Abstract
Transplantation tolerance induced by neonatal injection of semi-allogeneic spleen cells is associated with a pathological syndrome caused by T helper type 2 (Th2) differentiation of donor-specific CD4+ T lymphocytes. We have shown previously that this Th2-biased response is inhibited by host CD8+ T cells. Herein, we demonstrate that upon neonatal immunization with (A/J × BALB/c)F1 spleen cells, BALB/c mice expand a population of CD8+ T cells expressing both CD25 and forkhead box P3 (FoxP3) markers. In this setting, CD8+CD25+ T cells predominantly produce interferon (IFN)-γ and interleukin (IL)-10 and are efficient in controlling IL-4, IL-5 and IL-13 production by donor-specific CD4+ T cells in vitro. CD8+FoxP3- T cells are single producers of IFN-γ or IL-10, whereas CD8+FoxP3+ T cells are double producers of IFN-γ and IL-10. We further demonstrate that IFN-γ and IL-10 are two major cytokines produced by CD8+ T cells involved in the in vivo regulation of Th2-type pathology. In this setting, we conclude that neonatal alloimmunization induces the expansion of several regulatory CD8+ T cells which may control Th2 activities via IFN-γ and IL-10.
Keywords: IFN-γ, IL-10, neonates, regulatory CD8 T cells, Th2
Introduction
Pioneering experiments by Billingham and colleagues [1] established that transplantation tolerance across major histocompatibility complex (MHC) class I and class II barriers can be induced by neonatal inoculation of semi-allogeneic spleen cells. Deletion of donor-specific T cells was considered for several years to be the dominant mechanism for such unresponsiveness to specific antigens [2]. Subsequent studies recognized immunodeviation [3–5] and immunoregulation [6–8] as the crucial underlying mechanisms of this neonatal tolerance. Indeed, CD4+ T cells recognizing donor MHC class II antigens actually differentiate into T helper type 2 (Th2) cells in tolerant mice [3–5]. The consequence of this Th2 response is the development of an immunopathological syndrome which includes immunoglobulin (Ig)E overproduction, lymphoid organ hyperplasia, hypereosinophilia and increased production of Th2-type cytokines interleukin (IL)-4, IL-5, IL-13 and IL-10 [9,10].
Concerning donor-specific CD8+ T cells, the inability of neonatally tolerant mice to generate effector cytotoxic T cell activity was well established [11,12] but the mechanism by which functional CD8+ T cells may influence the unbalanced CD4+ T cells in early life is less explored. Indeed, it has been established in several experimental models that CD8+ T cells inhibit Th2 responses in adult animals [13–17]. Major effort still needs to be invested in order to characterize phenotypically those regulatory CD8+ T cells in early life, understand their effector mechanisms and the specificity of their effector phase. In this study we sought to characterize further the phenotype and effector mechanisms of regulatory CD8+ T cells occurring in the context of neonatal injection of allogeneic spleen cells. An important finding of this study is the demonstration that a subset of CD8+ T cells expressing CD25 displays a CD44high CD62Lhigh memory phenotype and expresses forkhead box P3 (FoxP3) at an early stage after neonatal immunization. We further demonstrate the crucial roles played by IFN-γ and IL-10, which are produced by these regulatory CD8+ T cells in down-regulation of the Th2 responses.
Materials and methods
Mice
BALB/c (H-2d) and C57BL/6 (H-2b) mice were obtained from Harlan (Zeist, the Netherlands). BALB/c β2-microglobulin (β2m−/−) was kindly provided by Dr J.-C. Guéry (Toulouse, France). A/J (H-2k), BALB/c IFN-γ-deficient mice (IFN-γ−/−) and BALB/c IL-10-deficient mice (IL-10−/−) were purchased from Jackson Laboratories (Bar Harbor, ME, USA), and along with (A/J × BALB/c)F1 hybrids were housed and bred in our specific pathogen-free animal facility.
In vivo treatments
Neonatal tolerance was induced in BALB/c mice by injection into the retro-orbital vein of 107 (A/J × BALB/c)F1 hybrid spleen cells within the first 24 h of life. For neonatal CD8+ T cell transfer experiments, 1 × 106 CD8+ T cells were injected intravenously (i.v.) along with the F1 spleen cells into β2m−/− BALB/c newborns.
Cell staining and flow cytometry analysis
Total lymph node (LN) cells were membrane-stained in fluorescence activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS) 1×, 0·5 % bovine serum albumin (BSA) serum ≥96% lyophilized powder] for 20 min at 4°C with the following conjugated antibodies: Pacific blue- or fluorescein isothiocyanate (FITC)-conjugated anti-CD8 monoclonal antibody (mAb), FITC-conjugated anti-CD62L mAb, phycoerythrin-cyanin 5 (PE-Cy5)-conjugated anti-CD44 mAb and allophycocyanin (APC)-conjugated anti-CD25 mAb, biotinylated anti-CD28 and anti-CD127 mAb, PE-conjugated anti-H-2Kk mAb and APC-Cy7- or PE-conjugated streptavidin were purchased from BD Biosciences (Erembodegem, Belgium). Data were obtained on a CyAnTM ADP LX9 flow cytometer and analysed using Summit version 4·2 software (DakoCytomation, Carpinteria, CA, USA). For intracellular cytokine staining, cells were stimulated with 50 ng/ml phorbol myristate acetate (PMA), 500 ng/ml ionomycin and 1 µg/ml Golgi Plug (BD Biosciences) for 4 h at 37°C or left unstimulated. After washing, cells were incubated for 10 min with Fc blocking mAb (2·4G2; BD Biosciences), labelled for surface markers, fixed and permeabilized in CytoFix/CytoPerm solution (BD Biosciences), washed with Perm/Wash buffer (BD Biosciences), and finally labelled with specific cytokine or FoxP3 mAbs or isotype controls. Anti-FoxP3-PE (eBioscience, Hatfield, UK), anti-IFN-γ-FITC (BD Biosciences), anti-IL-10-APC mAbs (BD Biosciences) and isotype control were used according to the manufacturer's instructions. For FoxP3 intracellular staining alone in CD8+CD25+ cells, the eBioscience FoxP3 staining buffer was used for fixation and permeabilization.
