Abstract
We recently showed that Nox4 NADPH oxidase is highly expressed in pancreatic ductal adenocarcinoma and that it is activated by growth factors and plays a pro-survival, anti-apoptotic role. Here we investigate the mechanisms through which insulin-like growth factor I and serum (FBS) activate NADPH oxidase in pancreatic cancer (PaCa) cells. We show that in PaCa cells, NADPH oxidase is composed of Nox4 and p22phox catalytic subunits, which are both required for NADPH oxidase activity. Insulin-like growth factor I and FBS activate NADPH oxidase through transcriptional up-regulation of p22phox. This involves activation of the transcription factor NF-κB mediated by Akt kinase. Up-regulation of p22phox by the growth factors results in increased Nox4-p22phox complex formation and activation of NADPH oxidase. This mechanism is different from that for receptor-induced activation of phagocytic NADPH oxidase, which is mediated by phosphorylation of its regulatory subunits. Up-regulation of p22phox represents a novel pro-survival mechanism through which growth factors and Akt inhibit apoptosis in PaCa cells.
Keywords: Akt PKB, Apoptosis, NF-kB Transcription Factor, Oxidase, Pancreas
Introduction
NADPH oxidases (1–3) are a major intracellular source of ROS.2 The phagocytic NADPH oxidase mediates oxidative burst, which kills the invading microorganisms. In non-phagocytic cells, NADPH oxidases play an important role in the regulation of various physiologic and patho-physiologic processes including proliferation, differentiation, and death.
Both phagocytic and non-phagocytic NADPH oxidases involve proteins of the NOX family. The phagocytic NADPH oxidase (1) is composed of the Nox2 (gp91phox) and p22phox catalytic membrane-associated subunits and several regulatory cytosolic subunits (2–4). Non-phagocytic NADPH oxidases also require both a Nox isoform and p22phox to generate ROS. However, the non-phagocytic and phagocytic NADPH oxidases employ different regulatory subunits; furthermore, some non-phagocytic NADPH oxidases do not require cytosolic regulatory subunits for their activity (5–7).
Activation of the phagocytic NADPH oxidase occurs through assembly of a multicomponent complex comprised of the membrane-bound Nox2-p22phox subunits and the cytosolic subunits. Receptor-induced phosphorylation of the regulatory p47phox and p67phox subunits causes their recruitment from cytosol, resulting in conformational changes in the membrane-bound Nox2-p22phox complex and oxidase activation. In phagocytes, the Akt kinase mediates p47phox phosphorylation and thus NADPH oxidase activation (1, 8). The mechanisms of non-phagocytic NADPH oxidase activation remain poorly defined. Differently from the phagocytic NADPH oxidase, non-phagocytic oxidase activation may not involve the regulatory subunits. For example, various receptors including IGF-I (9), insulin, TGFβ, TNFα, and TLR4 all activate Nox4 NADPH oxidase without engaging regulatory subunits (10–13).
We recently showed that Nox4 NADPH oxidase is highly expressed in pancreatic ductal adenocarcinoma (14, 15), and further, that it plays an important pro-survival role in pancreatic cancer (9, 14, 15). Growth factors (GFs) activate NADPH oxidase in PaCa cells, and the resulting ROS promote sustained activation of pro-survival kinases, such as JAK, thus suppressing apoptosis (15). Therefore, activation of NADPH oxidase is an important mechanism through which GFs protect PaCa cells from death. Of note, Nox4 NADPH oxidase was recently shown to promote endothelial tumor growth in mice (16).
The present study describes a novel mechanism whereby growth factors (in particular IGF-I) activate NADPH oxidase in PaCa cells, namely through transcriptional up-regulation of p22phox. We show that GFs stimulate Akt, which mediates activation of the transcription factor NF-κB to up-regulate p22phox expression. GF-induced p22phox up-regulation results in increased NADPH oxidase activity, leading to inhibition of apoptosis in PaCa cells.
MATERIALS AND METHODS
Reagents
Antibodies against Akt1, Nox4, and p22phox were from Santa Cruz Biotechnology (Santa Cruz, CA); antibody against Akt1 phosphorylated at Ser-473 was from Cell Signaling (Beverly, MA). Human IGF-I was from R&D Systems (Minneapolis, MN); Akt inhibitor VIII (isozyme selective Akti-1/2) was from Calbiochem. All other antibodies and chemicals were from Sigma-Aldrich.
Cell Culture
Human pancreatic ductal adenocarcinoma cells, the poorly differentiated MIA PaCa-2 and moderately differentiated PANC-1 cell lines, were obtained from the American Type Culture Collection (Manassas, VA). Cells were grown in 1/1 DMEM/F-12 medium (Invitrogen) supplemented with 15% FBS, 4 mm l-glutamine, and antibiotic/antimycotic solution (Omega Scientific, Tarzana, CA). Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO2 and used between passages 4 and 12. For the experiments, MIA PaCa-2 and PANC-1 cells were cultured for up to 48 h without and with 100 ng/ml IGF-I or 15% FBS.
Transfections
Transient transfections of MIA PaCa-2 and PANC-1 cells were performed using the electroporation Amaxa NucleofectorTM system (Amaxa Inc., Gaithersburg, MD) according to the manufacturer's protocol. pcDNA3-p22phox (wild type) was a kind gift from Dr. Tsukasa Kawahara (Emory University School of Medicine) (17). In knockdown experiments, we used p22phox siRNA (pool of three target-specific siRNAs, Santa Cruz Biotechnology); Nox4 siRNA (5′-AAACCGGCAGAGUUUACCCAG-3′), Nox3 and Nox5 siRNAs from MWG Biotechnology (High Point, NC); or Akt-1 siRNA (SmartPool, Ambion, Foster City, CA), each at 200 nm. For control transfections, we applied the Silencer negative control siRNA #1 (Ambion).
