Abstract
Degenerative eye diseases are the most common causes of untreatable blindness. Accumulation of lipofuscin (granular deposits) in the retinal pigment epithelium (RPE) is a hallmark of major degenerative eye diseases such as Stargardt disease, Best disease, and age-related macular degeneration. The intrinsic reactivity of vitamin A leads to its dimerization and to the formation of pigments such as A2E, and is believed to play a key role in the formation of ocular lipofuscin. We sought a clinically pragmatic method to slow vitamin A dimerization as a means to elucidate the pathogenesis of macular degenerations and to develop a therapeutic intervention. We prepared vitamin A enriched with the stable isotope deuterium at carbon twenty (C20-D3-vitamin A). Results showed that dimerization of deuterium-enriched vitamin A was considerably slower than that of vitamin A at natural abundance as measured in vitro. Administration of C20-D3-vitamin A to wild-type rodents with no obvious genetic defects in vitamin A processing, slowed A2E biosynthesis. This study elucidates the mechanism of A2E biosynthesis and suggests that administration of C20-D3-vitamin A may be a viable, long-term approach to retard vitamin A dimerization and by extension, may slow lipofuscin deposition and the progression of common degenerative eye diseases.
Keywords: Eye, Lipid Chemistry, Retina, Vision, Vitamin A
Introduction
The polyenes N-retinylidene-N-retinylethanolamine (A2E)2 and all-trans-retinaldehyde dimer (ATR-dimer) are dimers of vitamin A formed in the eye during the vitamin A cycle; the process in which vitamin A is used by the eye to enable vision. In the aged eye and in the eyes of people with certain genetic disorders such as Stargardt or Best disease, the concentrations of these dimers in the retinal pigment epithelium (RPE) cell layer of the eye can reach over 10 mol% of vitamin A (1–5). A2E and ATR-dimer have been extracted from lipofuscin granules and shown to be especially toxic to RPE cells via a variety of mechanisms (6–18). As a result, these vitamin A dimers have been theorized to contribute to the formation of granular lipofuscin deposits in the eye. Lipofuscin deposits are believed to play a decisive role in the aging of the eye and in the pathogenesis of age-related macular degeneration (AMD), which in the United States affects more than 12% of people over 80 years of age and is the leading cause of irreversible blindness (19).
Clinically amiable strategies to impede vitamin A dimerization are highly sought as potential interventions to prevent several forms of macular degeneration as well as to elucidate the mechanisms leading to vision loss and blindness. One strategy to retard the formation of these dimers relies on the development of small molecule visual cycle antagonists (20–22). In this approach, molecules are designed to slow the visual cycle in the hope of reducing the production of the dimers that are byproducts of the cycle. Another promising approach is to inhibit the delivery of vitamin A to the eye thereby lowering its concentration and thus its likelihood to dimerize (23). However, visual function and retinal survival are contingent upon an adequate supply of vitamin A and upon the unhindered processing of the visual cycle. Long-term modification or inhibition of the visual cycle might lead to photoreceptor cell death and vision loss (24–26), making these strategies suboptimal to study the link between vitamin A dimerization, lipofuscin, and vision loss, and potentially reducing their clinical benefit for long-term interventions.
In this work we demonstrate that the introduction of deuterium atoms at carbon twenty of vitamin A results in a kinetic isotope effect sufficient to slow the formation of vitamin A dimers in vitro. We then demonstrate a similar reduction in vitamin A dimer formation in a rodent model and compare this approach to a visual cycle antagonist (TDH) and a retinol-binding protein inhibitor (Fenretinide). Importantly, this strategy could be suitable for adaptation to the clinic as a means to prevent vitamin A dimerization in humans and potentially slow the progression of AMD and Stargardt disease.
EXPERIMENTAL PROCEDURES
Nomenclature
Herein, in accordance with recommendations set forth in 1981 by the International Union of Pure and Applied Chemistry (IUPAC), the term “vitamin A” is used as a generic descriptor for retinoids qualitatively exhibiting the biological activity of retinol. As such word vitamin A includes retinol, retinaldehyde or retinal, and retinyl ester, or retinyl acetate.