Cell purification
CD4+ T cells were purified from 6–8-week-old BALB/c wild-type mice using the anti-CD4 mAb-coupled magnetic antibody cell sorting (MACS) system (Miltenyi Biotec, Leiden, the Netherlands). CD8+ T cells were purified from 6–8-week-old wild-type, IL-10−/− or IFN-γ−/− BALB/c mice by negative selection from total LN using a Dynal mouse CD8-negative isolation kit, according to the manufacturer's instructions (Invitrogen, Merelbeke, Belgium). Purity >96% was assessed routinely by flow cytometry analysis. CD8+CD25+ T cell populations were obtained by cell sorting on a Moflo cytometer (DakoCytomation) to obtain a pure population of CD8+CD25- T cell and CD8+ CD25+ T cells (>99% purity).
Polymerase chain reaction (PCR) studies
Purified CD8+ T cells were frozen at −20°C after collection and mRNA was extracted using a MagnaPure LC mRNA Isolation Kit I (Roche Diagnostics, Brussels, Belgium). After reverse transcription (RT), quantitative real-time PCR was then carried out using LightCycler RNA Master Hybridization Probes on a Lightcycler device (Roche Diagnostics). The sequences of primers and probes are available on request (vflamand@ulb.ac.be).
Mixed lymphocyte culture and dosage of cytokines and IgE levels
Mixed lymphocyte culture, dosage of cytokines and IgE levels were performed as described previously [18].
Statistics
Data are expressed as mean ± standard error of the mean (s.e.m.). Group comparisons were made using a two-tailed non-parametric Mann–Whitney U-test (P < 0·05 considered significant).
Results
Neonatal injection of semi-allogeneic spleen cells results in the expansion of regulatory CD8+CD25+ T cells capable of controlling Th2-type responses
Immunodeviation of CD4+ T cells towards a Th2 phenotype after neonatal inoculation of semi-allogeneic spleen cells has been described intensively [3–5]. The emergence of regulatory CD4+ T cells in this setting has also been shown [19]. Much less attention was given to the CD8+ T cell subsets, although populations of CD8+ regulatory T cells capable of controlling neonatal responses of effector Th2-type T cells induced by self and alloantigens have been documented in rat and BALB/c mice [18,20]. Using flow cytometry we analysed the phenotype of the CD8+ T cells collected from 1- and 5-week-old mice immunized at birth with semi-allogeneic spleen cells and observed that several distinct populations of CD8+ T cells expanded. Over time, a relative expansion of CD8+CD127+ (not shown), CD8+CD28+ (not shown) and CD8+CD25+ (Fig. 1a) T cells was indeed observed in neonatally immunized mice compared to uninjected mice. Compared with untreated newborns, a significant increase in the absolute numbers of CD8+CD25+ T cells was already observed as early as 1 week after birth and persisted over time at 5 weeks of age (Fig. 1a). We could show that CD8+CD25+ T cells from immunized mice were CD28- and CD127- and were mainly CD44high and CD62Lhigh translating a central memory phenotype (Fig. 1b). Based on the expression of the donor-type MHC class I molecules (H-2k), we show in Fig. 1c that fewer than 3% of CD8+CD25+ T cells collected from BALB/c mice that have been injected with semi-allogeneic (A/J × BALB/c)F1 spleen cells would be of donor origin.
Fig. 1.