Western Blot Analysis
Cells were lysed in radioimmune precipitation phosphorylation buffer (50 mm NaCl, 50 mm Tris/HCl, pH 7.2, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 10 mm Na2HPO4 + NaH2PO4, 100 mm NaF, 2 mm Na3VO4, 80 μm glycerophosphate, 20% glycerol, 1 mm phenylmethylsulfonyl fluoride (PMSF), 5 μg/ml each of pepstatin, leupeptin, chymostatin, antipain, and aprotinin), sonicated, and centrifuged for 15 min at 16,000 × g at 4 °C. Proteins were separated by SDS-PAGE and electrophoretically transferred to nitrocellulose or PVDF membranes. Nonspecific binding was blocked for 1 h with 5% bovine serum albumin or nonfat dry milk in Tris-buffered saline (4 mm Tris base, 100 mm NaCl, pH 7.5) containing 0.05% Tween 20. Membranes were incubated with primary antibody for 2 h and then for 1 h with peroxidase-conjugated secondary antibody. Blots were developed using the SuperSignal chemiluminescent substrate (Pierce).
Immunoprecipitation
Cells were collected, washed twice in a buffer containing 20 mm Tris (pH 7.5) and 10 mm DTT, and then resuspended in a lysis buffer (50 mm Tris-HCl, 150 mm NaCl, 2 mm EGTA, 10 μg/ml each of leupeptin and aprotinin, 1 mm PMSF, 1% IGEPAL CA-630), and sonicated for 30 s. The lysates were clarified by centrifugation, and 500 μg of protein was subjected to immunoprecipitation with the indicated antibodies at room temperature according to the Catch and Release immunoprecipitation kit (Upstate Biotech Millipore, Temecula, CA). In this and other assays, protein concentration was measured by the Bradford assay (Bio-Rad Laboratories).
Measurement of Apoptosis
Internucleosomal DNA fragmentation was measured by using the Cell Death Detection ELISAPlus kit (Roche Applied Science, Manheim, Germany) according to the manufacturer's instructions (9, 14, 18).
Measurement of Intracellular ROS Production
Intracellular ROS levels were measured by flow cytometry in cells loaded with the redox-sensitive dye DCFH-DA as described previously (9, 14). Approximately 1 × 106 cells were incubated in the dark for 30 min at 37 °C with 10 μm DCFH-DA, harvested, and resuspended in the medium without DCFH-DA. Fluorescence was recorded on the FL-1 channel of a FACScan® (BD Biosciences). The data were analyzed with the Cell Quest program.
Measurement of NADPH Oxidase Activity
Superoxide production was measured in total cell homogenates by using lucigenin-derived chemiluminescence (12, 19), as described (14). Briefly, cells were collected, washed in PBS, and then lysed in 50 μm phosphate buffer containing 1 mm EGTA and 150 mm sucrose and homogenized in a Dounce homogenizer with 100 strokes. Homogenates were centrifuged, and the pellets (membrane fractions) were stored at −80 °C. For superoxide measurements, 50 μg of protein was diluted in 500 μl of the same lysis buffer. Dark-adapted lucigenin (15 μm) was added to the sample, and measurement of chemiluminescence immediately started. Chemiluminescence (in arbitrary units) was measured at 15-s intervals for 1 min in a Turner 20/20 luminometer (Turner Designs, Sunnyvale, CA). NADPH was used as a substrate.
Measurement of NF-κB DNA Binding Activity
Preparation of nuclear protein extracts and the electrophoretic mobility shift assay (EMSA) have been as described previously (20, 21). Briefly, MIA PaCa-2 cells were lysed in a hypotonic buffer, the nuclear protein was extracted and stored at −80 °C, and EMSA was performed as in Refs. 20 and 21.
For the DNA probes, we used the 5′-GCAGAGGGGACTTTCCGAGA-3′ oligonucleotide containing consensus κB binding motif (underlined), as well as several oligonucleotides containing putative NF-κB binding sites in the promoter region of human p22phox gene, in particular, 5′-ACAGAGGGGCTCCCCTCAAA-3′ (located 795 nucleotides upstream of the transcription start codon and hence termed “p22-795”) and 5′-AGCGCAGGAACTCCCCGCCT-3′ (correspondingly, termed “p22-241”). These sites were identified by analyzing the p22phox gene sequence (GenBankTM NG_007291) with the use of the TFSEARCH software (version 1.3). After annealing to their respective complementary oligonucleotides, the probes were end-labeled with T4 polynucleotide kinase.
In the cold competition experiment, a 100× molar excess of nonlabeled wild-type or mutated consensus NF-κB binding probe was added to the reaction together with the 32P-labeled probe. In the mutated oligonucleotide, the consensus κB binding motif was changed (lowercase) to GGccACTaaCC.
Measurement of NF-κB Transcriptional Activity
NF-κB transcriptional activity was assessed with the Dual-Luciferase reporter assay system (Promega, Madison WI). Briefly, MIA PaCa-2 cells were simultaneously co-transfected with the pGL3-4κB-Luc and pRL-TK plasmids by using the NucleofectorTM II system (Amaxa, Inc.) according to the manufacturer's protocol. pGL3-4κB-Luc contains the reporter gene encoding firefly luciferase driven by a promoter region containing four copies of NF-κB-responsive element. pRL-TK is a Renilla luciferase driven by a basic thymidine kinase promoter, thereby playing the role of a reference plasmid. To inhibit NF-κB, cells were transfected with the pcDNA3.1-IκBΔN plasmid expressing the NF-κB inhibitor IκBα (22). This plasmid expresses IκB lacking the N-terminal 36 amino acids and known to be resistant to phosphorylation and degradation.
Statistical Analysis
Results are expressed as means ± S.E. and represent data from at least three independent experiments. Differences between two groups were analyzed using Student's t test. p < 0.05 was considered statistically significant.
RESULTS
Growth Factors Stimulate NADPH Oxidase Activity in PaCa Cells by Up-regulating p22phox
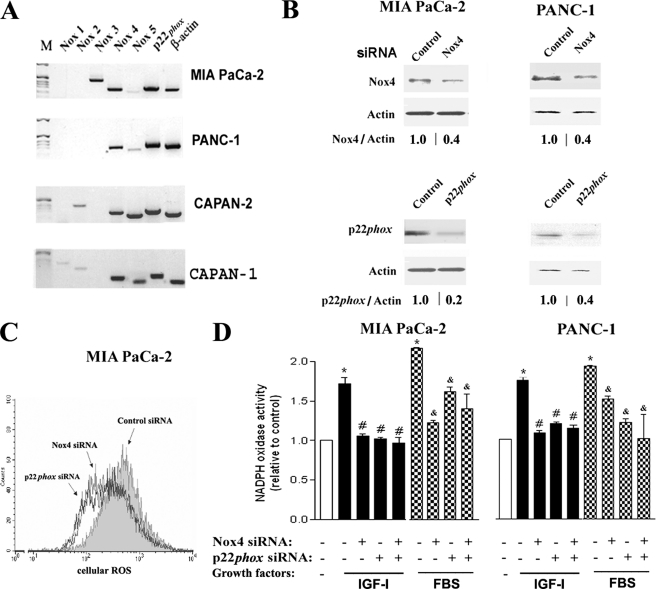
With RT-PCR, we assessed the expression of various catalytic subunits of NADPH oxidase in PaCa cell lines (Fig. 1A). Both Nox4 and p22phox mRNA were highly expressed in all the cell lines tested, whereas the expression of other Nox proteins varied.
FIGURE 1.
p22phox and Nox4 are both required for NADPH oxidase activity in pancreatic cancer cells. A, mRNA expression of NADPH oxidase components in PaCa cell lines was measured by reverse transcription PCR. M, marker. B–D, MIA PaCa-2 and PANC-1 cells were transfected with p22phox, Nox4, or control siRNA and cultured with 15% FBS (C) or as indicated (D) for 48 h. B, protein levels of p22phox and Nox4 were measured by immunoblotting; β-actin served as loading control. The intensities of p22phox and Nox4 bands were quantified by densitometry, and their ratio to β-actin was further normalized to that in control cells (values under the blots). C, changes in intracellular ROS were measured by FACS® analysis in cells labeled with DCFH-DA. The histogram is representative of at least three independent experiments. D, superoxide production was measured by lucigenin-derived chemiluminescence in total cell homogenate. The chemiluminescence values were normalized to those in cells cultured without GFs. Values are means ± S.E. from at least three independent experiments. *, p < 0.05 versus cells cultured without growth factors; #, p < 0.05 versus control cells cultured with IGF-I; &, p < 0.05 versus control cells cultured with FBS.
To determine the involvement of p22phox and Nox4 in ROS production, MIA PaCa-2 and PANC-1 cells were transfected with Nox4 or p22phox siRNA (Fig. 1B). Knocking down of either Nox4 or p22phox decreased ROS levels in cells cultured with FBS (Fig. 1C), indicating that both of these catalytic subunits are required for ROS generation.
NADPH oxidase in PaCa cells is activated by growth factors, IGF-I and FBS (9). Transfection with p22phox or Nox4 siRNA prevented this activation in both MIA PaCa-2 and PANC-1 cells (Fig. 1D). Knocking down p22phox and Nox4 together decreased NADPH oxidase activity to the same level as the knockdown of either Nox4 or p22phox alone (Fig. 1D), suggesting that both subunits are equally required for NADPH oxidase functional activity in PaCa cells.
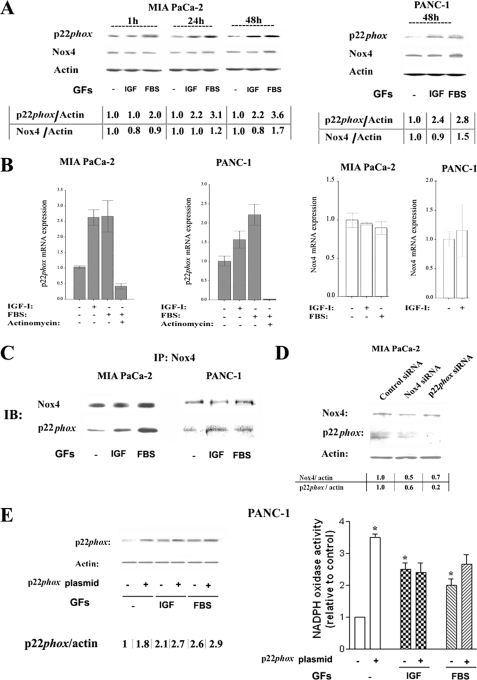
We next asked whether the up-regulation of Nox4 and/or p22phox could mediate the induction of NADPH oxidase activity by growth factors. Both IGF-I and FBS increased protein levels of p22phox in MIA PaCa-2 and PANC-1 cells at 24 and 48 h of incubation (Fig. 2A). IGF-I did not affect Nox4 protein level, whereas FBS moderately increased it at later times (48 h) (Fig. 2A). Similar effects were observed in PANC-1 cells (Fig. 2A).
FIGURE 2.
Growth factors up-regulate p22phox transcription and stimulate complex formation between p22phox and Nox4. MIA PaCa-2 and PANC-1 cells were cultured for 48 h or for indicated times in the absence and presence of IGF-I (100 ng/ml), FBS (15%), or the transcription inhibitor actinomycin D (10 μm). A, D, and E, protein levels of p22phox and Nox4 were measured by immunoblotting; β-actin served as loading control. The intensities of p22phox and Nox4 bands were quantified by densitometry, and their ratio to β-actin was further normalized to that in control cells (values under the blots). B, p22phox and Nox4 mRNA levels were measured using real time PCR and normalized to those in control cells (i.e. without growth factors and inhibitor). Values are means ± S.E. (n = 3). C, cell lysates were immunoprecipitated using Nox4 antibody and probed with p22phox and Nox4 antibodies. IP, immunoprecipitation; IB, immunoblotting. D, MIA PaCa-2 cells were transfected with Nox4, p22phox, or control siRNA and then cultured for 48 h in the presence of 15% FBS. Data in C and D are representative of three independent experiments. E, PANC-1 cells were transfected with 3 μg of pcDNA3-p22phoxWT plasmid or pcDNA empty vector and then cultured for 48 h in the presence of 100 ng/ml IGF-I or 15% FBS. Superoxide production was measured by lucigenin-derived chemiluminescence in total cell homogenate. The chemiluminescence values were normalized to those in cells cultured without GFs. Values are means ± S.E. (n = 3). *, p < 0.05 versus cells transfected with control vector and cultured without growth factors.
