Abstract
G protein-coupled receptors (GPCRs) represent the largest class of integral membrane protein receptors in the human genome. Despite the great diversity of ligands that activate these GPCRs, they interact with a relatively small number of intracellular proteins to induce profound physiological change. Both heterotrimeric G proteins and GPCR kinases are well known for their ability to specifically recognize GPCRs in their active state. Recent structural studies now suggest that heterotrimeric G proteins and GPCR kinases identify activated receptors via a common molecular mechanism despite having completely different folds.
Keywords: G Protein-coupled Receptors (GPCR), G Proteins, 7-Helix Receptor, Protein Kinases, Protein Structure, Signal Transduction
Introduction
A relatively small conformational change in G protein-coupled receptors (GPCRs)2 allows extracellular signals to regulate a vast number of physiological events in cells, principally through the activation of heterotrimeric G proteins (Gαβγ). The ability of GPCRs to transmute signals across biological membranes in this manner has enormous therapeutic potential, as evidenced by the fact that molecules targeting GPCRs represent a substantial fraction of drugs on the market today (1).
Many mechanistic details of heterotrimeric G protein function are now well established (2, 3). GPCR activation converts the receptor into a guanine nucleotide exchange factor (GEF) for heterotrimeric G proteins. Via this GEF activity, GDP is released from the inactive Gαβγ heterotrimer, and GTP binds to form the activated Gα-GTP and Gβγ components, which then interact with downstream effectors. Gα subunits are grouped into four subfamilies (Gαs, Gαi, Gαq, and Gα12/13) (4), which are characterized by their ability not only to couple to specific GPCRs but also to regulate distinct classes of enzymes and ion channels.
However, heterotrimeric G proteins are not the only family of proteins that form specific functional interactions with activated GPCRs. Eukaryotic cells regulate the strength and duration of GPCR signal transduction to adapt to changing external conditions and to avoid damage from sustained signaling. One such desensitization pathway is that of the GPCR kinases (GRKs) and arrestins. GRKs typically phosphorylate the third intracellular loop and/or C-terminal tails of activated receptors, allowing arrestins to bind and block the recoupling of heterotrimeric G proteins (5, 6). GRKs and arrestins preferentially interact with the active conformation of the receptor, are allosterically regulated by this interaction, and are capable of initiating their own G protein-independent signaling cascades. However, GRKs and arrestins generally lack the receptor selectivity exhibited by the various Gα subfamilies.
The ∼800 GPCRs in the human genome (1) are predicted to share the same seven-transmembrane helix topology and, from the perspective of the cytoplasm, exhibit a relatively conserved arrangement of structural features. Despite relatively low sequence homology, the cytoplasmic surfaces of activated receptors must have a common topography that a relatively small number of G proteins, GRKs, and arrestins can all recognize as being “active.” On the other hand, heterotrimeric G proteins, GRKs, and arrestins are structurally diverse (Fig. 1). They have different mechanisms by which they interact with the cell membrane and, presumably, distinct structural elements that interact directly with activated GPCRs. In this minireview, we summarize recent structural studies that suggest that heterotrimeric G proteins and GRKs utilize a similar mechanism to recognize activated receptors.
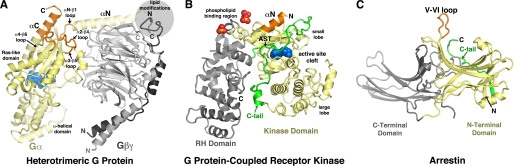
FIGURE 1.
Heterotrimeric G proteins, GRKs, and arrestins are three structurally diverse families of proteins that selectively recognize activated GPCRs. Each representative model is drawn to scale, with its expected receptor-binding elements oriented toward the top of the panel. The tertiary domain thought to form the primary interaction(s) with receptors is shown in yellow, and the structural elements within this domain most strongly implicated in directly binding to the core of the activated receptor are colored orange. A, heterotrimeric G proteins dock with activated GPCRs primarily with the αC helix, the C-terminal helix of Gα. GDP bound to the Ras-like domain of the Gα subunit is shown as blue spheres. The Gβ and Gγ subunits, which form an obligate heterodimer (Gβγ), are shown in gray and black, respectively. The model is a fusion of three atomic structures: the bulk of the model is that of Gαi1β1γ2 (Protein Data Bank code 1GG2) (66); the C terminus of Gαi1 is modeled from the RGS4-Gαi1 complex (code 1AGR) (47); and the C terminus of Gγ2 is from the GRK2-Gβγ complex (code 1OMW) (56). B, GRKs are predicted to interact with GPCRs primarily with residues in the αN helix. Residues in the C-tail of the kinase domain (green) may also contribute. The C-terminal tail or extended third intracellular loops of GPCRs, which contain the phosphoacceptor sites, necessarily bind in the kinase active-site cleft, but such interactions are of much lower affinity. In the GRK6 crystal structure, a basic region adjacent to αN binds three sulfate anions (yellow and red spheres) and is proposed to be an interaction site for anionic phospholipid headgroups (see also Ref. 60). The structure shown is that of GRK6 in complex with the adenosine analog sangivamycin (blue spheres) (code 3NYN) (53). C, current evidence suggests that arrestins recognize activated receptors using the extended V-VI “finger loop” (67). The phosphorylated loops or tails of GPCRs also bind to basic residues in the N-terminal domain (yellow), which helps to reorganize the so-called “polar core” of arrestin and release its C-terminal tail (green). The model shown is that of visual arrestin (arrestin-1; code 1CF1) (68).