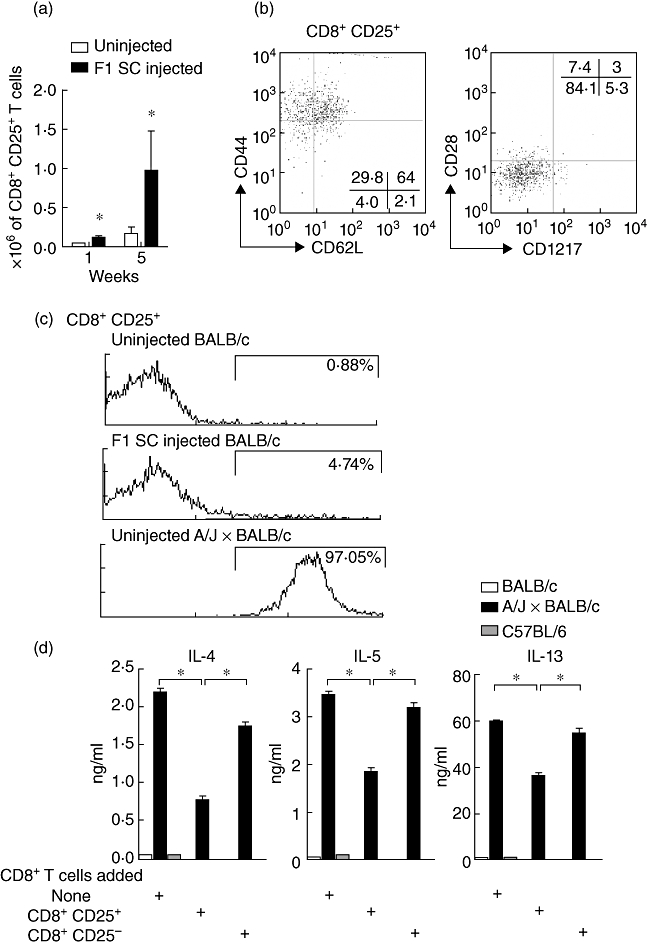
Phenotype of CD8+ T cells in F1 spleen cells neonatally injected mice and inhibition of T helper type 2 (Th2) cytokine production. (a) Absolute numbers of CD8+CD25+ T cells in total lymph nodes collected from uninjected and neonatally injected mice at 1 and 5 weeks of age. Results are mean values from five separate experiments, each involving a pool of five mice (*P < 0·004 compared with uninjected mice at 1 week of age and *P < 0·05 compared with uninjected mice at 5 weeks of age). (b) CD44/CD62L staining or CD28/CD127 staining on gated CD8+CD25+ T cells. Results are expressed as % of positively stained cells and are representative of three separate experiments, each involving a pool of five mice. (c) H-2Kk expression on CD8+CD25+ T cells collected from 5-week-old uninjected BALB/c mice, F1 spleen cells injected mice or donor uninjected A/J × BALB/c mice (pool of seven mice). (d) In vitro suppressive activity of purified CD8+CD25+ T cells versus purified CD8+CD25- T cells on Th2 cytokine production by CD4+ T cells from neonatally injected BALB/c wild-type mice; 2·5 × 106 CD8+ T cell subsets were cultured with 2·5 × 106 Th2 cytokine-producing CD4+ T cells and 2 × 106 irradiated allogeneic A/J × BALB/c spleen cells for 72 h or when indicated with 2 × 106 irradiated syngeneic BALB/c or third-party C57BL/6 spleen cells. Results are expressed as ng/ml of interleukin (IL)-4, IL-5 and IL-13 measured by enzyme-linked immunosorbent assay (ELISA). Results are representative of three individual experiments containing five mice per group (*P < 0·05).
We then tested in vitro whether CD8+CD25+ T cells from neonatally injected mice were efficient regulators of effector Th2-type CD4+ T cell responses to donor alloantigens. As illustrated in Fig. 1d, CD8+CD25+ T cells were significantly more potent in inhibition of IL-4, IL-5 and IL-13 production by CD4+ T cells in comparison to their CD8+CD25- counterparts.
CD8+CD25+FoxP3+ T cells expand in neonatally immunized mice and produce IL-10 and IFN-γ
We next analysed the mRNA profile of CD8+CD25+ T cells from neonatally injected mice and searched for known regulatory markers. We observed a significant increase of mRNA levels encoding for IFN-γ, IL-10, FoxP3, cytotoxic T lymphocyte antigen-4 (CTLA-4) and glucocorticoid-induced TNF receptor family-related gene (GITR) in CD8+CD25+ T cells compared with CD8+CD25- T cells collected from normal mice and mice injected with (A/J × BALB/c)F1 spleen cells at birth (Fig. 2a). IFN-γ mRNA levels were higher in CD8+CD25+ T cells collected from immunized mice compared with untreated mice. IL-10 and FoxP3 expression were also slightly increased in the immunized group but without reaching statistical significance (Fig. 2a). Flow cytometry analysis confirmed that the neonatal immunization with F1 spleen cells induced gradual expansion of the CD8+CD25+FoxP3+ T cell subset that is significant as early as after 1 week of life and that increased, on average, from 0·06 ± 0·034% to 0·67 ± 0·08% over a 5-week period (Fig. 2b). We wanted to characterize further these regulatory CD8+ T cells by intracellular cytokine measurement. Intracytoplasmic staining revealed that the percentage of CD8+CD25+ T cells producing IFN-γ or IL-10 was increased significantly in adult mice that were injected at birth with semi-allogeneic spleen cells compared to uninjected mice (2·9 ± 0·4-fold induction for the single producers of IFN-γ and 2·4 ± 0·5-fold induction for the single producers of IL-10). We noticed that a minor proportion of CD8+CD25+ T cells were producing both IFN-γ and IL-10 (Fig. 2c) and could demonstrate further that these double-producing cells belong exclusively to the CD8+FoxP3+ T cell population compared with the CD8+FoxP3- subset (Fig. 2c). As a control, monitoring of CD8+FoxP3+ T cells producing IFN-γ and IL-10 simultaneously was inhibited strongly in IFN-γ−/− and IL-10−/− mice that have been injected at birth with F1 spleen cells (Fig. 2c). It is important to note that this monitoring of cytokine-producing CD8+ T cells were performed under PMA/ionomycin stimulation and did not require donor-type alloantigen restimulation, suggesting a particular efficient activation of CD8+ T cell response in neonates.
Fig. 2.
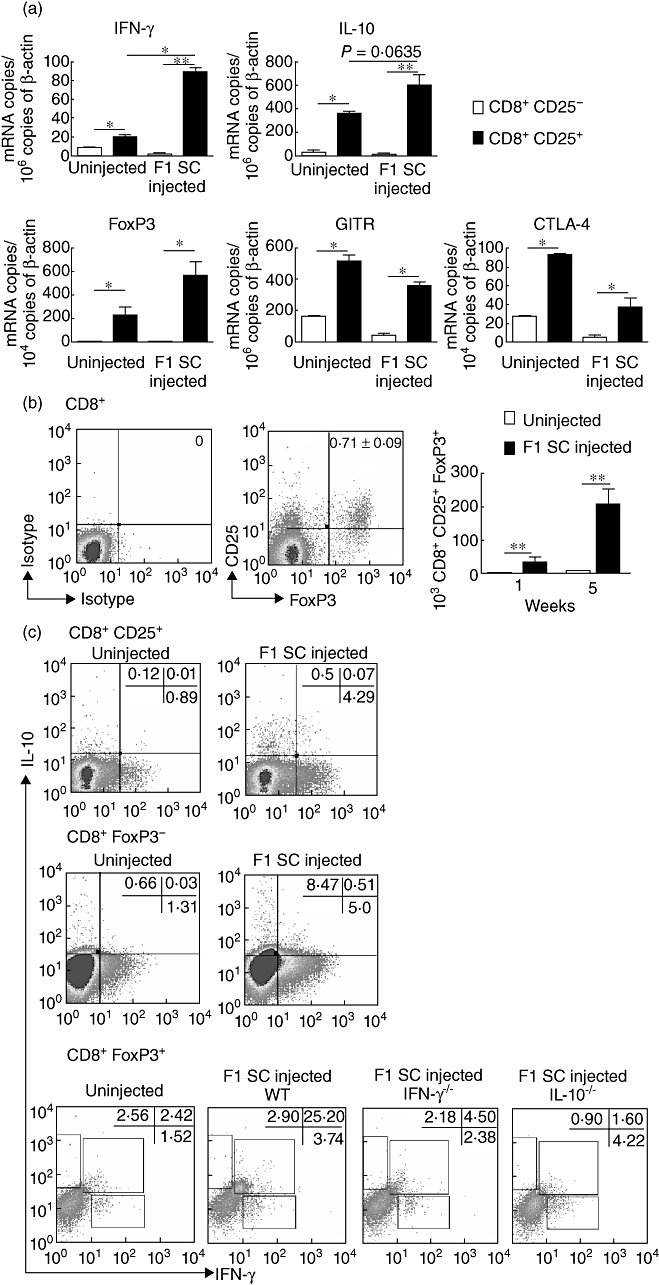
CD25 and forkhead box P3 (FoxP3) expression and interferon (IFN)-γ and interleukin (IL)-10 production by CD8+ T cells collected from F1 spleen cells injected mice. (a) mRNA quantification of cytokines and regulatory proteins on cell-sorted CD8+CD25+ T cells and CD8+CD25- T cells isolated from total lymph nodes (LN). Data are mean values normalized using β-actin as reference and are collected from three different experiments involving eight mice per experiment (*P < 0·05; **P < 0·001). (b) Intracytoplasmic staining of FoxP3 and surface expression of CD25 on CD8+ T cells on total LN collected from neonatally injected mice. Results are expressed as mean ± standard error of the mean percentages or absolute numbers of CD8+CD25+FoxP3+ T cells from six separate experiments each involving a pool of five mice (**P < 0·001). (c) Intracytoplasmic staining of IFN-γ and IL-10 on either CD8+CD25+, CD8+FoxP3- or CD8+FoxP3+ T cells from total lymph node cells collected from 6–8-week-old uninjected or neonatally injected wild-type, IFN-γ−/− or IL-10−/− mice. Results are expressed as percentage of positively stained cells and are representative of four separate experiments each involving a pool of four to 12 mice.
IFN-γ and IL-10 produced by CD8+ T cells play a dominant role in the regulation of Th2-associated immunopathology
We wanted to assess in vivo the respective role of IFN-γ and IL-10 produced by CD8+ T cells in the control of the Th2-type pathology. In mixed lymphocyte culture, we first checked the alloantigen specificity of the CD8+ T cells that were primed at birth to produce IFN-γ and IL-10. As shown in Fig. 3a, adult purified CD8+ T cells collected from mice that had been injected with F1 spleen cells produced a significantly increased amount of IFN-γ and IL-10 compared to uninjected mice only in response to donor-type alloantigen (A/J × BALB/c) recall and not under syngeneic (BALB/c) or third-party (C57BL/6) allostimulation.
Fig. 3.
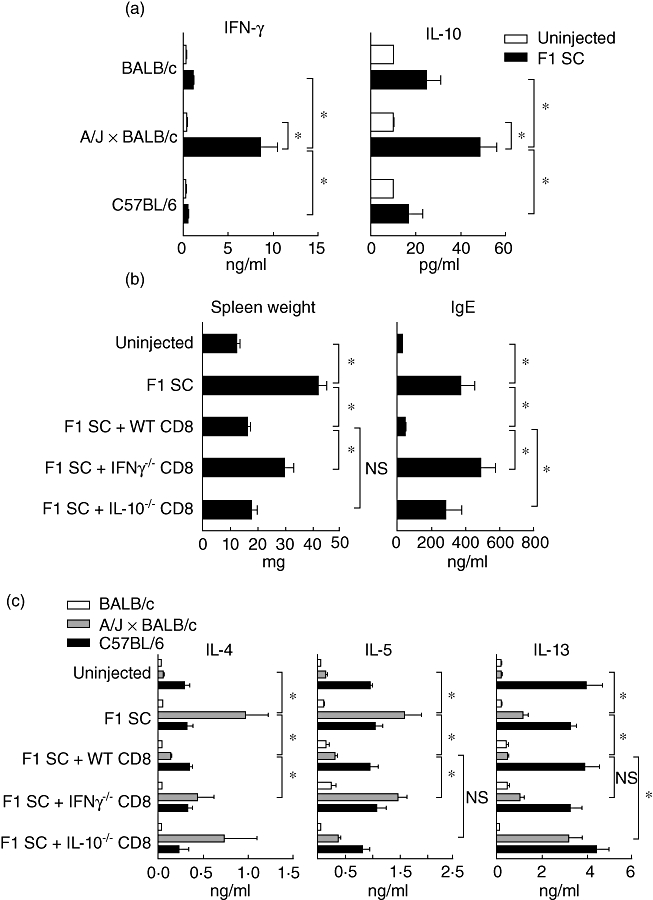
Role of interferon (IFN)-γ and interleukin (IL)-10 produced by CD8+ T cells in the control of the T helper type 2 (Th2) pathology. (a) Purified CD8+ T cells from uninjected and neonatally injected mice were cultured with irradiated syngeneic (BALB/c), allogeneic (A/J × BALB/c) or third-party (C57BL/6) spleen cells. Supernatants were collected after 72 h of culture and the presence of IFN-γ and IL-10 was determined by enzyme-linked immunosorbent assay (ELISA). Results are mean values of six pools of three mice per experimental group (*P < 0·05). (b,c) β2m−/− BALB/c mice were injected at birth with (A/J × BALB/c) F1 spleen cells and wild-type, IFN-γ−/− or IL-10−/− CD8+ T cells purified from neonatally injected BALB/c mice. (b) Spleen weight expressed in mg and seric immunoglobulin (Ig)E expressed in ng/ml monitored at 4 weeks of age. Results are mean values of at least 10 mice per group (*P < 0·05). (c) Total lymph node (LN) cells were cultivated with irradiated syngeneic (BALB/c), allogeneic (A/J × BALB/c) or third-party (C57BL/6) spleen cells. Supernatants were collected after 72 h and the presence of IL-4, IL-5 and IL-13 were determined by ELISA. Results are mean values of five to 14 pools of three mice per experimental group (*P < 0·05); n.s.: non-significant.
The pathology induced by neonatal inoculation of semi-allogeneic spleen cells includes lymphoid hyperplasia and serum hyper-IgE, the latter changes being related directly to overproduction of IL-4 and IL-5 [6–10]. To determine in vivo the role of IFN-γ and IL-10 production by CD8+ T cells, we monitored hyper-IgE and splenomegaly in CD8+ T cell-compromised recipient mice (β2m−/− BALB/c) that were co-administrated at birth with the F1 splenocyte inoculum and either wild-type, IFN-γ−/− or IL-10−/− CD8+ T cells that were primed at birth with the same F1 splenocytes. We first checked the establishment of chimerism in the β2 m−/− recipient mice, and using flow cytometry observed persistent donor B cell chimerism over time at 1 and 5 weeks of age (data not shown). We found that the splenomegaly induced by the injection of semi-allogeneic cells at birth was corrected completely by the co-transfer of wild-type primed CD8+ T cells (Fig. 3b). This regulatory effect was abolished if IFN-γ−/− primed CD8+ T cells but not if IL-10−/− primed CD8+ T cells were inoculated (Fig. 3b). Serum hyper-IgE-inhibition by CD8+ T cells was dependent on both IFN-γ and IL-10, as illustrated in Fig. 3b. Mixed lymphocyte culture (MLC) performed with total LN collected from 4–6-week-old β2 m−/− BALB/c mice co-transferred at birth with semi-allogeneic spleen cells and different types of primed CD8+ T cells showed that compared with wild-type primed CD8+ T cells, IFN-γ−/− primed CD8+ T cells failed to down-regulate IL-4 production and more predominantly IL-5 production, but not IL-13 production. Concerning the IL-10−/− primed CD8+ T cells, compared with wild-type primed CD8+ T cells, they mainly lost their capacity to inhibit IL-4 and IL-13 production, but not IL-5 production in response to donor-specific alloantigens. In vitro suppressive experiments confirmed a predominant role of IFN-γ produced by CD8+ T cells in the inhibition of IL-5 production, whereas IL-10 produced by CD8+ T cells had a predominant inhibitory effect on the production of IL-13 and to a lesser extent on IL-4 (data not shown). Similar results were obtained using antibodies against IFN-γ and IL-10 (data not shown). Altogether, these results highlighted that CD8+ T cells producing IFN-γ are potent regulators of IL-5 and lymphoid organ hypertrophy probably by reducing the drive for eosinophilic infiltration, whereas CD8+ T cells producing IL-10 mainly regulate the production of IL-13 and IL-4 by CD4+ T cells that would control B cell activation and IgE production.
Discussion
Over the past decade, significant advances have been made in our understanding of T cell function in neonates. Some studies pointed out that when varying the condition of antigen exposure at this period of life, partially or fully mature T cell responses can be achieved. Nevertheless, in murine newborns most of the stimulations intensify an imprinting of Th2 dominance that is maintained into adulthood. The prenatal and newborn period of life is thought to be a window of time in which tolerance to exogenously introduced antigens can be achieved with ease. Multiple mechanisms contribute to peripheral tolerance, including clonal deletion, anergy, immune deviation and active suppression by regulatory T cells. Th2 bias of CD4+ T cells was shown to be a predominant underlying mechanism of neonatal tolerance. Concerning CD8+ T cells, we demonstrated that neonatal CD8+ T cells induced specifically by donor-type alloantigens are poorly effective for cytotoxic functions, but are still capable of displaying regulatory function that inhibits the Th2 response [18].
CD8+ regulatory T cells occur naturally but are also induced by a variety of conditions, including manipulation of co-stimulatory molecules interaction, antigen processing or during viral infection [21]. Others have demonstrated that during periods of active inflammation, when growth-promoting cytokines are produced, regulatory CD8+ T cells which are distinct from conventional CD8+ T cells could be induced through IL-12- or IL-4-dependent pathways [22]. We observed that CD8+ regulatory T cells are inducible in early life upon alloantigen exposure by immunocompetent adult spleen cells. We have demonstrated previously that BALB/c mice that have been immunized at birth with (A/J × BALB/c)F1 spleen cells become chimeric mainly for the B cell subset [4,8,10]. It would be interesting to understand how constant stimulation of the immune system by the chimeric allogeneic B cells could influence the generation of memory and induced CD8+ regulatory T cells.
Several subsets of CD8+ regulatory T cells have been described in humans and mice, such as CD8+CD25+, CD8+CD122+, CD8αα+CD122+NKG2D+, CD8+CD28-, CD8+CD45RClow and IL-2/granulocyte–macrophage colony-stimulating factor (GM-CSF)-induced CD8+ regulatory T cells, all of which have been suggested to suppress immunity [23]. FoxP3 transcription factor expression, associated with CD4+CD25+ regulatory T cells, has also been shown to be present in CD8+CD28-, CD8+CD25+ and CD8+CD45RClow regulatory T cells [24–26]. In this study we have demonstrated the existence of a small population of CD8+ T cells expressing CD25 and FoxP3 that expanded after neonatal stimulation with alloantigens and that displayed a memory phenotype, as demonstrated by the expression of CD62Lhigh and CD44high. Consistent with our findings, most CD8+CD25+ regulatory T cells described in the literature share an activated CD44high phenotype and produce the immunosuppressive cytokine IL-10 upon in vitro stimulation [27,28].
The mechanisms of action of regulatory CD8+ T cells upon Th2 responses are multiple and may include direct killing, negative signalling directly on the CD4+ T cells or on APC through CTLA-4, B and T lymphocyte attenuator (BTLA) or programmed death 1 (PD1) and secretion of immunosuppressive cytokines such as IFN-γ, IL-10 and transforming growth factor (TGF)-β[29]. Indeed, the involvement of CD8+ T cell-derived IFN-γ in the control of Th2-polarized response has been well documented. IFN-γ plays a crucial role in either the induction or the expression of CD8+ regulatory T cells activity. IFN-γ might act as the end-stage suppressive molecule by inducing IL-12 and IL-18 synthesis by dendritic cells, resulting in the production of IFN-γ by CD4+ T cells, and thereby in inhibition of IL-4 and IgE synthesis [17]. IFN-γ might also function indirectly by activating other cells to produce immunomodulatory and anti-inflammatory molecules such as TGF-β, IL-10, indoleamine 2,3-dioxygenase (IDO) or by producing other Th1-polarizing factors. In the present study we show that both IFN-γ and IL-10 are critically involved in the regulation of cytokine production by Th2-orientated CD4+ T cells and in the regulation of Th2 pathology. Indeed, in vitro and in vivo experiments using IFN-γ and IL-10-deficient CD8+ T cells highlighted the role of CD8+ T cells producing IFN-γ in the control of IL-5 and lymphoid organ hypertrophy, most probably by reducing eosinophilia. CD8+ T cells producing IL-10 would mainly regulate the production of IL-13 and IL-4 by CD4+ T cells and in consequence would control B cell activation and IgE production. IL-10-producing CD8+ regulatory T cells blocking Th2 activation and IgE antibody response have been described in adult immunopathology [22,30]. An alternative way to suppress the Th2 response via CD8+ T cells in an IL-10-dependent manner could be through the activation of natural CD4+CD25+FoxP3+ T cells [31]. In addition, we were able to show that regulatory CD8+ T cells expressing CD25 and FoxP3 markers expanded in the neonatally injected mice. Further, we demonstrated that CD8+FoxP3+ T cells were unique producers of IFN-γ and IL-10 simultaneously and that CD8+FoxP3- T cells are single producers of IFN-γ or IL-10. Similar regulatory subsets were reported as regulatory CD4+ T cells expressing FoxP3 and producing both IL-10 and IFN-γ that were described to potently inhibit the development of airway hyper-reactivity [32]. We may therefore speculate that several CD8+ T cell subsets may regulate distinct parameters of the Th2-type response through IFN-γ and IL-10 that would be produced simultaneously or not by either CD8+CD25+FoxP3+ or CD8+CD25+FoxP3- cells, respectively. The present work suggests new therapeutic potential in order to reduce the occurrence of Th2-related diseases such as allergy in early life by designing strategies that favour CD8+CD25+ regulatory T cell expansion, as proposed in a recent report of Toll-like receptor (TLR)-2 agonists that enhance CD8+C25+FoxP3+ regulatory T cells and that suppress Th2 immune responses during allergen immunotherapy [33].
Acknowledgments
We thank Frédéric Paulart for technical assistance, Philippe Horlait, Laurent Depret, Christophe Notte and Grégory Waterlot for animal care, and Oberdan Leo for critical reading of the manuscript. The Institute for Medical Immunology is sponsored by the government of the Walloon Region and GlaxoSmithKline Biologicals. This study was also supported by the Fonds National de la Recherche Scientifique (FNRS, Belgium) and an Interuniversity Attraction Pole of the Belgian Federal Science Policy. B.A. is funded by the Belgian Kid's Foundation. A.D. is supported by FNRS grants, I.D. is a research fellow at the FNRS and V.F. is a research associate at the FNRS.
Disclosure
The authors have no financial conflict of interest.
References
- 1.Billingham R, Brent L, Medawar BP. Actively acquired tolerance to foreign cells. Nature. 1953;172:603–6. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Feng HM, Glasebrook AL, Engers HD, et al. Clonal analysis of T cell unresponsiveness to alloantigens induced by neonatal injection of F1 spleen cells into parental mice. J Immunol. 1983;131:2165–9. [PubMed] [Google Scholar]
- 3.Powell TJ, Streilein JW., Jr Neonatal tolerance induction by class II alloantigens activates IL-4-secreting, tolerogen-responsive T cells. J Immunol. 1990;144:854–9. [PubMed] [Google Scholar]
- 4.Abramowicz D, Vandervorst P, Bruyns C, et al. Persistence of anti-donor allohelper T cells after neonatal induction of allotolerance in mice. Eur J Immunol. 1990;20:1647–53. doi: 10.1002/eji.1830200805. [DOI] [PubMed] [Google Scholar]
- 5.Gao Q, Chen N, Rouse TM, et al. The role of interleukin-4 in the induction phase of allogeneic neonatal tolerance. Transplantation. 1996;62:1847–54. doi: 10.1097/00007890-199612270-00029. [DOI] [PubMed] [Google Scholar]
- 6.Le Moine A, Flamand V, de Lavareille A, et al. Hypereosinophilic syndrome induced by neonatal immunization against MHC class II alloantigen: critical role of IL-4. Eur J Immunol. 2002;32:174–81. doi: 10.1002/1521-4141(200201)32:1<174::AID-IMMU174>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Schurmans S, Heusser CH, Qin HY, et al. In vivo effects of anti-IL-4 monoclonal antibody on neonatal induction of tolerance and on an associated autoimmune syndrome. J Immunol. 1990;145:2465–73. [PubMed] [Google Scholar]
- 8.Donckier V, Wissing M, Bruyns C, et al. Critical role of interleukin 4 in the induction of neonatal transplantation tolerance. Transplantation. 1995;59:1571–76. [PubMed] [Google Scholar]
- 9.Streilein JW, Mohler K, Wood PJ. Mechanisms of neonatal transplantation tolerance. Transplant Proc. 1987;19:82–7. [PubMed] [Google Scholar]
- 10.Goldman M, Abramowicz D, Lambert P, et al. Hyperactivity of donor B cells after neonatal induction of lymphoid chimerism in mice. Clin Exp Immunol. 1988;72:79–83. [PMC free article] [PubMed] [Google Scholar]
- 11.Nossal GJ, Pike BL. Functional clonal deletion in immunological tolerance to major histocompatibility complex antigens. Proc Natl Acad Sci USA. 1981;78:3844–47. doi: 10.1073/pnas.78.6.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell TJ, Streilein JW. In vitro suppression of cytotoxic T cell generation by lymphocytes from mice rendered neonatally tolerant of class II MHC alloantigens. Transplantation. 1991;52:383–6. [PubMed] [Google Scholar]
- 13.Chan SY, DeBruyne LA, Goodman RE, et al. In vivo depletion of CD8+ T cells results in Th2 cytokine production and alternate mechanisms of allograft rejection. Transplantation. 1995;59:1155–61. [PubMed] [Google Scholar]
- 14.Srikiatkhachorn A, Braciale TJ. Virus-specific CD8+ T lymphocytes downregulate T helper cell type 2 cytokine secretion and pulmonary eosinophilia during experimental murine respiratory syncytial virus infection. J Exp Med. 1997;186:421–32. doi: 10.1084/jem.186.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmes BJ, MacAry PA, Noble A, et al. Antigen-specific CD8+ T cells inhibit IgE responses and interleukin-4 production by CD4+ T cells. Eur J Immunol. 1997;27:2657–65. doi: 10.1002/eji.1830271027. [DOI] [PubMed] [Google Scholar]
- 16.Foucras G, Coudert JD, Coureau C, et al. Dendritic cells prime in vivo alloreactive CD4 T lymphocytes toward type 2 cytokine- and TGFβ-producing cells in the absence of CD8 T cell activation. J Immunol. 2000;165:4994–5003. doi: 10.4049/jimmunol.165.9.4994. [DOI] [PubMed] [Google Scholar]
- 17.Thomas MJ, Noble A, Sawicka E, et al. CD8 T cells inhibit IgE via dendritic cell IL-12 induction that promotes Th1 T cell counter-regulation. J Immunol. 2002;168:216–23. doi: 10.4049/jimmunol.168.1.216. [DOI] [PubMed] [Google Scholar]
- 18.Gao Q, Rouse TM, Kazmerzak K, et al. CD4+CD25+ cells regulate CD8 cell anergy in neonatal tolerant mice. Transplantation. 1999;68:1891–7. doi: 10.1097/00007890-199912270-00013. [DOI] [PubMed] [Google Scholar]
- 19.Adams B, Nagy N, Paulart F, et al. CD8+T lymphocytes regulating Th2 pathology escape neonatal tolerization. J Immunol. 2003;171:5071–6. doi: 10.4049/jimmunol.171.10.5071. [DOI] [PubMed] [Google Scholar]
- 20.Field AC, Caccavelli L, Bloch MF, et al. Regulatory CD8+ T cells control neonatal tolerance to a Th2-mediated autoimmunity. J Immunol. 2003;170:2508–15. doi: 10.4049/jimmunol.170.5.2508. [DOI] [PubMed] [Google Scholar]
- 21.Niederkorn JY. Emerging concepts in CD8+ T regulatory cells. Curr Opin Immunol. 2008;20:327–31. doi: 10.1016/j.coi.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noble A, Giorgini A, Leggat JA. Cytokine-induced IL-10-secreting CD8 T cells represent a phenotypically distinct suppressor T-cell lineage. Blood. 2006;107:4475–83. doi: 10.1182/blood-2005-10-3994. [DOI] [PubMed] [Google Scholar]
- 23.Smith TRF, Kumar V. Revival of CD8+ Treg-mediated suppression. Trends Immunol. 2008;29:337–42. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Tugulea S, Cortesini R, et al. Specific suppression of T helper alloreactivity by allo-MHC class I-restricted CD8+CD28- regulatory T cells. Int Immunol. 1998;10:775–83. doi: 10.1093/intimm/10.6.775. [DOI] [PubMed] [Google Scholar]
- 25.Cosmi L, Liotta F, Lazzeri E, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–14. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 26.Xystrakis E, Dejean AS, Bernard I, et al. Identification of a novel natural regulatory CD8 T-cell subset and analysis of its mechanism of regulation. Blood. 2004;104:3294–301. doi: 10.1182/blood-2004-03-1214. [DOI] [PubMed] [Google Scholar]
- 27.Bienvenu B, Martin B, Auffray C, et al. Peripheral CD8+CD25+ T lymphocytes from MHC class II-deficient mice exhibit regulatory activity. J Immunol. 2005;175:246–53. doi: 10.4049/jimmunol.175.1.246. [DOI] [PubMed] [Google Scholar]
- 28.Pomié C, Ménager-Marcq I, van Meerwijk JP. Murine CD8+ regulatory T lymphocytes: the new era. Hum Immunol. 2005;69:708–14. doi: 10.1016/j.humimm.2008.08.288. [DOI] [PubMed] [Google Scholar]
- 29.Stock P, Kallinich T, Akbari O, et al. CD8+ T cells regulate immune responses in a murine model of allergen-induced sensitization and airway inflammation. Eur J Immunol. 2004;34:1817–27. doi: 10.1002/eji.200324623. [DOI] [PubMed] [Google Scholar]
- 30.Yamada A, Ohshima Y, Yasutomi M, et al. Antigen-primed splenic CD8+ T cells impede the development of oral antigen-induced allergic diarrhea. J Allergy Clin Immunol. 2009;123:889–94. doi: 10.1016/j.jaci.2008.12.1115. [DOI] [PubMed] [Google Scholar]
- 31.Joetham A, Takeda K, Miyahare N, et al. Activation of naturally occurring lung CD4+CD25+ regulatory T cells requires CD8 and MHC I interaction. Proc Natl Acad Sci USA. 2007;104:15057–62. doi: 10.1073/pnas.0706765104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stock P, Akbari O, Berry G, et al. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5:1149–56. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 33.Tsai Y-G, Yang K, Niu D-M, Chien J-W, Lin C-YJ. TLR2 agonists enhance CD8+Foxp3+ regulatory T cells and suppress Th2 responses during allergen immunotherapy. J Immunol. 2010;184:7229–37. doi: 10.4049/jimmunol.1000083. [DOI] [PubMed] [Google Scholar]


