Abstract
The chronicity of asthma results not only in persistent lung inflammation but also in changes in structure and composition of this vital organ. These changes are most commonly referred to as remodeling, and include epithelial dysplasia, angiogenesis, changes in the extracellular matrix and increased smooth muscle mass. In this review we summarize recent findings on the contribution of remodeling to the pathological phenotype of asthma. We discuss how and why current treatment (such as corticosteroids) options fail to adequately treat remodeling.
Keywords: asthma, remodeling, epithelium, vascular, smooth muscle
Asthma
Asthma is a chronic inflammatory disease of the airways, characterized by reversible airflow obstruction. In addition to chronic inflammation, the asthmatic airways have several structural changes, such as increased smooth muscle mass, differences in the extracellular matrix composition, increased vascularity and epithelium dysfunction. Collectively these changes are known as remodeling, and are thought to contribute to the airways’ increased ability to respond to a wide range of stimuli (hyper-responsiveness). Bronchial hyper-responsiveness (BHR) is quantified as the change in lung function, due to airway constriction, invoked by a range of agents. Agents are either non-specific, such as pharmacologically or chemically active molecules, or physical or physiochemical agents. Agents can also be specific, for example allergens. Worldwide the prevalence of asthma is increasing; however it remains to be seen if the increased prevalence observed over the last century continues.1,2 This may in part be due to better training of the medical community to recognize the symptoms of asthma, and the increase in environmental irritants and allergens such as the house dust mite.
Pathology of asthma
In 1922, a landmark study was carried out by Huber and Koessler on the airways of 21 patients who had died of asthma and seven patients who had died from other causes. The major findings from their report are still relevant today, in that they found that in bronchi with an external diameter of greater than 2 mm, the subepithelial layer, the smooth muscle layer and the total wall thickness were all increased.3 Endobronchial biopsies have confirmed that non fatal asthma is an inflammatory disease.4–6 Laitinen and others (1985) carried out a bronchoscopic study of various sites within the lungs of asthmatic subjects, and found that inflammation and tissue destruction were apparent at all sites sampled.7 In addition the inflammatory gene expression profile has been shown to be different in asthma using in-situ hybridization,8 and furthermore the specific source and expression of various cytokines differs in asthmatic tissue.9 Eosinophilia has for many years been considered a distinguishing feature of asthma, however eosinophilia has also been observed in other airways diseases such as eosinophilic bronchitis. Recently a report by Brightling et al highlighted the possible importance of mast cells located within smooth muscle bundles of subjects with asthma. Mast cell infiltration of the smooth muscle layer was found to be significantly higher in asthmatic subjects when compared to non-asthmatics, and to subjects with eosinophilic bronchitis.10 However mast cells located in the smooth muscle bundles may not be a distinguishing feature of asthma since this was also found in patients with chronic obstructive pulmonary disease (COPD) and subjects who were currently smoking.11,12 The role of the neutrophil in the pathology of asthma has for the most part been overlooked. Recent reports demonstrate that neutrophilia occurs in certain situations. Analysis of sputum from asthmatics reveals elevated numbers of neutrophils during exacerbation.13 This may however more likely reflect the immune response to respiratory tract infections and as such may not be a feature of stable asthma. On the other side, neutrophilia is also observed in sudden onset fatal asthma,14 indicating the role of the neutrophil in more severe manifestations of the disease. A report by Wenzel and co-workers found neutrophilia to be present in severe asthmatics that had been treated for five or more years with high doses of oral steroids, when compared to non-asthmatics and mild asthmatic subjects requiring treatment with inhaled β agonists alone.15 It is not known if neutrophilia observed in asthma is due to corticosteroid treatment alone, since corticosteroids reduce eosinophil numbers and inhibit neutrophil apoptosis.16 It is conceivable that the neutrophilia observed in asthma is due to factors other than steroid use or respiratory infection.
Airway remodeling
Histological examination has demonstrated that in asthma the airway wall is thickened, due to epithelial dysplasia, angiogenesis, increased extracellular matrix (ECM) deposition, and increased airway smooth muscle (ASM) mass; collectively termed remodeling. Increased mucous and mucous plugs are found in the asthmatic airways. This cannot only physically occlude the airways, but can increase the airway surface tension promoting airway collapse. Aikawa and colleagues (1992) carried out necroscopic morphometric analysis of subjects’ lungs who had died from an asthma attack in comparison to lungs from subjects with asthma who had died of other causes. A 30-fold increase in goblet cells and a three-fold increase in mucous production were observed in fatal asthma.17 Furthermore the mucin composition of mucous is altered in tissues from patients with fatal status asthmaticus in comparison to milder asthma, with an increase of mucin (MUC) gene 5 subtype AC and MUC5B found on the luminal side of the epithelium. In contrast, these were not present at this location in non-asthmatic tissue.18
Remodeling is a consistent feature of both fatal and milder manifestations of asthma. A report by Roche and colleagues described thickening below the basement membrane due to collagen deposition, and not increased basement membrane thickness as previously believed.19 The increased collagen deposition was not due to epithelial dysfunction, and is most likely due to fibroblast activation. This, as Roche and colleagues pointed out, is more correctly called subepithelial fibrosis.19 The subepithelial fibrosis is due to deposition of collagens type I, III, and V.
Smooth muscle cell remodeling
In 2004, Mitzner asked the question: “Airway smooth muscle: the appendix of the lung”? In this paper, Mitzner postulated that airway smooth muscle is a worthless structure with no essential physiological function that can lead to serious medical problems. Airway smooth muscle is largely involved in the bronchial remodeling that occurs in asthma.10,20–23 Many studies show an increase in airway smooth muscle mass in fatal asthma.20,24,25 Trian et al23 examined bronchus from severe asthmatics, (GINA 4)26 using specimens from biopsies, and showed an increase in smooth muscle area compared to non-asthmatics. However, this increase of airway smooth muscle mass has also been shown in non-fatal asthma, ie, mild and moderate. Carroll et al examined bronchial tissue from fatal asthma, those dying of other causes with a history of asthma (mild and moderate asthma) and those who died of other causes without any history of respiratory disease.27 They found a greater area of smooth muscle in the cartilaginous airways of fatal asthma compared to non-fatal and non-asthmatic. The inner wall area was greater in the fatal and non-fatal cases than in the control cases in the small cartilaginous airways and membranous bronchioles (MB). In small MB (perimeter <2 mm), the total and outer wall areas were greater (p < 0.05) in cases of fatal and non-fatal asthma than in control cases. Remodeling of the smooth muscle layer has a key role as an important effector of the pathology of asthma. For example, there is a correlation between BHR early phase amplitude and smooth muscle mass.28
Asthmatic smooth muscle remodeling is also characterized by an increase of extracellular matrix between smooth muscle cells. In a recent study, Begueret et al examined biopsies from severe asthmatics using electron microscopy.29 In this study, the authors found a higher degree of organization of the extracellular matrix between airway smooth muscle cells.