Compounds
C20-D3-retinaldehyde and C20-D3-retinyl acetate (containing 5% D0, 15% D2, and 80% D3 at the C20 position as measured using atmospheric pressure chemical ionization mass spectrometry (APCI MS) by calculating the ratio of peaks at the corresponding values of m/z) was prepared according to literature procedures (27, 28). Retinol and retinaldehyde were prepared from retinyl acetate according to literature procedures (29). 15-[(4-hydroxyphenyl)amino] retinal or Fenretinide (30), 3,7,11-trimethyldodeca-2,6,10-trienoic acid hexadecylamide or TDH (21), and A2E (2) were prepared according to the literature. The structures of all synthesized compounds were verified using 1H, 13C NMR spectroscopy, and/or mass spectrometry (MS).
Formulation of C20-D3-all-trans-retinal and All-trans-retinal for Intraperitoneal Injection
Crystalline C20-D3-all-trans-retinal or all-trans-retinal was heated in Tween-20 until it dissolved and then phosphate-buffered saline (PBS) was added to give final concentrations of 10% Tween-20 and 1.5 mg/0.20 ml of the respective vitamins. α-tocopherol (0.1 mg/0.20 ml) was added as an antioxidant and the solutions were stored in the dark at 4 °C.
Formulation of Fenretinide and TDH
Both drugs were emulsified with 1 g/L of Nu-rice (RIBUS, St. Louis, MO), a brown rice extract, and supplied in the drinking water. The compounds were dissolved in ethanol and blended into the water/Nu-rice mixture using a high sheer mixer, as per the manufacturer's instructions. The final concentrations of the drugs were 1.5 mg/25 ml; the mixtures contained less than 0.5% ethanol. These solutions were made fresh weekly and provided ad libitum.
General HPLC Procedure
All chromatographic separations were performed using an HPLC system featuring a Waters 996 photodiode array detector, Model 600 pump, and Model 600 controller equipped with an in-line degasser. Data were processed using Empower Pro (v 5.0) software. Reverse-phase HPLC was performed at room temperature using an Onyx Monolithic C18 column (100 cm × 3 mm I.D.). The mobile phase consisted of a gradient of acetonitrile, containing 0.05% trifluoroacetic acid, (from 50 to 100% over 10–15 min at 1.5 ml/min, then 100% at 3 ml/min for 15 min) and 0.05% trifluoroacetic acid.
In Vitro A2E Formation Rates
C20-D3-retinaldehyde or retinaldehyde (20 mg), ethanolamine (0.5 equiv), and glacial acetic acid (0.5 equiv) were mixed in 5 ml of absolute ethanol. The reaction mixtures were stirred at room temperature and kept in the dark, and aliquots were withdrawn periodically and analyzed by HPLC.
In Vitro ATR-dimer Formation Rates
C20-D3-all-trans-retinaldehyde or all-trans-retinaldehyde (10 mg), proline (2 equiv), and a catalytic amount of triethylamine were mixed in 5 ml of absolute ethanol. The reaction mixtures were stirred at room temperature and kept in the dark, and aliquots were withdrawn periodically and analyzed by HPLC.
In Vitro A2E Competition Study
C20-D3-retinaldehyde and retinaldehyde (10 mg each) were mixed with ethanolamine (0.25 equiv) and glacial acetic acid (0.25 equiv) in 5 ml of absolute ethanol; the reaction was monitored by MS.
A2E Quantification from Animal Tissue
Under a 10× stereomicroscope in dim lighting, the eyecups (with the retinas) were collected and homogenated in absolute ethanol. After clarifying the homogenate through centrifugation (13,000 rpm, 5 min), the supernatant (40 μl) was injected into an HPLC for A2E and ATR-dimer quantification. Total A2E was calculated at 445 nm by measuring the total area of the peaks that displayed the characteristic UV-Vis spectroscopic absorbance profile of A2E corresponding to either A2E or its cis or trans isomers. A2E was quantified by comparing the HPLC retention times and peak areas of unknown samples with those of known A2E prepared according to the literature (2). For mice, five eyecups were extracted with 80 μl of ethanol; for rats, two eyecups were extracted with 100 μl of ethanol. All extractions and subsequent manipulations were performed under >500 nm lighting (F40GO gold lights) to avoid potential photo-degradation of A2E.
Liver Retinyl Ester Isotopic Ratios
10 mg of liver was homogenized in butanol (100 μl). The homogenates were clarified through centrifugation and then analyzed directly using ACPI MS.
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University and complied with guidelines set forth by the Guide for the Care and Use of Laboratory Animals. Animals were housed under 12-h light/dark cycles in environmentally controlled rooms and given food and water ad libitum.
