Abstract
For treatment of several malignancies, transplantation of allogeneic haematopoietic stem cells (HSCT) derived from bone marrow or peripheral blood has been used as a therapeutic procedure for decades. In the past, HSCT has been suggested as a treatment option for infection with the human immunodeficiency virus type 1 (HIV-1), but these attempts were mostly unsuccessful. Today, after the introduction of an active anti-retroviral therapy, the lifetime expectancy of HIV-infected patients has improved substantially, but nevertheless the incidence rate of malignancies in these patients has increased considerably. Therefore, it can be assumed that there will be a rising necessity for HIV-1-infected patients with malignancies for allogeneic HSCT. At the same time, there is increasing interest in treatment methods which might target the HIV-1 reservoir more effectively, and the question has been raised as to whether allogeneic HSCT could be linked to such strategies. In this paper the data of more than 25 years experience with allogeneic HSCT in patients with HIV-1 are reviewed and analysed.
Keywords: allogeneic stem cell transplantation, gene therapy, HIV
Introduction
The human immunodeficiency virus type 1 (HIV-1) belongs to a subset of retroviruses called lentiviruses, and is causative for the acquired immunodeficiency syndrome (AIDS). The immune system of patients with infection of HIV-1 is characterized by an immunodeficiency developing in the setting of global immune activation and a complex array of HIV-specific immune responses, resulting in a decrease in both memory and naive CD4+ T cells [1].
The rate of malignancies in patients with HIV infection has increased and has became dramatically high, especially for B-non-Hodgkin's lymphomas [2,3]. According to the therapy standards in non-HIV-infected patients with leukaemia and relapsed lymphoma, there is a rising demand for curative treatment options such as high-dose chemotherapy with autologous or allogeneic haematopoietic stem cell transplantation (HSCT) [4]. Most recently, a retrospective trial could demonstrate a comparable survival between HIV-positive and HIV-negative non-Hodgkin's and Hodgkin's lymphoma patients who underwent autologous HSCT [5]. Nevertheless, prospective, randomized studies with HIV-1 infected patients receiving either autologous or allogeneic HSCT are still missing [6–8].
Before the introduction of any active anti-retroviral medication in individuals with HIV, the mortality rate of these patients was staggering. It is no surprise that at that time several efforts – even unusual efforts – were undertaken to influence the clinical course of infection [9,10]. Based on positive experiences within the field of allogeneic HSCT in children with severe combined immunodeficiency, this promising treatment option was considered to benefit the clinical course of HIV infection in the early 1980s [11]. It seemed likely that the lack of CD4+ T cells in the late stage of infection could be balanced easily by cell support from suitable healthy donors. However, it soon became obvious that this approach was not successful in improving the course of HIV infection. Irrespectively, in the following 25 years there were several allogeneic HSCT reports in HIV-infected patients with haematological diseases, including not only leukaemia and relapsed lymphoma but also successful treatments of non-malignant disorders such as aplastic anaemia (AA). This review summarizes the experiences from all reported cases and emphasizes major problems and particularities in this context.
Early experiences of allogeneic HSCT in patients with HIV
The first experimental attempt was made in the early 1980s by Hassett and coworkers (Table 1). They treated two patients with a history of Kaposi's sarcoma and severe opportunistic infections with human leucocyte antigen D-related (HLA-DR) matched haematopoietic progenitor cells from the bone marrow of related donors. Because of the severe wasting situation in these patients, Hassett abstained from giving a preparative conditioning regimen. Looking more closely, this attempt seemed to be more alike to a donor lymphocyte infusion (DLI) than to an allogeneic HSCT. Consequently, no stable engraftment was achieved despite the fact that huge amounts of marrow mononuclear cells were given. Clinically, the patients did not improve substantially from this procedure and the immunological condition remained stable or worsened [12]. A similar approach with DLIs was reported in the same year by Davis and coworkers [13].
Table 1.
Allogeneic stem cell transplantation in patients with human immunodeficiency virus (HIV) between 1983 and 1999.
| Reference | No. of patients | Graft | Conditioning regimen | Anti-retroviral therapy | Effect on HIV | Follow-up |
|---|---|---|---|---|---|---|
| Hasset et al. (1983) [12] | 2 | MUD | None | – | None | NR |
| Lane et al. (1984) [18] | 1 | SYN | None | – | CD4+ increased | Died after 12 months |
| Mitsuyasu et al. 1984 [19] | 2 | SYN | 1 × VPL, TBI | – | None | Died day +41 |
| Vilmer et al. (1987) [51] | 1 | SYN | NR | – | CD4+ increased | 7 years |
| Verdonck et al. (1988) [14] | 1 | SYN | CPM, TBI | – | None | Died after 56 months |
| Holland et al. (1989) [24] | 1 | MUD | CPM, TBI | – | PCR negative from day +41, AB declined | Died on day +47 |
| Saral & Holland (1994) [25] | 7 | 4 × MUD 3 × SYN | 6 × CPM and TBI 1 × CPM and BSN | – | 1 patient* PCR-negative from days 40–120, 1–2 log decrease in virus titre | *Died later on GVHD 1 patient died < 30 days |
| Lane et al. (1990) [26] | 16 | SYN | None | – | None | 10 alive (23·4 ± 4 months), 5 died (17·6 ± 5·5 months) |
| Furlini et al. (1988) [15]Bardini et al. (1991) [16] | 1 | MRD | CPM, TBI | – | None | Alive 3 years |
| Angelucci et al. (1991) [27] | 1 | MUD | BSN, CPM | – | None | Died on day +263 |
| Aboulafia et al. (1991) [28] | 1 | SYN | ETP, BCNU, CPM | – | None | Died on day +52 |
| Giri et al. (1992) [29] | 1 | MUD | CPM, ATG, TBI | – | None | Died after 13 months |
| Torlontano et al. (1992) [30] | 1 | MUD | BSN, CPM | – | Increased CD4/CD8 ratio | Died on day +48 |
| Turner et al. (1992) [65] | 1 | SYN | CPM, TBI | AZT | p24 antigene negative | Died on day +18 |
| Contu et al. (1993) [54] | 1 | MUD | BSN, CPM | AZT +IFN-α2 | PCR negative from day +30, anti-HIV-AB negative after 4 months | Died on day +301 |
| Campbell et al. (1999) [66] | 1 | SYN | CPM, BCNU, ETP | 3TC, d4T | None | Alive 12 months |
3TC, lamivudine; AB, antibodies; AZT, zidovudine; BCNU, carmustin; BSN, busulphan; CPM, cyclophosphamide; d4T, stavudine; ETP, etoposide; IFN-α2, interferon-alpha2; MRD, matched related donor; MUD, matched unrelated donor; NR, not reported; PCR, polymerase chain reaction; SYN, syngeneic donor; TBI, total body irradiation; VBL, vinblastine.
Probably the first allogeneic stem cell transplantation in a patient with HIV was performed in early 1982 with undiagnosed infection, as the test assay to detect HIV was not available at that time. Verdonck and coworkers reported the case of a 22-year-old man with acute lymphoblastic leukaemia who underwent syngeneic stem cell transplantation in 1982. One month before transplantation the patient had received a platelet concentrate from a donor who was later tested to be HIV+. Retrospectively, the patient displayed seroconversion at day +33 after HSCT, and 28 months after transplantation the patient developed AIDS and eventually died from severe pneumonia [14].
Another unusual case was reported by Furlini and coworkers in which a young woman with acute lymphoblastic leukaemia underwent HSCT from her brother, who was found later to be HIV-positive. The exact time-point of seroconversion in this patient was not known, but in the first available blood sample 5 months after engraftment, HIV antibody testing was positive [15]. Interestingly, although the allografted patient was heavily immunocompromised after transplantation and anti-retroviral medication was not available at that time, the patient did not progress towards the stage of AIDS in the following 8 years [16].
In 1984 another patient, who was in a better clinical condition and had an HLA identical twin, was treated by Lane and colleagues. Encouraged by experiences and previous reports that engraftment of stem cells from an identical twin can be achieved without conditioning treatment, over a period of 7 months the patient received six DLIs and a single stem cell boost from his twin [17]. This treatment regimen was associated with an increase of CD4+ T cell count with a peak at 3 months after allogeneic HSCT. Finally, however, the patient died of cytomegalovirus pneumonia 12 months after beginning treatment [18].
In the same year, Mitsuyasu and coworkers reported a small series of three patients with AIDS and Kaposi's sarcoma who were found to have HLA-identical siblings. Two patients received infusions of allogeneic bone marrow without previous conditioning, but no improvement in terms of T cell-mediated immunity could be observed. The third patient received a conditioning regimen containing vinblastine [0·3 mg/kg intravenously (i.v.), d1–2] and total body irradiation (TBI) (200 cGy, d1–5). Engraftment was achieved on day +10 and Kaposi's sarcoma showed a > 50% reduction. Nevertheless, the patient died on day +41 from severe pneumonia [19].
Based on these first investigations from 1982 to 1984, it can be assumed that although patients with AIDS display a high degree of immune deficiency, allogeneic transplantation without conditioning regimen did not lead to stable chimerism. Furthermore, one suggested rationale for allogeneic stem cell transplantation as a therapy in these patients was to target the reservoir of HIV-infected cells for degradation during the conditioning regimen, and secondly, to replace the old immune system with uninfected donor cells. The latter can be assumed, as not only are donor-derived circulating lymphocytes changed after allogeneic transplantation, but all lymphoid cells and tissues including alveolar macrophages in the lung, Kupffer's cells in the liver and even microglia cell in the central nervous system (CNS) were replaced during a long-term process [20–22]. However, this ignores a crucial problem, namely that as long as the HIV replication is not affected during this cytoablative therapy, the newly arising lymphoid cells are susceptible to infection after engraftment. Thus, anti-viral therapy seems to be an essential element to such an approach in elimination of the HIV-1 reservoir.
The introduction of anti-retroviral therapy
A promising solution for this problem was given after zidovudine received approval for HIV treatment in 1987 [23]. There was some hope that a suppression of viral replication by anti-retroviral medication during the set-up of allogeneic HSCT might protect the arising new immune system from reinfection. Indeed, this theory followed a first encouraging case reported from Holland and coworkers in 1989. A 41-year-old man with refractory lymphoma underwent allogeneic HSCT with concomitant zidovudine medication (5 mg/kg body weight). Engraftment was achieved on day +17 with complete donor chimerism. HIV-RNA polymerase chain reaction (PCR) was positive until day +32, and HIV culture experiments remained negative from day +25. Unfortunately, the patient died on day +47 after allogeneic HSCT due to a relapse of his lymphoma. Investigation of several tissues post-mortem revealed no evidence of HIV-RNA, except for a positive gag-specific signal for HIV-1 DNA from the recto-sigmoid under lowered stringency conditions for filter washing of this assay. The authors suggested that HIV had been eradicated secondary to the myeloablative chemoradiotherapy and that zidovudine may have prevented reinfection of the new donor haematopoetic stem cells [24].
Encouraged by these findings, the Hopkins and Emory group performed two allogeneic and three syngeneic stem cell transplantations in another five HIV+ patients with ongoing zidovudine. One of the patients who underwent allogeneic HSCT remained without evidence of viral rebound until his death from graft-versus-host-disease (GVHD) on day +120. The second allogeneic HSCT patient displayed no control of viraemia. Interestingly, all three recipients from syngeneic donors remained HIV culture-positive after this procedure [25]. This is the first compelling evidence for a possible allogeneic effect controlling HIV-1 replication.
In the same time-period, Lane and coworkers reported a series of 16 HIV+ patients who received syngeneic bone marrow transplantation and adoptive transfer of peripheral blood lymphocytes. Patients were randomized, receiving either zidovudine or placebo for a period of 12 months after transplantation. Lane et al. observed a rapid increase of the CD4+ cell count after infusions but without any notable clinical improvement. Furthermore, no difference in CD4+ T cell percentage, HIV isolate from blood or p24 antigenaemia was observed during follow-up in both groups [26]. In the following years, several groups tried to repeat this approach and four more HIV+ patients underwent HSCT, but all patients died during the first year of follow-up and none of them displayed improvement in HIV infection [27–30].
Allogeneic HSCT ongoing highly active anti-retroviral therapy (HAART)
Since the introduction of HAART for HIV the survival of infected patients has improved considerably and, in 1997, the first allogeneic HSCT was performed with ongoing HAART [31,32]. Subsequently, in further transplantations of patients with HIV, additional anti-retroviral medication became a standard treatment (Table 2).
Table 2.
Allogeneic stem cell transplantation with ongoing highly active anti-retroviral therapy (HAART).
| CD4+ count/µl | HIV load cop/ml | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Patient age and gender | Disease | before transplantation | Graft (CD34+ cells/kg) | Conditioning regimen | Day of engraftment | GVHD prophylaxis | HAART | Effect on HIV | Follow-up | |
| Schlegel et al. 2000 [32] | 34 male | CML | NR | 2 × 103 | MRD (NR) | BSN (16 × 1 mg/kg) CPM (2 × 60 mg/kg) | +17 | CsA MTX | DDI, d4T | Rebound ongoing HAART (day +90) | 353 days |
| Kang et al. (2002) [35] | (1) 42 male (2) 31 male | (1) AML (2) B-NHL | (1) 200 (2) 81 | (1) 494 (2) < det. | MRD (16·99 × 106) Both gene modified | CPM (60 mg/kg) FLUD (25 mg/m2) | NR | CsA | (1) IDV, d4T, 3TC (2) NFV, ABC, 3TC discont. day -7 | (2) Rebound after discont. HAART (day +60) | (1) 24 months (2) Died 12 months (relapse) |
| Sora et al. (2002) [36] | 33 female | AML | 294 | < det. | MRD (4·5 × 106) | BSN (16 × 1 mg/kg) CPM (2 × 60 mg/kg) | +9 | CsA | D4T, 3TC, IDV Discont. +5 until +14 (vomiting) | Rebound after discont. HAART (day +42) | 39 months |
| Shamansky et al. (2004) [67] | 47 male | AML | NR | NR | MRD (2·27 × 106) | FLUD (150 mg/m2) MLP (140 mg/m2) | +16 | CsA, MTX | NR | None | 250 days |
| Tomonari et al. (2005) [33] | 23 female | ALL | 72 | < det. | 1st MUD cord blood HLA-B/DR mismatch (0·7 × 105) 2nd +40 MUD one-locus Mismatch at HLA-DR (0·46 × 105) | 1st TBI (12 Gy) CPM (120 mg/kg) 2nd FLUD (3 × 40 mg/m2) | +27 | CSA MTX | d4T, 3TC, EFV Discont. days 0–28 | Rebound after discont. HAART (day +33) | 15 months |
| Binaghi et al. (2006) [68] | 31 male | AA | 383 | < 50 | Syngeneic (11·7 × 106) | CPM (4 × 5000 mg/day) | +11 | None | AZT, 3TC, EFV | None | 10 months |
| Wolf et al. (2007) [37] | 34 male | AA | 192 | < 400 | MUD (7·0 × 106) | FLUD (4 × 30 mg/m2) CPM (2 × 60 mg/kg) | +18 | ATG, CsA, MTX | ABC, 3TC, SQV Discont. 0–34 | Rebound after discont. HAART CNS reactivation | 8 months |
| Avettand-Fenoel et al. (2007) [39] | 17 male | AML | NR | < det. | MUD (NR) | IDA, FLUD, ARA-C | +19 | CsA, ATG | NR Discont. 114–134 | HIV-DNA negative Rebound after discont. HAART | Died +191 (infection) |
| Bryant & Milliken (2008) [34] | 34 male | PEL | NR | NR | MRD (2·38 × 106) | MLP (140 mg/m2), FLUD (5 × 30 mg/m2) | +13 | FK506, sirolimus, MTX | LPV, RTV, TDF, 3TC | none | 31 months |
| Woolfrey et al. (2008) [50] | (1) 39 male (2) 33 male | (1 and 2) AML | (1) 262 (2) 287 | (1 and 2) < det. | (1) MUD (4·58 × 106) (2) MUD (10·63 × 106) | (1 and 2) FLUD (90 mg/m2), TBI (200 cGy) | NR | (1 and 2) CsA, MMF | (1) EFV, ABC, TDF (2) EFV, ABC, 3TC | (1) HIV-DNA negative +56 (2) None | (1) Died +301 (relapse) (2) > 180 days |
| Hamnadani et al. (2009) [69] | (1–3) 39– 55 | (1) AML (2–3) B-NHL | (1–3) 189–457 | (1–2) < det. (3) 814 | (1) MRD, 2–3.) MUD (NR) | FLUD (30 mg/m2), BSN (8 × 0·8 mg/kg) | NR | MTX, MMF | NR | (1–2) None (3) increased viral load on +252 | (1–3) Median 375 days |
| Hütter et al. (2009) [63]; Hütter & Thiel (2010) [64] | 40 male | AML | 415 | < det. | 1st MUD (2·3 × 106) 2nd MUD (2·1 × 106) | 1st m-AMSA (4 × 100 mg/m2), FLUD (4 × 30 mg/m2), ARA-C (4 × 2000 mg/ m2), CPM (60 mg/kg), TBI (4 Gy) 2nd TBI (200 cGy) | +13 | 1st ATG, CsA, MMF 2nd CsA | EFV, FTC, TDF Discont. on day 0 | HAART discont. on day 0, no rebound | 42 months |
| Polizzotto et al. (2007; 2010) [38,70] | (1) 38 male (2) 41 male (3) 24 male | (1) MDS (2) AML (3) T-ALL | (1) 58 (2) 275 (3) 204 | (1–3) < det. | (1) MUD (NR) (2) MRD (NR) (3) MUD (NR) | (1 and 2) CPM (120 mg/kg), TBI (13·2 Gy) (3) ETP (60 mg/kg), TBI (13·2. Gy) | (1) +48 (2) +21 (3) +25 | (1) CsA (2 and 3) NR | (1) d4T, 3TC, TDF discont. days 0–365 (2) FTC, TDF, FosAPV (3) TDF, RTV, FosAPV | (1) Rebound on +30 after HAART discont. (2 and 3) None | (1) Died +730 (renal failure) (2) Died +78 (sepsis) (3) Died +105 (relapse) |
| Kamp et al. (2010) [49] | 34 male | AA | NR | NR | MUD (NR) | CPM, FLUD | NR | NR | NR discont. days 0–34 | Rebound after HAART discont. | +384 |
3TC, lamivudine; AA, aplastic anaemia; ABC, abacavir; ALL, acute lyphoblastic leukaemia; AML, acute myeloid leukaemia; ARA-C, cytarabine; ATG, anti-thymocyte globulin; AZT, zidovudine; BSN, busulfan; B-NHL, B-non-Hodgkin's lymphoma; BSN, busulphan; CML, chronic myeloid leukaemia; CPM, cyclophosphamide; CsA, cyclosporine A; DDI, didanosine; d4T, stavudine; EFV, efavirenz; FTC, emtricitabine; ETP, etoposide; FLUD, fludarabine; FosAPV, fosamprenavir; FT506, tacrolimus; IDA, idarubicin; HIV, human immunodeficiency virus; IDV, indinavir; LPV, lopinavir; m-AMSA, amsacrin; MDS, myelodysplastic syndrome; MLP, melphalan; MMF, mycophenolate mofetil; MRD, matched related donor; MTX, methotrexate; MUD, matched unrelated donor; NFV, nelfinavir; NR, not reported; PEL, HIV-related primary effusion lymphoma; RTV, ritonavir; SQV, ritonavir-boosted saquinavir; SYN, syngeneic donor; TBI, total body irradiation; TDF, tenofovir.
Tomonari reported transplantation of cord blood stem cells in a young Japanese girl with acute lymphoblastic leukaemia. After conditioning with 1200 cGy TBI and 120 mg/kg cyclophosphamide, there was only partial engraftment using a relatively small amount of 0·76 × 105 CD34+/kg stem cells, but there was successful engraftment 40 days after the first HSCT using a second unrelated donor with a one-locus mismatch at HLA-DR [33].
The first case of successful treatment of a patient with an AIDS-defining disease using allogeneic HSCT was reported in 2008 from Bryant and coworkers in a patient who had primary effusion lymphoma (PEL). PEL is associated with human herpesvirus 8 with poor prognosis, and this patient was treated with a reduced-intensity conditioning regimen followed by allogeneic HSCT. He remained in second complete remission regarding PEL over a period of 31 months after transplantation [34].
It is difficult to continue oral HAART during the post-transplant period, when there is severe mucositis or other complication which decreases oral intake. Thus, in seven patients noted in Table 2, HAART was discontinued in the early months after allogeneic HSCT. All such patients developed a rapid rebound in HIV-RNA, which reverted to low or undetectable levels after resumption of anti-retroviral therapy [33–39].
Feasibility of HSCT in patients with HIV
Because of the impaired immune system in HIV+ individuals there was great anxiety in the past that allogeneic HSCT – in the case of a haematological disease – would be an unforeseeable risk in these patients. Today, retrospective analysis of reported cases indicates that the outcome of allografted HIV-positive patients is probably only negligibly poorer in comparison to HIV-negative patients.
The median time to leucocyte engraftment was 19·7 days (Table 2). There are no data on the recovery of CD4+ T cell count after allogeneic HSCT in patients with HIV but, collating the data of 28 allografted patients with ongoing HAART, there might be a slight but continuous increase during engraftment and follow-up (Fig. 1). The infectious complications encountered during the phase of neutropenia are fairly similar to those seen in HIV-negative patients.
Fig. 1.
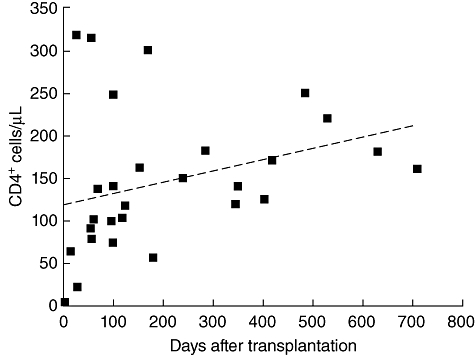
T cell recovery after allogeneic haematopoietic stem cell transplantation in patents with human immunodeficiency virus (HIV) infection ongoing highly active anti-retroviral therapy (HAART) (n = 28). The dashed line displays the median values of CD4+ T cell counts.
The summary of post-transplant complications implies no HIV-specific or unknown events in this patient group and the frequencies of the observed side effects are comparable to those in HIV-negative recipients of HSCT (Table 3) [40].
Table 3.
Allo haematopoietic stem cells (HSCT)-related toxicity and infectious complications according to Table 2.
| Post transplant complications (n = 22) |
|---|
| BK-virus associated hemorrhagic cystitis (×2) |
| Bronchiolitis obliterans with organizing pneumonia (BOOP) |
| Central nervous system toxoplasmosis |
| Cerebrospinal fluid pleocytosis |
| CMV enteritis |
| CMV reactivation |
| Critical illness polyneuropathy |
| Dental infection |
| Grade ≤ II acute GVHD (skin) (×9) |
| Grade II/III chronic GVHD (mucosal) (×2) |
| Grade III acute GVHD (skin) (×2) |
| Multi-organ failure (×2) |
| Neutropenic sepsis (×3) |
| Paresis of cranial nerves |
| Pericarditis |
| Post-transplant glomerulopathy (×2) |
| Systemic inflammatory response syndrome (SIRS) |
| Unclear neurological disease with myocloni |
Number of events is given in parentheses. CMV, cytomegalovirus; GVHD, graft-versus-host disease.
The Kaplan–Meier estimation of survival reveals a remarkable difference between patients with HIV ongoing anti-retroviral medication during the transplantation procedure and those who did not receive HAART (Fig. 2). From eight patients who died in the HAART group, in half the cases a relapse of the malignant diseases was causative. Restrictively, both these groups represent two historical populations, one group before HAART has been administered routinely between 1983 and 2000 and the second group from 2000 until today with routinely given HAART. During the last 27 years the steady optimization of the supportive therapy regarding treatment-related complications notably ameliorated the outcome of patients after allogeneic HSCT.
Fig. 2.
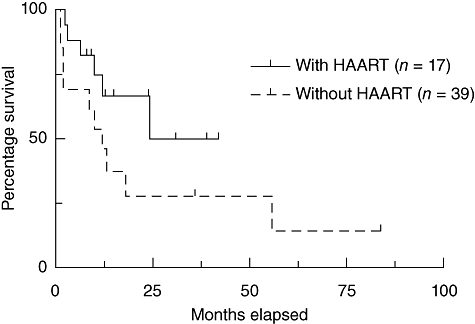
Kaplan–Meier estimation of the survival of human immunodeficiency virus (HIV)-infected patients after allogeneic haematopoietic stem cell transplantation reported from 1983 to 2010.
Specific problems in patients with HIV and allogeneic HSCT
Drug interactions
The pharmacokinetics and pharmacodyanamics of HAART agents, particularly the non-nucleoside reverse transcriptase inhibitors (NNRTIs) and the protease inhibitors (PIs), are influenced by the cytochrome P450 (CYP450) enzyme system [41]. CYP450 is altered significantly by the immunosuppressive agents and antibiotics used routinely after allogeneic HCST. Thus, we know little of the potential effects of HAART in the setting of allogeneic HSCT. These drug–drug interactions have been best studied in conventional chemotherapy regimens in AIDS patients with lymphoma, and although some interactions have been reported, adverse reactions have mainly been modest. Reverse transcriptase inhibitors known to alter marrow are the exception, and zidovudine has been incriminated in one transplant in which there was engraftment failure after autologous HSCT [42,43]. In the allogeneic HSCT setting, less is known only about the potential for drug interactions with anti-neoplastic agents used for conditioning regimen and concomitant medication [44]. Table 4 gives an overview on the previously described interactions of NNRTIs and PIs with agents used commonly during the allogeneic HSCT setting.
Table 4.
Interactions of agents commonly used during allogeneic haemopoietic stem cell transplantation and anti-retroviral medications.
| Agent | Anti-retroviral drug | Interaction on agent | Ref. |
|---|---|---|---|
| Cyclophosphamide | Indinavir | Indinavir AUC: increased 38% | [71] |
| Cyclosporine | Darunavir | Increased cyclosporine effects (increased immunosuppression, renal toxicity) | [72] |
| Lopinavir/ritonavir | [73] | ||
| Nelfinavir | [74] | ||
| Saquinavir | [75] | ||
| Mycophenolate mofetil | Nevirapine | Nevirapine clearance: increased 27% | [76] |
| Tacrolimus | Nelfinavir | Increased tacrolimus levels (e.g. increased bone marrow suppression) | [77] |
| Lopinavir/ritonavir | [78] |
AUC, area under the curve.
Infections
Infectious complications encountered during the phase of neutropenia are fairly similar to those seen in HIV-negative patients. Table 3 gives an overview of reported allogeneic HSCT-related events based on the cases listed in Table 2. Although these data are based upon retrospective analysis of case reports, the reported entities are comparable to non-HIV-infected patients after transplantation.
Graft failure
There has been controversy concerning the point as to whether haematopoietic progenitor cells can be infected by HIV [45,46]. In theory, the uncommitted haematopoietic multipotent progenitor lacks the receptors necessary for HIV infection, but at some point of lineage differentiation it probably develops the capability for infection. The published evidence which supports this, however, is incomplete due to the use of technologies which cannot ensure complete separation of lineage marker-negative progenitor cells [47].
Graft failure may have been the problem in the case of a 9-year-old boy with severe aplastic anaemia who was infected with HIV after blood cell transfusion and underwent allogeneic transplantation from his ABO HLA-identical brother. Post-transplant engraftment and T cell function 3 months after allogeneic transplantation were within normal range, but soon thereafter partial marrow failure occurred, with a rapid decline in absolute neutrophil counts. Thirteen months after allogeneic HSCT, AIDS was diagnosed and the patient subsequently died. It is not clear whether this rapid decline in T lymphocytes was due to a partial graft failure after reinfection of T cell progenitor cells, or was caused by acceleration of the progression into AIDS by the transplantation procedure [29]. Kang and coworkers reported another HIV+ patient who developed signs of an acute HIV infection after allogeneic transplantation. During this period HAART was discontinued and a viral rebound was measured. The authors suggested that these symptoms may have represented an infection of the new allograft [35]. In this case, the patient received reduced intensity conditioning prior to HSCT, and a critical question is whether ablative conditioning could, by itself, influence the virus reservoir and assist in eliminating HIV.
The effect of allogeneic HSCT on HIV
Successful allogeneic HSCT requires the use of a broad panel of additional medication and therapeutic techniques such as irradiation. The effect on HIV-1 replication and course of infection is somewhat speculative for single agents and, furthermore, completely unknown when given as combination. In particular, immunosuppressive medication has been considered to have a favourable effect on HIV. The most commonly used immunosuppressive drug in the clinical setting of allogeneic HSCT is cyclosporine A (CsA). CsA has been shown to inhibit T cell activation through a mechanism well defined at the molecular level. In terms of HIV infection, CsA is able to decrease the heightened state of T cell activation in order to limit the infection, and thus depletion of T lymphocytes that may contribute to a better long-term preservation CD4 T cell count. CsA has been tested during primary HIV infection. Thereby, CsA was not detrimental to virus-specific CD8+ or CD4+ T cell responses. At week 48, the proportion of interferon (IFN)-γ-secreting CD4+ and CD4+/CCR7- T cells was significantly higher in the CsA and HAART cohort than in the cohort where HAART had been given exclusively. The authors suggested that a rapid shutdown of T cell activation during the early phases of primary HIV-1 infection can have long-term beneficial effects and establish a more favourable immunological set-point. Appropriate, immune-based therapeutic interventions may represent a valuable complement to HAART for treating HIV infection [48]. Taken together, CsA and other immunosuppressive agents such as mycophenolate mofetil or thymoglobulin have only a marginal effect on HIV viraemia and no lasting effect on HIV replication.
Only few data are available to answer the question of how HSCT may influence the composition and the virus host interaction itself. During an observation period over 384 days, Kamp et al. reported on the change in HIV tropism after HSCT. To analyse viral dynamics, they studied viral diversity and phylogeny by means of maximum likelihood and Bayesian methods. Interestingly, viral diversity decreased after HSCT, although the patient developed HIV rebound during a short period of HAART discontinuation [49].
In another investigation, Woolfrey et al. focused their research on the development of HIV-specific T cells after allogeneic HSCT. There, CD8+ T cell responses targeting multiple epitopes were detected before transplantation and a different pattern of donor-derived HIV-specific CTL responses emerged a couple of months after transplantation. The authors suggested that HSCT offers the unique ability to characterize de novo HIV-1-specific immune responses [50].
Targeted strategies including gene therapy
As demonstrated by Hasset and Lane, donor lymphocyte infusions without prior conditioning regimens are not effective to enhance the CD4+ T cell count. An advancement of this idea was performed by Vilmer and coworkers, who reported a patient with AA who received syngeneic transplantation in 1979. Five years later AIDS was diagnosed, and a retrospective analysis of blood samples revealed a time-point of seroconversion 1 month before allogeneic transplantation. Vilmer decided to give DLI in combination with α/γ interferon. CD4+ T cell count rose rapidly from < 50 to 420/µl and declined to stable levels around 180/µl after 7 months following the last infusion without any additional treatment. Vilmers concluded from this that HIV had no immediately cytopathogenic effect for the transfused lymphocytes [51].
Nevertheless, there have been attempts to use genetic manipulation to ‘arm’ adoptively transferred T lymphocytes in immunotherapy studies in AIDS. An example of this idea is that developed by Morgan and Walker, who designed a gene transfer of HIV-1 anti-sense TAR and transdominat Rev protein genes into lymphocytes [52]. These modified cells from syngeneic twins of HIV-infected patients were transfused and showed a prolonged survival in comparison to untreated lymphocytes [53].
After the discouraging experiences with allogeneic stem cell transplantation on the natural course of HIV infection, there were some attempts to enhance the efficacy of this approach. In 1993 Contu and coworkers performed an HLA-identical allogeneic HSCT in a 25-year-old HIV+ woman and added a combination of zidovudine, IFN-α2 and HIV-1-specific T cell clones. Engraftment was achieved on day +18, but bone marrow function remained insufficient, leading to lasting depletion of CD4+ T and B lymphocytes. HIV-1 was undetectable by env-, gag-, pol- and ltr-specific DNA-PCR up to 30 days post-transplant, but the patient died on day +301 after allogeneic HSCT from an adult respiratory distress syndrome. Post-mortem investigation of several tissues was performed and found to be negative for HIV-1 DNA. Surprisingly, 4 months after transplantation this patient became HIV-1 seronegative as tested by enzyme-linked immunosorbent assay (ELISA) and Western blot assays [54,55].
Subsequently, Bex et al. attempted to generate HIV-specific T cells in a syngeneic adoptive setting by transferring syngeneic T cells following immunization of the donor. In this experiment, the HIV-negative identical twin brother of a terminally ill 38-year-old AIDS patient, who had failed single-agent therapy using zidovudine, was vaccinated with WR87, a recombinant HIV-1 envelope glycoprotein. Following infusion of these immunoreactive T cells, there was a slight increase in CD4+ and in activated CD8+/DR+ cell count. However, these effects were only transient and did not lead to a significant clinical change; in fact, a marked but transient increase in cellular and plasmatic virus loads was observed after the second adoptive T cell transfer [56].
Kang and coworkers performed the first allogeneic HSCT in patients with HIV using genetically modified allogeneic stem cells. They transduced the donor's CD34+ stem cells with GcsapSL3rd3, containing a dominant-mutant Rev (TdRev) engineered to inhibit viral replication through blocking of wild-type Rev, a key HIV regulatory protein. This trial was partially successful, because stable transfection of TdRev was measurable at least 2 years after allogeneic HSCT. The effect on HIV course was not clearly evaluable because HAART administration was not interrupted [35].
The combination of stem cell and gene-based therapies has been proposed as a long-lived alternative to anti-retroviral therapy. Today, the techniques of gene delivery and gene knock-down have progressed considerably, and a rising number of clinical data in patients undergoing gene therapy are available [57,58].
More recently, DiGiusto et al. have used a lentivirus vector encoding three anti-HIV RNAs (TAR decoy, siRNA targeting tat/rev and an anti-CCR5 ribozyme) to genetically modify autologous CD34 cells in patients undergoing marrow ablative therapy for AIDS lymphoma. Of four patients treated, all expressed the anti-viral RNA for up to 24 months [59].
HIV eradication by allogeneic HSCT?
For entry into host cells, HIV-1 uses CD4 and membrane-bound chemokine co-receptors such as CCR5 or CXCR4. Homozygosity towards a 32-base pair deletion (CCR5-delta32) in the CCR5 gene leads to an inactive receptor which is associated with a high but not complete HIV-1 resistance. It seemed likely that a CCR5-delta32-based stem cell-based approach could improve the course of HIV infection and, for example, in 2000 M. Lux and J. Stapleton attempted to pursue this approach. The relative lack of such donors, however, discouraged this effort (personal communication, M. Lux, Rochester, NY, USA). One year later R. Chow (founder of StemCyte Inc.) applied for a patent for stem cell donor screening for a beneficial gene to treat HIV infection (US patent 2003/0099621 A1). Chow's group built up a database with more than 10 000 cord blood units, genotyped for the CCR5-delta32 deletion. In this group, 30 donors were homozygous for the CCR5-delta32 deletion; this demonstrates a potential source of CCR5-negative donors for the future [60]. A similar approach was prepared by the M.D. Anderson CB Bank (Houston, Texas, USA), who have collected and CCR5-genotyped more than 10 000 cord blood units since 1995 as potential sources for allogeneic HSCT in HIV+ patients [61].
The problem of selection of donors has been addressed by genetic manipulation of CD34 cells to knock-down CCR5. DiGiusto et al. transplanted four AIDS lymphoma patients with autologous CD34 with a lentivirus vector encoding a triple anti-HIV RNA combination which included a ribozyme targeting CCR5 [59]. In a mouse model of transplantation of human CD34 cells treated with zinc finger nuclease targeted to the CCR5 gene, Holt et al. have shown that transplanted CCR5 knock-out CD34 cells will not only engraft, but are protective after HIV challenge [62]. If HSCT donor cells can be made routinely CCR5-negative by such genetic methods, then the problem of available CCR5-negative donors for HIV-related allogeneic HSCT would be solved.
The first allogeneic stem cell transplantation in an HIV+ patient with a donor selected to be homozygous for CCR5-delta32 was performed by Hütter and coworkers. The transplantation led to a complete donor chimerism and the patient's lymphocytes changed from a heterozygous into a homozygous genotype regarding the CCR5-delta32 allele. Although HAART was discontinued, HIV-1-load could not be detected as determined by RNA and proviral DNA PCR assays of peripheral blood, bone marrow and several other tissues during a 3·5-year follow-up period [63,64]. The question of why this patient has suppressed his HIV infection so completely is not known. Ultimately, the graft-versus-leukaemia effect probably produced a concomitant graft-versus-HIV-reservoir effect, which prevented any outgrowth of HIV after HAART was stopped. The lesson of this case is valuable, as it suggests that cellular therapy is likely to be important in producing a function cure for HIV (i.e. control of HIV without lifelong anti-viral chemotherapy). It will be important to confirm the ability of this proposed CCR5-negative cellular effect to alter the HIV reservoir in other patients
Conclusions
Patients with HIV infection have a considerably increased risk of developing different kinds of haematological malignancies. Effective anti-retroviral therapy has improved the life expectancy in these patients with consecutive increase in prevalence of malignancies. We assume that there will be a growing demand in intensified treatment options such as stem cell transplantation in the future. Since the early 1980s, several patients with HIV-1 infection underwent allogeneic stem cell infusion or transplantation procedures. Because of the relatively small number of patients and the non-systematic analysis of allogeneic HSCT in these patients, a conclusive statement regarding an altered procedure-related morbidity of the transplantation procedure in comparison to non-HIV-infected patients cannot be made. Nevertheless, the published data may suggest a lower rate of treatment-related mortality since HAART has been included during allogeneic HSCT. Finally, the survival rate for stem cell recipients has improved in these years from optimized supportive care and enhanced immunosuppressive and anti-microbiological medication. Thus, patients with HIV infection should be offered allogeneic HSCT if there is an indication to treat a haematological disease. Finally, allogeneic HSCT alone is not effective to treat HIV infection. Nevertheless, enhancement of this venture can probably be achieved with gene therapy approaches or special donor selection for HIV beneficial genes such as the CCR5-delta32 mutation.
Acknowledgments
We thank Susanne Ganepola for reading the manuscript.
Disclosure
The authors have nothing to disclose.
References
- 1.Fauci AS, Pantaleo G, Stanley S, Weissman D. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–63. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Besson C, Goubar A, Gabarre J, et al. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood. 2001;98:2339–44. doi: 10.1182/blood.v98.8.2339. [DOI] [PubMed] [Google Scholar]
- 3.Goedert JJ. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin Oncol. 2000;27:390–401. [PubMed] [Google Scholar]
- 4.Haioun C, Lepage E, Gisselbrecht C, et al. Benefit of autologous bone marrow transplantation over sequential chemotherapy in poor-risk aggressive non-Hodgkin's lymphoma: updated results of the prospective study LNH87-2. Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 1997;15:1131–7. doi: 10.1200/JCO.1997.15.3.1131. [DOI] [PubMed] [Google Scholar]
- 5.Diez-Martin JL, Balsalobre P, Re A, et al. Comparable survival between HIV positive and HIV negative non-Hodgkin and Hodgkin lymphoma patients undergoing autologous peripheral blood stem cell transplantation. Blood. 2009;113:6011–4. doi: 10.1182/blood-2008-12-195388. [DOI] [PubMed] [Google Scholar]
- 6.Wagner-Johnston ND, Ambinder RF. Blood and marrow transplant for lymphoma patients with HIV/AIDS. Curr Opin Oncol. 2008;20:201–5. doi: 10.1097/CCO.0b013e3282f5101e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krishnan A. HIV-infected patients. Biol Blood Marrow Transplant. 2009;15:142–5. doi: 10.1016/j.bbmt.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Tomblyn M, Pedersen TL, et al. Allogeneic hematopoietic cell transplantation in human immunodeficiency virus-positive patients with hematologic disorders: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:864–71. doi: 10.1016/j.bbmt.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krown SE, Real FX, Cunningham-Rundles S, et al. Preliminary observations on the effect of recombinant leukocyte A interferon in homosexual men with Kaposi's sarcoma. N Engl J Med. 1983;308:1071–6. doi: 10.1056/NEJM198305053081806. [DOI] [PubMed] [Google Scholar]
- 10.Oswald GA, Theodossi A, Gazzard BG, Byrom NA, Fisher-Hoch SP. Attempted immune stimulation in the gay compromise syndrome. BMJ (Clin Res Ed) 1982;285:1082. doi: 10.1136/bmj.285.6348.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masur H. The acquired immunodeficiency syndrome. Dis Mon. 1983;30:1–48. doi: 10.1016/0011-5029(83)90005-6. [DOI] [PubMed] [Google Scholar]
- 12.Hassett JM, Zaroulis CG, Greenberg ML, Siegal FP. Bone marrow transplantation in AIDS. N Engl J Med. 1983;309:665. doi: 10.1056/NEJM198309153091114. [DOI] [PubMed] [Google Scholar]
- 13.Davis KC, Hayward A, Ozturk G, Kohler PF. Lymphocyte transfusion in case of acquired immunodeficiency syndrome. Lancet. 1983;1:599–600. doi: 10.1016/s0140-6736(83)92855-6. [DOI] [PubMed] [Google Scholar]
- 14.Verdonck LF, de Gast GC, Lange JM, Schuurman HJ, Dekker AW, Bast BJ. Syngeneic leukocytes together with suramin failed to improve immunodeficiency in a case of transfusion-associated AIDS after syngeneic bone marrow transplantation. Blood. 1988;71:666–71. [PubMed] [Google Scholar]
- 15.Furlini G, Re MC, Bandini G, Albertazzi L, La Placa M. Antibody response to human immunodeficiency virus after infected bone marrow transplant. Eur J Clin Microbiol Infect Dis. 1988;7:664–6. doi: 10.1007/BF01964248. [DOI] [PubMed] [Google Scholar]
- 16.Bardini G, Re MC, Rosti G, Belardinelli AR. HIV infection and bone-marrow transplantation. Lancet. 1991;337:1163–4. doi: 10.1016/0140-6736(91)92831-l. [DOI] [PubMed] [Google Scholar]
- 17.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts) N Engl J Med. 1975;292:832–43. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 18.Lane HC, Masur H, Longo DL, et al. Partial immune reconstitution in a patient with the acquired immunodeficiency syndrome. N Engl J Med. 1984;311:1099–103. doi: 10.1056/NEJM198410253111706. [DOI] [PubMed] [Google Scholar]
- 19.Mitsuyasu R, Volberdin P, Groopman J, Champlin R. Bone marrow transplantation from identical twins in the treatment of AIDS and Kaposi's sarcoma. J Cell Biochem. 1984;26:22. [Google Scholar]
- 20.Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976;192:1016–8. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- 21.Gale RP, Sparkes RS, Golde DW. Bone marrow origin of hepatic macrophages (Kupffer cells) in humans. Science. 1978;201:937–8. doi: 10.1126/science.356266. [DOI] [PubMed] [Google Scholar]
- 22.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–2. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 23.Fischl MA, Richman DD, Grieco MH, et al. The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial. N Engl J Med. 1987;317:185–91. doi: 10.1056/NEJM198707233170401. [DOI] [PubMed] [Google Scholar]
- 24.Holland HK, Saral R, Rossi JJ, et al. Allogeneic bone marrow transplantation, zidovudine, and human immunodeficiency virus type 1 (HIV-1) infection. Studies in a patient with non-Hodgkin lymphoma. Ann Intern Med. 1989;111:973–81. doi: 10.7326/0003-4819-111-12-973. [DOI] [PubMed] [Google Scholar]
- 25.Saral R, Holland HK. Bone marrow transplantation for acquired immune deficiency syndrome. In: Forman SJ, Blume KG, Thomas ED, editors. Bone marrow transplantation. Boston: Blackwell Scientific Publications; 1994. pp. 654–64. [Google Scholar]
- 26.Lane HC, Zunich KM, Wilson W, et al. Syngeneic bone marrow transplantation and adoptive transfer of peripheral blood lymphocytes combined with zidovudine in human immunodeficiency virus (HIV) infection. Ann Intern Med. 1990;113:512–9. doi: 10.7326/0003-4819-113-7-512. [DOI] [PubMed] [Google Scholar]
- 27.Angelucci E, Lucarelli G, Baronciani D, et al. Bone marrow transplantation in an HIV positive thalassemic child following therapy with azidothymidine. Haematologica. 1990;75:285–7. [PubMed] [Google Scholar]
- 28.Aboulafia DM, Mitsuyasu RT, Miles SA. Syngeneic bone-marrow transplantation and failure to eradicate HIV. Aids. 1991;5:344. [PubMed] [Google Scholar]
- 29.Giri N, Vowels MR, Ziegler JB. Failure of allogeneic bone marrow transplantation to benefit HIV infection. J Paediatr Child Health. 1992;28:331–3. doi: 10.1111/j.1440-1754.1992.tb02681.x. [DOI] [PubMed] [Google Scholar]
- 30.Torlontano G, Di Bartolomeo P, Di Girolamo G, et al. AIDS-related complex treated by antiviral drugs and allogeneic bone marrow transplantation following conditioning protocol with busulphan, cyclophosphamide and cyclosporin. Haematologica. 1992;77:287–90. [PubMed] [Google Scholar]
- 31.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–60. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 32.Schlegel P, Beatty P, Halvorsen R, McCune J. Successful allogeneic bone marrow transplant in an HIV-1-positive man with chronic myelogenous leukemia. J Acquir Immune Defic Syndr. 2000;24:289–90. doi: 10.1097/00126334-200007010-00017. [DOI] [PubMed] [Google Scholar]
- 33.Tomonari A, Takahashi S, Shimohakamada Y, et al. Unrelated cord blood transplantation for a human immunodeficiency virus-1-seropositive patient with acute lymphoblastic leukemia. Bone Marrow Transplant. 2005;36:261–2. doi: 10.1038/sj.bmt.1705028. [DOI] [PubMed] [Google Scholar]
- 34.Bryant A, Milliken S. Successful reduced-intensity conditioning allogeneic HSCT for HIV-related primary effusion lymphoma. Biol Blood Marrow Transplant. 2008;14:601–2. doi: 10.1016/j.bbmt.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Kang EM, de Witte M, Malech H, et al. Nonmyeloablative conditioning followed by transplantation of genetically modified HLA-matched peripheral blood progenitor cells for hematologic malignancies in patients with acquired immunodeficiency syndrome. Blood. 2002;99:698–701. doi: 10.1182/blood.v99.2.698. [DOI] [PubMed] [Google Scholar]
- 36.Sora F, Antinori A, Piccirillo N, et al. Highly active antiretroviral therapy and allogeneic CD34(+) peripheral blood progenitor cells transplantation in an HIV/HCV coinfected patient with acute myeloid leukemia. Exp Hematol. 2002;30:279–84. doi: 10.1016/s0301-472x(01)00793-7. [DOI] [PubMed] [Google Scholar]
- 37.Wolf T, Rickerts V, Staszewski S, et al. First case of successful allogeneic stem cell transplantation in an HIV-patient who acquired severe aplastic anemia. Haematologica. 2007;92:e56–58. doi: 10.3324/haematol.11394. [DOI] [PubMed] [Google Scholar]
- 38.Polizzotto MN, Shortt J, Cole-Sinclair MF, Opat SS, Spencer A, Avery S. Allogeneic peripheral blood stem cell transplantation for hematological malignancies in patients with HIV. ASH Annu Meet Abstr. 2007;110:4941. [Google Scholar]
- 39.Avettand-Fenoel V, Mahlaoui N, Chaix ML, et al. Failure of bone marrow transplantation to eradicate HIV reservoir despite efficient HAART. Aids. 2007;21:776–7. doi: 10.1097/QAD.0b013e3280b01836. [DOI] [PubMed] [Google Scholar]
- 40.Jenq RR, van den Brink MR. Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer. Cancer. 2010;10:213–21. doi: 10.1038/nrc2804. [DOI] [PubMed] [Google Scholar]
- 41.Barry M, Mulcahy F, Merry C, Gibbons S, Back D. Pharmacokinetics and potential interactions amongst antiretroviral agents used to treat patients with HIV infection. Clin Pharmacokinet. 1999;36:289–304. doi: 10.2165/00003088-199936040-00004. [DOI] [PubMed] [Google Scholar]
- 42.Krishnan A, Molina A, Zaia J, et al. Durable remissions with autologous stem cell transplantation for high-risk HIV-associated lymphomas. Blood. 2005;105:874–8. doi: 10.1182/blood-2004-04-1532. [DOI] [PubMed] [Google Scholar]
- 43.Serrano D, Carrion R, Balsalobre P, et al. HIV-associated lymphoma successfully treated with peripheral blood stem cell transplantation. Exp Hematol. 2005;33:487–94. doi: 10.1016/j.exphem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 44.Mounier N, Katlama C, Costagliola D, Chichmanian RM, Spano JP. Drug interactions between antineoplastic and antiretroviral therapies: implications and management for clinical practice. Crit Rev Oncol Hematol. 2008;72:10–20. doi: 10.1016/j.critrevonc.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 45.Kaczmarski RS, Davison F, Blair E, et al. Detection of HIV in haemopoietic progenitors. Br J Haematol. 1992;82:764–9. doi: 10.1111/j.1365-2141.1992.tb06956.x. [DOI] [PubMed] [Google Scholar]
- 46.Stanley SK, Kessler SW, Justement JS, et al. CD34+ bone marrow cells are infected with HIV in a subset of seropositive individuals. J Immunol. 1992;149:689–97. [PubMed] [Google Scholar]
- 47.Zhang J, Crumpacker CS. Hematopoietic stem and progenitor cells in HIV/AIDS and immune reconstitution. Cell Res. 2010;20:745–7. doi: 10.1038/cr.2010.85. [DOI] [PubMed] [Google Scholar]
- 48.Rizzardi GP, Harari A, Capiluppi B, et al. Treatment of primary HIV-1 infection with cyclosporin A coupled with highly active antiretroviral therapy. J Clin Invest. 2002;109:681–8. doi: 10.1172/JCI14522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamp C, Wolf T, Bravo IG, et al. Decreased HIV diversity after allogeneic stem cell transplantation of an HIV-1 infected patient: a case report. Virol J. 2010;7:55. doi: 10.1186/1743-422X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woolfrey AE, Malhotra U, Harrington RD, et al. Generation of HIV-1-specific CD8+ cell responses following allogeneic hematopoietic cell transplantation. Blood. 2008;112:3484–7. doi: 10.1182/blood-2008-05-157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilmer E, Rhodes-Feuillette A, Rabian C, et al. Clinical and immunological restoration in patients with AIDS after marrow transplantation, using lymphocyte transfusions from the marrow donor. Transplantation. 1987;44:25–9. doi: 10.1097/00007890-198707000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Morgan RA, Walker R. Gene therapy for AIDS using retroviral mediated gene transfer to deliver HIV-1 antisense TAR and transdominant Rev protein genes to syngeneic lymphocytes in HIV-1 infected identical twins. Hum Gene Ther. 1996;7:1281–306. doi: 10.1089/hum.1996.7.10-1281. [DOI] [PubMed] [Google Scholar]
- 53.Morgan RA, Walker R, Carter CS, et al. Preferential survival of CD4+ T lymphocytes engineered with anti-human immunodeficiency virus (HIV) genes in HIV-infected individuals. Hum Gene Ther. 2005;16:1065–74. doi: 10.1089/hum.2005.16.1065. [DOI] [PubMed] [Google Scholar]
- 54.Contu L, La Nasa G, Arras M, et al. Allogeneic bone marrow transplantation combined with multiple anti-HIV-1 treatment in a case of AIDS. Bone Marrow Transplant. 1993;12:669–71. [PubMed] [Google Scholar]
- 55.La Nasa G, Carcassi C, Pizzati A, et al. Allogeneic bone marrow transplantation (BMT) combined with zidovudine (AZT), alpha 2 interferon (alpha 2-IFN) and donor HIV-1 specific T-cell clones in an AIDS patient. Int Conf AIDS. 1991;7:77. abstract no. TH.B.81. [Google Scholar]
- 56.Bex F, Hermans P, Sprecher S, et al. Syngeneic adoptive transfer of anti-human immunodeficiency virus (HIV-1)-primed lymphocytes from a vaccinated HIV-seronegative individual to his HIV-1-infected identical twin. Blood. 1994;84:3317–26. [PubMed] [Google Scholar]
- 57.Strayer DS, Akkina R, Bunnell BA, et al. Current status of gene therapy strategies to treat HIV/AIDS. Mol Ther. 2005;11:823–42. doi: 10.1016/j.ymthe.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 58.Hütter G, Ganepola S. Stem cell based treatment of HIV infection. In: Hajba L, editor. Treatment strategies: AIDS. London: The Cambridge Research Centre; 2010. pp. 19–21. [Google Scholar]
- 59.DiGiusto DL, Krishnan A, Li L, et al. RNA-based gene therapy for HIV with lentiviral vector-modified CD34(+) cells in patients undergoing transplantation for AIDS-related lymphoma. Sci Transl Med. 2010;2:36ra43. doi: 10.1126/scitranslmed.3000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen TK, Moore TB, Territo M, et al. The feasibility of using CCR5D32/D32 hematopoietic stem cell transplants for immune reconstitution in HIV-infected children. Biol Blood Marrow Transplant. 2008;14:Abstract 324. [Google Scholar]
- 61.Gonzalez G, Park S, Chen D, Armitage S, Shpall E, Behringer R. Identification and frequency of CCR5 d32/d32 HIV-resistant cord blood units from Houston area hospitals. HIV Med. 2011 doi: 10.1111/j.1468-1293.2010.00911.x. ISSN: 1468-1293 [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holt N, Wang J, Kim K, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–47. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 64.Hütter G, Thiel E. Allogeneic transplantation of CCR5 deficient progenitor cells in a patient with HIV infection – an update after 3 years and the search for patient no. 2. AIDS. 2011;25:273–4. doi: 10.1097/QAD.0b013e328340fe28. [DOI] [PubMed] [Google Scholar]
- 65.Turner ML, Watson HG, Russell L, Langlands K, Ludlam CA, Parker AC. An HIV positive haemophiliac with acute lymphoblastic leukaemia successfully treated with intensive chemotherapy and syngeneic bone marrow transplantation. Bone Marrow Transplant. 1992;9:387–9. [PubMed] [Google Scholar]
- 66.Campbell P, Iland H, Gibson J, Joshua D. Syngeneic stem cell transplantation for HIV-related lymphoma. Br J Haematol. 1999;105:795–8. doi: 10.1046/j.1365-2141.1999.01422.x. [DOI] [PubMed] [Google Scholar]
- 67.Shamansky S, Fedotova I, Popkov J, Burya A, Rukavicin O. Allogenic stem cell transplantation in a patient with acute myeloid leukaemia and HIV, hepatitis C infections. Bone Marrow Transplant. 2004;33:S344–345. abstract no. R1215. [Google Scholar]
- 68.Binaghi MA, Lasala MB, Longoni H, Corach D, Kohan A. Peripheral blood stem cell transplantation in a patient with acquired aplastic anemia and HIV infection. ASH Annu Meet Abstr. 2006;108:5368. [Google Scholar]
- 69.Hamnadani M, Baiocchi R, Lin T, et al. Feasibility of allogeneic peripheral blood stem cell transplantation (ALLO-SCT) following reduced intensity conditioning (RIC) in HIV+ patients with hematological malignancies. Biol Blood Marrow Transplant. 2009;15 abstract no. 314. [Google Scholar]
- 70.Polizzotto MN, Skinner M, Cole-Sinclair MF, Opat SS, Spencer A, Avery S. Allo-SCT for hematological malignancies in the setting of HIV. Bone Marrow Transplant. 2010;45:584–6. doi: 10.1038/bmt.2009.168. [DOI] [PubMed] [Google Scholar]
- 71.Gatti G, De Pascalis CR, Toffoli G. Case control study of indinavir pharmacokinetics in patients undergoing chemotherapy for AIDS related neoplasia. 2nd International Workshop on Clinical Pharmacology of HIV Therapy, Noordwijk, the Netherlands; 2001. Abstract no. 1.5.
- 72.El-Atrouni WI, Temesgen Z. Darunavir. Drugs Today (Barc) 2007;43:671–9. doi: 10.1358/dot.2007.43.10.1131764. [DOI] [PubMed] [Google Scholar]
- 73.Vogel M, Voigt E, Michaelis HC, et al. Management of drug-to-drug interactions between cyclosporine A and the protease-inhibitor lopinavir/ritonavir in liver-transplanted HIV-infected patients. Liver Transpl. 2004;10:939–44. doi: 10.1002/lt.20165. [DOI] [PubMed] [Google Scholar]
- 74.Frassetto LA, Baloum M, Roland ME, Carlson L, Stock P, Benet LZ. Two-year evaluation of the interactions between antiretroviral medication and cyclosporine in HIV+ liver and kidney transplant recipients. 10th Conference on retroviruses and opportunistic infections, Boston, USA; 2003. Abstract no. 540.
- 75.Brinkman K, Huysmans F, Burger DM. Pharmacokinetic interaction between saquinavir and cyclosporine. Ann Intern Med. 1998;129:914–5. doi: 10.7326/0003-4819-129-11_part_1-199812010-00022. [DOI] [PubMed] [Google Scholar]
- 76.Sankatsing S, Hoggard P, Back D, et al. Mycophenolate mofetil lowers plasma nevirapine concentrations but has no effect on intracellular triphosphate concentrations. 10th Conference on retroviruses and opportunistic infections, Boston, USA; 2003. Abstract no. 539.
- 77.Schvarcz R, Rudbeck G, Soderdahl G, Stahle L. Interaction between nelfinavir and tacrolimus after orthoptic liver transplantation in a patient coinfected with HIV and hepatitis C virus (HCV) Transplantation. 2000;69:2194–5. doi: 10.1097/00007890-200005270-00041. [DOI] [PubMed] [Google Scholar]
- 78.Teicher E, Taburet AM, Vincent I, et al. Management of drug-to-drug interactions between tacrolimus and HiAART. 12th Conference on retroviruses and opportunistic infections, Boston, USA; 2005. Abstract no. 662.