However, all these studies need to be viewed cautiously because ASM mass is quite difficult to measure due to problems in obtaining biopsies. Quantification of smooth muscle mass increase in asthma needs the involvement of new technology to assess specifically smooth muscle mass such as quantitative morphometry and/or laser dissection.30 Due to the difficulty in obtaining and assessing smooth muscle remodeling in asthma using human biopsies, many studies have been done using animal models. Using long-term ovalbumin sensitization models, smooth muscle asthma remodeling can be found, with an increase of the smooth muscle mass. Moreover, McMillan et al found that prolonged allergen challenge using ovalbumin in mice leads to persistent airway remodeling.31
Other changes in smooth muscle layer in asthma
As mentioned above, asthmatic smooth muscle features an inter-cellular increase of extracellular matrix. This increase of matrix seems to be an important characteristic. In a recent study, Slats32 found that airway hyperresponsiveness, FEV1% predicted, and airway responses to deep inspiration are associated with components of the extracellular matrix.32 In 2004, Johnson et al showed that an external protein matrix could modulate ASM proliferation in asthma.21 Another recent study using bovine tracheal smooth muscle shows that extracellular matrix proteins differentially regulate both phenotype and function of intact ASM in terms of contraction and proliferation.33
According to the literature, the mechanism of smooth muscle remodeling seems to involve both hyperplasia and hypertrophy. Indeed, there are some studies which involve hypertrophy in the increase of airway smooth muscle cell mass in asthma.20,34,35 In a recent study, Trian et al suggested that the increase of smooth muscle cell proliferation in asthma, previously shown by Johnson et al was due to an increase of mitochondrial mass in asthmatic airway smooth muscle.23 This increase of mitochondrial mass was found only in smooth muscle cells and only in severe asthmatic patients. In this paper, the authors also explained this increase by a deregulation of the mitochondrial biogenesis, with an up-regulation found in asthmatic airway smooth muscle cells. The cause of this activation seems to be a calcium deregulation involving the voltage operated channels (VOCs).
However, in an asthmatic bronchus there are many other factors that can induce smooth muscle cell proliferation (see Table 1). For example, one of the other characteristics of the smooth muscle layer remodeling that occurs in asthma, is the specific infiltration of mast cell.10 Airway smooth muscle layer mast cells found in asthma are in an activated state, primed to release mediators.29 One of these mediators, mast cell tryptase has been shown to be an activator of smooth muscle proliferation in vitro.36 This activation of ASM via the protease activated receptor 2 (PAR-2)37 is also involved in the secretion of TGFβ-1 and stem cell factor (SCF), two mast cell chemotactic factors that induce an activation loop between mast cell and ASM that could be involved in ASM remodeling by induction of smooth muscle cell (SMC) proliferation. Other factors have been shown to induce smooth muscle cell proliferation, for example, platelet derived growth factor (PDGF)38 or insulin-like growth factors 1 and 2 (IGF-1 and IGF-2).39 Smooth muscle proliferation is also induced by the interaction and the close relationship between all the cell types in the bronchus. One of the examples is the relationship between mast cells and SMC described above, but there is also a close interaction between epithelial cells and smooth muscle cells. For example, bronchial epithelial cells can release epidermal growth factors which has been shown to have a potential mitogenic activity on bronchial airway smooth muscle cell.40 Finally, there is also another hypothesis to explain this increase in SMC mass suggested by Ramos-Barbon who found a decrease in SMC apoptosis in an asthmatic rat model.41
Table 1.
Factors inducing airway smooth muscle cell proliferation
| Classification | Products | Origin |
|---|---|---|
| Growth factors | βFGF | ECM, Monocyte, SMC |
| IGF-1 | Epithelial cell, mesenchymal cell, macrophage | |
| EGF | Epithelial cell | |
| NGF | Eosinophil, fibroblast, epithelial cell, mast cell | |
| PDGF | Platelet, monocyte, SMC, epithelial cell | |
| Inflammatory products | Elastase | Neutrophil |
| β-Hexosaminidase | Mast cell | |
| Thrombin | Platelet | |
| Thromboxane A2 | Platelet | |
| Histamine | Mast cell | |
| Neutrophil elastase | Neutrophils | |
| Bradykinin | Mast cell | |
| Tryptase | Mast cell | |
| Cytokines | IGF-1 | Platelet, monocyte, SMC, epithelial cell |
| IL-1β | Lymphocyte, monocyte, epithelial cell | |
| IL-6 | Lymphocyte, monocyte, SMC, epithelial cell | |
| TNF-α | Lymphocyte, monocyte, SMC, epithelial cell | |
| TGF-β1 | Lymphocyte, monocyte, SMC, platelet, ECM | |
| Others | Endothelin-1 | Platelet, monocyte, epithelial cell |
| 5-HT | Platelet | |
| Substance P | Neuron, epithelial cell | |
| ATP | Mesenchymal cell | |
| Extra cellular matrix | Fibronectin | Mesenchymal cell |
| Collagen I | Mesenchymal cell | |
| MMP-2 | Endothelial cell, macrophage |
Abbreviations: SMC, smooth muscle cell; ECM, extracellular matrix.
However, the mechanism of smooth muscle increase in asthma is still controversial. Begueret et al used immunochemistry to assess the presence of Ki67 staining in airway smooth muscle cells. In this study, the authors could not find any staining of Ki67.29 Moreover, many studies involve hypertrophic mechanisms in smooth muscle remodeling in asthma. In 2004 Zheng et al showed that treatment of guinea pig airway explants with cardiotrophin, a member of the IL-6 family induces an increase of size and protein content of airway smooth muscle cells.42 Furthermore, TGF-β, which is mostly involved in asthma pathophysiology, has been shown to induce hypertrophy and increase in protein synthesis in airway smooth muscle cells.43 In a recent study, Deng et al suggested that inhibition of the GSK-3β/eIF2B translational control pathway contributes to airway smooth muscle hypertrophy in vitro and in vivo. However, they also demonstrated that TGF-β-induced hypertrophy does not depend on GSK-3β/eIF2B signaling.44
Vascular remodeling
Another component of asthmatic airway remodeling is alteration to the vasculature of the lung, including blood and lymphatic vessels. The cardinal function of the lung, gas exchange, requires a vast network of blood vessels ranging from macro- to submicro vascular vessels. Another well-appreciated function of this network is the transport of nutrients45 and the exchange of heat and water.46 Additionally, the blood vessel system provides extensive tracks for progenitor cells as well as immune cells.47,48 Furthermore, active distribution of airborne substances throughout the body has been described (eg, pharmacological agents and antigens49,50). An extensive valve and vascular smooth muscle system allows an active responding vascular system.
The lymphatic system on the other hand is thought to be of a less complex construction than the blood vessels.51 Simpler valve systems are supported passively by contraction and expansion of skeletal muscle or tissue.52 It is proposed that this function is maintained through filamentous structures anchored at the adjacent tissue.52 Through this passive pump mechanism interstitial fluid, containing among others lipids, proteins and cells, is drained away from the tissue. Accumulation of fluid can occur due to vasodilation (eg, caused by histamine) and leakage of blood vessels. To prevent severe edema, this fluid has to be drained away by the lymphatic system which therefore aids the collection of exogenous proteins such as inhaled antigens.53 In case of inflammation the lymphatic system represents the collecting (afferent) network for dentritic cells (DC) charged with foreign material (antigens) to be presented in the proximal lymph nodes to generate an adaptive immune response.52,54 Under healthy conditions accumulation and drainage represent a balanced process.55
Impaired growth of lymphatic vessels is reported to support bronchial lymphoedema in a mouse model of chronic lung inflammation (Mycoplasma pulmonis).56 Due to inflammation severe asthmatics experience mucosal edema, which may lead to further narrowing of the bronchial caliber, subsequently enhancing breathing difficulties.57,58 The current understanding of the importance of the lymphatic system in asthma is limited but it has recently gained more scientific interest.
The chronic inflammation occurring during asthma requires the support of the vascular system. In case of encounters with foreign material, DCs are charged with antigen and proceed to the lymph nodes for presentation. Replenishment with naïve DCs is maintained via the blood vessels. Additionally, to battle pathogenic viruses or bacteria the recruitment of activated leukocytes (neutrophils, eosinophils and macrophages) is achieved via the same gateway.
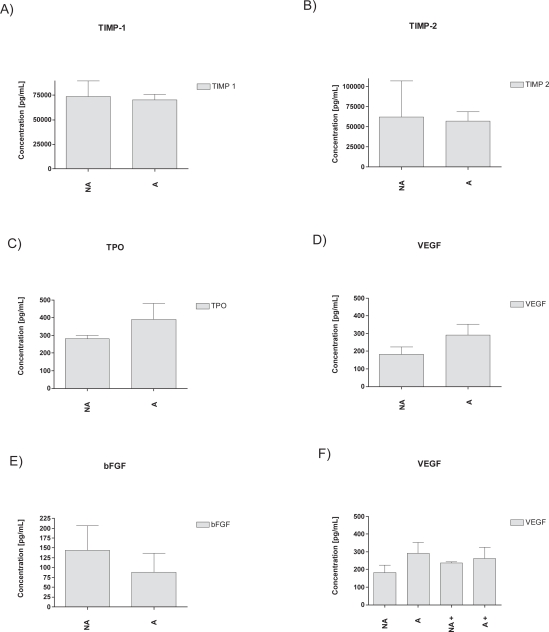
Resident structural cells such as lung epithelial cells (type I and II pneumocytes), smooth muscle cells and fibroblasts change expression profiles under inflammatory conditions supporting the growth and extension of the vascular system. ASM are able to produce a wide range of both angiogenic and anti-angiogenic factors such as tissue inhibitor of metalloproteinase (TIMP) 1 and 2, thrombopoietin (TPO), fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) (own unpublished data and Figure 1). VEGF has been shown in moderate or severe asthmatics to be elevated in sputum and submucosa59 and so was FGF,60 leading to a possible extension of the peri-bronchial vascular system. Simcock and colleagues recently demonstrated increased expression of VEGF by asthmatic airway smooth muscle cells which induced tubule formation of endothelial cells.61 Our findings support this, however as a trend only (Figure 1 D). Furthermore VEGF is a prominent and powerful modulator of endothelial proliferation.
Figure 1.
Secretion of the anti-angiogenic factors into cell culture medium by airway smooth muscle cells: TIMP-1 A), TIMP-2 B) as well as pro-angiogenic factors: VEGF C), TPO D), bFGF E). No statistical differences are observed between asthmatics (A) and non-asthmatics (NA). F) Treatment (+) with 10−8 M dexmethasone did not change significantly the expression levels of VEGF in ASM (A or NA) after 24 h. n = 4 for all.
The balance of angiogenic and anti-angiogenic stimuli is crucial and surely doesn’t rely solely on VEGF. However, the current understanding is not fully conclusive to how this balance is maintained. Interestingly it has been shown that in asthma an anti-angiogenic ECM component, derived from collagen XVIII α1 – endostatin – is reduced in steroid naïve asthmatics.59 Furthermore the levels of collagen IV are known to be decreased in the asthmatic lung,62 however, it is unknown which α – chain or chains are affected.
At least three of the collagen IV α-chain NC1 domains, α1–α3, have been reported to possess anti-angiogenic activity. Collagen IV α1 inhibits VEGF – induced proliferation and migration of endothelial cells through binding of the α1β1 integrin.63 As for collagen IV α2, inhibition of endothelial cell proliferation and migration is suggested to be mediated by a cell surface protein/receptor, the most likely candidates being αvβ3, α1β1 and α2β1 integrins.64 Lastly, collagen IV α3 induces apoptosis of proliferating endothelial cells.65–67 The mechanism of action is through the interaction with αvβ3 integrin, which leads to the inhibition of focal adhesion kinase (FAK), phosphatidylinositol (PI) −3 kinase, protein kinase B (Akt) and mammalian target of rapamycin (mTOR) as well as the prevention of the dissociation of the eukaryotic translation initiation factor 4E/eukaryotic initiation factor 4E-binding protein 1 (eIF4E/4E-BP1) complex, resulting in the inhibition of cap-dependent protein translation in endothelial cells.68
Is remodeling important?
