.3164. [DOI] [PubMed] [Google Scholar]
- 57.Kaiser H, Parasuraman B, Boggs R, Miller CJ, Leidy NK, O’Dowd L. Onset of effect of budesonide and formoterol administered via one pressurized metered-dose inhaler in patients with asthma previously treated with inhaled corticosteroids. Ann Allergy Asthma Immunol. 2008;101(3):295–303. doi: 10.1016/S1081-1206(10)60495-4. [DOI] [PubMed] [Google Scholar]
- 58.Harding G, Leidy NK, Meddis D, Kleinman L, Wagner S, O’Brien CD. Interpreting clinical trial results of patient-perceived onset of effect in asthma: methods and results of a Delphi panel. Curr Med Res Opin. 2009;25(6):1563–1571. doi: 10.1185/03007990902914403. [DOI] [PubMed] [Google Scholar]
- 59.Patrick DL, Martin ML, Bushnell DM, Meltzer EO, Parasuraman BM. Development of a treatment satisfaction measure for asthma patients: the asthma treatment satisfaction measure (ATSM) [ATS abstract 573] Am J Respir Crit Care Med. 2008;177(abstract issue):A573. [Google Scholar]
- 60.O’Connor RD, Patrick DL, Parasuraman BM, Martin P, Goldman M. Patient satisfaction during treatment with adjustable-dose budesonide/formoterol pressurized metered-dose inhaler (BUD/FM pMDI), fixed-dose BUD/FM pMDI, and fixed-dose fluticasone/salmeterol dry powder inhaler (FP/SM DPI) [ATS abstract 609] Am J Respir Crit Care Med. 2008;177(abstract issue):A609. [Google Scholar]
- 61.O’Byrne PM, Bisgaard H, Godard PP, et al. Budesonide/formoterol combination therapy as both maintenance and reliever medication in asthma. Am J Respir Crit Care Med. 2005;171(2):129–136. doi: 10.1164/rccm.200407-884OC. [DOI] [PubMed] [Google Scholar]
- 62.Scicchitano R, Aalbers R, Ukena D, et al. Efficacy and safety of budesonide/formoterol single inhaler therapy versus a higher dose of budesonide in moderate to severe asthma. Curr Med Res Opin. 2004;20(9):1403–1418. doi: 10.1185/030079904X2051. [DOI] [PubMed] [Google Scholar]
- 63.Rabe KF, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo UG. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomised controlled, double-blind study. Lancet. 2006;368(9537):744–753. doi: 10.1016/S0140-6736(06)69284-2. [DOI] [PubMed] [Google Scholar]
- 64.D’Urzo AD. Inhaled glucocorticosteroid and long-acting β2-adrenoceptor agonist single-inhaler combination for both maintenance and rescue therapy: a paradigm shift in asthma management. Treat Respir Med. 2006;5(6):385–391. doi: 10.2165/00151829-200605060-00003. [DOI] [PubMed] [Google Scholar]
- 65.Vogelmeier C, D’Urzo A, Pauwels R, et al. Budesonide/formoterol maintenance and reliever therapy: an effective asthma treatment option? Eur Respir J. 2005;26(5):819–828. doi: 10.1183/09031936.05.00028305. [DOI] [PubMed] [Google Scholar]