Abstract
Pleural tuberculosis (TB) remains a common presentation of Mycobacterium tuberculosis (MTB) infection in HIV/TB dually infected subjects, and both cellular and acellular components of the pleural milieu promote HIV-1 replication; however, they remain uncharacterized. Using cytokine array of pleural fluid and real-time reverse transcription–polymerase chain reaction (RT–PCR) and immunophenotype analysis, pleural fluid mononuclear cells (PFMC) were compared to systemic counterparts [i.e. plasma and peripheral blood mononuclear cells (PBMC)]. Significant increases in pleural fluid cytokines compared to plasma were limited to interleukin (IL)-6, IL-8, interferon (IFN)-γ and transforming growth factor (TGF)-β, and did not include other T helper type 1 (Th1) (IL-2, IL-15), Th2 or Th17 cytokines. Patterns and levels of cytokines were indistinguishable between pleural fluid from HIV/TB and TB patients. Forkhead box P3 (FoxP3) mRNA in PFMC was increased significantly and correlated highly with levels of IL-6 and IL-8, less with TGF-β, and not with IFN-γ. Among CD4 T cells, FoxP3-reactive CD25hi were increased in HIV/TB dually infected subjects compared to their PBMC, and up to 15% of FoxP3+ CD25hi CD4 T cells were positive for IL-8 by intracellular staining. These data implicate a dominant effect of MTB infection (compared to HIV-1) at pleural sites of dual HIV/TB infection on the local infectious milieu, that include IL-6, IL-8, IFN-γ and TGF-β and regulatory T cells (Treg). A correlation in expansion of Treg with proinflammatory cytokines (IL-6 and IL-8) in pleural fluid was shown. Treg themselves may promote the inflammatory cytokine milieu through IL-8.
Keywords: cytokines, HIV, regulatory T cells, transforming growth factor β, tuberculosis
Introduction
Tuberculosis (TB) remains the most prevalent opportunistic infection among HIV-1-infected subjects, and continues to be both fuelled by and fuel the HIV-1 epidemic worldwide [1]. Pleural TB is a common presentation of Mycobacterium tuberculosis (MTB) infection in HIV/TB dually infected subjects, affecting up to 10% in Africa [2]. The intense recruitment, activation and expansion of CD4 T cells at pleural sites of HIV/TB infection in turn underlies increased HIV-1 activity (reviewed in [3]). Evidence for augmented viral dynamics, including increased HIV-1 replication and spread of viral infection to uninfected mononuclear cells, has been established using this in situ disease model of HIV/TB co-infection [4,5]. Studies of HIV-1 heterogeneity indicate that at the time of diagnosis of pleural TB, HIV-1 sequences generated in situ are predominant in the systemic circulation [6]. HIV-1 production from both pleural fluid mononuclear cell (PFMC) CD4 T cells and macrophages has been shown before [7]. PFMC under cytokine pressure at pleural sites of HIV/TB display a specific profile of differentiation and HIV-1 production, which can be evaluated readily ex vivo. However, while in situ viral replication is the dominant source of HIV-1 during HIV/TB, it is in addition to the impact of systemic immune activation on the virus [8]. Studies differentiating the effect of these two compartments on HIV-1 activity are lacking. The role of the cytokines and cellular profile at pleural sites of TB on HIV activity is unclear.
The importance of proinflammatory cytokines, in particular tumour necrosis factor (TNF)-α, in support of HIV-1 replication at sites of TB [4] has been well established. Transcriptional activation of HIV-1 by proinflammatory cytokines [TNF-α, interleukin (IL)-1 β, IL-6, and IL-8] is based on induction of nuclear factors, namely nuclear factor (NF)-κB and NF-IL-6 [a member of the CCAAT-enhancer-binding proteins (C/EBP)-β family][9]. On the other hand IFN-γ, a premier T helper type 1 (Th1) cytokine found at high concentrations at sites of TB, promotes transcriptional activation of HIV-1 through activation of other nuclear factors [e.g. class II major histocompatibility complex transactivator (CIITA) and interferon (IFN) regulatory factor 1 (IRF-1)] and through co-operation with TNF-α . In turn, TNF-α supports IFN-γ expression and may promote CD4 depletion [10]. A role for Th2 cytokines on HIV-1 activity is more controversial [11,12]. Transforming growth factor (TGF)-β, a prominent anti-inflammatory cytokine is abundant during TB and may promote viral activity by counteraction to anti-HIV-1 immune responses either directly or through support of expansion of regulatory T cells (Treg) [13,14]. While Treg have been shown to be restrictive of viral activity through control of immune activation [15], evidence for HIV-1 production by Treg has also been shown recently [16]. Treg expansion at sites of TB [17] and HIV/TB [18] has been documented previously.
Here, we evaluated plasma and pleural fluid from HIV/TB infected subjects diagnosed with pleural TB by cytokine arrays to understand the effect of the cytokine milieu on HIV-1 viral activity. A group of HIV-1-uninfected subjects with pleural TB were studied simultaneously to distinguish effects of TB compared to dual HIV/TB infection on cytokine activities.
Methods
Study subjects
Patients with signs and symptoms consistent with TB who had moderate to large pleural effusions were recruited at Mulago Hospital, Kampala, Uganda. The study protocol was approved by the Ugandan National AIDS Research Subcommittee and the Institutional Review Board for Human Investigations at University Hospitals Case Medical Center, Cleveland, OH, USA. Twenty HIV-1 infected subjects and 20 HIV-1 uninfected subjects (control group) with culture-confirmed pleural TB met inclusion and exclusion criteria and were enrolled. All patients underwent thoracentesis for diagnosis of pleural TB. The median age of patients was 31 (range 23–45 years) and the male/female ratio was 3:1. HIV-1 infected and uninfected subjects had a mean day to positive pleural fluid culture positivity indistinguishable from one another. In the HIV-1-infected group, median CD4 count was 86/µl (range, 17–805/µl). HIV-1 viral load was assessed in plasma and pleural fluid by Amplicor assay (Roche, Fullerton, CA, USA).
Cytokine assay
Cytokine array (Quansys/Licor Biosciences, Logan, UT, USA) was performed on pleural fluid and plasma according to the manufacturer. Cytokines assessed included IL-1 α, IL-1β, TNF-α, TNF-β, IL-6, IL-8, IFN-γ, IL-2, IL-15, IL-4, IL-5, IL-13 and IL-17. Measurement of TGF-β, IL-12p70 and IL-23 were by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN, USA).
Total and bioactive TGF-β in pleural fluid was also assessed by a sensitive bioassay, which is based on TGF-β responsiveness [19]. Briefly, MFB-F11 cell lines that contain a secreted alkaline phosphatase (SEAP) reporter coupled to a Smad (of TGF-β signalling) binding element are seeded at 104/well in 96-well flat-bottomed plates in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen/Gibco, Carslbad, CA, USA). After overnight culture, cells are washed and receive recombinant TGF-β or pleural fluid in dilution. Supernatants are harvested after 24 h and assessed for SEAP activity by chemiluminescence (Great EscAPE ™) (Mountainview, CA, USA).
Cell isolation and characterization
PBMC and PFMC were prepared by Ficoll Hypaque (Pharmacia Fine Chemicals, Piscataway, NJ, USA) density gradient centrifugation as described previously [20]. Cell viability was > 98% as assessed by trypan blue exclusion. Frequencies of macrophages and T cells in PFMC were assessed by immunostaining and fluorescence activated cell sorter (FACS) analysis using conjugated antibodies to CD14, CD4 and CD3 as before [21]. Frequencies of macrophages (CD3-CD14+) in PFMC were a mean of 1–3%, and those of CD3+CD4+ T cells were 45–55% of PFMC. Frequencies of both PFMC macrophages and T cells were similar in HIV/TB and TB patients.
Forkhead box P3 (FoxP3) mRNA and HIV-1 expression in PFMC
Total RNA was obtained from PFMC as before [22], and real-time reverse transcription–polymerase chain reaction (RT–PCR) using the Taqman methodology by an ABI 7700 thermocycler (Applied Biosystems, Foster City, CA, USA) was used to quantify mRNA. Taqman primers and probes for FoxP3 and HIV-1 gag/pol mRNA were as described before [22,23]. Quantities of mRNA were determined by using a dilution series of target cDNA in each assay. FoxP3 and HIV-1 mRNA copies were corrected to the copy numbers of ribosomal 18s (R18) in the same sample as before and expressed as copies/1010 copies of R18 (equivalent to 1 × 106 cells) [22].
Immunostaining for FoxP3 and IL-8-reactive Treg
Intracellular content of FoxP3 and IL-8 was assessed using a commercially available FoxP3 staining kit (eBioscience, San Diego, CA, USA), as per the manufacturer's instructions. Anti-IL-8 and isotype control antibodies were purchased from BD Biosciences (San Jose, CA, USA). For analysis, 15 000 events were acquired in the physical T cell gate. First, CD4 T cells were identified versus forward-scatter (FSC) and subdivided further into CD25-[defined by immunoglobulin G1 (IgG1) mouse isotype antibody], CD25low+ (IgG1 cut-off to log2 of fluorescence intensity) and CD25hi+ Treg (> log2 fluorescence intensity). The Treg phenotype of CD4 T cell subsets then was confirmed further by identifying the subset of CD4 T cells in each gate that stained positive for FoxP3 (defined by rat IgG2a isotype antibody). Finally, IL-8 expression among subsets of FoxP3+ CD4 T cells was assessed (defined by a mouse IgG1 isotype antibody).
Statistical analysis
All statistical analyses were performed with SAS/STAT version 9·2 (SAS Institute Inc., Cary, NC, USA). Wilcoxon's rank sum test was used to analyse differences in medians between independent samples and the signed-rank test was used to analyse paired (plasma versus pleural fluid) median differences. Correlation was assessed using Spearman's rank correlation coefficient. All P-values are two-sided with P < 0·05 considered statistically significant.
Results
Cytokine activity in plasma and pleural fluid
Results of cytokines in plasma and pleural fluid in HIV-1-infected (Fig. 1a) and uninfected (Fig. 1b) subjects with pleural TB are shown. Cytokines were separated according to known functional profile into proinflammatory/anti-inflammatory (IL-1α, IL-1β, TNF-α, IL-6, IL-8, IL-10), Th1 (IFN-γ, IL-2, IL-15, TNF-β), Th2 (IL-4, IL-5, IL-13) and Th17 (IL-17). Levels of IL-23 and IL-12p70 were negligible in both plasma and pleural fluid (not shown).
Fig. 1.
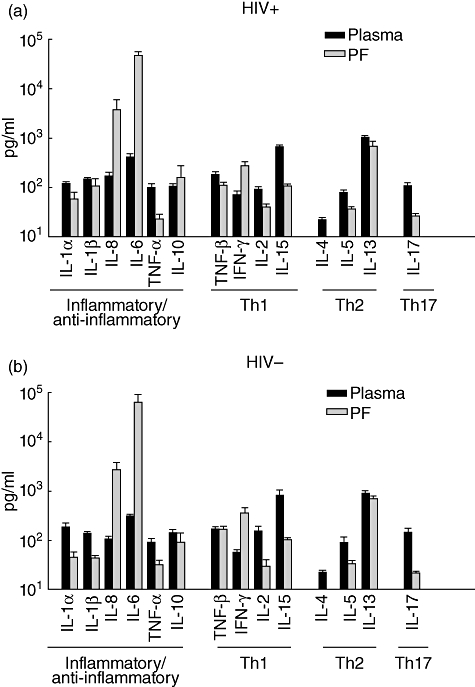
Cytokine activity in plasma and pleural fluid. Results of cytokines in plasma and pleural fluid (PF) in HIV-1-infected (a) and uninfected (b) subjects with pleural tuberculosis (TB) are shown. No significant differences in pattern or level of cytokines in pleural fluid was found between the two patient groups. Cytokines significantly higher in pleural fluid than in plasma in HIV/TB patients included interleukin (IL)-6 (P < 0·0001), IL-8 (P < 0·005) and interferon (IFN)-γ (P < 0·0001).
In the HIV/TB patients (Fig. 1a) and among the proinflammatory/anti-inflammatory cytokine group, only IL-6 and IL-8 were significantly higher in pleural fluid compared to plasma (P < 0·0001 and P < 0·005, respectively). By contrast, levels of IL-1α and TNF-α were higher in plasma (P < 0·001). Levels of IL-10 were similar in both plasma and pleural fluid. In the Th1 cytokine group higher IFN-γ was seen in pleural fluid (P < 0·0001), whereas both IL-2 and IL-15 were higher in plasma (P < 0·001). Levels of the Th2 cytokines IL-4 and IL-5 were higher in plasma (P < 0·0001 and P < 0·001, respectively). IL-13 was also higher in plasma compared to pleural fluid (P < 0·02). IL-17 was also compartmentalized to plasma (P < 0·0001).
Notably, the significant compartmentalization of cytokines between plasma and pleural fluid in HIV-1 infected and uninfected patients were similar and no differences in pattern or levels of cytokines in pleural fluid or plasma compartments were found, except for slightly higher IL-8 in plasma of dually infected subjects (173 ± 29 pg/ml) compared to TB alone (109 ± 12 pg/ml) (P < 0·04).
Concentrations of TGF-β assessed by ELISA were high (14 ± 1·9 ng/ml) in pleural fluid from HIV/TB patients, however, were similar to that of TB patients. Next, total (i.e. acid activated) and bioactive TGF-β were assessed by a bioassay [19]. While total TGF-β bioactivity in pleural fluid was similar in the two patient groups (Fig. 2a), bioactive TGF-β was higher in HIV/TB patients compared to TB patients (P < 0·02) (Fig. 2b).
Fig. 2.
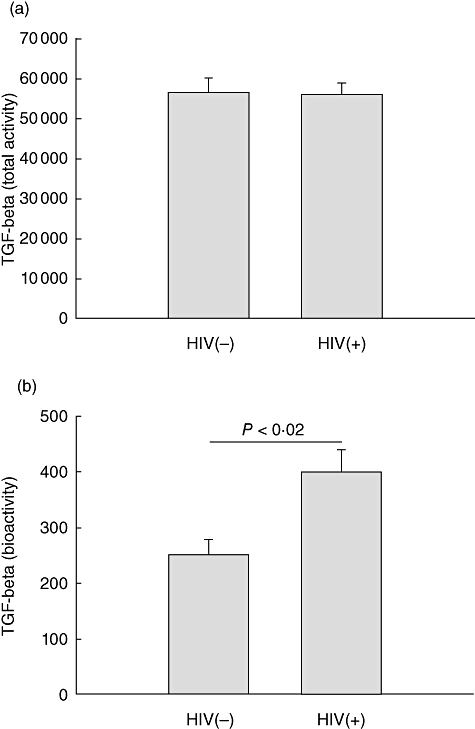
Transforming growth factor (TGF)-β levels in pleural fluid. TGF-β bioactivity in pleural fluid from HIV/tuberculosis (TB) and TB alone patients is shown. Whereas total TGF-β activity was similar in both populations (a), bioactive TGF-β is higher in pleural fluid from HIV/TB patients compared to TB patients (b) (P < 0·02).
Cytokine basis of Treg expansion in pleural TB
Treg activity was assessed by measurement of FoxP3 mRNA in PFMC and PBMC of a subgroup of HIV/TB (n = 11) and TB (n = 10) patients. FoxP3 mRNA levels were similar between HIV/TB and TB patients. In HIV/TB patients, FoxP3 mRNA expression was significantly higher in PFMC compared to PBMC (P < 0·002) (Fig. 3a). Interestingly, expression of FoxP3 mRNA in PFMC of HIV/TB patients correlated strongly with IL-6 and IL-8 levels in pleural fluid (r = 0·7, P < 0·02 for both), less with TGF-β (r = 0·5) and not at all with IFN-γ (r = 0·2).
Fig. 3.
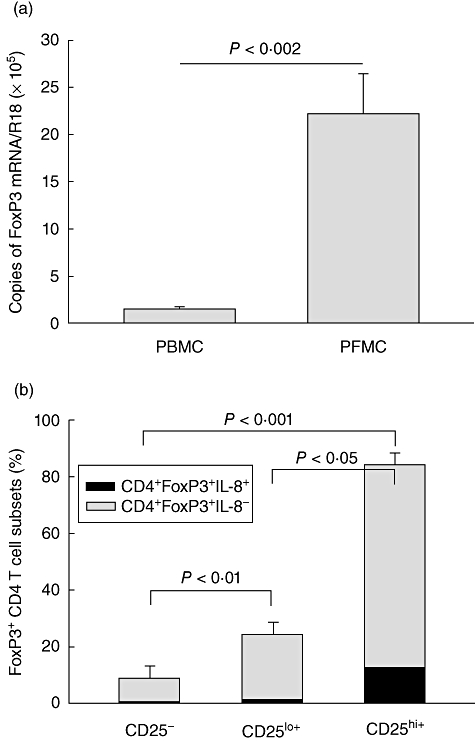
Expression of forkhead box (Fox) mRNA in pleural fluid mononuclear cells (PFMC) and interleukin (IL)-8+ FoxP3+ Treg. FoxP3 mRNA levels in peripheral blood mononuclear cells (PBMC) and PFMC of HIV/tuberculosis (TB) patients assessed by real-time polymerase chain reaction (PCR) are shown in (a). FoxP3 mRNA was significantly higher in PFMC compared to PBMC (P < 0·002). In (b), IL-8 expression in FoxP3+ T cells is compared between CD4+CD25hiT cells [regulatory T cells (Treg)] and CD4+CD25lo and CD4+CD25- T cells. Intracellular IL-8 was significantly higher in Treg than the other two populations (P < 0·05 and < 0·001, respectively).
Recently, IL-8 expression by a regulatory T cell line has been described [24]. We examined IL-8 expression in FoxP3-reactive CD4+CD25hi T cells in PFMC from HIV/TB patients by intracellular immunostaining and FACS analysis. Up to 15% of FoxP3-reactive CD4+CD25hi T cells were also IL-8 reactive (Fig. 3b). This level of IL-8 expression was higher than that among CD4+CD25lo and CD4+CD25- T cells (P < 0·05 and 0·001).
Impact of pleural milieu on HIV-1 activity
In 19 of 20 subjects, viral load was available both in plasma and pleural fluid. Overall HIV-1 activity in pleural fluid was significantly higher than that in plasma (by a mean fold of 4·7 ± 1·08, P < 0·05), and there was a significant correlation between the viral load in pleural fluid and plasma (r = 0·8, P < 0·005). A significant correlation was also found between pleural fluid viral load and HIV-1 gag/pol mRNA in PFMC (r = 0·81, P < 0·005). However, HIV-1 viral load in pleural fluid or HIV-1 mRNA in PFMC did not correlate with cytokines found in excess in pleural fluid (IL-6, IL-8, IFN-γ, TGF-β), either when analysed singly or in combination. A modest (r = 0·5), but not significant, correlation of FoxP3 mRNA in PFMC was found with both pleural fluid viral load and PFMC HIV-1 gag/pol mRNA.
Discussion
A Th1/proinflammatory, but not Th2 or Th17, cytokine profile at sites of pleural HIV/TB was established in this study. However, the cytokine profile in plasma or pleural fluid were indistinguishable between dually infected HIV/TB and singly infected TB subjects, except for slightly higher IL-8 in the plasma and bioactive TGF-β in pleural fluid of HIV/TB subjects. These findings are in contrast to a mixed Th1/Th2 cytokine pattern by in situ hybridization of pleural biopsies [25], and the significantly lower plasma IL-1α, IL-10 and TNF-α in HIV/TB compared to TB patients demonstrated more recently [26]. The basis for higher bioactive TGF-β in pleural fluid of dually infected subjects is unclear. Conversely, higher circulating IL-8 levels may possibly be related to the more ‘acute’ nature of TB in HIV-1 infected subjects. However, small numbers of patients studied (i.e. 20 HIV-infected and 20 HIV-1 uninfected patients) may have skewed the results.
As before [7], viral load in pleural fluid was higher than that in plasma, and correlated with both plasma viral load and with HIV-1 gag/pol mRNA activity in PFMC, implicating that at time of diagnosis of pleural TB, HIV-1 generated in situ contributes to viral activity. Four cytokines (IL-6, IL-8, IFN-γ, TGF-β) characterized the pleural compartment, yet none correlated with HIV-1 activity in this compartment. These data implicate a more complex relationship between HIV-1 replication and the pleural infectious milieu in HIV-1-infected patients with pleural TB.
High FoxP3 mRNA expression and FoxP3 +CD4CD25hi T cells were identified in PFMC. FoxP3 mRNA expression was increased significantly in PFMC compared to PBMC. These data are confirmatory of data by Guyot-Revol et al. [27]; however, small numbers of patients were analysed in our study. Collectively these data indicate an expansion of Treg at pleural sites of TB. Interestingly, a strong correlation of proinflammatory (IL-6 and IL-8) cytokines (but not TGF-β or IFN-γ) with FoxP3 mRNA expression in PFMC was found. Recently, IL-8 has been found to be produced by a Treg cell line [24]. Here we found that up to 15% of FoxP3+ CD4+CD25hi in HIV/TB patients stained positive for intracellular IL-8. A relationship between IL-8 and Treg in primary cells has not been described previously. It is intriguing to postulate that expanded PFMC Treg, either solely or together with other immune cells, constitute the source of excess IL-8 in pleural fluid of TB patients. This is likely as the number of mononuclear phagocytes, the main source of IL-8, is not increased over that of PBMC (see Methods). In addition, IL-8 is a mediator of recruitment of neutrophils and lymphocytes. It is possible that IL-8 maintains the inflammatory milieu in pleural fluid of patients with TB pleuritis. Whether IL-8 is an early or late marker of Treg differentiation among PFMC, the overwhelming majority of which are probably adaptive rather than natural Treg, is currently unknown.
Conversely, it was surprising that a strong pleural Th17 cytokine presence was not seen here in the face of high levels of pleural fluid IL-6 and TGF-β. These data confirm those from a recent study from China, indicating that active TB is associated with low Th17 responses [28]. Lack of a Th17 response may, however, be due to increased IFN-γin situ[29] or a reciprocal effect of increased pleural Treg activity [30]in situ. This lack of Th17 and uncontrolled proinflammatory profile probably contributes to increased HIV-1 activity [31] in pleural fluid of HIV/TB patients with pleural TB. The latter needs to be investigated thoroughly in these subjects.
A correlation of the pleural cytokines and Treg expansion with clinical or virological outcomes was not sought due to the small numbers of HIV/TB subjects in this study. However, it appears that clinical parameters were not different among HIV-infected and uninfected patients.
In summary, effects of MTB infection predominate over that of HIV-1 at sites of dual HIV/TB infection in pleural TB. The cellular (expanded Treg) and cytokine (IL-6, IL-8, TGF-β, IFN-γ) characteristics at pleural sites of HIV/TB are distinct from these parameters in the systemic circulation. Expansion of Treg and specific patterns in the pleural cytokine profile, which also include limitation in IL-17, may promote HIV-1 expansion in pleural TB.
Acknowledgments
This study was supported by NIH grants HL-51636 and AI-95383, by the CFAR at CWRU (AI-36219), and by funding from the James B. Pendleton Charitable Trust to CFAR. We thank Dr S. Sieg for helpful comments.
Disclosure
The authors declare no financial or commercial conflict of interest.
References
- 1.Dye C, Lonnroth K, Jaramillo E, Williams BG, Raviglione M. Trends in tuberculosis incidence and their determinants in 134 countries. Bull World Health Organ. 2009;87:683–91. doi: 10.2471/BLT.08.058453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richter C, Perenboom R, Mtoni I, et al. Clinical features of HIV-seropositive and HIV-seronegative patients with tuberculous pleural effusion in Dar es Salaam, Tanzania. Chest. 1994;106:1471–5. doi: 10.1378/chest.106.5.1471. [DOI] [PubMed] [Google Scholar]
- 3.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis. 2003;188:1146–55. doi: 10.1086/378676. [DOI] [PubMed] [Google Scholar]
- 4.Toossi Z, Johnson JL, Kanost RA, et al. Increased replication of HIV-1 at sites of Mycobacterium tuberculosis infection: potential mechanisms of viral activation. J Acquir Immune Defic Syndr. 2001;28:1–8. doi: 10.1097/00042560-200109010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Toossi Z, Mayanja-Kizza H, Baseke J, et al. Inhibition of human immunodeficiency virus-1 (HIV-1) by beta-chemokine analogues in mononuclear cells from HIV-1-infected patients with active tuberculosis. Clin Exp Immunol. 2005;142:327–32. doi: 10.1111/j.1365-2249.2005.02913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collins KR, Quinones-Mateu ME, Wu M, et al. Human immunodeficiency virus type 1 (HIV-1) quasispecies at the sites of Mycobacterium tuberculosis infection contribute to systemic HIV-1 heterogeneity. J Virol. 2002;76:1697–706. doi: 10.1128/JVI.76.4.1697-1706.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn SD, Pisell TL, Hirsch CS, Wu M, Butera ST, Toossi Z. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J Infect Dis. 2001;184:1127–33. doi: 10.1086/323649. [DOI] [PubMed] [Google Scholar]
- 8.Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Isshiki H, Sugita T, et al. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An P, Vlahov D, Margolick JB, et al. A tumor necrosis factor-alpha-inducible promoter variant of interferon-gamma accelerates CD4+ T cell depletion in human immunodeficiency virus-1-infected individuals. J Infect Dis. 2003;188:228–31. doi: 10.1086/376455. [DOI] [PubMed] [Google Scholar]
- 11.Goletti D, Kinter AL, Coccia EM, et al. Interleukin (IL)-4 inhibits phorbol-ester induced HIV-1 expression in chronically infected U1 cells independently from the autocrine effect of endogenous tumour necrosis factor-alpha, IL-1beta, and IL-1 receptor antagonist. Cytokine. 2002;17:28–35. doi: 10.1006/cyto.2001.0989. [DOI] [PubMed] [Google Scholar]
- 12.Li YG, Iwabu Y, Warachit J, et al. Interleukin-4 up-regulates T-tropic human immunodeficiency virus type 1 transcription in primary CD4+ CD38+ T-lymphocyte subset. Microbiol Immunol. 2005;49:155–65. doi: 10.1111/j.1348-0421.2005.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 13.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25– T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 14.Yamazaki S, Bonito AJ, Spisek R, Dhodapkar M, Inaba K, Steinman RM. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood. 2007;110:4293–302. doi: 10.1182/blood-2007-05-088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggena MP, Barugahare B, Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol. 2005;174:4407–14. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 16.Hryniewicz A, Boasso A, Edghill-Smith Y, et al. CTLA-4 blockade decreases TGF-beta, IDO, and viral RNA expression in tissues of SIVmac251-infected macaques. Blood. 2006;108:3834–42. doi: 10.1182/blood-2006-04-010637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro-Rodrigues R, Resende Co T, Rojas R, et al. A role for CD4CD25 T cells in regulation of the immune response during human tuberculosis. Clin Exp Immunol. 2006;144:25–34. doi: 10.1111/j.1365-2249.2006.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch CS, Baseke J, Mayanja-Kizza H, Wu M, Boom WH, Toossi Z. T-regs and suppression of CD4 T-cell responses during HIV/TB (abstract) Am J Respir Med. 2006;(Suppl.):173. [Google Scholar]
- 19.Tesseur I, Zou K, Berber E, Zhang H, Wyss-Coray T. Highly sensitive and specific bioassay for measuring bioactive TGF-beta. BMC Cell Biol. 2006;7:15. doi: 10.1186/1471-2121-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 21.Hertoghe T, Wajja A, Ntambi L, et al. T cell activation, apoptosis and cytokine dysregulation in the (co)pathogenesis of HIV and pulmonary tuberculosis (TB) Clin Exp Immunol. 2000;122:350–7. doi: 10.1046/j.1365-2249.2000.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayanja-Kizza H, Wu M, Aung H, et al. The interaction of monocyte chemoattractant protein-1 and tumour necrosis factor-alpha in Mycobacterium tuberculosis-induced HIV-1 replication at sites of active tuberculosis. Scand J Immunol. 2009;69:516–20. doi: 10.1111/j.1365-3083.2009.02246.x. [DOI] [PubMed] [Google Scholar]
- 23.Yagi H, Nomura T, Nakamura K, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643–56. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 24.Harashima A, Toraya T, Okochi A, et al. Interleukin-8 and RANTES are signature cytokines made by HOZOT, a new type of regulatory T cells. Mol Immunol. 2009;46:3310–19. doi: 10.1016/j.molimm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Bezuidenhout J, Roberts T, Muller L, van Helden P, Walzl G. Pleural tuberculosis in patients with early HIV infection is associated with increased TNF-alpha expression and necrosis in granulomas. PLoS ONE. 2009;4:e4228. doi: 10.1371/journal.pone.0004228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djoba Siawaya JF, Chegou NN, van den Heuvel MM, et al. Differential cytokine/chemokines and KL-6 profiles in patients with different forms of tuberculosis. Cytokine. 2009;47:132–6. doi: 10.1016/j.cyto.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in tuberculosis patients. Am J Respir Crit Care Med. 2005;173:803–10. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Zhang M, Liao M, et al. Reduced Th17 response in patients with tuberculosis correlates with IL-6R expression on CD4+ T Cells. Am J Respir Crit Care Med. 2010;181:734–42. doi: 10.1164/rccm.200909-1463OC. [DOI] [PubMed] [Google Scholar]
- 29.Nakagome K, Okunishi K, Imamura M, et al. IFN-gamma attenuates antigen-induced overall immune response in the airway as a Th1-type immune regulatory cytokine. J Immunol. 2009;183:209–20. doi: 10.4049/jimmunol.0802712. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–91. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prendergast A, Prado JG, Kang YH, et al. HIV-1 infection is characterized by profound depletion of CD161+ Th17 cells and gradual decline in regulatory T cells. AIDS. 2010;24:491–502. doi: 10.1097/QAD.0b013e3283344895. [DOI] [PubMed] [Google Scholar]