Asthma is characterized by reversible airflow obstruction. While airflow obstruction is often variable, it is now thought that it may not be fully reversible in all asthmatic patients due to chronic airway remodeling.69 Whether remodeling initiates the development of asthma or occurs secondarily is not known. In children with asymptomatic bronchial hyper-responsiveness, longitudinal studies have demonstrated that the subsequent development of asthma is closely associated with the development of remodeling.70,71 In adults, the natural decline in airway function which occurs with age is known to occur at an increased rate in patients with asthma.72,73 This accelerated loss of lung function is likely to be due to remodeling. Furthermore recent studies have shown that the severity of asthma clearly correlates with the presence of remodeling.74,75 In contrast, others have postulated that airway remodeling which results in a thickened airway wall may however protect against excessive airway narrowing.76
The etiology of remodeling
In vivo histological studies of airways from patients with and without asthma describe enhanced deposition of collagen I, III, and V, fibronectin, tenascin, hyaluronan, versican, laminin α2/β2, lumican, and biglycan,77 whereas levels of collagen IV and elastin are decreased.77 Data from our group and others have demonstrated that the ECM produced by lung cells in vitro differs significantly when cells from asthmatic and non-asthmatic individuals are compared.78,79 The interaction of the ECM and lung cells is dynamic. In response to different ECM components, such as fibronectin, collagen and perlecan, the proliferation, survival and inflammatory mediator release from smooth muscle cells can be altered.80–83 Lung cells also possess the ability to modulate the surrounding ECM in response to factors such as physical stress and soluble mediators.77,84,85 Moreover, we have previously shown that the ECM produced by asthmatic cells can induce cells from non-asthmatic subjects to assume the characteristics of those from asthmatic subjects.77
Role of remodeling and inflammation in the progression of asthma
It is not known if remodeling initiates inflammation, or vice versa, in the airways of asthmatics. A recent study from Saglani et al demonstrated that neither remodeling nor inflammation were present in atopic infants with reversible airflow obstruction, whilst they did occur in older children.70 However, in a longitudinal study in older children, both preceded the development of asthma.71 Chronic airway remodeling is likely to be the result of an aberrant repair cycle which occurs in response to chronic inflammation.86 The interactions between many cell types, including epithelial cells, fibroblasts and smooth muscle cells play an important role in this process. The bronchial epithelium is a rich source of profibrotic growth factors, and it is likely that the interaction between these and the underlying mesenchymal cells controls remodeling. Furthermore, some groups hypothesize that altered signaling in the “epithelial-mesenchymal trophic unit” drives chronic remodeling in asthma.86 It is likely that these mediators can act via both an autocrine and paracrine mechanism to increase remodeling.
Therapeutic options for the treatment of remodeling
Inflammation can to some degree be treated in asthma, however the current therapeutic regimes may not reverse the remodeling process. Short term treatment of asthmatic subjects with steroids (6 weeks) can reduce the thickness of the basement membrane,87 however long term treatment with steroids (mean 3.1 years) did not reduce the total thickness of the airways when compared to non-steroid treated asthmatics.76
Recently leukotriene receptor antagonists have shown some therapeutic potential for the treatment of airway remodeling in asthma. In a murine model of allergic airways disease blockage of the CysLT1 receptor reduced allergen-induced mucus plug formation, smooth muscle hyperplasia, and airway fibrosis (assessed by collagen deposition).88 Interestingly, leukotriene receptor antagonists have also been shown to inhibit bleomycin-induced lung fibrosis in mice.89
Leukotriene receptor antagonists have also been shown to inhibit human lung myofibroblast proliferation ex vivo from patients with fibrotic lung diseases90 and there is some evidence that allergen-induced myofibroblasts are inhibited in vivo following treatment with leukotriene receptor antagonists in asthmatics.91 The mechanism of action by which leukotriene receptor antagonists inhibit airway remodeling is still not known, however since CysLTC4 induces the production of TGFβ from epithelial cells92 presumably inhibition of the leukotriene receptor would result in decreased TGFβ production. However a recent study by Altraja et al suggests that leukotrienes induce the production of ECM proteins directly from epithelial cells adding further weight to the potential for leukotriene receptor antagonists to treat remodeling. Randomized controlled trials in humans examining the effect of leukotriene receptor antagonists upon airway remodeling in asthma have not been done; however a case report by Pifferi et al demonstrated the therapeutic potential of montelukast to reverse airway remodeling in children.93 We have shown that a phosphodiesterase 4 inhibitor inhibited the deposition of extracellular matrix proteins ex vivo in human bronchus.94
There are no therapeutics available to reverse the increased airway smooth muscle that is observed in asthma. One potential reason why corticosteroids do not decrease the amount of airway smooth muscle in asthma was proposed by Roth et al.95 They demonstrated an abnormality in the corticosteroid receptor cofactor C/EBPα which results in the loss of antiproliferative effect of the corticosteroids on asthmatic smooth muscle cell proliferation.
Recently, however, a new technique has increased interest to find therapeutics against smooth muscle cell remodeling in asthma. Laviolette‘s group,96,97 treated moderate to severe asthmatics using a bronchoscopic procedure, bronchial thermoplasty, to reduce ASM mass. In this study, the authors have shown that the reduction of ASM mass improves asthma control. Indeed, this “treatment” reduced exacerbation frequency, and increased morning peak expiratory flow and symptom scores. This study showed the importance of smooth muscle remodeling in the physiopathology of asthma. However, there are no therapeutics to specifically treat endogenous causes of ASM remodeling in asthma.
In contrast it may be possible to specifically treat vascular abnormalities in the asthmatic airway. The pro-angiogenic molecule mostly studied in asthma is VEGF and studies indicate its susceptibility to corticosteroid treatment. Chetta and colleagues observed reduction of VEGF after inhalation of high doses of fluticasone in a subgroup of asthmatic patients. Accompanied by the reduction of VEGF, the vascular area was significantly decreased and fewer vessel numbers were found.98 Structural and non-resident cells are capable of producing VEGF and other pro-angiogenic factors. However, in ASM expression of VEGF appears to be dexamethasone insensitive unless TGF-β is added as a stimulus (regardless of disease state) supporting a direct, but interlinked effect of corticosteroids on the reduction of this factor (own unpublished data, Figure 1D, F). Furthermore collagen IV expression was increased back to normal (baseline levels of sham treated animals) after dexamethasone treatment in an acute mouse model of allergic airway disease,99 suggesting that corticosteroids not only reduce pro-angiogenic but also increase potential ECM derived anti-angiogenic factors. In humans endostatin (NC domain of collagen XVIII) has been described to be uninfluenced by corticosteroids (beclomethasone dipropionate) treatment, suggesting a shift from a pro-angiogenic towards an anti-angiogenic environment in asthmatics.59 Long- or short-acting β antagonists studies on vascular remodeling are rare and effects range from reduction of vascular permeability to antiangiogenic properties of salmeterol.100
Conclusion
Remodeling affects airway function in asthma, and is likely to contribute to the increased rate of lung function decline in asthmatics.73 The current therapies used in humans for the treatment of asthma do not fully reverse the remodeling process, and, in fact, the findings of reports of in vivo treatment are conflicting. In vitro, we and others have demonstrated that ECM deposition may in fact be increased by corticosteroids.101 Mechanisms of the remodeling process need to be better understood to allow the development of new therapeutics, which can target this important aspect of airways disease.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.Bernardi E, Prandoni P, Lensing AW, et al. D-dimer testing as an adjunct to ultrasonography in patients with clinically suspected deep vein thrombosis: prospective cohort study. The Multicentre Italian D-dimer Ultrasound Study Investigators Group. Bmj. 1998 Oct 17;317(7165):1037–1040. doi: 10.1136/bmj.317.7165.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verlato G, Corsico A, Villani S, et al. Is the prevalence of adult asthma and allergic rhinitis still increasing? Results of an Italian study. J Allergy Clin Immunol. 2003;111(6):1232–1238. doi: 10.1067/mai.2003.1484. [DOI] [PubMed] [Google Scholar]
- 3.Huber H, Koessler K. The Pathology of Bronchial Asthma. 1922;30:689–760. [Google Scholar]
- 4.Laitinen LA, Laitinen A, Haahtela T. Airway mucosal inflammation even in patients with newly diagnosed asthma. Am Rev Respir Dis. 1993;147(3):697–704. doi: 10.1164/ajrccm/147.3.697. [DOI] [PubMed] [Google Scholar]
- 5.Azzawi M, Bradley B, Jeffery PK, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142(6 Pt 1):1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 6.Bradley BL, Azzawi M, Jacobson M, et al. Eosinophils, T-lymphocytes, mast cells, neutrophils, and macrophages in bronchial biopsy specimens from atopic subjects with asthma: comparison with biopsy specimens from atopic subjects without asthma and normal control subjects and relationship to bronchial hyperresponsiveness. J Allergy Clin Immunol. 1991;88(4):661–674. doi: 10.1016/0091-6749(91)90160-p. [DOI] [PubMed] [Google Scholar]
- 7.Laitinen LA, Heino M, Laitinen A, Kava T, Haahtela T. Damage of the airway epithelium and bronchial reactivity in patients with asthma. Am Rev Respir Dis. 1985;131(4):599–606. doi: 10.1164/arrd.1985.131.4.599. [DOI] [PubMed] [Google Scholar]
- 8.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;30326(5):298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 9.Ackerman V, Marini M, Vittori E, Bellini A, Vassali G, Mattoli S. Detection of cytokines and their cell sources in bronchial biopsy specimens from asthmatic patients. Relationship to atopic status, symptoms, and level of airway hyperresponsiveness. Chest. 1994;105(3):687–696. doi: 10.1378/chest.105.3.687. [DOI] [PubMed] [Google Scholar]
- 10.Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi: 10.1056/NEJMoa012705. [DOI] [PubMed] [Google Scholar]
- 11.Berger P, Lavallee J, Rouiller R, Laurent F, Marthan R, Tunon-de-Lara JM. Assessment of bronchial inflammation using an automated cell recognition system based on colour analysis. Eur Respir J. 1999;14(6):1394–1402. doi: 10.1183/09031936.99.14613949. [DOI] [PubMed] [Google Scholar]
- 12.Berger P, Laurent F, Begueret H, et al. Structure and function of small airways in smokers: relationship between air trapping at CT and airway inflammation. Radiology. 2003;228(1):85–94. doi: 10.1148/radiol.2281020187. [DOI] [PubMed] [Google Scholar]
- 13.Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputum from subjects with asthma exacerbation. J Allergy Clin Immunol. 1995;95(4):843–852. doi: 10.1016/s0091-6749(95)70128-1. [DOI] [PubMed] [Google Scholar]
- 14.Sur S, Crotty TB, Kephart GM, et al. Sudden-onset fatal asthma. A distinct entity with few eosinophils and relatively more neutrophils in the airway submucosa? Am Rev Respir Dis. 1993;148(3):713–719. doi: 10.1164/ajrccm/148.3.713. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel SE, Szefler SJ, Leung DY, Sloan SI, Rex MD, Martin RJ. Bronchoscopic evaluation of severe asthma. Persistent inflammation associated with high dose glucocorticoids. Am J Respir Crit Care Med. 1997;156(3 Pt 1):737–743. doi: 10.1164/ajrccm.156.3.9610046. [DOI] [PubMed] [Google Scholar]
- 16.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol. 1995;154(9):4719–4725. [PubMed] [Google Scholar]
- 17.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101(4):916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 18.Groneberg DA, Eynott PR, Lim S, et al. Expression of respiratory mucins in fatal status asthmaticus and mild asthma. Histopathology. 2002;40(4):367–373. doi: 10.1046/j.1365-2559.2002.01378.x. [DOI] [PubMed] [Google Scholar]
- 19.Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989;1(8637):520–524. doi: 10.1016/s0140-6736(89)90067-6. [DOI] [PubMed] [Google Scholar]
- 20.Ebina M, Takahashi T, Chiba T, Motomiya M. Cellular hypertrophy and hyperplasia of airway smooth muscles underlying bronchial asthma. A 3-D morphometric study. Am Rev Respir Dis. 1993;148(3):720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- 21.Johnson PR, Roth M, Tamm M, et al. Airway smooth muscle cell proliferation is increased in asthma. Am J Respir Crit Care Med. 2001;164(3):474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- 22.Lambert RK, Wiggs BR, Kuwano K, Hogg JC, Pare PD. Functional significance of increased airway smooth muscle in asthma and COPD. J Appl Physiol. 1993;74(6):2771–2781. doi: 10.1152/jappl.1993.74.6.2771. [DOI] [PubMed] [Google Scholar]
- 23.Trian T, Benard G, Begueret H, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204(13):3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James AL, Pare PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis. 1989;139(1):242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- 25.Sobonya RE. Quantitative structural alterations in long-standing allergic asthma. Am Rev Respir Dis. 1984;130(2):289–292. doi: 10.1164/arrd.1984.130.2.289. [DOI] [PubMed] [Google Scholar]
- 26.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 27.Carroll N, Elliot J, Morton A, James A. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis. 1993;147(2):405–410. doi: 10.1164/ajrccm/147.2.405. [DOI] [PubMed] [Google Scholar]
- 28.Armour CL, Black JL, Berend N, Woolcock AJ. The relationship between bronchial hyperresponsiveness to methacholine and airway smooth muscle structure and reactivity. Respir Physiol. 1984;58(2):223–233. doi: 10.1016/0034-5687(84)90150-6. [DOI] [PubMed] [Google Scholar]
- 29.Begueret H, Berger P, Vernejoux JM, Dubuisson L, Marthan R, Tunon-de-Lara JM. Inflammation of bronchial smooth muscle in allergic asthma. Thorax. 2007;62(1):8–15. doi: 10.1136/thx.2006.062141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodruff PG, Dolganov GM, Ferrando RE, et al. Hyperplasia of smooth muscle in mild to moderate asthma without changes in cell size or gene expression. Am J Respir Crit Care Med. 2004;169(9):1001–1006. doi: 10.1164/rccm.200311-1529OC. [DOI] [PubMed] [Google Scholar]
- 31.McMillan SJ, Lloyd CM. Prolonged allergen challenge in mice leads to persistent airway remodelling. Clin Exp Allergy. 2004;34(3):497–507. doi: 10.1111/j.1365-2222.2004.01895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slats AM, Janssen K, van Schadewijk A, et al. Expression of smooth muscle and extracellular matrix proteins in relation to airway function in asthma. J Allergy Clin Immunol. 2008;121(5):1196–1202. doi: 10.1016/j.jaci.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Dekkers BG, Schaafsma D, Nelemans SA, Zaagsma J, Meurs H. Extracellular matrix proteins differentially regulate airway smooth muscle phenotype and function. Am J Physiol Lung Cell Mol Physiol. 2007;292(6):L1405–L1413. doi: 10.1152/ajplung.00331.2006. [DOI] [PubMed] [Google Scholar]
- 34.Hossain S. Quantitative measurement of bronchial muscle in men with asthma. Am Rev Respir Dis. 1973;107(1):99–109. doi: 10.1164/arrd.1973.107.1.99. [DOI] [PubMed] [Google Scholar]
- 35.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167(10):1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 36.Berger P, Perng DW, Thabrew H, et al. Tryptase and agonists of PAR-2 induce the proliferation of human airway smooth muscle cells. J Appl Physiol. 2001;91(3):1372–1379. doi: 10.1152/jappl.2001.91.3.1372. [DOI] [PubMed] [Google Scholar]
- 37.Trian T, Girodet PO, Ousova O, Marthan R, Tunon-de-Lara JM, Berger P. RNA interference decreases PAR-2 expression and function in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2006;34(1):49–55. doi: 10.1165/rcmb.2005-0187OC. [DOI] [PubMed] [Google Scholar]
- 38.Hirst SJ, Barnes PJ, Twort CH. PDGF isoform-induced proliferation and receptor expression in human cultured airway smooth muscle cells. Am J Physiol. 1996;270(3 Pt 1):L415–L428. doi: 10.1152/ajplung.1996.270.3.L415. [DOI] [PubMed] [Google Scholar]
- 39.Noveral JP, Bhala A, Hintz RL, Grunstein MM, Cohen P. Insulin-like growth factor axis in airway smooth muscle cells. Am J Physiol. 1994;267(6 Pt 1):L761–L765. doi: 10.1152/ajplung.1994.267.6.L761. [DOI] [PubMed] [Google Scholar]
- 40.Stewart AG, Grigoriadis G, Harris T. Mitogenic actions of endothelin-1 and epidermal growth factor in cultured airway smooth muscle. Clin Exp Pharmacol Physiol. 1994;21(4):277–285. doi: 10.1111/j.1440-1681.1994.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 41.Ramos-Barbon D, Presley JF, Hamid QA, Fixman ED, Martin JG. Antigen-specific CD4+ T cells drive airway smooth muscle remodeling in experimental asthma. J Clin Invest. 2005;115(6):1580–1589. doi: 10.1172/JCI19711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng X, Zhou D, Seow CY, Bai TR. Cardiotrophin-1 alters airway smooth muscle structure and mechanical properties in airway explants. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1165–L1171. doi: 10.1152/ajplung.00171.2004. [DOI] [PubMed] [Google Scholar]
- 43.Goldsmith AM, Bentley JK, Zhou L, et al. Transforming growth factor-beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol. 2006;34(2):247–254. doi: 10.1165/rcmb.2005-0166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deng H, Dokshin GA, Lei J, et al. Inhibition of glycogen synthase kinase-3beta is sufficient for airway smooth muscle hypertrophy. J Biol Chem. 2008;283(15):10198–10207. doi: 10.1074/jbc.M800624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McIlveen SA. Bronchovascular role in pulmonary congestion. Clin Exp Pharmacol Physiol. 2000;27(12):1045–1048. doi: 10.1046/j.1440-1681.2000.03374.x. [DOI] [PubMed] [Google Scholar]
- 46.Serikov VB, Fleming NW. Pulmonary and bronchial circulations: contributions to heat and water exchange in isolated lungs. J Appl Physiol. 2001;91(5):1977–1985. doi: 10.1152/jappl.2001.91.5.1977. [DOI] [PubMed] [Google Scholar]
- 47.Simper D, Stalboerger PG, Panetta CJ, Wang S, Caplice NM. Smooth muscle progenitor cells in human blood. Circulation. 2002;3106(10):1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 48.Hollingsworth JW, Li Z, Brass DM, et al. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2007;37(2):248–253. doi: 10.1165/rcmb.2006-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roach P. New insulin analogues and routes of delivery : pharmacodynamic and clinical considerations. Clin Pharmacokinet. 2008;47(9):595–610. doi: 10.2165/00003088-200847090-00003. [DOI] [PubMed] [Google Scholar]
- 50.Braley JF, Peterson LB, Dawson CA, Moore VL. Effect of hypersensitivity on protein uptake across the air-blood barrier of isolated rabbit lungs. J Clin Invest. 1979;63(6):1103–1109. doi: 10.1172/JCI109402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marchetti C, Poggi P, Clement MG, Aguggini G, Piacentini C, Icaro-Cornaglia A. Lymphatic capillaries of the pig lung: TEM and SEM observations. Anat Rec. 1994;238(3):368–373. doi: 10.1002/ar.1092380311. [DOI] [PubMed] [Google Scholar]
- 52.Hong YK, Shin JW, Detmar M. Development of the lymphatic vascular system: a mystery unravels. Dev Dyn. 2004;231(3):462–473. doi: 10.1002/dvdy.20179. [DOI] [PubMed] [Google Scholar]
- 53.Swartz MA, Hubbell JA, Reddy ST. Lymphatic drainage function and its immunological implications: from dendritic cell homing to vaccine design. Semin Immunol. 2008;20(2):147–156. doi: 10.1016/j.smim.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 54.Bienenstock J, McDermott MR. Bronchus- and nasal-associated lymphoid tissues. Immunol Rev. 2005;206:22–31. doi: 10.1111/j.0105-2896.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 55.Swartz MA, Kaipainen A, Netti PA, et al. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech. 1999;32(12):1297–1307. doi: 10.1016/s0021-9290(99)00125-6. [DOI] [PubMed] [Google Scholar]
- 56.Baluk P, Tammela T, Ator E, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115(2):247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vrugt B, Aalbers R. Inflammation and bronchial hyperresponsiveness in allergic asthma and chronic obstructive pulmonary disease. Respir Med. 1993;87(Suppl B):3–7. doi: 10.1016/0954-6111(93)90117-i. [DOI] [PubMed] [Google Scholar]
- 58.Ebina M. Remodeling of airway walls in fatal asthmatics decreases lymphatic distribution; beyond thickening of airway smooth muscle layers. Allergol Int. 2008;57(2):165–174. doi: 10.2332/allergolint.O-07-497. [DOI] [PubMed] [Google Scholar]
- 59.Asai K, Kanazawa H, Otani K, Shiraishi S, Hirata K, Yoshikawa J. Imbalance between vascular endothelial growth factor and endostatin levels in induced sputum from asthmatic subjects. J Allergy Clin Immunol. 2002;110(4):571–575. doi: 10.1067/mai.2002.127797. [DOI] [PubMed] [Google Scholar]
- 60.Hoshino M, Takahashi M, Aoike N. Expression of vascular endothelial growth factor, basic fibroblast growth factor, and angiogenin immunoreactivity in asthmatic airways and its relationship to angiogenesis. J Allergy Clin Immunol. 2001;107(2):295–301. doi: 10.1067/mai.2001.111928. [DOI] [PubMed] [Google Scholar]
- 61.Simcock DE, Kanabar V, Clarke GW, et al. Induction of angiogenesis by airway smooth muscle from patients with asthma. Am J Respir Crit Care Med. 2008;178(5):460–468. doi: 10.1164/rccm.200707-1046OC. [DOI] [PubMed] [Google Scholar]
- 62.Bousquet J, Chanez P, Lacoste JY, et al. Asthma: a disease remodeling the airways. Allergy. 1992;47(1):3–11. doi: 10.1111/j.1398-9995.1992.tb02242.x. [DOI] [PubMed] [Google Scholar]
- 63.Colorado PC, Torre A, Kamphaus G, et al. Anti-angiogenic cues from vascular basement membrane collagen. Cancer Res. 2000;60(9):2520–2526. [PubMed] [Google Scholar]
- 64.Kamphaus GD, Colorado PC, Panka DJ, et al. Canstatin, a novel matrix-derived inhibitor of angiogenesis and tumor growth. J Biol Chem. 2000;275(2):1209–1215. doi: 10.1074/jbc.275.2.1209. [DOI] [PubMed] [Google Scholar]
- 65.Maeshima Y, Colorado PC, Torre A, et al. Distinct antitumor properties of a type IV collagen domain derived from basement membrane. J Biol Chem. 2000;275(28):21340–21348. doi: 10.1074/jbc.M001956200. [DOI] [PubMed] [Google Scholar]
- 66.Maeshima Y, Manfredi M, Reimer C, et al. Identification of the anti-angiogenic site within vascular basement membrane-derived tumstatin. J Biol Chem. 2001;276(18):15240–15248. doi: 10.1074/jbc.M007764200. [DOI] [PubMed] [Google Scholar]
- 67.Maeshima Y, Yerramalla UL, Dhanabal M, et al. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J Biol Chem. 2001;276(34):31959–31968. doi: 10.1074/jbc.M103024200. [DOI] [PubMed] [Google Scholar]
- 68.Yamamoto Y, Maeshima Y, Kitayama H, et al. Tumstatin peptide, an inhibitor of angiogenesis, prevents glomerular hypertrophy in the early stage of diabetic nephropathy. Diabetes. 2004;53(7):1831–1840. doi: 10.2337/diabetes.53.7.1831. [DOI] [PubMed] [Google Scholar]
- 69.Boulet L, Belanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med. 1995;152(3):865–871. doi: 10.1164/ajrccm.152.3.7663797. [DOI] [PubMed] [Google Scholar]
- 70.Saglani S, Malmstrom K, Pelkonen AS, et al. Airway remodeling and inflammation in symptomatic infants with reversible airflow obstruction. Am J Respir Crit Care Med. 2005;171(7):722–727. doi: 10.1164/rccm.200410-1404OC. [DOI] [PubMed] [Google Scholar]
- 71.Pohunek P, Warner JO, Turzikova J, Kudrmann J, Roche WR. Markers of eosinophilic inflammation and tissue re-modelling in children before clinically diagnosed bronchial asthma. Pediatr Allergy Immunol. 2005;16(1):43–51. doi: 10.1111/j.1399-3038.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 72.Bai TR, Cooper J, Koelmeyer T, Pare PD, Weir TD. The effect of age and duration of disease on airway structure in fatal asthma. Am J Respir Crit Care Med. 2000;162(2 Pt 1):663–669. doi: 10.1164/ajrccm.162.2.9907151. [DOI] [PubMed] [Google Scholar]
- 73.Lange P, Parner J, Vestbo J, Schnohr P, Jensen G. A 15-year follow-up study of ventilatory function in adults with asthma. N Engl J Med. 1998;339(17):1194–1200. doi: 10.1056/NEJM199810223391703. [DOI] [PubMed] [Google Scholar]
- 74.Pepe C, Foley S, Shannon J, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol. 2005;116(3):544–549. doi: 10.1016/j.jaci.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Chetta A, Foresi A, Del Donno M, Bertorelli G, Pesci A, Olivieri D. Airways remodeling is a distinctive feature of asthma and is related to severity of disease. Chest. 1997 Apr;111(4):852–857. doi: 10.1378/chest.111.4.852. [DOI] [PubMed] [Google Scholar]
- 76.Niimi A, Matsumoto H, Takemura M, Ueda T, Chin K, Mishima M. Relationship of airway wall thickness to airway sensitivity and airway reactivity in asthma. Am J Respir Crit Care Med. 2003;168(8):983–988. doi: 10.1164/rccm.200211-1268OC. [DOI] [PubMed] [Google Scholar]
- 77.Johnson PR, Burgess JK, Underwood PA, et al. Extracellular matrix proteins modulate asthmatic airway smooth muscle cell proliferation via an autocrine mechanism. J Allergy Clin Immunol. 2004;113(4):690–696. doi: 10.1016/j.jaci.2003.12.312. [DOI] [PubMed] [Google Scholar]
- 78.Johnson PR, Burgess JK, Ge Q, et al. Connective tissue growth factor induces extracellular matrix in asthmatic airway smooth muscle. Am J Respir Crit Care Med. 2006;173(1):32–41. doi: 10.1164/rccm.200406-703OC. [DOI] [PubMed] [Google Scholar]
- 79.Westergren-Thorsson G, Chakir J, Lafreniere-Allard MJ, Boulet LP, Tremblay GM. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol. 2002;34(10):1256–1267. doi: 10.1016/s1357-2725(02)00058-4. [DOI] [PubMed] [Google Scholar]
- 80.Hirst SJ, Twort CH, Lee TH. Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol. 2000;23(3):335–344. doi: 10.1165/ajrcmb.23.3.3990. [DOI] [PubMed] [Google Scholar]
- 81.Peng Q, Lai D, Nguyen TT, Chan V, Matsuda T, Hirst SJ. Multiple beta 1 integrins mediate enhancement of human airway smooth muscle cytokine secretion by fibronectin and type I collagen. J Immunol. 2005;174(4):2258–2264. doi: 10.4049/jimmunol.174.4.2258. [DOI] [PubMed] [Google Scholar]
- 82.Nguyen TT, Ward JP, Hirst SJ. beta1-Integrins mediate enhancement of airway smooth muscle proliferation by collagen and fibronectin. Am J Respir Crit Care Med. 2005;171(3):217–223. doi: 10.1164/rccm.200408-1046OC. [DOI] [PubMed] [Google Scholar]
- 83.Walker HA, Whitelock JM, Garl PJ, Nemenoff RA, Stenmark KR, Weiser-Evans MC. Perlecan up-regulation of FRNK suppresses smooth muscle cell proliferation via inhibition of FAK signaling. Mol Biol Cell. 2003;14(5):1941–1952. doi: 10.1091/mbc.E02-08-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hasaneen NA, Zucker S, Cao J, Chiarelli C, Panettieri RA, Foda HD. Cyclic mechanical strain-induced proliferation and migration of human airway smooth muscle cells: role of EMMPRIN and MMPs. Faseb J. 2005;19(11):1507–1509. doi: 10.1096/fj.04-3350fje. [DOI] [PubMed] [Google Scholar]
- 85.Salerno FG, Fust A, Ludwig MS. Stretch-induced changes in constricted lung parenchymal strips: role of extracellular matrix. Eur Respir J. 2004;23(2):193–198. doi: 10.1183/09031936.03.00025403. [DOI] [PubMed] [Google Scholar]
- 86.Holgate ST. Tissue injury. Clinical and Experimental Allergy Reviews. 2001;1:102–106. [Google Scholar]
- 87.Olivieri D, Chetta A, Del Donno M, et al. Effect of short-term treatment with low-dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo-controlled study. Am J Respir Crit Care Med. 1997;155(6):1864–1871. doi: 10.1164/ajrccm.155.6.9196087. [DOI] [PubMed] [Google Scholar]
- 88.Henderson WR, Jr, Chiang GK, Tien YT, Chi EY. Reversal of allergen-induced airway remodeling by CysLT1 receptor blockade. Am J Respir Crit Care Med. 2006;173(7):718–728. doi: 10.1164/rccm.200501-088OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Izumo T, Kondo M, Nagai A. Cysteinyl-leukotriene 1 receptor antagonist attenuates bleomycin-induced pulmonary fibrosis in mice. Life Sci. 2007;80(20):1882–1886. doi: 10.1016/j.lfs.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 90.Fireman E, Schwartz Y, Mann A, Greif J. Effect of montelukast, a cysteinyl receptor antagonist, on myofibroblasts in interstitial lung disease. J Clin Immunol. 2004;24(4):418–425. doi: 10.1023/B:JOCI.0000029110.11097.4d. [DOI] [PubMed] [Google Scholar]
- 91.Kelly MM, Chakir J, Vethanayagam D, et al. Montelukast treatment attenuates the increase in myofibroblasts following low-dose allergen challenge. Chest. 2006;130(3):741–753. doi: 10.1378/chest.130.3.741. [DOI] [PubMed] [Google Scholar]
- 92.Perng DW, Wu YC, Chang KT, et al. Leukotriene C4 induces TGF-beta1 production in airway epithelium via p38 kinase pathway. Am J Respir Cell Mol Biol. 2006;34(1):101–107. doi: 10.1165/rcmb.2005-0068OC. [DOI] [PubMed] [Google Scholar]
- 93.Pifferi M, Caramella D, Ragazzo V, De Marco E, Pietrobelli A, Boner AL. Montelukast and airway remodeling in children with chronic persistent asthma: an open study. Pediatr Allergy Immunol. 2004;15(5):472–473. doi: 10.1111/j.1399-3038.2004.00184.x. [DOI] [PubMed] [Google Scholar]
- 94.Burgess JK, Oliver BG, Poniris MH, et al. A phosphodiesterase 4 inhibitor inhibits matrix protein deposition in airways in vitro. J Allergy Clin Immunol. 2006;118(3):649–657. doi: 10.1016/j.jaci.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 95.Roth M, Johnson PR, Borger P, et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med. 2004;351(6):560–574. doi: 10.1056/NEJMoa021660. [DOI] [PubMed] [Google Scholar]
- 96.Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356(13):1327–1337. doi: 10.1056/NEJMoa064707. [DOI] [PubMed] [Google Scholar]
- 97.Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176(12):1185–1191. doi: 10.1164/rccm.200704-571OC. [DOI] [PubMed] [Google Scholar]
- 98.Chetta A, Zanini A, Foresi A, et al. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005;35(11):1437–1442. doi: 10.1111/j.1365-2222.2005.02360.x. [DOI] [PubMed] [Google Scholar]
- 99.Kumagai K, Ohno I, Imai K, et al. The involvement of matrix metalloproteinases in basement membrane injury in a murine model of acute allergic airway inflammation. Clin Exp Allergy. 2002;32(10):1527–1534. doi: 10.1046/j.1365-2745.2002.01491.x. [DOI] [PubMed] [Google Scholar]
- 100.Kwan ML, Gomez AD, Baluk P, Hashizume H, McDonald DM. Airway vasculature after mycoplasma infection: chronic leakiness and selective hypersensitivity to substance P. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L286–297. doi: 10.1152/ajplung.2001.280.2.L286. [DOI] [PubMed] [Google Scholar]
- 101.Johnson PR, Black JL, Carlin S, Ge Q, Underwood PA. The production of extracellular matrix proteins by human passively sensitized airway smooth-muscle cells in culture: the effect of beclomethasone. Am J Respir Crit Care Med. 2000;162(6):2145–2151. doi: 10.1164/ajrccm.162.6.9909111. [DOI] [PubMed] [Google Scholar]