Intraperitoneal Administration of Vitamin A and C20-D3-vitamin A to Wild-type Mice
Ten 8-week-old ICR mice (from Charles River, Wilmington, MA) were divided into two groups of five, and each group was administered 1.5 mg of ether all-trans-retinal or C20-D3-all-trans-retinal through intraperitoneal (IP) injection, twice a week for 6 weeks. During this time, the animals were placed on a diet poor in vitamin A (Harlan Teklad, Madison, WI). At the end of the 6-week period, each mouse was given a total of 60,000 IU (18 mg) of either vitamin A or C20-D3-vitamin A. Animals were sacrificed 5 days after the last vitamin injection, the retinas and eyecups were analyzed for A2E, and liver samples were collected for vitamin A analysis.
Comparison of A2E in Wild-type Rats Administered Fenretinide, TDH, C20-D3-vitamin A, or Vitamin A
15 45–50-day-old, female, CD IGS rats (Charles River, Wilmington, MA) were divided into five groups of three. One group was given a standard rodent diet containing 20,000 IU of vitamin A/kg feed. The other four groups were fed vitamin A poor diets, three of these four groups were supplemented with (IP) all-trans-retinal and the fourth group supplemented with C20-D3-all-trans-retinal. Each injection provided 3 mg of the vitamins, which was given two or three times per week, for 8 weeks (resulting in 20, 3-mg injections). Of the three groups administered all-trans-retinal, one group was given the retinol binding protein inhibitor Fenretinide and the other the retinal pigment epithelium-specific 65 kDa protein (RPE65) antagonist TDH. The above treatments are summarized in Table 1. At the end of the 8 weeks, animals were sacrificed (5 days after the last vitamin injection), the retinas and eyecups analyzed for A2E, and liver samples were collected for vitamin A analysis.
TABLE 1.
Treatment summary
IP, intraperitoneal.
| Group | Treatment |
|---|---|
| Control | 20,00 IU retinyl acetate/kg diet |
| Vitamin A | IP retinaldehyde |
| C20-D3-vitamin A | IP C20-D3-retinadehyde |
| Fenretinide | IP retinaldehyde + daily fenretinide in water |
| TDH | IP retinaldehyde + daily TDH in water |
Dietary Administration of C20-D3-vitamin A in Wild-type Mice
C20-D3-retinyl acetate was blended with sucrose and added to a purified diet (at 100,000 IU/kg diet) that was deficient in vitamin A (Harlan Teklad, Madison, WI). This diet provided five times the amount of vitamin A available in most commercial diets. Three, 2-month-old ICR animals, raised on standard rodent chow, were placed on the C20-D3-vitamin A diet for one month; the liver ratio of retinyl ester to C20-D3-retinyl ester was then measured using ACPI MS.
Statistical Analysis
Comparisons between groups were made using two-tailed, unpaired t-tests. A p value of less than 0.05 was considered statistically significant. Data are reported as means along with standard deviations.
RESULTS
Retardation of Vitamin A Dimerization in Vitro
We used HPLC to compare the rates of in vitro formation of A2E by retinaldehyde or by C20-D3-retinaldehyde (Fig. 1). Fig. 1C presents plots for the formation of A2E over time for reaction mixtures containing either all-trans-retinaldehyde or C20-D3-all-trans-retinaldehyde. Typical HPLC traces are displayed in Fig. 1, A and B for the all-trans-retinaldehyde and C20-D3-all-trans-retinaldehyde reaction mixtures after 40 h. The area under the curve at 15–17 min corresponds to the amount of A2E, formed as a mixture of cis and trans isomers. By measuring the rate of appearance of A2E in the two reaction media, we found that A2E formation occurred seven times slower for C20-D3-retinaldehyde than it did for retinaldehyde. In the retinaldehyde reaction mixture, in addition to A2E, we also observed the formation of an unidentified product having a retention time of ∼14.5 min; this material was not detected in the C20-D3-retinaldehyde reaction mixture. This observation is consistent with C20-D3-retinaldehyde being less reactive than retinaldehyde. To determine whether H-D exchange occurred during the course of the reaction, we used MS to analyze the reaction mixture after 50 h; although we detected the presence of the trideuterated retinaldehyde, trideuterated retinaldehyde hydroxylamine Schiff base and tetradeuterated A2E, we did not observe any evidence for H-D exchange.
FIGURE 1.
Replacing C20 hydrogen atoms of all-trans-retinaldehyde with deuteriums slows the formation of A2E. A, HPLC profiles of reaction mixtures containing C20-D3-retinaldehyde or retinaldehyde, plus ethanolamine and acetic acid after 30 h. B, expanded section of panel A. Gray line: retinaldehyde reaction; Black line: C20-D3-retinaldehyde reaction. C, plots of A2E formation over time for the reactions in panel A (kH/kD = 7 ± 1; from an average of three runs).
Similarly, we used HPLC to compare the ability of C20-D3-retinaldehyde to form ATR-dimer relative to that of retinaldehyde, using proline as a catalyst (31) (Fig. 2). We calculated that C20-D3-retinaldehyde formed ATR-dimer twelve times less rapidly than did the unlabeled retinaldehyde (kH/kD = 12 ± 2; from an average of three runs) and did not observe any H-D exchange in the corresponding mass spectra.
FIGURE 2.
Replacing C20 hydrogen atoms of all-trans-retinaldehyde with deuteriums slows the formation of ATR-dimer. A, HPLC profiles recorded after 1 h of reaction mixtures containing proline and either C20-D3-all-trans-retinaldehyde or all-trans-retinaldehyde to form ATR-dimer. B, plot of ATR-dimer formation over time for the reactions in panel A.
To further evaluate the difference in reactivity between the native and C20-D3-Schiff base, we performed a competition experiment in which we followed the disappearance of the two Schiff bases using MS (Fig. 3). We prepared a solution containing a nearly equal mixture of retinaldehyde and C20-D3-retinaldehyde and added ethanolamine and acetic acid. The deuterated and non-deuterated Schiff bases formed immediately as evidenced in mass spectra recorded within 2 min of the commencement of the reaction (Fig. 3A). These mass spectra featured peaks of nearly equal intensity at m/z 328 and 331, corresponding to the retinaldehyde/ethanolamine and the C20-D3-retinaldehyde/ethanolamine Schiff bases respectively. In addition peaks at 329 and 330 m/z were observed corresponding to the Schiff base with one (D1) and two (D2) deuteriums, respectively, which reflected the deuterium distribution at the start of the reaction. We compared the relative rates of disappearance of the Schiff bases by measuring the peak ratios at m/z 328 and 331. After 2 weeks, the peak at m/z 328 was half as intense as that at m/z 331, confirming that the non-deuterated Schiff base reacted faster than the deuterated Schiff base. In addition, the peak for the D1-Schiff base decreased by 10% relative to that of the D3-Schiff base, which was also in accord with slower reactivity with more deuterium incorporation. There was a negligible decrease in the peak corresponding to the D2-Schiff base relative to that of the D3-Schiff base.
FIGURE 3.
APCI MS of a reaction mixture of retinaldehyde and C20-D3-retinaldehyde with ethanolamine after (A) 2 min and (B) 2 weeks. Mass to charge ratios are shown and ion intensities are shown as relative percents. The peak at m/z 328 corresponds to the Schiff base at natural abundance and the peak at m/z 331 corresponding to the deuterated Schiff base.
C20-D3-vitamin A Slows A2E Biosynthesis in Wild-type Mice
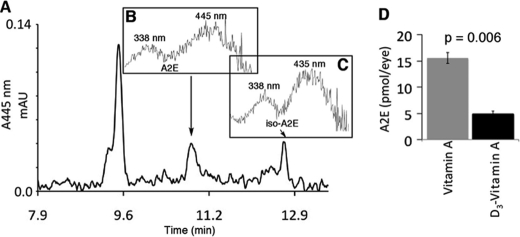
We administered vitamin A or C20-D3-vitamin A to two groups of wild-type mice for 6 weeks. Fig. 4A shows a representative HPLC chromatogram of an eyecup ethanol extract for the C20-D3-vitamin A treated animals. Fig. 4, B and C display the UV-Vis absorbance spectra of the peaks at 18.8 and 11.8 min respectively; these spectra are characteristic of A2E (Fig. 4B) and iso-A2E (Fig. 4C), a cis isomer of A2E. We confirmed the identities of these compounds through co-elution with A2E and iso-A2E standards. The limit of detection for A2E was 5 pmol. A2E was defined as the amount of all-trans-A2E combined with iso-A2E. Fig. 4D summarizes the concentrations of A2E found in the eyes of mice given C20-D3-vitamin A or vitamin A. Mice administered C20-D3-vitamin A had 68% less A2E relative to mice administered normal vitamin A (p = 0.006, standard deviations <10%).
FIGURE 4.
Administration of C20-D3-vitamin A to wild-type mice slows A2E synthesis in vivo. A, representative HPLC trace of extracted retinoids from eyecups of mice administered C20-D3-vitamin A. B and C, UV-Vis absorbance spectra of the peaks at B ∼10.8, representative of A2E and C, ∼11.8 min, representative of iso-A2E. D, average A2E levels in animals given either vitamin A at natural abundance or C20-D3-vitamin A. Five animals per group were used. p: p value.
Inhibition of A2E Biosynthesis in Wild-type Rats in Response to a Visual Cycle Antagonist, a Retinol-binding Protein Inhibitor and C20-D3-Vitamin A
Fig. 5 displays A2E concentrations measured in wild-type rats administered either retinaldehyde, C20-D3-all-trans-retinaldehyde, Fenretinide or TDH along with age-matched animals raised on a standard rodent chow containing 20,000 IU of vitamin A/kg of food (control group). The control group and those given extra vitamin A (retinaldehyde group), had equal amounts of A2E (p = 0.22). On the other hand, animals receiving C20-D3-vitamin A (C20-D3-all-trans-retinaldehyde) had 45% less A2E compared with the control group (p = 0.007). Likewise, animals receiving Fenretinide or TDH had 58 and 40% less A2E respectively, relative to the control group. This difference was significant for Fenretinide (p = 0.004) but not for TDH (p = 0.95) for which there was more variation in A2E levels in the eyecups of these animals. There was no statistically significant difference in the relative average decrease in A2E among all three inhibitors of A2E biosynthesis.
FIGURE 5.

Comparison of A2E in age-matched animals given different inhibitors of A2E biosynthesis. All groups were raised on a standard rodent diet for the first 50 days. The 100-day-old controls were raised on a standard diet throughout. While the Fenretinide, TDH, vitamin A, and C20-D3-vitamin A groups were given high doses of the amount of vitamin A for the remaining 50 days, except that the last mentioned group was given vitamin A in the form of C20-D3-vitamin A. Three animals per group were used.
Liver Vitamin A Stores Can Be Swapped with C20-D3-vitamin A
To estimate the time it would take to swap the existing hepatic vitamin A with C20-D3-vitamin A, we fed a diet containing 100,000 IU of deuterated vitamin A/kg feed to three, two-month-old mice for 30 days. We then used MS to investigate the extent to which hepatic vitamin A had been replaced with C20-D3-vitamin A. Fig. 6 displays representative ACPI mass spectra of the liver ethanol extracts of mice on the diet for two and 4 weeks. Under the MS conditions we employed, retinyl esters fragmented through elimination of the fatty acyl chain, providing a base peak at m/z 269 (32). After 2 weeks on the diet containing C20-D3-vitamin A, a new peak appeared at m/z 272, corresponding to the C20-D3-retinyl ester fragment. We confirmed the identities of the peaks at m/z 269 and 272 through comparisons with retinyl acetate and C20-D3-retinyl acetate, respectively, which have the same fragmentation patterns as their corresponding longer-chain esters such as, retinyl palmitate that are present in the liver. By comparing the ratio of the peaks at m/z 269 and 272, we estimated the ratio of normal to deuterated retinyl esters in the liver. After 2 weeks on this diet, 20 ± 11% of the liver was in the deuterated form; at 4 weeks, this number increased to 33 ± 14%. Similarly, we used MS to measure the percentage of liver C20-D3-vitamin A in the above groups of mice and rats administered C20-D3-vitamin A through intraperitoneal injections. In these animals, 43 ± 12 (for rats) and 50 ± 18% (for mice) of the hepatic vitamin A had been converted.
FIGURE 6.
Liver vitamin A in response to dietary C20-D3-vitamin A. Representative ACPI mass spectra of liver extracts of animals on a diet containing 100,000 IU of C20-D3-vitamin A/kg of diet for (A) 2 and (B) 4 weeks. The peaks labeled “269” and “272” represent fragments of vitamin A and C20-D3-vitamin A, respectively.
DISCUSSION
In the eye, A2E- and ATR-dimer are putatively formed through a multistep reaction sequence involving two molecules of all-trans-retinaldehyde (Fig. 7) (2, 33). In the first step, retinaldehyde, which is released from opsin after photon recognition, condenses with phosphatidylethanolamine, which is abundant in the lipid-rich environment of the disk membrane, to form a Schiff base (34). In principle, this Schiff base can be converted, upon dissociation of the C20-H bond, into either a cis or trans enamine through the transition state of a (1, 5) hydrogen shift or deprotonation (step 2, Fig. 7). Subsequent reaction with another molecule of retinaldehyde, either through amine condensation or 1,4-addition followed by cyclization (or direct Diels-Alder cycloaddition) yields the reduced forms of A2E and ATR-dimer, respectively. A2E is then formed through aromatization of the dihydropyridine; ATR-dimer, through deamination. We speculated that enamine formation through transition states for either (1, 5) hydrogen atom migration (35) or deprotonation may require more energy than the subsequent condensation, 2-azatriene electrocyclization, and aromatization to form A2E or 1,4-condensation, intramolecular Mannich condensation (or direct Diels-Alder reaction), and deamination to form ATR-dimer (36). If so, replacing the three C20-H bonds with C20-D bonds should result in a primary kinetic isotope effect slowing the formation of both of these lipofuscin chromophores. This result was observed in vitro with a seven times slower formation of A2E and twelve times slower formation of ATR-dimer.
FIGURE 7.
Biosynthesis of A2E and ATR-dimer from all-trans-retinaldehyde. Hydrogen atoms are presented at the C20 position of all-trans-retinaldehyde and on its Schiff base. Inset: possible transition states (TS) leading to the cis- or trans-enamines.
The larger kinetic isotope effect for ATR-dimer, relative to that found for A2E formation, suggests that enamine formation in the presence of triethylamine and proline catalysts may occur via δ-proton abstraction rather than (1, 5) hydrogen atom migration. The related α-proton abstraction from an iminium ion gives large deuterium isotope effects (37), a result that is consistent with the large isotope effect observed here. Prior to (1, 5) hydrogen atom migration, the retinaldehyde Schiff base must reorganize into the cisoid geometry, placing the migrating hydrogen atom proximal to the nitrogen atom. Steric hindrance around the nitrogen atom, provided by proline, may increase the energy of the (1, 5) hydrogen atom migration TS, thereby resulting in a preference for the deprotonation TS leading to the trans-enamine. After condensation with another molecule of retinaldehyde, the cis-enamine can adopt a geometry that favors the 2-azatriene electrocyclization leading to A2E; the corresponding geometry of the trans-enamine causes the 2-azatriene electrocyclization to be energetically unfavorable. Steric interactions around the amine in vivo may lead to a preference for the formation of ATR-dimer over A2E, while a lack of steric crowding may favor the formation of A2E.
If vitamin A dimerizes via the above, non-enzymatic mechanism in vivo, then the observed decreased reactivity of the deuterated vitamin to dimerize should translate to the eye. To verify this we compared A2E levels in wild-type mice given vitamin A or C20-D3-vitamin A at the same concentration. Mice given C20-D3-vitamin A had less (68% decrease) A2E than control animals given normal vitamin A. Similarly, we found 45% less A2E in rats given C20-D3-vitamin A over 8 weeks compared with a control group that received an estimated six times less (assuming a 230 g rat consumes 23 g of food per day (38)) of standard vitamin A from a commercial diet. We were not able to detect ATR-dimer in these young, wild-type animals.
Of note, rats raised on vitamin A from a standard diet and those given six times more vitamin A, had the same amount of A2E. This observation was expected since the concentration of all-trans-retinaldehyde, the precursor to A2E, depends on visual pigment turnover which is thought to not be affected by increasing vitamin A intake. It has however been observed in the mouse that after prolonged feeding of 60 times the mouse nutrient requirement of vitamin A, there was a slight increase in A2E despite a similar concentration of retinaldehyde (39). The relevance of this finding to humans is difficult to interpret as such equivalent doses of vitamin A in humans would presumably be toxic.
We also compared the ability of C20-D3-vitamin A to inhibit A2E biosynthesis to that of other known small-molecule inhibitors, mainly TDH and Fenretinide. TDH is an RPE65 antagonist. The drug Fenretinide lowers retinaldehyde concentrations by inhibiting serum retinol-binding protein (RBP) thus limiting the amount of vitamin A that enters the eye. In clinical trials, late-AMD patients taking 300 mg of Fenretinide for 18 months exhibited a 45% reduction in median growth rate of geographic atrophy (GA) lesions, relative to that of placebo groups (40). In this work, we gave wild-type animals 45 mg/kg/week of either TDH or Fenretinide and compared their abilities to slow A2E biosynthesis with that of C20-D3-vitamin A given at 33 mg/kg/week. The TDH and Fenretinide groups both received supplementary vitamin A so as to be given the same total amount of vitamin A as the C20-D3-vitamin A-treated animals. Considering that vitamin A supplementation itself had no effect on A2E levels at one month, the absence of such supplementation would probably not have changed the outcome of the experiment. Administration of both Fenretinide and C20-D3-vitamin A decreased A2E by the same order of magnitude (∼ 50%).
TDH and Fenretinide retard vitamin A dimerization by binding to retinoid proteins and impeding the visual cycle. However, molecules that bind to retinoid-binding proteins can exhibit potent and often toxic biological activities, particularly if these molecules also bind to retinoid receptors, which regulate gene transcription (41). Although such side effects may be limited with prudent dosing regiments, the method introduced herein could be considered attractive, as deuterium incorporation at the C20 position should not interfere with the endogenous role of vitamin A in the body, during vision or gene transcription, because the C20-hydrogen bond is not believed to be cleaved during its use or metabolism (42). Thus, we would not expect C20-D3-vitamin A to inhibit the visual cycle or cause any greater toxicity than that caused by normal vitamin A at widely recognized safe doses. C20-D3-vitamin A can, in theory, inhibit vitamin A dimerization without inhibiting the visual cycle, and as a result, C20-D3-vitamin A could be advantageous as a life-long treatment for dry-AMD, Stargardt disease and other macular dystrophies associated with vitamin A dimerization and lipofuscin accumulation.
Lipofuscin pigment synthesis occurs in the outer segments of the neural retina. The pigments are then delivered through phagocytosis to the RPE layer, where they accumulate. The successful translation of the use of deuterated vitamin A to slow lipofuscin formation in humans depends on the extent to which non-labeled vitamin A can be replaced with C20-D3-vitamin A in the neural retina (outer segments). The practical extent to which this “swap” can occur depends on two factors: (1) the patient's steady-state intake of vitamin A versus C20-D3-vitamin A intake and (2) the time it takes to replace RPE vitamin A stores with the deuterated vitamin upon its intake.
In the United States, the median daily dietary intake of retinol is ∼0.3 mg (43). Upon additional administration of 3 mg of C20-D3-vitamin A/day, 91% of the body's vitamin A would be replaced by C20-D3-vitamin A at steady-state. If consumption of unlabeled retinol is halved the percentage of enriched body retinol would increase to 95%. In these treatment scenarios, the total vitamin A intake would approximately be 3.3 mg per day, which is widely considered to be a safe amount for chronic consumption (44). There remains the question of how long it might take to swap native RPE vitamin A with ingested C20-D3-vitamin A.
The fact that we observed a significant decrease in A2E before liver reserves had been completely swapped to the deuterated form of the vitamin, suggests that lipofuscin pigment biosynthesis can be slowed soon after administration of C20-D3-vitamin A and may be the result of faster exchange of dietary vitamin A with the RPE versus liver reserves. Body stores of vitamin A represent a complex and not completely understood balance between its absorption and elimination. In mammals, 90% of the body's vitamin A is stored in the liver as retinyl esters. To investigate the extent to which hepatic vitamin A had been replaced with C20-D3-vitamin A, we analyzed liver extracts using MS. For animals supplemented with five times their normal amount of dietary vitamin A, as C20-D3-vitamin A, for one month, 33% of the liver retinyl esters existed in the D3-deuterated form. The percentage of hepatic C20-D3-vitamin A incorporation was similar for the animals that were administered C20-D3-vitamin A via intraperitoneal injections. Through a survey of rodent diets we found that most rodent diets already provide 5–10 times the nutrient requirement of vitamin A (45). This may explain why laboratory rodents, raised on most commercial diets, seem to have large liver stores of vitamin A. For example, a 2-year-old rat raised on a diet of 24,000 IU of vitamin A/kg of food has a liver content of ∼1.77 mg of vitamin A/g of liver (46). By comparison, the average, healthy, nourished adult, human has a median liver vitamin A store of ∼0.066 mg/g of liver, about 4% the concentration of vitamin A in the liver of rats (47, 48). Because a majority of humans appear to have less stored vitamin A, swapping out vitamin A for C20-D3-vitamin A might occur at a rate faster than that found here in rodents. Further studies, perhaps in a different animal model, might be needed to predict how long this swap may take in the human retina. Alternatively, local delivery of C20-D3-vitamin A to the eye (e.g. through local injections) might decrease the time required for C20-D3-vitamin A to accumulate in the RPE and slow A2E biosynthesis.
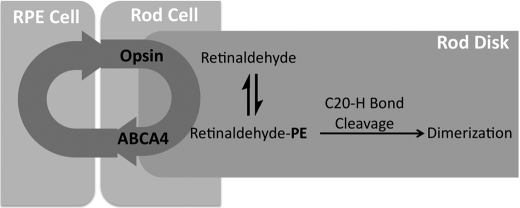
In the normal functioning eye, retinaldehyde released from opsin after photon absorption, may condense with phosphatidylethanolamine (PE) to form a Schiff base (retinaldehyde-PE, Fig. 8). This work suggests that the rate-limiting step in the formation of vitamin A dimers from retinaldehyde-PE is the cleavage of a C20-H bond. After deuterium incorporation at the C20 position, more energy is required to break this bond. Decreasing the reactivity of retinaldehyde-PE to form lipofuscin pigments should in theory allow more time for the Schiff base to be incorporated back into the visual cycle. Reincorporation may occur through active (ABCR mediated) or passive transport of retinaldehyde or retinaldehyde-PE to the cytosolic disk face, where retinaldehyde can be reduced to retinol.
FIGURE 8.
Inhibition of A2E biosynthesis by C20-D3-vitamin A in wild-type animals. PE, phosphatidylethanolamine. The circular arrows represent the vitamin A cycle; two proteins are displayed. Upon photon recognition in the outer segment of the neural retina (rod cell shown), retinaldehyde is released from the opsin protein. A portion of this retinaldehyde condenses with PE to form retinaldehyde-PE, leading to the formation of vitamin A dimers. Deuteration of the C20-H bonds of vitamin A inhibits dimerization allowing more time for retinaldehyde-PE to be processed by ABCA4.
In summary, age-related macular degeneration, which typically develops around the seventh decade of life, is thought to be the result of a lifetime of accumulative damage. A slight slowing of the life-long molecular mechanisms responsible for macular degeneration might postpone the onset of the disease for decades. Because A2E is believed to contribute to declining retinal health, it is often used as an early marker for evaluating the efficacy of therapeutic interventions to treat AMD in murine models (20, 21, 23, 49). A2E levels are often taken as a quantitative measure of ocular lipofuscin (50, 51) as the levels of A2E coincide with granular lipofuscin deposits in animal models, in elderly humans and in individuals with certain genetic eye disorders (1, 2, 52, 53). Importantly, methods that retard A2E biosynthesis have been shown to lower the concentration of lipofuscin granules in animal models of macular degeneration and are being tested in human clinical trials with an RBP antagonist and an RPE65 inhibitor. In this study we showed that C20-D3-vitamin A could slow A2E formation in animals with no obvious defects in retinoid processing. We believe that this situation replicates the age related biosynthesis of A2E in the healthy human eye. Our data elucidate the mechanism of A2E biosynthesis and suggest that C20-D3-vitamin A could perhaps be a realistic approach to slow the age-related biosynthesis of lipofuscin pigments in the human retina. As such, C20-D3-vitamin A has the potential to be used as a clinical tool for studying the relationship between vitamin A dimerization, lipofuscin, and vision and as a possible therapeutic for lipofuscin-mediated macular degenerations.
Acknowledgment
We thank Dr. Leonide Saad for comments during the preparation of the manuscript.
This work was supported by the International Retinal Research Foundation.
- A2E
- N-retinylidene-N-retinylethanolamine
- ATR-dimer
- all-trans-retinaldehyde dimer
- RPE
- retinal pigment epithelium
- AMD
- age-related macular degeneration
- GA
- geographic atrophy
- RBP
- retinol-binding protein.
REFERENCES
- 1. