Abstract
Mycoplasma pneumoniae is a human pathogen causing respiratory infections that are also associated with serious exacerbations of chronic lung diseases. Membranes and lipoproteins from M. pneumoniae induced a 4-fold increase in arachidonic acid (AA) release from RAW264.7 and a 2-fold increase in AA release from primary human alveolar macrophages. The bacterial lipoprotein mimic and TLR2/1 agonist Pam3Cys and the TLR2/6 agonist MALP-2 produced effects similar to those elicited by M. pneumoniae in macrophages by inducing the phosphorylation of p38MAPK and p44/42ERK1/2 MAP kinases and cyclooxygenase-2 (COX-2) expression. M. pneumoniae induced the generation of prostaglandins PGD2 and PGE2 from RAW264.7 cells and thromboxane B2 (TXB2) from human alveolar macrophages. Anti-TLR2 antibody completely abolished M. pneumoniae-induced AA release and TNFα secretion from RAW264.7 cells and human alveolar macrophages. Disruption of the phosphorylation of p44/42ERK1/2 or inactivation of cytosolic phospholipase A2α (cPLA2α) completely inhibited M. pneumoniae-induced AA release from macrophages. The minor pulmonary surfactant phospholipid, palmitoyl-oleoyl-phosphatidylglycerol (POPG), antagonized the proinflammatory actions of M. pneumoniae, Pam3Cys, and MALP-2 by reducing the production of AA metabolites from macrophages. The effect of POPG was specific, insofar as saturated PG, and saturated and unsaturated phosphatidylcholines did not have significant effect on M. pneumoniae-induced AA release. Collectively, these data demonstrate that M. pneumoniae stimulates the production of eicosanoids from macrophages through TLR2, and POPG suppresses this pathogen-induced response.
Keywords: Cyclooxygenase (COX) Pathway, Inflammation, MAP Kinases (MAPKs), Prostaglandins, Toll-like Receptors (TLR), Thromboxane
Introduction
Mycoplasmas are the smallest free-living organisms, belonging to the phylogenetic class of Mollicutes. Mycoplasmas lack a cell wall, and as compared with eubacteria, have an unusually small genome and a unique genetic code (1). Mycoplasma pneumoniae is an important agent of human diseases, producing pharyngitis, bronchiolitis, bronchitis, and community-acquired pneumonia, as well as extrapulmonary disorders such as encephalitis, encephalomyelitis, and hemolysis (2–6). Although M. pneumoniae is a well established cause of asthma exacerbations, more recent data have suggested that it may also contribute to the pathogenesis of asthma (7–12). The lipoprotein constituents of M. pneumoniae membrane play a critical role in immune recognition by the host and induction of the inflammatory response (13, 14). The membrane components are recognized mainly by the Toll-like receptors, TLR1,2 TLR2, and TLR6, that initiate downstream signaling events including activation of NF-κB and the mitogen-activated protein kinases (MAPKs) (15).
Eicosanoids have important regulatory roles in human inflammatory diseases and modulate innate immunity in response to microbial infections (19, 32). Eicosanoids are generated in a multistep process that begins with the release of arachidonic acid (AA) from membrane phospholipids by the catalytic action of cytosolic phospholipase A2α (cPLA2α) (16, 17). AA can be metabolized by cyclooxygenase (COX) and cell-specific enzymes to generate five primary prostanoids, PGD2, PGE2, PGF2a, PGI2, and thromboxane A2 (TXA2). AA is also metabolized by 5-lipoxygenase to generate leukotrienes. Prostaglandins exert proinflammatory effects by increasing vascular permeability but also exert immunosuppressive effects (18). Leukotrienes induce increased vascular permeability and recruitment of granulocytes (19, 20). Prostanoids can act as either bronchodilators or bronchoconstrictors by binding to a family of G-protein-coupled prostanoid receptors (21). Previous studies demonstrated that COX-2 expression and PGE2 production were enhanced in asthmatics with sputum eosinophilia (22). TXA2 is known to have a role in the pathogenesis of asthma as a consequence of its potent bronchoconstrictive activity (23), induced through an M3 muscarinic acetylcholine receptor-dependent mechanism (24).
Phospholipids are the major components of pulmonary surfactant, accounting for 90% of its composition by weight. The most abundant phospholipid class in pulmonary surfactant is phosphatidylcholine, mainly in the form of dipalmitoyl phosphatidylcholine (DPPC), and the second most abundant surfactant lipid class is phosphatidylglycerol (PG), with palmitoyl-oleoyl phosphatidylglycerol (POPG) as the dominant molecular species (25). Surfactant lipids maintain the low surface tension required to prevent alveolar collapse during expiration (26). In addition, surfactant lipids also prevent inflammatory fibrosis by suppressing migration of macrophages (27). It has been reported that the major surfactant lipid, DPPC, modulates the inflammatory functions of human monocytic cells (28). PG from Treponema inhibited pathogen-associated molecular pattern-induced immune responses in mouse peritoneal macrophages and alveolar macrophages. In addition, PG reduced the proinflammatory cytokine production in serum of lipopolysaccharide (LPS)-injected mice and decreased abscess formation in Treponema-infected mice (29). More recently, POPG has been shown to inhibit activation of MAP kinases and NF-κB by binding to CD-14 and MD-2 and blocking CD-14 interactions with LPS and MD-2 interactions with TLR4 (30). Additional studies also demonstrate that POPG disrupts LPS-binding protein interactions with LPS (31). In addition to disrupting TLR4 signaling processes, recent findings suggest that POPG also interferes with TLR2 and TLR3 signaling in BEAS2B cells.3 A recent in vivo study from our laboratory demonstrates that POPG has the ability to inhibit the propagation and pro-inflammatory signaling of respiratory syncytial virus in mice (33). There is now growing evidence that identifies PG within pulmonary surfactant as an important regulator of innate immunity and inflammation (30, 32, 33).
The mechanism by which M. pneumoniae causes asthma exacerbation is not well understood, but one plausible pathway is via the production of eicosanoids (prostaglandins and leukotrienes) from host cells. In this study, we show that membrane components of M. pneumoniae and live M. pneumoniae stimulate the release of eicosanoids from macrophages. We sought to characterize the eicosanoids elicited by M. pneumoniae via TLR2 receptors and quantify the role of POPG as an antagonist of this process. Our findings demonstrate that M. pneumoniae and its membrane components elicit a strong eicosanoid response from macrophages that is abrogated by the anionic surfactant phospholipid, POPG.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
The cPLA2α inhibitor (N-([2S,4R]-4-(biphenyl-2-ylmethyl-isobutyl-amino)-1-[2-(2,4-difluorobenzoyl)-benzoyl]-pyrrolidin-2-ylmethyl)-3-[4-(2,4-dioxothiazolidin-5-ylidenemethyl)-phenyl]acrylamide,HCl), p38MAPK inhibitor, SB203580, and MEK1 inhibitor, U0126, were purchased from Calbiochem. LPS from Escherichia coli serotype 0111:B4 was purchased from Sigma. Pam3Cys and MALP-2 were obtained from Alexis Biochemicals. The neutralizing antibody against mouse and human TLR2 was obtained from eBioscience. Polyclonal antibodies against phospho-p38MAPK, p38MAPK, phospho-p44/42ERK1/2, p44/42ERK1/2, phospho-Ser505cPLA2α, cPLA2α, and mouse β-actin were purchased from Cell Signaling Technology. Polyclonal antibody to murine COX-2 was purchased from Cayman Biochemicals, Ann Arbor, MI. PhosphoSafe buffer for cell lysis was purchased from Novagen. [5,6,8,9,11,12,14,15-3H]AA (specific activity 100 Ci/mmol) was from PerkinElmer Life Sciences. Human serum albumin was obtained from Sigma. Mouse and human TNFα CytoSetTM ELISA kits were obtained from BIOSOURCE, Camarillo, CA. Bovine growth serum was obtained from HyClone and heat-inactivated at 56 °C for 30 min. Dulbecco's modified Eagle's medium (DMEM) was purchased from Lonza. Phospholipids were obtained from Avanti Biochemicals.
Cell Culture
The mouse macrophage cell line RAW264.7 was obtained from the ATCC and maintained in DMEM supplemented with 10% bovine growth serum. RAW264.7 cells were grown on 24- or 96-well plates (5 × 105cells/well or 1 × 105 cells/well) and treated as described in legends for Figs. 1, 3, 5, 6, and 8–12. Human alveolar macrophages were isolated from the bronchoalveolar lavage fluid of human volunteers and cultured on 48 well plates (5 × 104cells/well) for arachidonic acid release assays. The procedures used for isolating human macrophages from healthy volunteers were performed using protocols approved by the National Jewish Health Human Subjects Institutional Review Board.
FIGURE 1.
Multiple TLR ligands stimulate AA release from RAW264.7 cells. In A, [3H]AA-labeled cells were stimulated with LPS (20 ng/ml), Pam3Cys (1 μg/ml), M. pneumoniae (Mpn) membrane (0.5 μg/ml), or M. pneumoniae lipoprotein (0.5 μg/ml) for 3 h. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are expressed as the mean ± S.E. value for three independent experiments, each with duplicate samples. The asterisk indicates p < 0.05. In B, [3H]AA-labeled RAW264.7 cells were stimulated with M. pneumoniae membrane (0.5 μg/ml) and M. pneumoniae LPP (0.5 μg/ml), individually, for the indicated times, and the released AA was quantified. In C, [3H]AA-labeled RAW264.7 cells were stimulated with M. pneumoniae membrane and LPP at the indicated concentrations for 45 min. In D, [3H]AA-labeled RAW264.7 cells were stimulated with M. pneumoniae membrane in the presence or absence of 5 mm EGTA for 3 h. Data are expressed as the mean ± S.E. value for three independent experiments, each with duplicate samples. The asterisk indicates p < 0.05. In E, the indicated agonists (unstimulated (Con); LPS; Pam3Cys (Pam); M. pneumoniae membrane (Mem); and LPP were added, and the cells were analyzed for the phosphorylation (P) of p38MAPK and p44/42ERK1/2 after 30 min. The cPLA2α and COX-2 analyses were performed after 4 h. Phosphorylation of p38MAPK, p44/42ERK1/2, and cPLA2α at Ser505 and the expression of COX-2 were determined by probing the cellular extracts with specific antibodies in Western blots. As a loading control, the extracts were probed for total p38MAPK, p44/42ERK1/2, cPLA2α, and β-actin.
FIGURE 3.
U0126 and pyrrolidine significantly block M. pneumoniae membrane- and MALP-2-induced AA release from RAW264.7 cells. [3H]AA-labeled RAW264.7 cells were preincubated with SB203580 (10 μm), U0126 (25 μm), and pyrrolidine (2 μm) for 30 min and then incubated in the presence of M. pneumoniae (Mpn) membrane (A) or MALP2 (B) agonists for 3 h. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are expressed as the mean ± S.E. value from three independent experiments, each performed with duplicate samples. The asterisk indicates p < 0.05. To analyze the phosphorylation (P) of p44/42ERK1/2, RAW264.7 cells were stimulated for 30 min. To measure phosphorylation of cPLA2α and COX-2 expression, the cells were stimulated for 4 h. Phosphorylation of p44/42ERK1/2 and cPLA2α and the expression of COX-2 were determined by probing the cellular extracts with specific antibodies in Western blots. As a loading control, the extracts were probed for total p44/42ERK1/2, total cPLA2α, and β-actin. Quantification of band intensity was performed using the National Institutes of Health ImageJ program.
FIGURE 5.
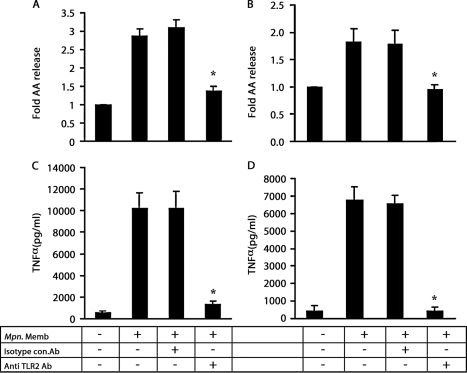
Anti-TLR2 antibody blocks M. pneumoniae membrane-mediated AA release and TNFα secretion from macrophages. [3H]AA-labeled RAW264.7 cells (A) or human alveolar macrophages (B) were preincubated with antibody (50 μg/ml) for 30 min in a total volume of 50 μl. The preincubated macrophages were stimulated with M. pneumoniae membrane (Mpn. Memb) using 0.1 μg/ml for mouse cells and 1.0 μg/ml for human cells. Mouse cells were stimulated for 3 h, and human cells were stimulated for 4 h. The amount of [3H]AA released into the medium is expressed as a -fold increase as compared with the unstimulated cells (A and B). The recovered culture supernatants were used to measure secreted TNFα from RAW264.7 cells (C) or human alveolar macrophages (D). Data are expressed as the mean ± S.E. values for three independent experiments, each with duplicate samples. The asterisks indicate p < 0.05. Isotype con.Ab, isotype control non-immune antibody; anti TLR2 ab, anti-TLR2 antibody.
FIGURE 6.
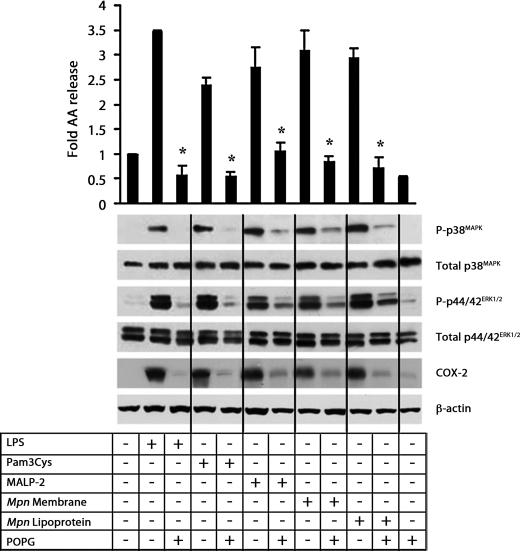
POPG blocks multiple TLR ligand-induced AA release from RAW264.7 cells. [3H]AA-labeled RAW264.7 cells were stimulated with LPS (20 ng/ml), Pam3Cys (1 μg/ml), MALP-2 (0.2 ng/ml), M. pneumoniae (Mpn) membrane (0.2 μg/ml), or M. pneumoniae lipoprotein (0.2 μg/ml) for 3 h in the absence or presence of POPG (200 μg/ml). Cells were preincubated with POPG (200 μg/ml) for 30 min prior to stimulation. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are expressed as the mean ± S.E. values from three independent experiments. The asterisks indicate p < 0.05. To analyze the phosphorylation (P) of p38MAPK and p44/42ERK1/2, cells were stimulated for 30 min. COX-2 expression was measured after 4 h. Phosphorylation of p38MAPK and p44/42ERK1/2 and the expression of COX-2 were determined by probing the cellular extracts with specific antibodies and Western blots. As a loading control, the extracts were probed for total p38MAPK, total p44/42ERK1/2, and β-actin.
FIGURE 8.
POPG inhibition of M. pneumoniae membrane-induced AA release and intracellular signaling from RAW264.7 cells depends upon phospholipid class and species. In A, [3H]AA-labeled RAW264.7 cells were incubated with M. pneumoniae (Mpn) membrane (0.2 μg/ml) in either the absence or the presence of POPG, dipalmitoyl phosphatidylglycerol (DPPG), dioleoyl phosphatidylglycerol (DOPG), dipalmitoyl phosphatidylcholine (DPPC), or palmitoyl-oleoyl phosphatidylcholine (POPC) at concentrations of 200 μg/ml for 3 h. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are presented as means ± S.E. values from four independent experiments, performed with duplicate samples. The asterisks indicate p < 0.05. PL, phospholipid. In B, cells were stimulated for 30 min to analyze p38MAPK and p44/42ERK1/2. Phosphorylation of p38MAPK and p44/42ERK1/2 was determined by Western blots. As a loading control, the extracts were probed for β-actin.
FIGURE 9.
PG derived from pulmonary surfactant suppresses M. pneumoniae membrane-elicited AA release from RAW264.7 cells. Lipids were extracted from alveolar proteinosis fluid recovered by lavage. The B-PC and B-PG classes present in lavage were isolated by thin layer chromatography and liposomes composed of B-PC or B-PG, or defined ratios of B-PC and B-PG present as mixtures within individual liposomes were prepared by sonication. The [3H]AA-labeled RAW264.7 cells were incubated with M. pneumoniae (Mpn) membrane (0.2 μg/ml) in either the absence or the presence of liposomes of the specified composition at concentrations of 200 μg/ml for 3 h. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are presented as means ± S.E. values from three independent experiments, performed with duplicate samples. The asterisks indicate p < 0.05.
FIGURE 10.
POPG blocks AA release and TNFα secretion from RAW264.7 cells induced by live M. pneumoniae. In A, freshly prepared M. pneumoniae (Mpn) was diluted in medium to obtain the desired multiplicity of infection (MOI). [3H]AA-labeled RAW264.7 cells were stimulated with live M. pneumoniae in the presence or absence (NO PLs) of POPG (200 μg/ml) for 3 h. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are expressed as the mean ± S.E. values from three independent experiments, performed with duplicate samples. In B, the recovered culture supernatants were used to measure TNFα secreted from RAW264.7 cells by ELISA. In C, cells were stimulated for 30 min to analyze p38MAPK and p44/42ERK1/2. The time period for inducing COX-2 expression was 4 h. Phosphorylated (P) p38MAPK, p44/42ERK1/2, and COX-2 expression was determined by probing blots of the cellular extracts with specific antibodies. As a loading control, the extracts were probed for total p38MAPK, total p44/42ERK1/2, and β-actin. The asterisks indicate p < 0.05.
FIGURE 11.
POPG blocks macrophage signaling but not cell surface binding of Mycoplasma. In A, freshly prepared M. pneumoniae (Mpn) harboring p30-YFP was diluted in medium to obtain the desired multiplicity of infection (MOI). RAW264.7 cells were stimulated with varying MOI of the bacteria in either the absence or the presence of POPG (200 μg/ml) for 4 h. The cells were washed with ice-cold PBS three times, and sample buffer was added to the cells. In B and C, the amount of cell-bound YFP-M. pneumoniae (B) and the expression of COX-2 (C) were determined by probing the whole extracts with specific antibodies in Western blots. As a loading control, the extracts were probed with β-actin antibody. The intensity of the bands was analyzed using the National Institutes of Health ImageJ program. Data are expressed as the mean ± S.E. values from three independent experiments, and the asterisks indicate p < 0.05. NO PLs, absence of POPG.
FIGURE 12.
M. pneumoniae membrane and LPP stimulate the production of PGD2 and PGE2 from RAW264.7 cells and TXA2/TXB2 from human alveolar macrophages. In A, RAW264.7 cells were incubated with 1 μg/ml AA and stimulated with M. pneumoniae (Mpn) membrane or LPP in the absence or presence of POPG (200 μg/ml) for 6 h. The culture supernatants were collected by centrifugation at 5,000 rpm for 5 min. The released AA metabolites were analyzed by mass spectrometry. Data are expressed as the mean ± S.D. values of two independent experiments performed with duplicate samples; the single asterisks indicate p < 0.05, and the double asterisks indicate p < 0.005. In B, human alveolar macrophages were incubated with 1 μg/ml AA and stimulated with M. pneumoniae membrane in the absence or presence of POPG (200 μg/ml) for 24 h. The supernatant was collected by centrifugation at 5,000 rpm for 5 min. The released AA metabolites were analyzed by mass spectrometry. Data are expressed as the mean ± S.E. values from three independent experiments, performed with duplicate samples, and the asterisks indicate p < 0.05.
Preparation of M. pneumoniae Membrane and LPP
M. pneumoniae (strain FH, ATCC 15531) was grown in polystyrene flasks containing 100 ml of SP-4 medium, at 37 °C for 5 days. Adherent bacteria were harvested by scraping the flask with a rubber spatula and centrifugation at 8,000 × g for 15 min at 4 °C. The pellet was washed with phosphate-buffered saline (PBS; pH 7.4) and centrifuged twice. The bacterial pellet was resuspended in PBS and layered on a discontinuous sucrose gradient (60, 52, 48, and 40%) and centrifuged at 10,000 rpm (SW28) for 30 min at 4 °C. The cells were recovered from 48–52% interface, mixed with PBS, and centrifuged at 8,000 × g for 15 min at 4 °C. The purified cells were either used to challenge macrophages or further processed to produce membranes and lipoproteins. For membrane preparation, the purified M. pneumoniae pellet was resuspended in PBS, mixed with 3 volumes of distilled water, and incubated on ice for 30 min. The cells were lysed completely by probe sonication on ice for a total of 2 min, using 30-s sonication and 30-s cooling cycles. Polymyxin B (100 μg/ml) was added to the lysed M. pneumoniae cells, and the preparation was centrifuged at 100,000 × g for 1 h at 4 °C to sediment membranes. The pellet was washed twice with polymyxin B and centrifuged at 100,000 × g for 1 h at 4 °C. The final M. pneumoniae membrane pellet was resuspended in PBS, homogenized, and used as the bacterial membrane fraction.
For bacterial lipoprotein preparation, M. pneumoniae membrane was solubilized by the addition of 1% Triton X-114 and incubated at 4 °C for 2 h with gentle shaking. Triton X-114-treated M. pneumoniae membrane was centrifuged at 12,000 × g for 5 min at 4 °C, and the supernatant was collected and incubated at 37 °C for 5 min. The resulting cloudy solution was centrifuged at 8,000 × g for 5 min at room temperature to separate the aqueous and detergent phases. The aqueous phase was again incubated with 1% Triton X-114 at 37 °C for 5 min and centrifuged to separate the phases. The last step was repeated once again. The Triton X-114 phases were pooled, and 3 volumes of methanol were added. The preparation was incubated at −80 °C overnight and then centrifuged at 12,000 × g for 5 min at 4 °C. The pellet was dried and suspended in PBS and used as bacterial lipoprotein (LPP).
Western Blot Analysis
RAW264.7 cells were plated at a density of 5 × 105 cells/well in a 24-well plate. After stimulation, macrophages were washed twice with ice-cold PBS and lysed with PhosphoSafe lysis buffer containing Sigma protease inhibitor mixture. After incubation on ice for 15 min, cell lysates were centrifuged at 15,000 rpm for 15 min, and the protein concentration in the supernatant was determined using BCA reagents. Lysates were boiled for 5 min after the addition of Laemmli buffer, and then proteins (10 μg) were resolved on 8–16% gradient Tris-SDS-polyacrylamide gels. The separated proteins were transferred to nitrocellulose membranes and subsequently incubated in blocking buffer containing 20 mm Tris-HCl buffer, pH 7.6, 137 mm NaCl, 0.1% Tween 20, and 5% nonfat milk (TBS) and then incubated overnight at 4 °C with primary antibody. The membranes were washed with TBS containing 0.1% Tween 20 for 30 min, replacing the buffer every 10 min. The membranes were incubated with horseradish peroxidase-linked secondary antibody (1:4,000) in blocking buffer for 1 h at room temperature and washed with buffer for 30 min, replacing the buffer every 10 min. The immunoreactive proteins were detected using coumaric acid and luminol reagents (34).
[3H]AA Release
Mouse macrophages were plated at 2.5 × 105 cells/well (48-well plate) or 1 × 105 cells/well (96-well plate), and primary human alveolar macrophages were plated at 5 × 104 cells/well (48-well plate), and both human and mouse macrophages were incubated for 4 h at 37 °C in a humidified atmosphere of 5% CO2 in air. Macrophages were cultured in supplemented DMEM containing [3H]AA (0.1 μCi/250 μl/well or 0.04 μCi/100 μl/well) for 16–18 h at 37 °C. The cells were washed twice with DMEM-high glucose medium containing 0.1% human albumin to remove unincorporated [3H]AA and then infected with M. pneumoniae or stimulated with TLR ligands, M. pneumoniae membrane, or LPP in medium. The culture medium was removed at the indicated times after stimulation and centrifuged, and the amount of radioactivity in the supernatant was measured by scintillation counting. The cell monolayer was solubilized with 0.1% Triton X-100. The amount of radioactivity released into the culture medium was expressed as the percentage of the total radioactivity incorporated in the cells.
Mass Spectrometry Analysis
An equal volume of methanol containing 2 ng each of internal standards [d4]LTB4 and [d8]5-HETE and 5 ng each of [d4]TXB2, [d4]PGE2,and LTF4 was added to each macrophage sample (500 μl). Samples were then centrifuged at 2,500 rpm for 10 min to precipitate proteins. Supernatants were diluted with water to a final methanol concentration lower than 15% and then extracted using a solid phase extraction cartridge (Strata C18-E, 100 mg/1 ml, Phenomenex, Torrance, CA). The eluate (1 ml methanol) was dried under N2 and solubilized in 40 μl of HPLC solvent A (8.3 mm acetic acid buffered to pH 5.7 with ammonium hydroxide) plus 20 μl of HPLC solvent B (acetonitrile/methanol 65:35, v/v).
A 25-μl aliquot of each sample was injected onto a C18-HPLC column (Columbus 150 × 1 mm, 5 μm, Phenomenex) and eluted at a flow rate of 50 μl/min with a linear gradient from 25 to 100% of HPLC solvent B, which was increased from 25 to 85% at 24 min and then to 100% at 26 min and held at 100% for a further 12 min. The HPLC system was directly interfaced into the electrospray source of a triple quadrupole mass spectrometer (Sciex API 3000, PE-Sciex, Thornhill, Ontario, Canada) where mass spectrometric analysis was performed in the negative ion mode using multiple reaction monitoring of the specific transitions m/z 319 → 115 for 5-HETE, m/z 335 → 195 for LTB4 and Δ6–trans–LTB4s, m/z 335 → 115 for 5,6-diHETEs, m/z 624 → 272 for LTC4, m/z 495 → 177 for LTD4, 438 → 333 for LTE4, 351 → 233 for PGD2, 351 → 271 for PGE2, m/z 353 → 309 for PGF2α, 369 → 169 for TXB2, m/z 319 → 219 for 15-HETE, m/z 319 → 179 for 12-HETE, 327 → 116 for [d8]5-HETE, m/z 339 → 197 for [d4]LTB4, 567 → 171 for LTF4, 373 → 173 for [d4]TXB2, and 355 → 275 for [d4]PGE2. Quantification was performed using standard isotope dilution curves.
Analysis of TNFα Secretion
An aliquot of sample collected for AA release was also used for TNFα measurement. The quantification of secreted TNFα was performed using human or mouse TNFα CytoSetTM ELISA kit from BIOSOURCE according to the manufacturer's protocol.
Statistical Analysis
All results are expressed as mean ± S.E. Data were analyzed in Excel using analysis of variance. The p value for significance was set at 0.05.
RESULTS
M. pneumoniae Membrane and Lipoprotein Induce AA Release from RAW264.7 Cells
We first examined whether M. pneumoniae membrane fractions and LPPs could stimulate cPLA2α activation and eicosanoid production by RAW264.7 mouse macrophages. Activation of cPLA2α was determined by measuring the release [3H]AA from RAW264.7 cells. Analysis of culture supernatants demonstrated a 3–4-fold increase in the release of [3H]AA from cells stimulated with M. pneumoniae constituents (Fig. 1A). The stimulation of AA release by M. pneumoniae membrane and LPP was comparable with that elicited by the TLR4 ligand LPS (35) and the TLR2/1 ligand Pam3Cys.
The release of AA induced by 0.5 μg/ml M. pneumoniae membrane or LPP occurred over a period of 60 min following a 15-min lag (Fig. 1B). The enhancement of AA release occurred at concentrations of M. pneumoniae membrane or LPP as low as 7.8 ng/ml and showed evidence of plateauing at 125 ng/ml (Fig. 1C). cPLA2α plays a major role in the release of AA from membrane phospholipids, and the activity is dependent upon increases in cytosolic Ca2+. When incubated in the presence of 5 mm EGTA, M. pneumoniae-mediated AA release was completely blocked, indicating that calcium is required for the process (Fig. 1D).
M. pneumoniae Membrane and LPP Induce Activation of p38MAPK and p44/42ERK1/2 and COX-2 Expression
To determine whether the MAPKs participate in the regulation of M. pneumoniae-stimulated AA release from RAW264.7 cells, we examined the effect of the bacterial membrane and LPP on the phosphorylation of p38MAPK and p44/42ERK1/2 at 30 min after stimulation. As shown in Fig. 1E, M. pneumoniae membrane and LPP stimulated phosphorylation of both p38MAPK and p44/42ERK1/2 in RAW264.7 cells. The TLR4 ligand, LPS, and the TLR2/1 ligand, Pam3Cys, also stimulated the phosphorylation of both p38MAPK and p44/42ERK1/2. Fig. 1E also demonstrates that the level of phospho-cPLA2α in the stimulated cells was not increased as compared with the unstimulated cells. This latter finding is explained by the presence of constitutively phosphorylated cPLA2α in RAW264.7 cells. However, COX-2 expression was enhanced in macrophages stimulated with either the M. pneumoniae membrane fraction or LPP (Fig. 1E).
M. pneumoniae Membrane and LPP Stimulate AA Release from Primary Human Alveolar Macrophages
Primary human alveolar macrophages were stimulated with M. pneumoniae membrane or LPP for 4 h and analyzed for [3H]AA release. As shown in Fig. 2, human alveolar macrophages, isolated from normal subjects, responded to M. pneumoniae membrane and LPP stimulation with a 2–3-fold increase in AA release as compared with unstimulated cells. Similarly, stimulation of primary human alveolar macrophages with TLR4, TLR2/1, or TLR2/6 ligands resulted in a 2–3-fold increase in AA release (Fig. 2).
FIGURE 2.
M. pneumoniae membrane and TLR agonists induce AA release from human alveolar macrophages. [3H]AA-labeled primary human alveolar macrophages were stimulated with LPS (20 ng/ml, Pam3Cys (1 μg/ml), M. pneumoniae (Mpn) membrane (1 μg/ml), or M. pneumoniae lipoprotein (1 μg/ml) for 4 h. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data are presented as means ± S.E. values from three independent experiments, each prepared with duplicate samples. The asterisk indicates p < 0.05.
Inhibition of the MEK1-ERK1/2 or cPLA2α Activity Blocks M. pneumoniae-induced AA Release
To examine the role of p38MAPK and p44/42ERK1/2 in the regulation of M. pneumoniae- or MALP-2-mediated AA release, RAW264.7 cells were preincubated with the selective p38MAPK inhibitor SB203580 or the MEK-ERK inhibitor U0126 for 30 min followed by stimulation with M. pneumoniae membrane or MALP-2 for 3 h. As shown in Fig. 3, A and B, M. pneumoniae membrane and MALP-2 stimulated AA release by 4- and 2.5-fold, respectively. The p38MAPK inhibitor SB203580 did not alter M. pneumoniae membrane or MALP-2-mediated AA release from RAW264.7 cells. However, the MEK1-ERK inhibitor completely attenuated AA release. To confirm a role for cPLA2α in M. pneumoniae-induced AA release from RAW264.7 cells, the cells were preincubated with the specific cPLA2α inhibitor, pyrrolidine (36), for 30 min followed by stimulation with M. pneumoniae membrane or MALP-2. As shown in Fig. 3, A and B, pyrrolidine completely blocked M. pneumoniae membrane or MALP-2-stimulated AA release from RAW264.7 cells, indicating that cPLA2α is the major, if not the sole, enzyme involved in this response.
To determine the specificity of the inhibitors, the levels of phospho-p44/42, phospho-cPLA2α, and COX-2 expression were analyzed in M. pneumoniae membrane- or MALP-2-stimulated cells. As shown in Fig. 3, A and B, incubation of RAW264.7 cells with M. pneumoniae membrane or MALP-2 induced the phosphorylation of p44/42ERK1/2, and the addition of U0126 completely blocked this phosphorylation. The total amounts of p44/42ERK1/2 were equal among the different conditions. As expected, neither SB203580 nor pyrrolidine affected the level of phospho-p44/42ERK1/2. As further expected, the addition of the MEK1-ERK inhibitor, U0126, significantly reduced the amount of phospho-cPLA2α in M. pneumoniae membrane or MALP-2-stimulated RAW264.7 cells by a factor of 2–3, consistent with previous reports that p44/42ERK1/2 phosphorylate cPLA2α at Ser505 (37). Also shown in Fig. 3, A and B, the expression of COX-2 was enhanced in M. pneumoniae membrane-stimulated RAW264.7, and its expression was not affected by either SB203580 or pyrrolidine. The expression of COX-2 was also increased by MALP-2, and it was not affected by pyrrolidine but moderately inhibited by SB203580. The addition of U0126 reduced COX-2 expression significantly in either the M. pneumoniae membrane-stimulated or the MALP-2-stimulated macrophages by a factor of 2–3. These results indicate that the p44/42ERK1/2 signaling pathway plays a major role in M. pneumoniae membrane- or MALP-2-mediated activation of cPLA2α, the expression of COX-2, and eicosanoid release from RAW264.7 cells.
Using a similar approach, we found that U0126 and pyrrolidine, but not SB203580, suppressed M. pneumoniae membrane-mediated AA release from human alveolar macrophages (Fig. 4) to levels comparable with that of untreated cells. These results demonstrate that M. pneumoniae membrane-mediated induction of eicosanoids is also dependent on p44/42ERK1/2 and cPLA2α in human tissue.
FIGURE 4.
U0126 and pyrrolidine block M. pneumoniae-mediated AA release from human alveolar macrophages. [3H]AA-labeled human alveolar macrophages were preincubated with SB203580 (10 μm), U0126 (25 μm), or pyrrolidine (2 μm) for 30 min. M. pneumoniae (Mpn) membrane was added as the stimulus in the presence or absence of inhibitors for 4 h. The amount of [3H]AA released into the medium is expressed as the -fold increase in release as compared with the unstimulated cells. Data are presented as the mean ± S.E. for three independent experiments, each with duplicate samples. The asterisk indicates p < 0.05.
Anti-TLR2 Antibody Blocks M. pneumoniae Membrane-stimulated AA Release from RAW264.7 Cells and Human Alveolar Macrophages
Previous studies showed that TLR2 is involved in Mycoplasma signaling (15, 38). To examine the role of TLR2 in AA release, RAW264.7 cells were preincubated with an isotype control non-immune antibody or anti-TLR2 antibody for 30 min and stimulated with M. pneumoniae membrane (0.125 μg/ml) for 3 h. The addition of anti-TLR2 antibody completely blocked M. pneumoniae membrane-induced [3H]AA release from RAW 264.7 cells (Fig. 5A), consistent with previous studies (15, 38). The addition of anti-TLR2 antibody also completely inhibited the secretion of TNFα from RAW264.7 cells (Fig. 5C). Isotype control antibody had no effect on either [3H]AA release or TNFα secretion. In additional control experiments, we also determined that the anti-TLR2 antibody had no inhibitory effect upon TNFα secretion by RAW264.7 cells stimulated with LPS.
Anti-TLR2 antibody also completely blocked the [3H]AA release and TNFα secretion from primary human alveolar macrophages elicited by the bacterial membrane (Fig. 5, B and D), whereas the isotype control antibody was without effect. We conclude from these studies that M. pneumoniae membrane-induced AA release is TLR2-dependent.
POPG Inhibits AA Release from RAW264.7 Cells and Human Alveolar Macrophages Stimulated with TLR Ligands
Because M. pneumoniae membrane and LPP were able to stimulate the release of AA metabolites from mouse and human macrophages through activation of TLR2, we examined whether this stimulation could be blocked by POPG. POPG, one of the minor anionic pulmonary surfactant phospholipids, has recently been shown to disrupt TLR4 signaling by direct interactions with CD14 and MD-2. POPG has also been shown to block the inflammatory cytokine response elicited by respiratory syncytial virus. RAW264.7 cells were stimulated with various TLR ligands, including M. pneumoniae membrane and LPP, for 3 h in the presence or absence of 200 μg/ml POPG. This level of POPG is ∼5–10% of the level found in human pulmonary surfactant. As shown in Fig. 6, POPG completely inhibited AA release from RAW264.7 cells stimulated with LPS, Pam3Cys, MALP-2, M. pneumoniae membrane, or LPP.
POPG also markedly inhibited the phosphorylation of p38MAPK and p44/42ERK1/2 and the expression of COX-2 stimulated by the TLR ligands including M. pneumoniae membrane and LPP (Fig. 6). Previous studies have established that treatment of cells with POPG does not induce the expression of MKP-1, which dephosphorylates MAP kinases (30). Additional experiments also demonstrate that POPG does not pleiotropically inhibit all TLR signaling pathways because the lipid does not antagonize TLR5 or TNFα-induced MAP kinase phosphorylation (30).
POPG also completely blocked the [3H]AA release from human alveolar macrophages stimulated with TLR ligands (Fig. 7). We conclude from these studies that POPG acts as a potent inhibitor of [3H]AA release from mouse and primary human alveolar macrophages stimulated by various TLR ligands. This finding implicates POPG as an important regulator of TLR2-dependent inflammatory processes, in addition to its role in regulating TLR4-dependent processes.
FIGURE 7.
POPG inhibits TLR ligand-induced AA release from human alveolar macrophages. [3H]AA-labeled human alveolar macrophages were stimulated with LPS (20 ng/ml), Pam3Cys (1 μg/ml), MALP-2 (1 ng/ml), M. pneumoniae (Mpn) membrane (1 μg/ml), or M. pneumoniae lipoprotein (1 μg/ml) for 4 h in the presence or absence of POPG (200 μg/ml) as indicated. The amount of [3H]AA released into the medium is expressed as a -fold increase in release as compared with the unstimulated cells. Data shown are the mean ± S.E. values from three independent experiments, performed with duplicate samples. The asterisks indicate p < 0.02.
Inhibition of AA Release Is Specific to POPG
To determine whether inhibition by POPG is specific for this surfactant phospholipid, RAW264.7 cells were stimulated with M. pneumoniae membrane in the presence of phospholipids related to POPG but differing in either fatty acid composition or polar head group structure. As shown in Fig. 8 A, M. pneumoniae membrane induced a 3-fold increase in [3H]AA release, and POPG suppressed this response to the level of untreated control cells. PG molecular species containing two palmitic (dipalmitoyl phosphatidylglycerol or two oleic acid (dioleoyl phosphatidylglycerol substituents had a very modest and insignificant reduction in [3H]AA release. The major lung surfactant phospholipid, DPPC (dipalmitoyl phosphatidylcholine), and a PC species related to POPG, palmitoyl-oleoyl phosphatidylcholine (POPC), did not have any significant effect on [3H]AA release from RAW264.7 cells stimulated with M. pneumoniae membrane. POPC also did not have any inhibitory effect on M. pneumoniae membrane-induced phosphorylation of p38MAPK and p44/42ERK1/2 or expression of COX-2 (Fig. 8B). In contrast, POPG blocked phosphorylation of p38MAPK and p44/42ERK1/2 (Fig. 8B).
The PG Pool from Pulmonary Surfactant Suppresses TLR2 Activation
POPG is the most abundant molecular species (∼35% of total) of the PG class present in pulmonary surfactant. We isolated the entire biological PG pool (B-PG) and the entire biological PC pool (B-PC) from human alveolar proteinosis fluid and tested the suppression of TLR2 activation by these lipids. The data presented in Fig. 9 show activation of RAW 264.7 cells by M. pneumoniae membrane and its antagonism by surfactant lipids, individually and in combination. Surfactant PC was ineffective as an inhibitor of AA release induced by M. pneumoniae membrane. In contrast, surfactant PG, at ∼6% of the concentration present in the alveolar hypophase (i.e. 200 μg/ml), completely suppressed the induced AA release. Mixtures of B-PC and B-PG did not suppress TLR2 activation until the PG content reached 75% of the total lipid. These findings are similar to those previously reported for TLR4/CD14/MD2 antagonism, which indicate that segregated populations of PG, rather than random mixtures of PG with surfactant lipids, are the most effective TLR antagonists (30).
POPG Blocks AA Release from RAW264.7 Cells Challenged with Live M. pneumoniae
RAW264.7 macrophages were incubated with freshly isolated bacteria at different multiplicities of infection (MOI) in the presence or absence of POPG for 3 h. As shown in Fig. 10A, M. pneumoniae induced the release of AA from RAW264.7 cells in an inoculum-dependent response. At a multiplicity of infection of 10, M. pneumoniae induced a 4-fold increase in [3H]AA release as compared with the uninfected cells. Similarly, a dose-dependent increase in TNFα secretion was observed with M. pneumoniae challenge of macrophages (Fig. 10B). POPG strongly inhibited both AA release and TNFα secretion at multiplicities of infection ranging from 0.04 to 2.5. At a multiplicity of infection of 10 M. pneumoniae, the blocking effect of POPG was incomplete, suggesting that the lipid competitively antagonizes the bacterial activation of the effector cells.
As shown in Fig. 10C, infection of RAW264.7 cultures with M. pneumoniae stimulated phosphorylation of p38MAPK and p44/42ERK1/2 in a dose-dependent manner. Activation of these MAPKs at bacterial multiplicities of infection of 1 and 10 was strongly inhibited by POPG. The expression of COX-2 was increased by live M. pneumoniae infection in an inoculum-dependent manner, and this response was likewise suppressed to near basal levels by POPG (Fig. 10C).
POPG Does Not Affect the Binding of M. pneumoniae to the Cell Surface
To determine whether POPG acts by preventing the binding of M. pneumoniae to macrophages, we used p30-YFP-tagged M. pneumoniae for binding assays. RAW264.7 cells were incubated with different doses of YFP-tagged M. pneumoniae in the presence or absence of POPG. Cell-bound YFP-M. pneumoniae was analyzed by Western blot analysis using YFP antibody. As shown in Fig. 11A, there is a dose-dependent increase in the binding of YFP-M. pneumoniae to RAW264.7 cells. The addition of POPG under these conditions did not affect the intensity of the bands, indicating that the amount of YFP-M. pneumoniae bound to the cells was unaffected by POPG. The YFP-M. pneumoniae binding curves were superimposable in either the absence or the presence of POPG (Fig. 11B), clearly demonstrating that the lipid did not block the attachment interaction between the bacteria and the cell surface. Analysis of COX-2 expression showed that there is a dose-dependent increase in COX-2 protein level in YFP-M. pneumoniae-treated macrophages, and the addition of POPG almost completely reversed the COX-2 level to that of unchallenged macrophages. There was a 20-fold increase in COX-2 protein level in macrophages infected at a multiplicity of infection of 5 with YFP-M. pneumoniae, and this increase was inhibited 90% by treatment with POPG (Fig. 11C). The results of this experiment demonstrate that POPG does not block the binding of M. pneumoniae to RAW264.7, but it effectively blocks M. pneumoniae-mediated signaling through TLR2.
M. pneumoniae Membrane and LPP Stimulate the Production of PGD2 and PGE2 from RAW264.7 Cells and TXA2 from Human Alveolar Macrophages
To identify the AA metabolites released from RAW264.7 cells stimulated with Mycoplasma components, the culture supernatants were analyzed by mass spectrometry. As shown in Fig. 12A, M. pneumoniae membrane or LPP induced the secretion of PGD2 and PGE2, which was completely inhibited by POPG.
The eicosanoids secreted from primary human alveolar macrophages stimulated with M. pneumoniae membrane for 24 h were also analyzed by mass spectrometry. As shown in Fig. 12B, primary human alveolar macrophages secrete 3-fold higher TXA2/TXB2 in response to the M. pneumoniae membrane as compared with the unstimulated macrophages. TXA2 undergoes spontaneous conversion to TXB2 in aqueous environments with a t½ of seconds, so the identification is reported as the combined TXA2/TXB2. M. pneumoniae membrane stimulated the production of PGD2 and PGE2 from the human alveolar macrophages, but this response was much weaker than observed for mouse cells (data not shown). The stimulated synthesis and secretion of TXA2/TXB2 from primary human cells was completely suppressed by the addition of POPG. These results suggest that POPG present within the airways and alveoli of the lung may play an important role in regulating eicosanoid levels under basal and TLR agonist-induced conditions.
DISCUSSION
In this study, we demonstrate that M. pneumoniae membrane, LPP, and the live bacteria activate p38MAPK and p44/42ERK1/2 signaling pathways and stimulate the production of prostanoids from RAW264.7 cells and primary human alveolar macrophages. Pharmacologic inhibition of phosphorylation of the ERK pathway or cPLA2α activity abolished M. pneumoniae-stimulated prostanoid release from the macrophages. In contrast to our study, Rawadi et al. (39) reported that lipid-associated membrane proteins derived from M. pneumoniae failed to induce the activation of MAPK or TNFα production from RAW264.7 cells. However, several subsequent reports indicate that M. pneumoniae or its cell membrane components stimulate the production of cytokines (14, 40–43). Previous reports showed that live M. pneumoniae infection induces IL-8 expression from human lung epithelial cells and macrophages. Additionally, our recent study showed that M. pneumoniae membrane and LPP induced IL-8 production from BEAS-2B cells and that NF-κB and to a lesser extent the MAPK pathways played an important role in the induction (14).
Previous studies have also shown that cPLA2α is phosphorylated on Ser505 by p38MAPK and p44/42ERK1/2 in response to stimulation (37, 44). Phosphorylated cPLA2α then binds to calcium through its C2 domain, translocating the enzyme to the Golgi apparatus, endoplasmic reticulum, or nuclear envelope to catalyze the release of AA (45–48). Earlier studies showed that inhibition of the MEK1/ERK pathway partially inhibited the phosphorylation of cPLA2α at the Ser505 site but did not affect enzyme translocation of cPLA2α to the Golgi in the Madin-Darby canine kidney cell line (49). Our data suggest that M. pneumoniae acts through TLR2-induced phosphorylation of p44/42ERK1/2, Ser505 phosphorylation of cPLA2α, and increased Ca2+ signals that promote full activation of cPLA2α.
Several studies provide evidence that two anionic phospholipids uniquely associated with the environmental interface of the bronchoalveolar compartment of the lung, PG and phosphatidylinositol, can markedly suppress the activation of TLR4 by bacterial LPS (29–31). Phosphatidylinositol acts by inhibiting the recognition of LPS by CD14. PG antagonizes TLR4 activation by inhibiting LPS recognition by CD14 and MD2 and disrupting MD2/TLR4 interaction (30). Among the PG molecular species antagonizing TLR4 activation, POPG, which is the major species within this lipid class in the lung, is especially potent. In this report, we demonstrate that POPG also disrupts the TLR2 activation by M. pneumoniae, its membrane, and derived LPP. Additional TLR2 agonists, Pam3Cys and MALP2, are also antagonized by POPG. The antagonism by POPG suppresses signaling from TLR2, involving phosphorylation of p38MAPK and p44,42ERK1/2, and prevents the release of TNFα and the cPLA2α-dependent release of AA, expression of COX2, and eicosanoid production.
The effects of M. pneumoniae and POPG upon TXA2/TXB2 production are interesting from several perspectives. Several studies have shown that TXA2 plays an important role in airway hyper-responsiveness and that asthmatic subjects excrete very high levels of TXB2 and its metabolites 2,3-dinor- and 11-dehydro-TXB2 in their urine (50, 51). Moreover, the TXA2 antagonist BAYu0345 reduced airway hyper-responsiveness in human asthmatic subjects (52). In another study, AA-2414, a TXA2 antagonist, reduced the infiltration of eosinophil and T lymphocytes into the lungs. AA-2414-treated human subjects had reduced numbers of MCP-3-, regulated upon activation normal T cell expressed and presumably secreted (RANTES), MIP-1α-, and eotaxin-positive cells in the epithelium and submucosa, suggesting that TXA2 may play a role in airway inflammation and bronchoconstriction by recruiting eosinophils and T lymphocytes (53). In addition, previous studies have shown that TXA2 receptor polymorphism was associated with asthma and atopy (54, 55). The above findings are consistent with the idea that TXA2 secreted from M. pneumoniae-stimulated primary human alveolar macrophages plays an important role in the exacerbation of asthma in humans.
Although PG is a minor lipid of pulmonary surfactant, its absolute concentration in the extracellular compartment of the lung is extraordinarily high (∼3.5 mg/ml), and it has not been reported at mucosal surfaces for other organs. In most tissues, PG is a minor constituent of mitochondrial lipids, and it appears to function principally as a precursor for cardiolipin. Several studies have identified PG as an antagonist of TLR4 activation (29–31), and recent work demonstrates its interactions with MD2 and the LPS binding site of CD14 (30). Additional work also reveals that the lipid suppresses both the infectivity of respiratory syncytial virus and the inflammatory sequelae of infection (33). The work in this report provides evidence for a broader role for POPG and other molecular species of surfactant PG as antagonists of TLR2 activation. The current data support the concept that PG plays a protective role in the bronchoalveolar compartment of the lung by suppressing inflammation and viral infection. The findings in this and other recent studies suggest that PG sets a high threshold for activation of TLR2 and TLR4 in the lung. This threshold effect likely prevents the activation of the TLRs in response to casual environmental stimuli present on microparticulate material that is inspired on a daily basis. By constitutively inhibiting TLR activation, PG prevents the development of chronic inflammation within the airways and alveoli of the organ. The data in Fig. 10, A and B, also suggest that the suppression of inflammation by POPG is competitive, insofar as it can be overcome by increasing the concentration of TLR agonists. Competition between TLR-activating ligands and POPG enables the lung to ignore ambient levels of agonists, such as LPS or M. pneumoniae membrane components, while also maintaining the ability to respond when the burden becomes too high, as occurs during an active infection.
One element of the findings in this and previous reports (30, 33), which requires further study, is the status of the PG pool in situ within the lung. The data show that pure PG liposomes antagonize TLR2 and TLR4, whereas liposomes containing mixtures of surfactant PG and PC are much less effective. We speculate that in vivo, surfactant PG can exist in segregated domains that enable potent suppression of TLR2 and TLR4 activation and respiratory syncytial virus infection. The requirement for segregated domains is minimal, insofar as only 6% of the total PG pool would be required to produce maximal TLR or respiratory syncytial virus antagonism. Biophysical studies have previously established that the DPPC pool within the alveolar compartment forms segregated domains at the air liquid interface (56), so the idea that this phenomenon could also apply to PG is not without precedent. Indeed, the process of selective enrichment of PC at the air liquid interface of the alveolar compartment would also be expected to enrich the alveolar hypophase with PG. Currently, there is no method available to visualize PG-enriched domains within the alveolar hypophase.
In summary, this study provides evidence for a robust eicosanoid response by macrophages to M. pneumoniae and its membrane constituents through TLR2 activation. For primary human alveolar macrophages, the main product of AA metabolism is TXA2/TXB2. The TLR2 activation is markedly suppressed by POPG, which suggests that the lipid may have useful therapeutic properties for controlling lung inflammation.
Acknowledgment
We thank Dr. Duncan C. Krause, Department of Microbiology, University of Georgia, for the generous gift of M. pneumoniae expressing p30-YFP.
This work was supported, in whole or in part, by National Institutes of Health Grants HL094629, HL073907 (to D. R. V.), HL34303 (to C. C. L. and R. C. M.), and infrastructure Grants M01 RR00051, and Clinical and Translational Science Award (CTSA) 1UL1 RR025780.
M. Nakamura, H. W. Chu, E. D. Chan, and D. R. Voelker, poster presented at the Thomas L. Petty Aspen Lung Conference 50th Annual Meeting, Aspen, CO (June 6–9, 2007).
- TLR
- Toll-like receptor
- MAPKs
- mitogen activated protein kinases
- AA
- arachidonic acid
- TXA2
- thromboxane A2
- TXB2
- thromboxane B2
- cPLA2α
- cytosolic phospholipase A2α
- LPP
- lipoprotein
- PG
- prostaglandin
- PC
- phosphatidylcholine
- POPG
- palmitoyl-oleoyl-phosphatidylglycerol
- POPC
- palmitoyl-oleoyl phosphatidylcholine
- DPPC
- dipalmitoyl phosphatidylcholine
- LT
- leukotriene
- B-PC
- biological PC pool
- B-PG
- biological PG pool
- HETE
- hydroxyeicosatetraenoic acid.
REFERENCES
- 1. Himmelreich R., Hilbert H., Plagens H., Pirkl E., Li B. C., Herrmann R. (1996) Nucleic Acids Res. 24, 4420–4449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beskind D. L., Keim S. M. (1994) Ann. Emerg. Med. 23, 1375–1378 [DOI] [PubMed] [Google Scholar]
- 3. Chan E. D., Kalayanamit T., Lynch D. A., Tuder R., Arndt P., Winn R., Schwarz M. I. (1999) Chest 115, 1188–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gil J. C., Cedillo R. L., Mayagoitia B. G., Paz M. D. (1993) Ann. Allergy 70, 23–25 [PubMed] [Google Scholar]
- 5. Stamm B., Moschopulos M., Hungerbuehler H., Guarner J., Genrich G. L., Zaki S. R. (2008) Emerg. Infect. Dis. 14, 641–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsiodras S., Kelesidis I., Kelesidis T., Stamboulis E., Giamarellou H. (2005) J. Infect. 51, 343–354 [DOI] [PubMed] [Google Scholar]
- 7. Johnston S. L., Martin R. J. (2005) Am. J. Respir. Crit. Care Med. 172, 1078–1089 [DOI] [PubMed] [Google Scholar]
- 8. Kraft M., Cassell G. H., Henson J. E., Watson H., Williamson J., Marmion B. P., Gaydos C. A., Martin R. J. (1998) Am. J. Respir. Crit. Care Med. 158, 998–1001 [DOI] [PubMed] [Google Scholar]
- 9. Lieberman D., Lieberman D., Printz S., Ben-Yaakov M., Lazarovich Z., Ohana B., Friedman M. G., Dvoskin B., Leinonen M., Boldur I. (2003) Am. J. Respir. Crit. Care Med. 167, 406–410 [DOI] [PubMed] [Google Scholar]
- 10. Martin R. J. (2006) Clin. Chest Med. 27, 87–98 [DOI] [PubMed] [Google Scholar]
- 11. Nisar N., Guleria R., Kumar S., Chand Chawla T., Ranjan Biswas N. (2007) Postgrad. Med. J. 83, 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Singh A. M., Busse W. W. (2006) Thorax 61, 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wieslander A., Boyer M. J., Wroblewski H. (1992) in Mycoplasmas: Molecular Biology and Pathogenesis (Maniloff J. M., McElhaney R. N., Finch L. R., Baseman J. B. eds) pp. 93–112, American Society for Microbiology, Washington, D.C [Google Scholar]
- 14. Chmura K., Bai X., Nakamura M., Kandasamy P., McGibney M., Kuronuma K., Mitsuzawa H., Voelker D. R., Chan E. D. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 295, L220–L230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shimizu T., Kida Y., Kuwano K. (2005) J. Immunol. 175, 4641–4646 [DOI] [PubMed] [Google Scholar]
- 16. Clark J. D., Schievella A. R., Nalefski E. A., Lin L. L. (1995) J. Lipid Mediat. Cell Signal 12, 83–117 [DOI] [PubMed] [Google Scholar]
- 17. Leslie C. C. (1997) J. Biol. Chem. 272, 16709–16712 [DOI] [PubMed] [Google Scholar]
- 18. Rocca B., FitzGerald G. A. (2002) Int. Immunopharmacol. 2, 603–630 [DOI] [PubMed] [Google Scholar]
- 19. Peters-Golden M., Canetti C., Mancuso P., Coffey M. J. (2005) J. Immunol. 174, 589–594 [DOI] [PubMed] [Google Scholar]
- 20. Christie P. E., Barnes N. C. (1996) Thorax 51, 1171–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore M. L., Peebles R. S., Jr. (2006) J. Allergy Clin. Immunol. 117, 1036–1039 [DOI] [PubMed] [Google Scholar]
- 22. Profita M., Sala A., Bonanno A., Riccobono L., Siena L., Melis M. R., Di Giorgi R., Mirabella F., Gjomarkaj M., Bonsignore G., Vignola A. M. (2003) J. Allergy Clin. Immunol. 112, 709–716 [DOI] [PubMed] [Google Scholar]
- 23. O'Byrne P. M., Fuller R. W. (1989) Eur. Respir. J. 2, 782–786 [PubMed] [Google Scholar]
- 24. Allen I. C., Hartney J. M., Coffman T. M., Penn R. B., Wess J., Koller B. H. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 290, L526–L533 [DOI] [PubMed] [Google Scholar]
- 25. Wright S. M., Hockey P. M., Enhorning G., Strong P., Reid K. B., Holgate S. T., Djukanovic R., Postle A. D. (2000) J. Appl. Physiol. 89, 1283–1292 [DOI] [PubMed] [Google Scholar]
- 26. Pattle R. E. (1955) Nature 175, 1125–1126 [DOI] [PubMed] [Google Scholar]
- 27. Tanaka F., Suga M., Nishikawa H., Muranaka H., Ando M. (1997) Respirology 2, 119–126 [DOI] [PubMed] [Google Scholar]
- 28. Tonks A., Morris R. H., Price A. J., Thomas A. W., Jones K. P., Jackson S. K. (2001) Clin. Exp. Immunol. 124, 86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hashimoto M., Asai Y., Ogawa T. (2003) J. Biol. Chem. 278, 44205–44213 [DOI] [PubMed] [Google Scholar]
- 30. Kuronuma K., Mitsuzawa H., Takeda K., Nishitani C., Chan E. D., Kuroki Y., Nakamura M., Voelker D. R. (2009) J. Biol. Chem. 284, 25488–25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mueller M., Brandenburg K., Dedrick R., Schromm A. B., Seydel U. (2005) J. Immunol. 174, 1091–1096 [DOI] [PubMed] [Google Scholar]
- 32. Harizi H., Gualde N. (2005) Tissue Antigens 65, 507–514 [DOI] [PubMed] [Google Scholar]
- 33. Numata M., Chu H. W., Dakhama A., Voelker D. R. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diaz N. A., Sanchez G. F., Gonzalez G. A. (1996) Anal. Chim. Acta 327, 161–165 [Google Scholar]
- 35. Qi H. Y., Shelhamer J. H. (2005) J. Biol. Chem. 280, 38969–38975 [DOI] [PubMed] [Google Scholar]
- 36. Seno K., Okuno T., Nishi K., Murakami Y., Watanabe F., Matsuura T., Wada M., Fujii Y., Yamada M., Ogawa T., Okada T., Hashizume H., Kii M., Hara S., Hagishita S., Nakamoto S., Yamada K., Chikazawa Y., Ueno M., Teshirogi I., Ono T., Ohtani M. (2000) J. Med. Chem. 43, 1041–1044 [DOI] [PubMed] [Google Scholar]
- 37. Lin L. L., Wartmann M., Lin A. Y., Knopf J. L., Seth A., Davis R. J. (1993) Cell 72, 269–278 [DOI] [PubMed] [Google Scholar]
- 38. Chu H. W., Jeyaseelan S., Rino J. G., Voelker D. R., Wexler R. B., Campbell K., Harbeck R. J., Martin R. J. (2005) J. Immunol. 174, 5713–5719 [DOI] [PubMed] [Google Scholar]
- 39. Rawadi G., Ramez V., Lemercier B., Roman-Roman S. (1998) J. Immunol. 160, 1330–1339 [PubMed] [Google Scholar]
- 40. Fonseca-Aten M., Ríos A. M., Mejías A., Chávez-Bueno S., Katz K., Gómez A. M., McCracken G. H., Jr., Hardy R. D. (2005) Am. J. Respir. Cell Mol. Biol. 32, 201–210 [DOI] [PubMed] [Google Scholar]
- 41. Hardy R. D., Jafri H. S., Olsen K., Wordemann M., Hatfield J., Rogers B. B., Patel P., Duffy L., Cassell G., McCracken G. H., Ramilo O. (2001) Infect. Immun. 69, 3869–3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shimizu T., Kida Y., Kuwano K. (2008) Infect. Immun. 76, 270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang J., Hooper W. C., Phillips D. J., Talkington D. F. (2002) Infect. Immun. 70, 3649–3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nemenoff R. A., Winitz S., Qian N. X., Van Putten V., Johnson G. L., Heasley L. E. (1993) J. Biol. Chem. 268, 1960–1964 [PubMed] [Google Scholar]
- 45. Evans J. H., Spencer D. M., Zweifach A., Leslie C. C. (2001) J. Biol. Chem. 276, 30150–30160 [DOI] [PubMed] [Google Scholar]
- 46. Glover S., de Carvalho M. S., Bayburt T., Jonas M., Chi E., Leslie C. C., Gelb M. H. (1995) J. Biol. Chem. 270, 15359–15367 [DOI] [PubMed] [Google Scholar]
- 47. Hirabayashi T., Kume K., Hirose K., Yokomizo T., Iino M., Itoh H., Shimizu T. (1999) J. Biol. Chem. 274, 5163–5169 [DOI] [PubMed] [Google Scholar]
- 48. Schievella A. R., Regier M. K., Smith W. L., Lin L. L. (1995) J. Biol. Chem. 270, 30749–30754 [DOI] [PubMed] [Google Scholar]
- 49. Evans J. H., Fergus D. J., Leslie C. C. (2002) BMC Biochem. 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oosaki R., Mizushima Y., Kawasaki A., Kashii T., Mita H., Shida T., Akiyama K., Kobayashi M. (1997) Int. Arch. Allergy Immunol. 114, 373–378 [DOI] [PubMed] [Google Scholar]
- 51. Taylor I. K., Ward P. S., O'Shaughnessy K. M., Dollery C. T., Black P., Barrow S. E., Taylor G. W., Fuller R. W. (1991) Am. Rev. Respir. Dis. 143, 119–125 [DOI] [PubMed] [Google Scholar]
- 52. Aizawa H., Shigyo M., Nogami H., Hirose T., Hara N. (1996) Chest 109, 338–342 [DOI] [PubMed] [Google Scholar]
- 53. Hoshino M., Sim J., Shimizu K., Nakayama H., Koya A. (1999) J. Allergy Clin. Immunol. 103, 1054–1061 [DOI] [PubMed] [Google Scholar]
- 54. Kim S. H., Choi J. H., Park H. S., Holloway J. W., Lee S. K., Park C. S., Shin H. D. (2005) Clin. Exp. Allergy 35, 585–590 [DOI] [PubMed] [Google Scholar]
- 55. Shin H. D., Park B. L., Jung J. H., Wang H. J., Park H. S., Choi B. W., Hong S. J., Lee Y. M., Kim Y. H., Park C. S. (2003) J. Allergy Clin. Immunol. 112, 454–457 [DOI] [PubMed] [Google Scholar]
- 56. Nag K., Perez-Gil J., Ruano M. L., Worthman L. A., Stewart J., Casals C., Keough K. M. (1998) Biophys. J. 74, 2983–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]