Active Conformation of a GPCR
The crystal structure of dark-state bovine rhodopsin was reported in 2000 and represents a milestone for understanding the molecular basis of GPCR signaling (7). Now a decade later, a flurry of new GPCR structures has been reported, including the human β2-adrenergic (8, 9), turkey β1-adrenergic (10), human A2A adenosine (11), human CXCR4 (12), and human dopamine D3 (13) receptors. Structures are also available for squid rhodopsin (14) and bovine opsin (rhodopsin without the retinal chromophore) (15, 16). A detailed comparison of all but the most recent of these structures is covered by several thorough reviews (17–19). These models provide a wealth of new formation about the structure and function of GPCRs, in particular about their specific interactions with antagonists or inverse agonists.
In these structures, the seven transmembrane-spanning helices (TM1–TM7) of these receptors form a characteristic bundle that presents three intracellular loops (IL1–IL3) and a long C-terminal tail to the cytoplasm of the cell. In all but the CXCR4 receptor, TM7 is followed by a cytoplasmic amphipathic helix (H8) that runs parallel to the plasma membrane (Fig. 2, A and B). Ligands typically bind in a deep pocket formed by the transmembrane spans at a depth of about one-third of the way through the lipid bilayer from the extracellular side (Fig. 2A), whereas the cytoplasmic ends of the transmembrane helices contain many of the residues thought to be important for interacting with G proteins, GRKs, and arrestins (20–22). However, most of these receptors were crystallized in an inhibited state, and hence, their cytoplasmic surfaces adopt conformations that are nonpermissive for binding G proteins or GRKs.3
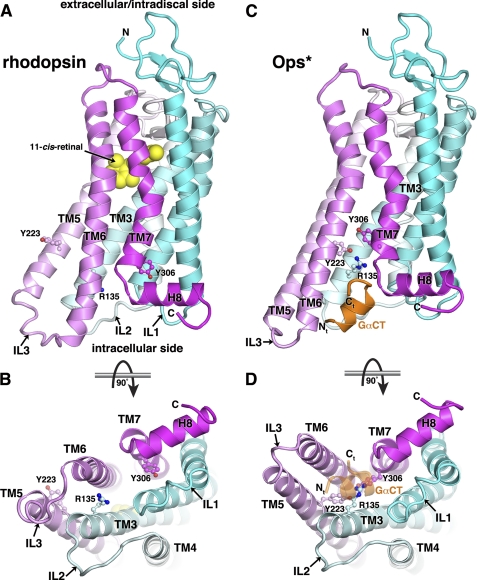
FIGURE 2.
Conformational changes expected to occur upon GPCR activation. Receptors are colored in a gradient from the N terminus (cyan) to the C terminus (magenta). A, structure of rhodopsin, a GPCR in an inactive state. Three highly conserved residues among GPCRs, Arg135 in TM3, Tyr233 in TM5, and Tyr306 in TM7, are well separated (ball-and-stick models). The photosensitive 11-cis-retinal chromophore (yellow spheres), which serves as an inverse agonist, demarks the approximate location of the ligand-binding pocket. The model is that of bovine rhodopsin (Protein Data Bank code 1GZM) (69). Twenty-one amino acids are disordered at the C terminus, which includes all of the GRK phosphorylation sites. B, the cytoplasmic surface of an inactive GPCR. The view is rotated 90° around a vertical access from that in A. In this conformation, IL2 and IL3 are in close proximity and block access to the core of the transmembrane bundle. C, structure of Ops*, a GPCR proposed to be in a relatively active conformation. Conformational changes in the TM5–TM7 segments allow Arg135, Tyr233, and Tyr306 to interact and form the roof of a pocket on the cytoplasmic side of the receptor. Residues equivalent to Tyr223 and Tyr306 in other reported structures of GPCRs are in a conformation intermediate to that observed in rhodopsin and Ops*. A peptide derived from the sequence of the extreme C terminus of the αC helix in Gαt (GαCT orange; cf. Fig. 1A) binds in the pocket as an amphipathic helix. The structure is that of bovine Ops* (code 3DQB) (15). D, the cytoplasmic surface of Ops*. Comparison with B reveals the outward motion of TM6 and the extension of TM5, leading to separation of IL2 and IL3 and the creation of a pocket that can be detected by proteins that specifically interact with activated GPCRs. The bound GαCT peptide is shown as a transparent ribbon.
The apparent exception is the structure of bovine opsin (Fig. 2, C and D) (15, 16). Although opsin has very low activity relative to the activated form of rhodopsin (Rho*), Fourier transform infrared spectroscopy shows that it exists in two conformational states. One of these (called Ops*) bears spectral properties very similar to those of Rho* and is stabilized by low pH and/or via binding a peptide derived from the C-terminal tail of transducin (23). The most profound changes exhibited by the opsin structure compared with that of dark-state rhodopsin are an extension of TM5, an outward movement of TM6, and a slight inward movement of TM7 relative to the helical core of the protein (Fig. 2, compare B and D). These motions are consistent with fluorescence (24), EPR (25), and cross-linking (26, 27) studies that, along with other studies, suggest a rigid-body movement of TM6 relative to TM3 upon activation of rhodopsin. The same activation-dependent conformational change was suggested by fluorescence studies of the β2-adrenergic receptor (28). The cytoplasmic ends of the transmembrane helices in the opsin structure are similar in conformation to those observed in structures of an agonist-bound β2-adrenergic receptor4 and a constitutively active recombinant form of rhodopsin.5 Thus, the opsin structure likely corresponds to Ops*, and if so, it represents the best model of an activated GPCR currently available.
The conformational change exhibited by Ops* relative to rhodopsin creates a solvent-accessible pocket on the intracellular side of the GPCR. Conserved hydrophobic residues on TM3, TM5, and TM6 form one wall of the pocket. Arg135 of the (E/D)RY motif in TM3, one of the most highly conserved sequences in the class A family of GPCRs (29), rearranges from its conformation in dark-state rhodopsin (where it forms the so-called “ionic lock” that helps stabilize the inactive state) to form a new hydrogen bond with Tyr223 on TM5, which has rotated from an external to an internal position. Tyr306 of the conserved NPXXY motif in TM7 rotates into the central region of the GPCR to pack against Arg135 and Tyr223 (Fig. 2, compare A and C with B and D). Together, these residues coalesce to form the “roof” of the pocket. Many of the residues that line the observed pocket are known to be important for coupling to G proteins (see, for example, Ref. 20 and 24), and as such, the pocket likely represents the primary site used by G proteins, GRKs, and arrestins to probe the activation status of a GPCR.
There is considerable evidence that GPCRs can form homo- or hetero-oligomers that help dictate transport and/or function (as reviewed in Ref. 30). From a crystallographic standpoint, the recent CXCR4 receptor structures provide the most convincing case for a functional (and allosteric) GPCR oligomer, as a similar dimer interface was observed in multiple crystal forms. However, the residues in this interface are not conserved even within the chemokine receptor family (12), and thus, it remains unclear in what manner other GPCR oligomers are assembled. Regardless, studies of monomeric GPCRs reconstituted in high density lipoprotein-like particles strongly suggest that receptor oligomers are not required for efficient functional interactions with heterotrimeric G proteins (31–34), GRKs (35), or arrestins (35, 36). Thus, in terms of coupling to its cytoplasmic partners, a monomeric GPCR is sufficient, at least for class A receptors.
Structural Features of Heterotrimeric G Proteins Responsible for Receptor Recognition
Heterotrimeric G proteins are composed of a Gα subunit, which contains a Ras-like domain that binds guanine nucleotides, and a Gβγ heterodimer, which bind with high affinity to Gα in its GDP-bound state (Fig. 1A). As of yet, there are no structures of the Gαβγ heterotrimer in a nucleotide-free state that clearly mimics its conformation when in complex with a GPCR.6 Various studies have implicated two regions of Gαβγ as being involved in receptor interactions (Ref. 37; Ref. 2 and references therein). The first is the C-terminal helix and the adjacent αN-β1, α2-β4, α3-β5, and α4-β6 loops of the Gα subunit. The second is the C-terminal region of Gγ (Fig. 1A). The two regions are separated by ∼55 Å in the Gαβγ heterotrimer, suggesting that both could not be in contact with the same receptor (∼45 Å at its widest) at the same time. Because Gβγ subunits have been shown to be dispensable, at least under certain conditions, for the formation of complexes between Gα subunits and activated receptors (38), the primary role of the C terminus of Gγ, which is either farnesylated or geranylgeranylated, may instead be to work in conjunction with the adjacent N terminus of Gα, which is myristoylated and/or palmitoylated (Fig. 1A), in targeting heterotrimeric G proteins to the membrane surface and perhaps in helping to dictate a particular orientation of the complex (2).7 On the other hand, the C-terminal helix of Gα (in particular, its last four amino acids) is well known to dictate the selectivity of the GPCR-Gαβγ interaction (39–41), and the binding of activated rhodopsin induces structural changes in the C-terminal helix of Gα that are likely coupled to nucleotide exchange (42, 43). Therefore, the C terminus and adjacent structural elements of Gα are expected to form the primary docking site for activated GPCRs.
The structure determination of a GPCR-Gαβγ complex is an important objective because it would illuminate the process by which signals are transferred from receptors to heterotrimeric G proteins. This turns out to be a formidable task not only because it involves a conformationally flexible, integral membrane protein (28) but also because the Gα subunit seems to become more disordered when undergoing nucleotide exchange mediated either by GPCRs (44) or by the soluble GEF Ric8A (45).8 As a result, structural studies aimed at understanding the interaction between GPCRs and heterotrimeric G proteins have thus far relied on studying complexes with peptide fragments derived from either the receptor or G protein. Even so, interpreting these studies can be perilous because peptide fragments often bind with lower affinity and in a manner that is potentially inconsistent with how they would when constrained in the context of a full-length protein.
However, a consistent story has emerged from NMR and x-ray studies of peptides derived from the C terminus of Gαt. Kisselev et al. (46) used transfer nuclear Overhauser effect spectroscopy to show that light-activated rhodopsin induces order in a peptide corresponding to the last 11 amino acids of Gαt (residues 340–350). The peptide forms a helix from residues 340 to 346, followed by a C-terminal cap involving Gly348, whose backbone adopts a left-handed helical conformation (46). This structure is remarkably similar to the C terminus observed in a prior crystal structure of Gαi (47), which belongs to the same subfamily of G proteins as Gαt.9 Most recently, the same peptide but with an affinity-enhancing K341L substitution (GαCT) was crystallized in complex with Ops* (15). The peptide adopts the same conformation observed in the NMR and Gαi structures and docks into the pocket created on the cytoplasmic domain of the receptor (Fig. 2, C and D). Hydrophobic residues from the helical peptide interact with the hydrophobic residues in TM3, TM5, and TM6, whereas other elements of the peptide form specific contacts with Arg135 from the (E/D)RY motif and with the N terminus of H8. To an extent, the structure helps to explain how G protein selectivity can be achieved because a similar C-terminal cap structure has not yet been observed in structures of other Gα subunits, and other Gα subfamilies do not have a residue equivalent to Gly348, which can more readily adopt a left-handed helical conformation. Residues of the receptor known to be important for coupling with heterotrimeric G proteins (20, 24) cluster around the bound peptide, so convincingly, in fact, that one is led to question whether GPCRs routinely form strong interactions with any other region of a Gαβγ heterotrimer. Indeed, the C terminus of Gαt can be extended by three helical turns and retain functional interactions with rhodopsin, even though this would seem to require displacement of the remainder of the heterotrimer away from the GPCR (43).
The Gαi structure with the ordered C terminus can be superimposed on the Ops*-bound GαCT peptide to generate a model of Gα bound to an activated GPCR, and then a model of Gαβγ bound to Ops* can be generated by superimposing the Gα subunit from the structure of the Gαtβ1γ1 heterotrimer (Fig. 3A). This simple docking exercise yields some interesting quandaries. First of all, IL3 of the receptor collides with the β1-β2 loop of Gα, IL2 collides with the αN-β1 and α2-β4 loops, and no direct interactions are formed with the α3-β5 and α4-β6 loops, which have been reported to serve as receptor interaction sites (2). Another major collision implied by the model is that of the lipid bilayer with Gβγ and the N-terminal helix of Gα (Fig. 3A). Consequently, one must conclude that either (i) the structure of Ops* is not that of a fully activated receptor; or (ii) upon binding to a Gαβγ heterotrimer, as opposed to a small peptide fragment, the receptor adopts a distinct conformation that alleviates the observed collisions in this model; or (iii) receptor-bound Gαβγ undergoes a conformation change, one that is perhaps imposed on Gα by the steric constraints imposed by the topology of the activated receptor and by the membrane surface. The last conclusion is consistent with the idea that Gα must undergo a conformational change to eject the bound nucleotide, in particular of its C-terminal helix, whose C-terminal end binds directly to GPCRs and whose preceding loop directly contacts the guanine base of GDP. It is not clear how large this conformational change has to be to provoke nucleotide release (48). The Ops*-Gαβγ docking exercise may also provide an explanation for why lipid modification of either the extreme N terminus of Gα or the C terminus of Gγ is required for efficient nucleotide exchange (38): conformational strain imposed by the requirement to maintain simultaneous and favorable interactions with both the GPCR and the lipid bilayer could be used to help stabilize the transition state for nucleotide exchange on Gα (15).
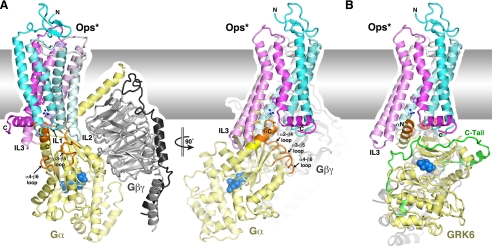
FIGURE 3.
Hypothetical models of a heterotrimeric G protein and a GRK bound to an activated GPCR. The hydrophobic phase of the lipid bilayer is indicated by the gray rectangle. A, the Ops*-Gαβγ docking model suggests that, when activated receptors engage Gαβγ subunits, there is likely a conformational change in the heterotrimeric G protein and perhaps in the receptor. To create this model, the Gαβγ structure shown in Fig. 1A was superimposed on the GαCT peptide in complex with Ops* (Fig. 2C). The model on the left highlights the resulting collision of Gβγ and the N terminus of Gα with the lipid bilayer. IL2 of the receptor also collides with the αN-β1 and α2-β4 loops on the Gα subunit. These collisions would be alleviated, at least in part, by rotation of the bulk of the heterotrimer with respect to the αC helix by ∼60° (clockwise in this view), similar to that proposed previously (70). Collisions could also be avoided if the αC helix were kinked upon receptor binding, but current data suggest that an intact αC helix is required for functional receptor interactions (42, 43). The view on the right shows the receptor in the same orientation as Fig. 2C. B, GRK6 in an active conformation can be docked with Ops* without significant steric overlap and in a manner that positions the C terminus (or an extended IL3 loop) of the GPCR in closed proximity to the active-site cleft of the kinase domain (demarked by blue spheres) (53). To create this model, the side chains of outward-facing hydrophobic residues in the αN helix of GRK6 (Leu3, Ile6, and Val7) were superimposed on those of conserved hydrophobic positions in the GαCT peptide (Leu349, Cys347, and Leu344, respectively) bound to Ops*. This model suggests that there may be additional interactions formed between H8 of the receptor and the kinase C-tail of the GRK (green).
Structural Features of GRKs Responsible for Receptor Recognition
Activated GPCRs are both substrates and allosteric activators of GRKs, and receptors are far better substrates for GRKs than peptide substrates derived from the same receptors, with 2–3 orders of magnitude improvement in Km and Vmax. Moreover, peptide phosphorylation can be enhanced in the presence of activated receptors (49). Such data strongly argue that there is an allosteric receptor-docking site distinct from the active site that, when occupied, helps to stabilize the kinase in an active state. Each GRK consists of an N-terminal helical element of ∼20 amino acids, followed by an RH (regulator of G protein signaling homology) domain. A protein kinase domain is inserted into a loop of the RH domain. Following the RH domain is a variable C-terminal region that is typically involved in membrane targeting (Fig. 1B). As in heterotrimeric G proteins, the membrane-binding domains of GRKs facilitate but are not absolutely required for their function because GRKs can phosphorylate GPCRs in the absence of these elements in vitro. The kinase domain of GRK is closely related to that of PKA and therefore consists of small and large lobes, followed by the “C-tail,” a meandering extension of the protein kinase fold that spans both lobes and contributes residues to the active-site cavity (Fig. 1B). Functional studies have identified residues in the N terminus, the small lobe, the αD helix, and the C-tail of the kinase domain as being important for phosphorylation of activated GPCRs (50–55).
Crystal structures representing each of the three vertebrate GRK subfamilies have now been determined, including those of GRK1, GRK2, and GRK6 (53, 56–58). However, with one exception, the kinase domains in these structures adopt a relatively open, inactive conformation that is not permissive for phosphotransfer, even in cases in which the structures were determined in complex with nucleotides or nucleotide analogs. Furthermore, the functionally important N-terminal and C-tail regions were either absent or poorly ordered, suggesting that kinase domain closure and formation of the GPCR-docking site are coupled events. In support of this model, the central segment of the PKA C-tail, called the active-site tether, becomes fully ordered only when the kinase domain adopts a relatively closed, active conformation (59).
In a recently reported structure of GRK6, the kinase domain adopts a conformation that is expected to be close to its fully active state (53). The N terminus is ordered, forming an ∼4.5-turn helix (αN) that packs in a groove formed between the small lobe and the ordered active-site tether of the kinase domain (Fig. 1B). The residues that form the interfaces between αN and the kinase domain are highly conserved, and alteration of these residues impairs both GPCR and peptide phosphorylation, implying a role in stabilizing the active conformation of the kinase domain (52, 53, 55). A highly conserved, hydrophobic patch of residues oriented away from the kinase domain is found at the N-terminal end of αN. Mutation of these residues decreases the catalytic efficiency of receptor but not peptide phosphorylation (33), consistent with the idea that this patch is involved in direct interactions with activated GPCRs. A relatively flat, positively charged region immediately adjacent to αN (Fig. 1B) is well situated to bind anionic phospholipid headgroups, as suggested previously by functional studies of GRK5 (60).
A Similar Mechanism for the Recognition of Activated GPCRs by G Proteins and GRKs?
Comparison of the GαCT peptide bound to Ops* and the GRK6 N terminus suggests that both Gα subunits and GRKs utilize an amphipathic helix to form the primary interaction with activated receptors, even though this helix has the opposite polarity in each protein. Indeed, both the C-terminal helix of Gα and the αN helix of GRKs protrude from surfaces of their respective domains that are anticipated to be in close proximity to the cell membrane. A hypothetical GRK-GPCR docking model can thus be generated by superimposing the side chains of residues in the hydrophobic patch on the GRK6 αN helix with those of conserved hydrophobic residues in the GαCT helix such that they similarly interact with the hydrophobic wall of the pocket in the activated GPCR (Fig. 3B). The resulting model is satisfying due the lack of severe steric collisions and because it places the proposed membrane-interacting region of GRK6 (and of other GRKs) adjacent to the lipid bilayer and the C terminus of Ops* in close proximity to the active-site cleft of the kinase domain. However, there are a number of ways one could dock the GRK6 structure on the GαCT peptide,10 and thus, the exact details of this intermolecular complex must await structural characterization.
Both G proteins and GRKs seem to require at least two discrete interactions for efficient coupling to activated GPCRs because, in either case, direct contacts with lipids are required for optimal activity. In heterotrimeric G proteins, this lipid dependence may play an important role in the GEF exchange mechanism, as discussed above. In GRKs, there may be insufficient free energy from receptor binding alone to stabilize the active state of the kinase. Thus, GRKs may identify activated receptors by looking for an appropriately sized hydrophobic pocket adrift in a sea of negatively charged lipid headgroups, a topology that is complementary in shape and charge to the active configuration of the kinase domain (62).
Future Directions
We now eagerly await atomic structures of heterotrimeric G proteins, GRKs, and arrestins in complex with GPCRs. It is likely that, in each complex, the receptor will adopt a distinct conformation that is optimized to interact with each partner, as is implied by the existence of biased ligands for GPCRs (63–65). Structures of these complexes would provide valuable mechanistic insights into how selectivity for heterotrimeric G proteins is achieved and how the same extracellular signal can be used to catalyze nucleotide exchange in one family of proteins, stimulate kinase activity in another, and facilitate high affinity receptor sequestration in a third.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants HL086865 and HL071818 (to J. J. G. T.). This minireview will be reprinted in the 2011 Minireview Compendium, which will be available in January, 2012.
A low resolution “photoactivated” structure of rhodopsin is available from crystals of dark-adapted rhodopsin exposed to light (71), but it is unclear if the requirement to maintain crystal contacts, and hence diffraction, limited the conformational changes that could be observed in this experiment. The resulting structure is qualitatively very similar to that of rhodopsin and other inactive GPCRs.
B. K. Kobilka, personal communication.
G. F. Schertler, personal communication.
A structure exists for the fast exchanging A326S mutant of Gαi1 in complex with Gβγ wherein the bound GDP is clearly at low occupancy (48). However, because this structure was crystallized under conditions identical to those of the wild-type heterotrimer, it is not clear if it closely approximates a receptor-bound conformation or merely one possible state that is trapped by crystal lattice contacts.
Gβγ is, however, important for GPCR-mediated GEF activity. Both the “lever arm” (72) and the “gear shift” (61) hypotheses suggest distinct mechanisms by which Gβγ could help dislodge guanine nucleotides from Gα.
C. Thomas and S. R. Sprang, personal communication.
This is not meant to imply that the Gαi subunit in this structure would be a substrate for a GPCR because it is in an active conformation and is not bound to Gβγ.
An alternative docking model using different residues in the GαCT peptide was presented previously (53). In either case, the C terminus of Ops* is positioned near the active site of GRK6, and there are no significant steric collisions between GRK and receptor.
- GPCR
- G protein-coupled receptor
- GEF
- guanine nucleotide exchange factor
- GRK
- GPCR kinase
- TM
- transmembrane helix
- IL
- intracellular loop
- H8
- helix 8.
REFERENCES
- 1. Gruber C. W., Muttenthaler M., Freissmuth M. (2010) Curr. Pharm. Des. 16, 3071–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oldham W. M., Hamm H. E. (2006) Q. Rev. Biophys. 39, 117–166 [DOI] [PubMed] [Google Scholar]
- 3. Sprang S. R., Chen Z., Du X. (2007) Adv. Protein Chem. 74, 1–65 [DOI] [PubMed] [Google Scholar]
- 4. Hepler J. R., Gilman A. G. (1992) Trends Biochem. Sci. 17, 383–387 [DOI] [PubMed] [Google Scholar]
- 5. Pitcher J. A., Freedman N. J., Lefkowitz R. J. (1998) Annu. Rev. Biochem. 67, 653–692 [DOI] [PubMed] [Google Scholar]
- 6. Gurevich V. V., Gurevich E. V. (2006) Pharmacol. Ther. 110, 465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palczewski K., Kumasaka T., Hori T., Behnke C. A., Motoshima H., Fox B. A., Le Trong I., Teller D. C., Okada T., Stenkamp R. E., Yamamoto M., Miyano M. (2000) Science 289, 739–745 [DOI] [PubMed] [Google Scholar]
- 8. Cherezov V., Rosenbaum D. M., Hanson M. A., Rasmussen S. G., Thian F. S., Kobilka T. S., Choi H. J., Kuhn P., Weis W. I., Kobilka B. K., Stevens R. C. (2007) Science 318, 1258–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rasmussen S. G., Choi H. J., Rosenbaum D. M., Kobilka T. S., Thian F. S., Edwards P. C., Burghammer M., Ratnala V. R., Sanishvili R., Fischetti R. F., Schertler G. F., Weis W. I., Kobilka B. K. (2007) Nature 450, 383–387 [DOI] [PubMed] [Google Scholar]
- 10. Warne T., Serrano-Vega M. J., Baker J. G., Moukhametzianov R., Edwards P. C., Henderson R., Leslie A. G., Tate C. G., Schertler G. F. (2008) Nature 454, 486–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jaakola V. P., Griffith M. T., Hanson M. A., Cherezov V., Chien E. Y., Lane J. R., Ijzerman A. P., Stevens R. C. (2008) Science 322, 1211–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu B., Chien E. Y., Mol C. D., Fenalti G., Liu W., Katritch V., Abagyan R., Brooun A., Wells P., Bi F. C., Hamel D. J., Kuhn P., Handel T. M., Cherezov V., Stevens R. C. (2010) Science 330, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chien E. Y., Liu W., Zhao Q., Katritch V., Han G. W., Hanson M. A., Shi L., Newman A. H., Javitch J. A., Cherezov V., Stevens R. C. (2010) Science 330, 1091–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Murakami M., Kouyama T. (2008) Nature 453, 363–367 [DOI] [PubMed] [Google Scholar]
- 15. Scheerer P., Park J. H., Hildebrand P. W., Kim Y. J., Krauss N., Choe H. W., Hofmann K. P., Ernst O. P. (2008) Nature 455, 497–502 [DOI] [PubMed] [Google Scholar]
- 16. Park J. H., Scheerer P., Hofmann K. P., Choe H. W., Ernst O. P. (2008) Nature 454, 183–187 [DOI] [PubMed] [Google Scholar]
- 17. Rosenbaum D. M., Rasmussen S. G., Kobilka B. K. (2009) Nature 459, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hofmann K. P., Scheerer P., Hildebrand P. W., Choe H. W., Park J. H., Heck M., Ernst O. P. (2009) Trends Biochem. Sci. 34, 540–552 [DOI] [PubMed] [Google Scholar]
- 19. Mustafi D., Palczewski K. (2009) Mol. Pharmacol. 75, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Natochin M., Gasimov K. G., Moussaif M., Artemyev N. O. (2003) J. Biol. Chem. 278, 37574–37581 [DOI] [PubMed] [Google Scholar]
- 21. Shi W., Osawa S., Dickerson C. D., Weiss E. R. (1995) J. Biol. Chem. 270, 2112–2119 [DOI] [PubMed] [Google Scholar]
- 22. Raman D., Osawa S., Gurevich V. V., Weiss E. R. (2003) J. Neurochem. 84, 1040–1050 [DOI] [PubMed] [Google Scholar]
- 23. Vogel R., Siebert F. (2001) J. Biol. Chem. 276, 38487–38493 [DOI] [PubMed] [Google Scholar]
- 24. Janz J. M., Farrens D. L. (2004) J. Biol. Chem. 279, 29767–29773 [DOI] [PubMed] [Google Scholar]
- 25. Farrens D. L., Altenbach C., Yang K., Hubbell W. L., Khorana H. G. (1996) Science 274, 768–770 [DOI] [PubMed] [Google Scholar]
- 26. Sheikh S. P., Zvyaga T. A., Lichtarge O., Sakmar T. P., Bourne H. R. (1996) Nature 383, 347–350 [DOI] [PubMed] [Google Scholar]
- 27. Cai K., Klein-Seetharaman J., Hwa J., Hubbell W. L., Khorana H. G. (1999) Biochemistry 38, 12893–12898 [DOI] [PubMed] [Google Scholar]
- 28. Kobilka B. K., Deupi X. (2007) Trends Pharmacol. Sci. 28, 397–406 [DOI] [PubMed] [Google Scholar]
- 29. Rovati G. E., Capra V., Neubig R. R. (2007) Mol. Pharmacol. 71, 959–964 [DOI] [PubMed] [Google Scholar]
- 30. Milligan G. (2008) Br. J. Pharmacol. 153, S216–S229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Whorton M. R., Bokoch M. P., Rasmussen S. G., Huang B., Zare R. N., Kobilka B., Sunahara R. K. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7682–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Whorton M. R., Jastrzebska B., Park P. S., Fotiadis D., Engel A., Palczewski K., Sunahara R. K. (2008) J. Biol. Chem. 283, 4387–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leitz A. J., Bayburt T. H., Barnakov A. N., Springer B. A., Sligar S. G. (2006) BioTechniques 40, 601–2, 604,, 606, passim [DOI] [PubMed] [Google Scholar]
- 34. Bayburt T. H., Leitz A. J., Xie G., Oprian D. D., Sligar S. G. (2007) J. Biol. Chem. 282, 14875–14881 [DOI] [PubMed] [Google Scholar]
- 35. Bayburt T. H., Vishnivetskiy S. A., McLean M. A., Morizumi T., Huang C. C., Tesmer J. J., Ernst O. P., Sligar S. G., Gurevich V. V. (2011) J. Biol. Chem. 286, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsukamoto H., Sinha A., Dewitt M., Farrens D. L. (2010) J. Mol. Biol. 399, 501–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Onrust R., Herzmark P., Chi P., Garcia P. D., Lichtarge O., Kingsley C., Bourne H. R. (1997) Science 275, 381–384 [DOI] [PubMed] [Google Scholar]
- 38. Herrmann R., Heck M., Henklein P., Hofmann K. P., Ernst O. P. (2006) J. Biol. Chem. 281, 30234–30241 [DOI] [PubMed] [Google Scholar]
- 39. Conklin B. R., Farfel Z., Lustig K. D., Julius D., Bourne H. R. (1993) Nature 363, 274–276 [DOI] [PubMed] [Google Scholar]
- 40. Natochin M., Muradov K. G., McEntaffer R. L., Artemyev N. O. (2000) J. Biol. Chem. 275, 2669–2675 [DOI] [PubMed] [Google Scholar]
- 41. Hamm H. E., Deretic D., Arendt A., Hargrave P. A., Koenig B., Hofmann K. P. (1988) Science 241, 832–835 [DOI] [PubMed] [Google Scholar]
- 42. Oldham W. M., Van Eps N., Preininger A. M., Hubbell W. L., Hamm H. E. (2006) Nat. Struct. Mol. Biol. 13, 772–777 [DOI] [PubMed] [Google Scholar]
- 43. Natochin M., Moussaif M., Artemyev N. O. (2001) J. Neurochem. 77, 202–210 [DOI] [PubMed] [Google Scholar]
- 44. Abdulaev N. G., Ngo T., Ramon E., Brabazon D. M., Marino J. P., Ridge K. D. (2006) Biochemistry 45, 12986–12997 [DOI] [PubMed] [Google Scholar]
- 45. Tall G. G., Krumins A. M., Gilman A. G. (2003) J. Biol. Chem. 278, 8356–8362 [DOI] [PubMed] [Google Scholar]
- 46. Kisselev O. G., Kao J., Ponder J. W., Fann Y. C., Gautam N., Marshall G. R. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 4270–4275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tesmer J. J., Berman D. M., Gilman A. G., Sprang S. R. (1997) Cell 89, 251–261 [DOI] [PubMed] [Google Scholar]
- 48. Posner B. A., Mixon M. B., Wall M. A., Sprang S. R., Gilman A. G. (1998) J. Biol. Chem. 273, 21752–21758 [DOI] [PubMed] [Google Scholar]
- 49. Palczewski K., Buczyłko J., Kaplan M. W., Polans A. S., Crabb J. W. (1991) J. Biol. Chem. 266, 12949–12955 [PubMed] [Google Scholar]
- 50. Noble B., Kallal L. A., Pausch M. H., Benovic J. L. (2003) J. Biol. Chem. 278, 47466–47476 [DOI] [PubMed] [Google Scholar]
- 51. Pao C. S., Barker B. L., Benovic J. L. (2009) Biochemistry 48, 7325–7333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sterne-Marr R., Leahey P. A., Bresee J. E., Dickson H. M., Ho W., Ragusa M. J., Donnelly R. M., Amie S. M., Krywy J. A., Brookins-Danz E. D., Orakwue S. C., Carr M. J., Yoshino-Koh K., Li Q., Tesmer J. J. (2009) Biochemistry 48, 4285–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boguth C. A., Singh P., Huang C. C., Tesmer J. J. (2010) EMBO J. 29, 3249–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Palczewski K., Buczyłko J., Lebioda L., Crabb J. W., Polans A. S. (1993) J. Biol. Chem. 268, 6004–6013 [PubMed] [Google Scholar]
- 55. Huang C. C., Yoshino-Koh K., Tesmer J. J. (2009) J. Biol. Chem. 284, 17206–17215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lodowski D. T., Pitcher J. A., Capel W. D., Lefkowitz R. J., Tesmer J. J. (2003) Science 300, 1256–1262 [DOI] [PubMed] [Google Scholar]
- 57. Singh P., Wang B., Maeda T., Palczewski K., Tesmer J. J. (2008) J. Biol. Chem. 283, 14053–14062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lodowski D. T., Tesmer V. M., Benovic J. L., Tesmer J. J. (2006) J. Biol. Chem. 281, 16785–16793 [DOI] [PubMed] [Google Scholar]
- 59. Kannan N., Haste N., Taylor S. S., Neuwald A. F. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 1272–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pitcher J. A., Fredericks Z. L., Stone W. C., Premont R. T., Stoffel R. H., Koch W. J., Lefkowitz R. J. (1996) J. Biol. Chem. 271, 24907–24913 [DOI] [PubMed] [Google Scholar]
- 61. Cherfils J., Chabre M. (2003) Trends Biochem. Sci. 28, 13–17 [DOI] [PubMed] [Google Scholar]
- 62. Palczewski K. (1997) Eur. J. Biochem. 248, 261–269 [DOI] [PubMed] [Google Scholar]
- 63. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kenakin T., Miller L. J. (2010) Pharmacol. Rev. 62, 265–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Reiner S., Ambrosio M., Hoffmann C., Lohse M. J. (2010) J. Biol. Chem. 285, 36188–36198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wall M. A., Coleman D. E., Lee E., Iñiguez-Lluhi J. A., Posner B. A., Gilman A. G., Sprang S. R. (1995) Cell 83, 1047–1058 [DOI] [PubMed] [Google Scholar]
- 67. Hanson S. M., Francis D. J., Vishnivetskiy S. A., Kolobova E. A., Hubbell W. L., Klug C. S., Gurevich V. V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 4900–4905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirsch J. A., Schubert C., Gurevich V. V., Sigler P. B. (1999) Cell 97, 257–269 [DOI] [PubMed] [Google Scholar]
- 69. Li J., Edwards P. C., Burghammer M., Villa C., Schertler G. F. (2004) J. Mol. Biol. 343, 1409–1438 [DOI] [PubMed] [Google Scholar]
- 70. Scheerer P., Heck M., Goede A., Park J. H., Choe H. W., Ernst O. P., Hofmann K. P., Hildebrand P. W. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 10660–10665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Salom D., Lodowski D. T., Stenkamp R. E., Le Trong I., Golczak M., Jastrzebska B., Harris T., Ballesteros J. A., Palczewski K. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16123–16128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rondard P., Iiri T., Srinivasan S., Meng E., Fujita T., Bourne H. R. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6150–6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.