Further, IGF-I and FBS significantly increased p22phox mRNA levels in both MIA PaCa-2 and PANC-1 cells (Fig. 2B). These increases in mRNA were completely abolished in the presence of actinomycin, indicating that the GFs up-regulate the protein level of p22phox through stimulating its transcription. Neither IGF-I nor FBS up-regulated Nox4 mRNA level (Fig. 2B).
We determined (Fig. 2C) that Nox4 and p22phox co-immunoprecipitate and that both IGF-I and FBS markedly increase the amount of p22phox co-immunoprecipitated with Nox4. Complex formation between Nox proteins and p22phox is known to increase the stability of both proteins (4, 23, 24). This provides an explanation for our finding (Fig. 2D) that Nox4 siRNA reduced the protein level of not only Nox4 but also p22phox in MIA PaCa-2 cells. Similarly, p22phox siRNA decreased somewhat the level of Nox4 (Fig. 2D). Of note, neither Nox4 nor p22phox siRNAs had any effect on the levels of other Nox proteins tested, i.e. Nox3 and Nox5 (data not shown).
These results indicate that activation of NADPH oxidase by growth factors in PaCa cells is mediated by transcriptional up-regulation of p22phox. In support of this conclusion, overexpression of p22phox stimulated NADPH oxidase activity (Fig. 2E), mimicking the effect of the growth factors. Further, there was little additional stimulation of NADPH oxidase activity by the GFs in cells overexpressing p22phox (Fig. 2E).
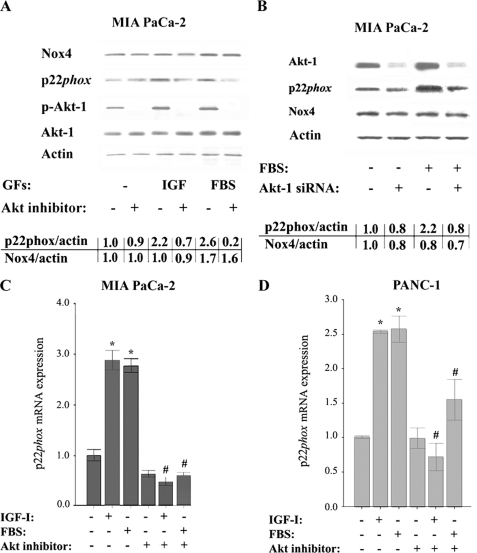
Akt Kinase Mediates the Up-regulation of p22phox by Growth Factors
We next assessed the role of Akt kinase, a key mediator of GF receptor signaling, in the activation of NADPH oxidase in PaCa cells. To inhibit Akt, we applied a specific inhibitor of Akt isoforms 1/2, which greatly decreased the active (phosphorylated) Akt (Fig. 3A), and Akt-1 siRNA, which decreased the total Akt-1 level (Fig. 3B). Both the Akt1/2 inhibitor and the Akt-1 siRNA prevented the up-regulation of p22phox protein induced by IGF-I or FBS (Fig. 3, A and B). Further, the Akt1/2 inhibitor prevented the increases in p22phox mRNA induced by IGF-I or FBS in both MIA PaCa-2 and PANC-1 cells (Fig. 3, C and D).
FIGURE 3.
Inhibition of Akt kinase prevents p22phox up-regulation by growth factors. MIA PaCa-2 and PANC-1 cells were cultured for 48 h in the absence or presence of IGF-I (100 ng/ml), FBS (15%), or Akt inhibitor VIII (50 μm). A and B, protein levels of p22phox, Nox4, phosphorylated (p-Akt-1), and total Akt-1 were measured by immunoblotting; β-actin served as loading control. The intensities of p22phox and Nox4 bands were quantified by densitometry, and their ratio to β-actin was further normalized to that in control cells (values under the blots). Values are means ± S.E. (n = 3). B, cells were transfected with control or Akt-1 siRNA and cultured for 48 h with and without FBS. C and D, p22phox mRNA levels were measured using real time PCR. Values are means ± S.E. (n = 3). *, p < 0.05 versus cells cultured in the absence of the GFs and Akt inhibitor. #, p < 0.05 versus cells cultured in the same conditions but without Akt inhibitor.
The effect of Akt inhibition on p22phox expression depended on whether cells were cultured with or without GFs. That is, Akt inhibition greatly down-regulated p22phox protein and mRNA in cells cultured with the GFs, whereas p22phox down-regulation was minimal in cells cultured without GFs (i.e. in cells with a low level of Akt activity). Of note, the GFs significantly increased active (i.e. phosphorylated) Akt in PaCa cells (Fig. 3, A and B) (15). Neither the Akt1/2 inhibitor nor the Akt-1 siRNA affected Nox4 protein (Fig. 3, A and B) nor mRNA (not illustrated) levels.
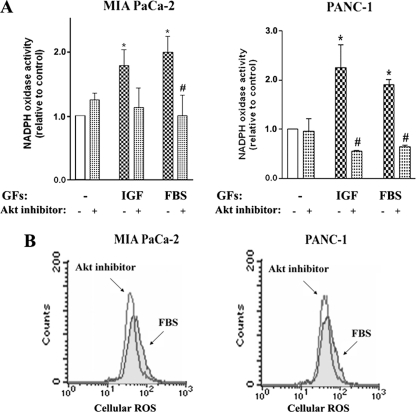
Akt inhibition prevented the activation of NADPH oxidase by IGF-I and FBS (Fig. 4A) and decreased intracellular ROS (Fig. 4B) in both PaCa cell lines. The combined data in Figs. 3 and 4 indicate that Akt mediates GF-induced transcriptional up-regulation of p22phox, resulting in NADPH oxidase activation. In contrast, Nox4 expression is not regulated by Akt. Interestingly, the results suggest that Akt is not involved in the regulation of p22phox expression in cells cultured without growth factors.
FIGURE 4.
Akt inhibition prevents stimulation of NADPH oxidase by growth factors. MIA PaCa-2 and Panc-1 cells were cultured for 48 h in the absence or presence of IGF-I (100 ng/ml), FBS (15%), or the Akti-1/2 inhibitor (50 μm). A, superoxide production was measured by lucigenin-derived chemiluminescence in total cell homogenate. The chemiluminescence values were normalized to those in cells cultured in the absence of the GFs and Akt inhibitor. Values are means ± S.E. (n = 3). *, p < 0.05 versus cells cultured in the absence of the GFs and Akt inhibitor. #, p < 0.05 versus cells cultured in the same conditions but without Akt inhibitor. B, changes in intracellular ROS level were measured by FACS® analysis using the redox-sensitive dye DCFH-DA in cells cultured with FBS. Data are representative of two independent experiments that gave similar results.
NF-κB Mediates the Up-regulation of p22phox Expression Induced by IGF-I and FBS
In search for transcription factors mediating p22phox up-regulation, we tested the involvement of NF-κB because the p22phox gene has putative NF-κB binding sites in its promoter (see below) and because growth factors activate NF-κB in PaCa cells (25). Moreover, it has been recently reported that NF-κB mediates p22phox expression in smooth muscle cells (26).
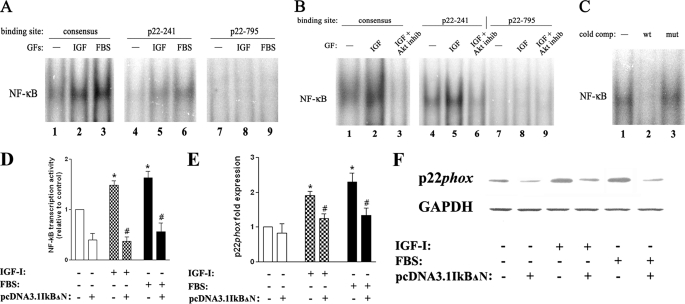
Both IGF-I and FBS stimulated NF-κB DNA binding activity in PaCa cells, measured by EMSA both at 48 h and at shorter incubation times (Fig. 5, A and B). Importantly, the increase in NF-κB activity was not only on a consensus NF-κB binding site but also on DNA sequences containing putative NF-κB binding sites in the human p22phox gene promoter. These sites were identified with the use of the TFSEARCH software (see ”Materials and Methods“). Of note, we detected NF-κB binding on some, but not all, of the tested putative sites in p22phox promoter: for example, the p22-241 site (located 241 nucleotides upstream of the transcription start) but not the p22-795 site (Fig. 5, A and B). The specificity of binding was confirmed in a cold competition experiment (Fig. 5C) in which nuclear protein bound to the p22-241 site was competed away by a 100× excess of unlabeled NF-κB probe, whereas the binding was not decreased with the same excess of mutated NF-κB probe.
FIGURE 5.
NF-κB activation mediates p22phox up-regulation by growth factors. MIA PaCa-2 cells were cultured for 48 h (A, D, E, and F) or 4 h (B and C) in the absence and presence of IGF-I (100 ng/ml), FBS (15%), or the Akti-1/2 inhibitor (inhib, 50 μm). A–C, nuclear protein extracts were prepared from these cells and subjected to EMSA for NF-κB, using a consensus NF-κB oligonucleotide probe and the p22-241 and p22-795 probes corresponding to putative NF-κB binding sites in p22phox gene promoter (see ”Materials and Methods“). In the cold competition (cold comp.) experiment (C), the binding reaction containing nuclear protein from IGF-I-treated cells and the p22-241 probe (lane 1) was repeated in the presence of a 100× molar excess of unlabeled wild-type (wt) or mutated (mut) consensus NF-κB oligonucleotide. Data are representative of two independent experiments that gave similar results. D–F, NF-κB transcriptional activity (D) and p22phox mRNA (E) and protein (F) levels were measured in MIA PaCa-2 cells transfected with pcDNA3.1IkBΔN or empty plasmid. D, NF-κB transcriptional activity was measured in cells co-transfected with pGL3-4kB-Luc and pRL-TK using the Dual-Luciferase reporter assay system according to the manufacturer's protocol. E, p22phox mRNA levels were measured using real time PCR. Values in D and E are means ± S.E. (n = 3). *, p < 0.05 versus cells transfected with empty pcDNA3.1 and cultured without GFs. #, p < 0.05 versus cells transfected with empty pcDNA3.1 and cultured in the same conditions. F, protein levels of p22phox were measured by immunoblotting; GAPDH served as loading control. Data are representative of two independent experiments that gave similar results.
GF-induced NF-κB binding was abolished by the specific Akt1/2 inhibitor (Fig. 5B). Importantly, the Akt1/2 inhibitor blocked NF-κB binding not only to the consensus DNA sequence but also to the p22phox promoter p22-241 binding site (Fig. 5B).
To further confirm that NF-κB mediates p22phox expression, we measured the effect of NF-κB inhibition on p22phox mRNA and protein levels. To inhibit NF-κB, MIA PaCa-2 cells were transfected with pcDNA3.1IκBΔN plasmid encoding for non-degradable IκBα. IκBs are a family of physiologic NF-κB inhibitory proteins that keep NF-κB proteins inactive in the cytosol (27). Upon activation, IκBα is degraded in the proteasome, thus allowing NF-κB translocation to the nucleus. Overexpression of IκBα inhibited NF-κB transcriptional activity in MIA PaCa-2 cells measured with a luciferase reporter assay (Fig. 5D). Importantly, it prevented the up-regulation of p22phox mRNA and protein levels by IGF-I and FBS (Fig. 5, E and F). The results in Fig. 5 strongly indicate that NF-κB mediates the up-regulation of p22phox transcription induced by GFs in PaCa cells.
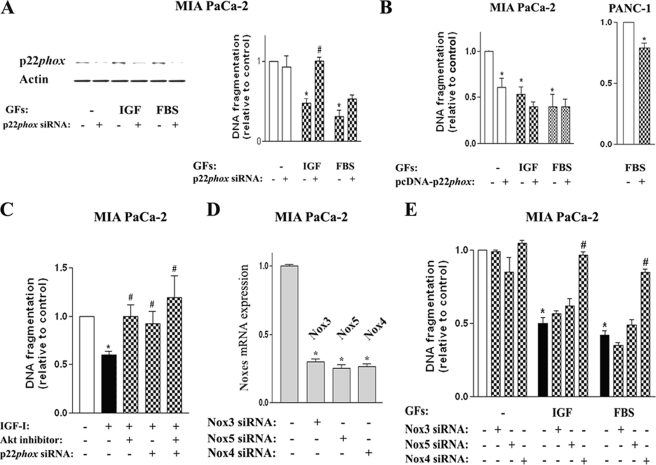
p22phox Mediates the Anti-apoptotic Effect of the Growth Factors
Knocking down p22phox with siRNA markedly increased apoptosis in cells cultured with growth factors (Fig. 6A), but it had little effect on apoptosis in GF-free conditions (i.e. at a relatively low p22phox level) (Fig. 6A). Conversely, p22phox overexpression had little effect on apoptosis in cells cultured with growth factors (i.e. in conditions resulting in a relatively high level of p22phox) but greatly decreased apoptosis in cells cultured in GF-free conditions (Fig. 6B).
FIGURE 6.
p22phox and Nox4 (but not other Nox proteins) mediate the anti-apoptotic effect of growth factors in pancreatic cancer cells. MIA PaCa-2 and PANC-1 cells were cultured for 48 h in the absence and presence of IGF-I (100 ng/ml), FBS (15%), or the Akt1/2 inhibitor (50 μm). Cells were transfected with p22phox or control siRNA (A and C); pcDNA-p22phoxWT or control pcDNA plasmid (B); or Nox3, Nox4, Nox5, or control siRNA (D and E). A, protein levels of p22phox were measured by immunoblotting; β-actin served as loading control. A–C and E, DNA fragmentation was measured using cell death ELISA. D, Nox3, Nox4, and Nox5 mRNA levels were measured using real time PCR. In all panels, values are means ± S.E. (n = 3). *, p < 0.05 versus cells transfected with control siRNA (or control pcDNA plasmid) and cultured without growth factors. #, p < 0.05 versus cells cultured in the same condition without inhibitor or siRNA transfection.
Although siRNA knockdown of p22phox stimulated apoptosis in cells cultured with either IGF-I or FBS, this stimulation was much greater for IGF-I, completely reversing its anti-apoptotic effect (Fig. 6A). This suggests that p22phox-mediated mechanism(s) is largely responsible for the anti-apoptotic action of IGF-I in PaCa cells. In comparison, the effect of p22phox siRNA on apoptosis in cells cultured with FBS was less pronounced (Fig. 6A), indicating that FBS inhibits apoptosis through both p22phox-dependent and p22phox-independent mechanisms.
The anti-apoptotic effect of IGF-I was also abrogated by the Akt inhibitor (Fig. 6C). Interestingly, the stimulation of apoptosis in IGF-I-treated cells by the Akt inhibitor and p22phox siRNA was non-additive (Fig. 6C). That is, Akt inhibition did not further increase apoptosis in cells in which apoptosis had been stimulated by p22phox siRNA transfection (Fig. 6C). These results indicate that p22phox mediates the pro-survival effect of Akt in PaCa cells.
We and others previously showed the anti-apoptotic role for Nox4 in PaCa cells (9, 14, 15, 28). Here, we tested whether Nox proteins other than Nox4 also have an anti-apoptotic effect in PaCa cells. Knockdown of Nox3 or Nox5 with corresponding siRNAs (Fig. 6D) did not reverse the anti-apoptotic effects of IGF-I and FBS in MIA PaCa-2 cells, in contrast to Nox4 siRNA knockdown (Fig. 6E). This indicates that the pro-survival, anti-apoptotic role of Nox proteins is isoform-specific; in PaCa cells, it is mediated by Nox4, but not the other Nox proteins. The combined results in Fig. 6, together with our previous findings (9, 14, 15), indicate that NADPH oxidase composed of Nox4 and p22phox mediates the resistance of PaCa cells to death.
DISCUSSION
One reason why pancreatic cancer (i.e. pancreatic adenocarcinoma) is so aggressive and unresponsive to chemo- and radiotherapy is its resistance to apoptosis. Growth factors play a key role in pancreatic tumorigenesis; in particular, IGF-I is overexpressed in PaCa cells, suppressing apoptosis and stimulating growth both in cell lines and in animal models of pancreatic cancer (29, 30).
We have recently shown (9, 15) that the pro-survival, anti-apoptotic effect of IGF-I and FBS in PaCa cells is mediated by Nox4 NADPH oxidase. The present study investigates the mechanisms through which the GFs activate NADPH oxidase.
Our previous findings demonstrated a critical role of Nox4 in ROS production in PaCa cells (9, 14, 15). The present study shows that both Nox4 and p22phox catalytic subunits are required for NADPH oxidase activity in PaCa cells. In other cell types, Nox4 similarly requires p22phox, but not the cytosolic regulatory subunits, for oxidase activity (5–7). Our results show that growth factors transcriptionally up-regulate p22phox, resulting in increased level of p22phox protein, complex formation between p22phox and Nox4, and oxidase activity.
Of note, little is known on transcriptional regulation of p22phox expression. In phagocytes, p22phox transcription is likely mediated through blood cell-specific transcription factor PU.1 (31), and NF-κB was recently suggested to regulate p22phox transcription in smooth muscle cells (26). The transcription factors that control p22phox expression in epithelial cells remain undefined.
Several lines of evidence indicate that in PaCa cells, NF-κB mediates transcriptional up-regulation of p22phox by the GFs. (i) NF-κB is activated by GFs in PaCa cells. (ii) GFs specifically stimulate NF-κB binding at NF-κB binding sites in the p22phox promoter. (iii) Inhibiting NF-κB prevents the GF-induced up-regulation of p22phox.
Akt kinase is an important effector of GF receptors and is activated by both IGF-I and FBS in MIA PaCa-2 and PANC-1 cells (15). In the present study, we show that Akt mediates GF-induced NF-κB activation in PaCa cells through which the GFs stimulate p22phox expression (Fig. 7). Based on findings in other cell types, the effect of Akt on NF-κB activity is likely through phosphorylation of the p65 NF-κB subunit (32).
FIGURE 7.
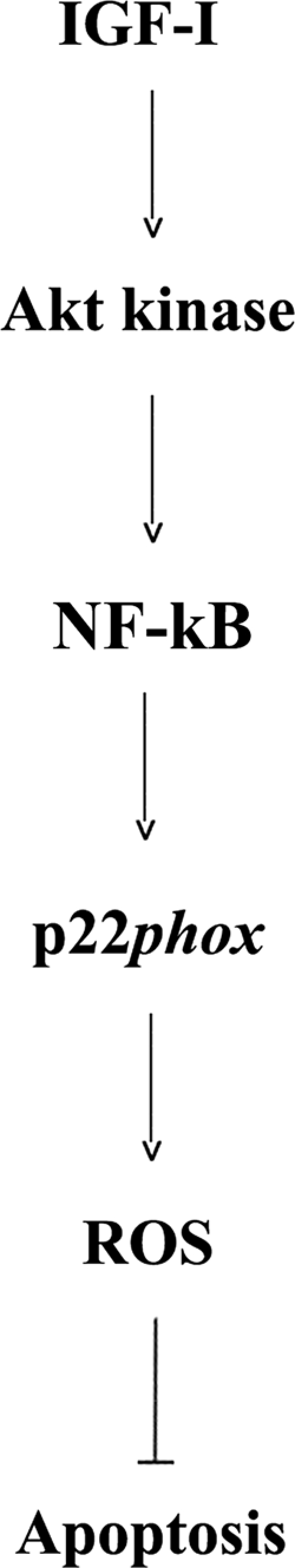
Schematic illustrating the proposed mechanism whereby p22phox mediates the anti-apoptotic effect of growth factors (i.e. IGF-I) in cancer cells.
Our results show that the mechanism of NADPH oxidase activation in PaCa cells is different from that of phagocytic NADPH oxidase. In PaCa cells, the GF-induced activation of NADPH oxidase is through transcriptional up-regulation of p22phox, whereas in phagocytes, NADPH oxidase activation requires receptor-induced phosphorylation of its regulatory subunits. Of note, although Akt plays a critical role in NADPH oxidase activation in both PaCa cells and phagocytes, the underlying mechanisms are quite different. In particular, in phagocytes, Akt-mediated phosphorylation of the p47 regulatory subunit is important for NADPH oxidase activation (1, 8).
As we have shown, Nox4 is abundantly expressed in human pancreatic ductal adenocarcinomas (15) and plays a pro-survival role in PaCa cells (Refs. 9 and 15 and this study). Here, we show that Nox4 and p22phox both contribute to the pro-survival effect of the GFs and that knocking down either of these subunits stimulates apoptosis in PaCa cells.
Our data further indicate that up-regulation of p22phox, leading to NADPH oxidase activation, is critical for the anti-apoptotic effect of growth factors. Up-regulation of p22phox fully mediates the anti-apoptotic effect of IGF-I, and to a lesser extent, that of FBS. The fact that overexpressing p22phox by itself greatly inhibits apoptosis in PaCa cells further demonstrates the anti-apoptotic, pro-survival role of NADPH oxidase and ROS in these cells, supporting our previous findings (9, 14, 15, 18). Moreover, the results show that the pro-survival effect of Akt is mediated, at least in part, through up-regulation of p22phox (Fig. 7).
In sum, the results establish a novel pathway for receptor-mediated activation of Nox4 NADPH oxidase. We find that both Nox4 and p22phox are required for NADPH oxidase activity in PaCa cells. IGF-I and FBS activate NADPH oxidase by up-regulating p22phox expression through activation of NF-κB, which is mediated by Akt. The p22phox-mediated activation of NADPH oxidase, in turn, leads to inhibition of apoptosis and thus promotes pancreatic cancer cell survival.
Acknowledgment
We thank Dr. Tsukasa Kawahara (Emory University School of Medicine) for providing us with the pcDNA3-p22phox plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grants R01CA119025 through the NCI (to A. S. G.) and AT003960 through the NCCAM (to S. J. P.). This study was also supported by the Department of Veterans Affairs Merit Review (to A. S. G.) and the CURE-Digestive Diseases Research Center Pilot and Feasibility Award (to M. E.).
- ROS
- reactive oxygen species
- GF
- growth factor
- IGF-I
- insulin-like growth factor-I
- NOX
- non-phagocytic NADPH oxidase
- PaCa
- pancreatic cancer
- DCFH-DA
- 2′,7′-dichlorofluorescein-diacetate.
REFERENCES
- 1. Babior B. M., Lambeth J. D., Nauseef W. (2002) Arch. Biochem. Biophys. 397, 342–344 [DOI] [PubMed] [Google Scholar]
- 2. Bedard K., Krause K. H. (2007) Physiol. Rev. 87, 245–313 [DOI] [PubMed] [Google Scholar]
- 3. Lambeth J. D. (2007) Free Radic. Biol. Med. 43, 332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. von Löhneysen K., Noack D., Jesaitis A. J., Dinauer M. C., Knaus U. G. (2008) J. Biol. Chem. 283, 35273–35282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martyn K. D., Frederick L. M., von Loehneysen K., Dinauer M. C., Knaus U. G. (2006) Cell. Signal. 18, 69–82 [DOI] [PubMed] [Google Scholar]
- 6. Xu H., Goettsch C., Xia N., Horke S., Morawietz H., Förstermann U., Li H. (2008) Free Radic. Biol. Med. 44, 1656–1667 [DOI] [PubMed] [Google Scholar]
- 7. Govindarajan B., Sligh J. E., Vincent B. J., Li M., Canter J. A., Nickoloff B. J., Rodenburg R. J., Smeitink J. A., Oberley L., Zhang Y., Slingerland J., Arnold R. S., Lambeth J. D., Cohen C., Hilenski L., Griendling K., Martínez-Diez M., Cuezva J. M., Arbiser J. L. (2007) J. Clin. Invest. 117, 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoyal C. R., Gutierrez A., Young B. M., Catz S. D., Lin J. H., Tsichlis P. N., Babior B. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 5130–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vaquero E. C., Edderkaoui M., Pandol S. J., Gukovsky I., Gukovskaya A. S. (2004) J. Biol. Chem. 279, 34643–34654 [DOI] [PubMed] [Google Scholar]
- 10. Ismail S., Sturrock A., Wu P., Cahill B., Norman K., Huecksteadt T., Sanders K., Kennedy T., Hoidal J. (2009) Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L489–L499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maloney E., Sweet I. R., Hockenbery D. M., Pham M., Rizzo N. O., Tateya S., Handa P., Schwartz M. W., Kim F. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1370–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahadev K., Motoshima H., Wu X., Ruddy J. M., Arnold R. S., Cheng G., Lambeth J. D., Goldstein B. J. (2004) Mol. Cell. Biol. 24, 1844–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moe K. T., Aulia S., Jiang F., Chua Y. L., Koh T. H., Wong M. C., Dusting G. J. (2006) J. Cell. Mol. Med. 10, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edderkaoui M., Hong P., Vaquero E. C., Lee J. K., Fischer L., Friess H., Buchler M. W., Lerch M. M., Pandol S. J., Gukovskaya A. S. (2005) Am. J. Physiol. Gastrointest. Liver Physiol. 289, G1137–G1147 [DOI] [PubMed] [Google Scholar]
- 15. Lee J. K., Edderkaoui M., Truong P., Ohno I., Jang K. T., Berti A., Pandol S. J., Gukovskaya A. S. (2007) Gastroenterology 133, 1637–1648 [DOI] [PubMed] [Google Scholar]
- 16. Bhandarkar S. S., Jaconi M., Fried L. E., Bonner M. Y., Lefkove B., Govindarajan B., Perry B. N., Parhar R., Mackelfresh J., Sohn A., Stouffs M., Knaus U., Yancopoulos G., Reiss Y., Benest A. V., Augustin H. G., Arbiser J. L. (2009) J. Clin. Invest. 119, 2359–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kawahara T., Ritsick D., Cheng G., Lambeth J. D. (2005) J. Biol. Chem. 280, 31859–31869 [DOI] [PubMed] [Google Scholar]
- 18. Edderkaoui M., Hong P., Lee J. K., Pandol S. J., Gukovskaya A. S. (2007) J. Biol. Chem. 282, 26646–26655 [DOI] [PubMed] [Google Scholar]
- 19. Maccarrone M., Melino G., Finazzi-Agrò A. (2001) Cell Death Differ. 8, 776–784 [DOI] [PubMed] [Google Scholar]
- 20. Gukovsky I., Cheng J. H., Nam K. J., Lee O. T., Lugea A., Fischer L., Penninger J. M., Pandol S. J., Gukovskaya A. S. (2004) Gastroenterology 126, 554–566 [DOI] [PubMed] [Google Scholar]
- 21. Mouria M., Gukovskaya A. S., Jung Y., Buechler P., Hines O. J., Reber H. A., Pandol S. J. (2002) Int. J. Cancer. 98, 761–769 [DOI] [PubMed] [Google Scholar]
- 22. Brockman J. A., Scherer D. C., McKinsey T. A., Hall S. M., Qi X., Lee W. Y., Ballard D. W. (1995) Mol. Cell. Biol. 15, 2809–2818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ambasta R. K., Kumar P., Griendling K. K., Schmidt H. H., Busse R., Brandes R. P. (2004) J. Biol. Chem. 279, 45935–45941 [DOI] [PubMed] [Google Scholar]
- 24. Dinauer M. C., Pierce E. A., Bruns G. A., Curnutte J. T., Orkin S. H. (1990) J. Clin. Invest. 86, 1729–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Niu J., Chang Z., Peng B., Xia Q., Lu W., Huang P., Tsao M. S., Chiao P. J. (2007) J. Biol. Chem. 282, 6001–6011 [DOI] [PubMed] [Google Scholar]
- 26. Manea A., Manea S. A., Gafencu A. V., Raicu M. (2007) Arch. Physiol. Biochem. 113, 163–172 [DOI] [PubMed] [Google Scholar]
- 27. Oeckinghaus A., Ghosh S. (2009) Cold Spring Harb. Perspect. Biol. 1, a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mochizuki T., Furuta S., Mitsushita J., Shang W. H., Ito M., Yokoo Y., Yamaura M., Ishizone S., Nakayama J., Konagai A., Hirose K., Kiyosawa K., Kamata T. (2006) Oncogene 25, 3699–3707 [DOI] [PubMed] [Google Scholar]
- 29. Korc M. (1998) Surg. Oncol. Clin. N. Am. 7, 25–41 [PubMed] [Google Scholar]
- 30. Min Y., Adachi Y., Yamamoto H., Ito H., Itoh F., Lee C. T., Nadaf S., Carbone D. P., Imai K. (2003) Cancer Res. 63, 6432–6441 [PubMed] [Google Scholar]
- 31. Dusi S., Donini M., Lissandrini D., Mazzi P., Bianca V. D., Rossi F. (2001) Eur. J. Immunol. 31, 929–938 [DOI] [PubMed] [Google Scholar]
- 32. Madrid L. V., Mayo M. W., Reuther J. Y., Baldwin A. S., Jr. (2001) J. Biol. Chem. 276, 18934–18940 [DOI] [PubMed] [Google Scholar]