Abstract
Mast cell tryptase (MCT) is a key diagnostic test for mastocytosis and anaphylaxis. High serum tryptase levels are also one of the risk factors for adverse reaction in venom immunotherapy, yet occasional patients are seen with raised levels in the absence of either diagnosis. False positive results can be due to assay interference by heterophilic antibodies such as rheumatoid factor (RF) and human anti-mouse antibodies (HAMA). We therefore investigated heterophilic antibody interference by rheumatoid factor activity and HAMA as a cause of raised MCT results in the Phadia tryptase assay. Serum samples from 83 patients were assayed for MCT and rheumatoid factor before and after the use of heterophilic antibody blocking tubes (HBT). Samples with more than 17% reduction in MCT with detectable RF were then assayed for HAMA. Fourteen (17%) of the 83 samples with positive RF showed a >17% decrease in mast cell tryptase after HBT blocking. Post-HBT, eight of 14 (57%) reverted from elevated to normal range values with falls of up to 98%. RF levels were also decreased significantly (up to 75%). Only one of the 83 tested was apparently affected by HAMA in the absence of detectable IgM RF. In conclusion, any suspicious MCT result should be checked for heterophilic antibodies to evaluate possible interference. False positive MCT levels can be caused by rheumatoid factor. We suggest a strategy for identifying assay interference, and show that it is essential to incorporate this caveat into guidance for interpretation of MCT results.
Keywords: HAMA, heterophilic antibodies, interference, rheumatoid factor, tryptase
Introduction
Immunoassay results inform many diagnostic pathways and patient management algorithms. However, they can also lead to inappropriate treatment due to errors caused by interference from heterophile antibodies, typically human anti-mouse antibodies (HAMA) or rheumatoid factor (RF).
Heterophilic antibodies are antibodies which can bind to immunoglobulins of other species and interfere in immunoassays, causing a spurious elevation of measured value that is independent of the true analyte concentration. Heterophile interference has been reported to affect up to 27% of immunoassay results [1,2].
Sandwich assays use at least two antibodies directed against different epitopes of an antigen; one antibody is bound to a solid-phase, while the other is in solution and tagged with a signal moiety. Normally, antigen present in the sample ‘bridges’ the two antibodies so that the amount of labelled antibody which becomes bound to the solid-phase is proportional to the antigen concentration in the sample. Heterophilic antibodies can ‘bridge’ the two antibodies independently of antigen, resulting in an increase in bound labelled antibody concentration.
RFs are autoantibodies of immunoglobulin (Ig)G, IgA and IgM class. The pentavalent structure of the IgM isotype can cross-link the Fc portion of human or animal IgG, causing falsely elevated results in sandwich assays. Some RFs have the capacity to bind Fc regions of other species and may also have HAMA-like activity.
HAMA may occur because of treatment with animal products (such as murine monoclonal antibodies) or contact with animals. They interfere with tests by binding the detector and capture antibodies even in the absence of the specific antigen that the assay is designed to detect. This can cause an increase or decrease in the apparent signal [3]. HAMA may also interfere in assays using anti-sera from multiple species due to interspecies cross-reactivity.
A number of techniques have been used to remove heterophile interference, including ultracentrifugation, removal of the interference with protein A or protein G, precipitation with trichloroacetic acid and pre-treatment with ethanol, polyethylene glycol 6000, sulphydryl agents and detergents. A commonly used approach is the use of a modified assay buffer containing blocking agents such as bovine immunoglobulins or irrelevant murine antibodies [4].
Heterophilic interference due to HAMA and RF can be blocked by the stearic hinderance effect of the heterophilic antibody blocking tube (HBT) tube treatment.
Measurement of MCT is one of the diagnostic criteria for systemic mastocytosis (SM) and anaphylactic reactions. Raised tryptase has also been proposed as a risk factor for adverse reactions in venom immunotherapy, with many such patients being thought to have occult mastocytosis [5]. An unpublished retrospective case-note review of patients at our Clinical Immunology and Allergy Unit (2005–9) showed that 14 patients had persistently elevated MCT. None had features of SM on investigation [World Health Organization (WHO criteria], but all had idiopathic urticaria and angioedema.
There is a single report of reductions in MCT in 30 RF-positive sera following the use of heterophilic antibody blocking tubes (HBT), suggesting the potential for heterophilic antibody interference in the assay, but the numbers of raised tryptases were low [6].
The manufacturer of Immunocap 250 tryptase assay (Phadia AB, Uppsala, Sweden) states that the assay is not affected significantly by heterophile antibodies. The Immunocap 100 kit reportedly does not incorporate such agents and the assay therefore may be compromised by the presence of HAMA in serum samples [6]. Validation carried out prior to moving the assay from the Immunocap 100 to Immunocap 250 in our Sheffield laboratory (using 50 randomly selected patient samples with MCT concentrations between 2·7 and 180 µg/l) showed excellent correlation between the platforms (n = 50, r2 = 0·99).
We intended to determine whether the unexplained raised MCT results in our patient cohort was secondary to heterophilic interference; whether the Immunocap 250 MCT assay was affected by the presence of heterophilic antibodies (HAMA or RF); and if HBT blocking would minimize any interference.
Materials and methods
Sample selection
Eighty-three different patient samples were investigated. Of these, 49 were selected randomly from tryptase batches run previously on the Immunocap 250 (values from less than 1 to 319 µg/l). Fourteen were patient samples from the clinical unit with raised MCT and no apparent SM. None of these 63 samples had had RF measured prior to this study. A further 20 randomly selected samples with high RF levels (40–4690 IU/ml) were identified from RF assays run on the BN II analyser (Siemens Medical Solutions, Bracknell, UK), without prior knowledge of the tryptase levels.
Mast cell tryptase assay
The Immunocap 250 tryptase assay measures total tryptase using two monoclonal antibodies (B12 and G4) that recognize both pro- and mature forms of α-tryptase and β-tryptase [7]. The assay has a mean within-run coefficient of variation of 3% and a between-run coefficient of variation (CV) of 12% (in-house data determined at three concentration levels of 3·8, 17·8 and 22·8 µg/l). The analyser was run and maintained according to the manufacturer's instructions.
Rheumatoid factor assay
RF was measured by nephelometry on the BNII analyser reading at a wavelength of 840 nm. The analyser was serviced and operated as directed by the manufacturer.
Assay validation
All assay results were validated using third-party internal controls in conjunction with the Biorad QC Oncall package. Appropriate Westgard rules were determined by Westgards’ QC Validator software package version 2·0 (Westgard QC, Madison, WI, USA) to monitor assay performance.
HAMA ELISA assay
Human anti-mouse antibodies (HAMA) were measured using the Alpha Diagnostic International (ADI) enzyme-linked immunosorbent assay (ELISA) kit (Autogen Bioclear, Calne, UK). HAMA in the patients’ serum is detected by a sandwich ELISA technique using immobilized mouse IgG and horseradish peroxidase-conjugated anti-human IgG. The concentrations of HAMA were determined against standards supplied with the kit. Patient samples with a mean absorbance of 0·088 at 450 nm are negative, and patients treated with mouse monoclonal antibodies have a mean absorbance of around 0·559. The manufacturers claim intra-assay coefficient of variations of between 4·2 and 8·3% (mean 6·0%), suggesting that the maximum upper limit of negativity has an A450 of 0·095. A positive serum control from the manufacturer was run with each batch of patient samples. The manufacturers state that RF does not interfere with the measurement of HAMA, although clearly any RF may bind potentially to mouse IgG Fc and therefore behave as a form of HAMA.
HBT tube assay
Heterophilic antibody blocking tubes (HBT) tubes (Scantibodies® Laboratories Inc., Laboratoire Scantibodies, Villebon/Yvette, France) have been reported to block heterophile antibodies (HAMA and RF) in serum [8]. Five hundred µl of serum is added to the HBT tube, mixed gently by inversion and incubated for 1 h, before re-analysis.
The Scantibodies HBT (http://www.scantibodies.com/scanhbr.html) contains a blocking reagent composed of specific binders which inactivate heterophilic interference from HAMA, human anti-goat antibodies, human anti-sheep antibodies, human anti-rabbit antibodies and RF by stearic hinderance effect.
Study method
Each of the 83 samples was separated into two aliquots. One aliquot was treated with HBT blocking tubes to remove heterophile antibodies. Both treated and untreated aliquots were assayed for MCT and RF on a single run. Five samples containing tryptase with values of less than 1·0 µg/l and RF with values of less than 9·8 IU/ml were assayed in the same way to act as negative controls. The presence of HAMA was determined on pre- and post-blocked sera and used to validate the blocking performance of the HBT tubes.
Results
Throughout the study we have used the clinically accepted cut-off for MCT in the UK of 14 µg/l as the ‘upper limit’ of normal, and have designated a RF of less than 14 IU/ml as negative.
Of the 83 samples, 56 of 83 (67%) had MCT > 14 µg/l (Figs 1 and 2). Of these, 14 were patient samples from the clinical unit with raised MCT and no apparent mastocytosis, 24 samples from patients with anaphylaxis and 13 samples from patients with mastocytosis. Five of 20 (25%) samples from the raised RF group (no prior knowledge of MCT) had raised MCT. Twenty-seven of 83 (33%) samples were RF-positive (Fig. 1). One of the WHO criteria for systemic mastocytosis is MCT > 20 µg/l. There were 51 of 83 patients with MCT > 20 µg/l. Five of these became MCT < 20 µg/l after HBT treatment.
Fig. 1.
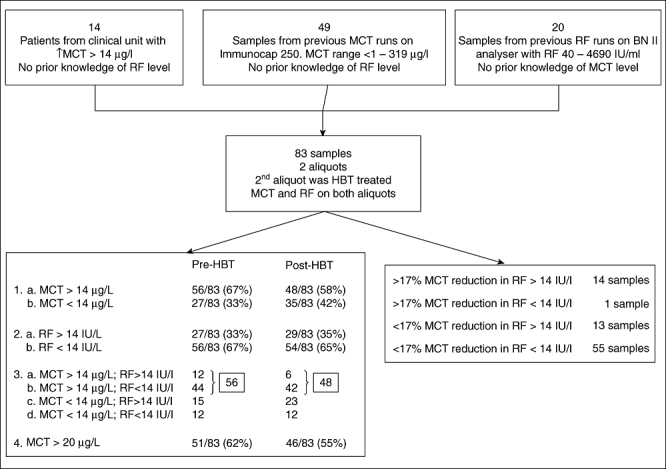
Sample selection, results pre- and post-heterophilic antibody blocking tubes (HBT) treatment.
Fig. 2.
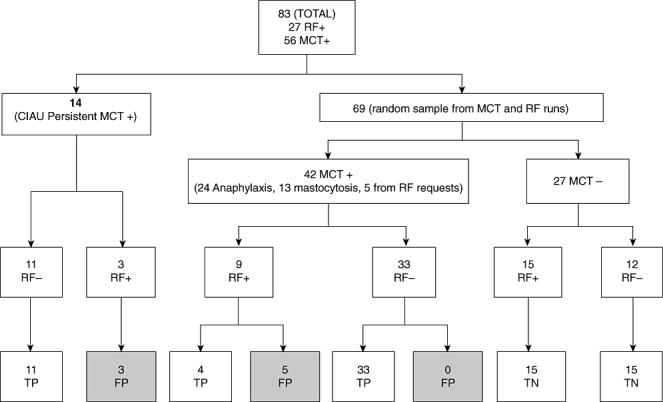
Presence of rheumatoid factor (RF) and its effect on mast cell tryptase (MCT) measurement (CIAU: Clinical Immunology and Allergy Unit; FP: false positive – if the MCT became negative after HBT treatment; TP: true positive – MCT remained high after HBT treatment; TN: true negative – MCT-negative pre-and post-HBT).
Toorenenbergen et al. [6] used a value of 12% (four times the within-run CV%) to indicate any significant change in tryptase following treatment with the HBT tubes. However, in the samples with no detectable levels of RF (<9·8 IU/ml), a change in tryptase level (both positive and negative) of up to 17% (independent of baseline tryptase levels) was seen following HBT treatment. This suggested that there was a wide range of non-specific blocking taking place and/or a number of summative errors within the analytical technique itself. A value of 17% was therefore chosen as the cut-off level above which any change was attributed to heterophile activity. Clearly, this may underestimate the true contribution of heterophilic antibodies to observed assay values.
Of the samples, 14% had false-positive MCT results – eight of 56 (14%) had raised levels pre-HBT which became normal following HBT blocking; these samples were deemed to be falsely elevated due to assay interference.
Almost half the RF factor-positive patients had raised tryptase: 27 of 83 (32%) patients were RF-positive with a range of 15·3 to 4690 IU/ml; 12 of 27 (44%) RF-positive patients had raised tryptase values (>14 µg/l).
Half the tryptase values in RF-positive sera showed evidence of heterophile antibody interference: 14 of 27 (52%) RF-positive patients had a decrease (>17%) in their tryptase concentration following treatment with the HBT. In the RF-negative cohort only one sample had >17% reduction.
Of the raised tryptases in the RF-positive cohort, 57% were false positives: eight of 14 (57%) RF-positive samples had raised MCT levels (>14 µg/l) pre-HBT which became normal (<14 µg/l) post-block (false positives). Six of 14 RF-positive samples had a reduction of >17% in their MCT value but the pre- and post-tryptase values were <14 µg/l and so remained within the normal range at all times, even though there was evidence of heterophilic interference. The IgM RF concentrations were also variably reduced by up to 75% (Table 1).
Table 1.
Effect of heterophilic antibody blocking tubes (HBT) blocking on mast cell tryptase (MCT), rheumatoid factor (RF) and human anti-mouse antibodies (HAMA) in patients with raised RF and reduction of MCT >17%. Results in bold type highlight the specimens which became negative after HBT treatment and corresponding RF and HAMA levels.
| Pre-HBT tryptase µg/l | Post-HBT tryptase µg/l | Δ% tryptase | Pre-HBT RF IU/ml | Post-HBT RF IU/ml | Δ% RF | Pre-HBT HAMA OD (450) | Post-HBT HAMA OD (450) | |
|---|---|---|---|---|---|---|---|---|
| 1 | 9·1 | 7·5 | 18 | 321 | 140 | 56 | 0·064 | 0·058 |
| 2 | 7·07 | 5·56 | 21 | 316 | 109 | 66 | 0·104 | 0·074 |
| 3 | 12·5 | 7·9 | 37 | 393 | 124 | 68 | 0·095 | 0·08 |
| 4 | 13·9 | 7·3 | 47 | 2120 | 1510 | 29 | 0·067 | 0·065 |
| 5 | 6·22 | 2·76 | 56 | 525 | 347 | 34 | 0·086 | 0·09 |
| 6 | 10·6 | 4·6 | 57 | 350 | 219 | 37 | Insufficient | Insufficient |
| 7 | 14·8 | 8·4 | 43 | 341 | 94·2 | 72 | 0·107 | 0·056 |
| 8 | 25 | 12·9 | 48 | 364 | 104 | 71 | 0·127 | 0·08 |
| 9 | 29·3 | 13·9 | 53 | 374 | 112 | 70 | 0·105 | 0·072 |
| 10 | 18·9 | 8·9 | 53 | 1940 | 1570 | 19 | 0·108 | 0·091 |
| 11 | 31·9 | 6·7 | 79 | 260 | 63·8 | 75 | 0·107 | Insufficient |
| 12 | 41·8 | 2·6 | 94 | 3200 | 2670 | 17 | 0·104 | 0·088 |
| 13 | 160 | 5·2 | 97 | 550 | 338 | 39 | 0·132 | 0·077 |
| 14 | 200 | 4·1 | 98 | 4690 | 3730 | 20 | 0·197 | 0·057 |
OD: optical density.
A significant association was observed between the presence of the IgM RF and heterophile interference. A χ2 test (Table 2) was performed and gave a value of 30·84 (P < 0·0001), suggesting a significant relationship between changes in tryptase level and the presence of RF in the patients’ serum, but clearly not all RF isotypes are bound by the HBT treatment and a perfect correlation would not be expected.
Table 2.
Effect of rheumatoid factor (RF) positivity on mast cell tryptase (MCT) values following heterophilic antibody blocking tubes (HBT) treatment in relation to pre-HBT RF levels (P < 0·0001).
| <17% change in tryptase value | >17% change in tryptase value | Total | |
|---|---|---|---|
| RF negative | 55 | 1 | 56 |
| RF positive | 13 | 14 | 27 |
Of the samples with normal RF levels, 38% had trace levels of HAMA: of the 56 samples with negative RF values in the study, 53 contained undetectable levels (<9·8 IU/ml), 13 of which were selected randomly and analysed for the presence of HAMA: five (38%) were found to have contained trace levels of HAMA with the remainder being negative.
Any level of elevated MCT may be a falsely elevated, even very high MCT: three samples with very high IgM RF values were reduced by 17 to 39% following HBT treatment. The MCT levels became normal in all three (41·8 to 2·6 µg/l; 160 to 5·2 µg/l; 200 to 4·1 µg/l) with 94%, 97% and 98% reduction, respectively. These patients had diagnoses of rheumatoid arthritis in the first two cases and non-Hodgkin lymphoma in the latter, respectively; none had any clinical history of mast cell increase or activation. Another sample with a raised RF (in a patient with rheumatoid arthritis) had a 47% reduction in MCT (13·9 to 7·3 µg/l).
Overall, there was no clear correlation between the measured IgM RF levels and the degree of reduction in MCT. This is due probably to variability in binding of mouse IgG Fc or to the variability in the relative total amounts of IgG RF and IgA RF in individual sera (which are not measured in the IgM RF assay).
HAMA interference can also occur in the absence of RF but appears uncommon: one sample (systemic mastocytosis) with significantly raised tryptase level (319 µg/l) had almost undetectable levels of RF but raised levels of IgG HAMA (A450 0·115). Following blocking treatment, the tryptase result remained elevated (246 µg/l) but reduced by more than 17%, but the IgG HAMA dropped to normal levels (A450 0·087).
Nine of 13 samples with a >17% reduction in tryptase after HBT absorption had positive HAMA (A450 > 0·095) and eight of these became negative for HAMA after HBT treatment (one sample insufficient for HBT treatment) (Table 1).
Heterophile antibodies can also lead potentially to false negative results, but we found little evidence for this in our cohort. In one RF-negative sample there was an apparent increase in MCT level >17% after HBT treatment (18·8 to 22·2 µg/l).
In two RF-positive samples analysed, there was an apparent increase in MCT following HBT treatment (43·3 to 49·2 and 128 to 143 µg/l), 14% and 12%, respectively. Both samples showed a decrease in RF level (314 to 102 and 129 to 82). HAMA was not detected in the first of these samples and there was insufficient material to measure HAMA in the second sample.
We needed to ensure that the apparent presence of IgM RF was not itself caused by HAMA. Of the 14 samples with raised IgM RF, 13 had sufficient serum remaining to allow the analysis of HAMA. Of these, three were negative for IgG HAMA with the remaining samples having very low levels (A450 values between 0·095 and 0·197), and the blocking experiments revealed no samples that appeared to have false positive RF levels due to HAMA (Table 1).
There is a cohort of patients with persistently raised tryptase, but no evidence of mastocytosis or anaphylaxis.
Table 3 shows the results in the 14 patients without acute mast cell mediator release or evidence of mastocytosis from the Sheffield Allergy Clinic. Three of 14 were falsely elevated and had evidence of RF and some HAMA activity. Eleven of 14 samples with undetectable IgM RF levels had tryptase concentrations which were not affected by the action of the HBT tubes. This suggests a lack of heterophile interference and demonstrates the existence of a cohort of patients in whom unexpectedly raised tryptase levels appear to be real.
Table 3.
Results from the Sheffield allergy unit showing the effect of raised rheumatoid factor (RF) and human anti-mouse antibodies (HAMA) concentrations on the levels of mast cell tryptase (MCT). Results in bold type highlight the specimens which became negative after HBT treatment and corresponding RF and HAMA levels.
| Pre-HBT tryptase µg/l | Post-HBT tryptase µg/l | Tryptase Δ% | Pre-HBT RF IU/ml | Post-HBT RF IU/ml | RF Δ% | HAMA A450 pre-HBT | HAMA A450 post-HBT | |
|---|---|---|---|---|---|---|---|---|
| 1 | 18·9 | 8·9 | 53 | 1940 | 1570 | 19 | 0·108 | 0·091 |
| 2 | 25 | 12·9 | 48 | 364 | 104 | 71 | 0·127 | 0·08 |
| 3 | 29·3 | 13·9 | 53 | 374 | 112 | 70 | 0·105 | 0·072 |
| 4 | 42·4 | 40·4 | 5 | <9·8 | <9·8 | 0 | Insufficient | Insufficient |
| 5 | 29·4 | 28·6 | 3 | <9·8 | <9·8 | 0 | Insufficient | Insufficient |
| 6 | 24 | 23·7 | 1 | <9·8 | <9·8 | 0 | 0·074 | 0·065 |
| 7 | 32 | 33·5 | 5 | <9·8 | <9·8 | 0 | 0·102 | 0·068 |
| 8 | 51·8 | 49·4 | 5 | <9·8 | <9·8 | 0 | 0·119 | 0·049 |
| 9 | 20·2 | 18·7 | 7 | <9·8 | <9·8 | 0 | 0·098 | 0·101 |
| 10 | 25·1 | 24·7 | 2 | <9·8 | <9·8 | 0 | 0·087 | 0·05 |
| 11 | 38·2 | 35·6 | 7 | <9·8 | <9·8 | 0 | 0·07 | 0·069 |
| 12 | 14·8 | 14·3 | 3 | <9·8 | <9·8 | 0 | 0·057 | 0·07 |
| 13 | 23·7 | 24·4 | 3 | <9·8 | <9·8 | 0 | Insufficient | Insufficient |
| 14 | 21·6 | 22·6 | 5 | <9·8 | <9·8 | 0 | Insufficient | Insufficient |
Discussion
Care should be exercised in the interpretation of MCT results due to the significant potential for interference by heterophilic antibodies including RF.
This study shows that eight of 56 sera (14%) with MCT > 14 µg/l were confirmed as having falsely elevated MCT. Five of 51 (10%) with MCT > 20 µg/l (WHO minor criteria for SM) were falsely elevated. All false positives had raised levels of IgM RF.
Of the cohort with unexplained raised MCT, 20% were false positives due to assay interference but 80% were not, and had truly elevated stable increases of uncertain clinical significance. None of these patients had evidence of mastocytosis on extensive investigation.
The persistently raised tryptase in this cohort of patients who do not have any clinical features of mastocytosis is interesting, but any attempts to explain it are speculative. Three of these were false positive elevations due to heterophilic interference from rheumatoid factor activity. There do not appear to be any obvious clinical differences that would distinguish these patients from most of our cohort with idiopathic urticaria and angioedema. Longer-term follow-up may be revealing.
MCT is an important marker of acute mast cell mediator release in severe allergic reactions or mastocytosis [9]. It is recognized increasingly that there are some individuals who have persistently elevated tryptase using the current assay but in whom no evidence of either disorder can be found, leading to suspicion of assay interference [1,2,6,10]. However, the manufacturer states that the assay is not affected significantly by heterophile interference.
We confirm that the presence of IgM RF correlates with interference in the Phadia tryptase assay and results in overestimation of tryptase or false positivity.
This study demonstrates that IgM RF or a HAMA-like activity associated with IgM RF interferes with the assay and leads usually to overestimation of the true MCT value. We confirm that there are patients with persistently raised MCT who appear to be unaffected by HAMA or RF blocking, and these cases are not rare.
It is important to note that the values produced following HBT treatment must be interpreted with caution, as this may not remove all the interfering heterophile activity and still give a misleading raised value for the analyte being measured [3]. A comment indicating the presence of interference by heterophile antibodies should be added to the report.
Among the heterophilic antibodies, IgM RF was associated most closely with interference in the measurement of tryptase (P < 0·0001). We have not assessed the potential interference associated with IgG and IgA RF activity, which may be important. HAMA detected without RF rarely caused interference.
Interpretation of laboratory results should always be made in light of the clinical features. Test results are almost worthless without context. We recommend checking IgM RF levels and consider HBT treatment in all specimens where there is doubt about the significance of the MCT result. This may avoid unnecessary invasive investigations for mastocytosis or inappropriate diagnosis of anaphylaxis.
In anaphylaxis, this step may not be necessary provided that there are consecutive samples showing appropriate rise and fall of MCT values in an acute release pattern which cannot be mimicked by stable heterophile activity.
The positive predicted value (PPV) of a rise and fall of tryptase in the context of an acute allergic reaction will not change, because the pretest probability is high and heterophilic interference is unlikely to change within 24 h. In this study cohort there were 24 raised MCT samples from anaphylaxis, which remained elevated post-HBT treatment.
However, the PPV of a persistently raised MCT > 20 µg/l as a screen for mastocytosis is likely to be impaired significantly. The positive predictive value of raised MCT alone is not as high as generally assumed when used as a surrogate screen for underlying mastocytosis or acute allergic reactions.
Box 1. Key messages.
Tryptase measurement must be interpreted in the clinical context
Consider possibility of heterophile interference in tryptase assay and other immunometric assays
There is a cohort of patients with raised MCT who do not obviously have mastocytosis
Clinical implications
Raised MCT values may be due to heterophile interference from RF rather than mast cell degranulation. All samples with unexplained or incongruous raised MCT values should be re-tested after treatment with heterophile blocking tubes.
Acknowledgments
None.
Disclosure
None.
References
- 1.Hennig C, Rink L, Fagin U, Jabs WJ, Kirchner H. The influence of naturally occurring heterophilic anti-immunoglobulin antibodies on direct measurement of serum proteins using sandwich ELISAs. J Immunol Methods. 2000;235:71–80. doi: 10.1016/s0022-1759(99)00206-9. [DOI] [PubMed] [Google Scholar]
- 2.Ismail AA, Walker PL, Cawood ML, Barth JH. Interference in immunoassay is an underestimated problem. Ann Clin Biochem. 2002;39:366–73. doi: 10.1258/000456302760042128. [DOI] [PubMed] [Google Scholar]
- 3.Levinson SS. Antibody multispecificity in immunoassay interference. Clin Biochem. 1992;25:77–87. doi: 10.1016/0009-9120(92)80048-l. [DOI] [PubMed] [Google Scholar]
- 4.Levinson SS, Miller JJ. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays. Clin Chim Acta. 2002;325:1–15. doi: 10.1016/s0009-8981(02)00275-9. [DOI] [PubMed] [Google Scholar]
- 5.Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341–6. doi: 10.1097/00130832-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 6.van Toorenenbergen AW, Heerenbrinck GK, Dufour-van den Goorbergh DM. Heterophilic antibody interference in a tryptase immunoassay. Clin Biochem. 2008;41:331–4. doi: 10.1016/j.clinbiochem.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Schwartz LB, Bradford TR, Rouse C, et al. Development of a new, more sensitive immunoassay for human tryptase: use in systemic anaphylaxis. J Clin Immunol. 1994;14:190–204. doi: 10.1007/BF01533368. [DOI] [PubMed] [Google Scholar]
- 8.Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45:942–56. [PubMed] [Google Scholar]
- 9.Valent P, Sperr WR, Schwartz LB, Horny HP. Diagnosis and classification of mast cell proliferative disorders: delineation from immunologic diseases and non- mast cell hematopoietic neoplasms. J Allergy Clin Immun. 2004;114:3–11. doi: 10.1016/j.jaci.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 10.Ward G, McKinnon L, Badrick T, Hickman PE. Heterophilic antibodies remain a problem for the immunoassay laboratory. Am J Clin Pathol. 1997;108:417–21. doi: 10.1093/ajcp/108.4.417. [DOI] [PubMed] [Google Scholar]


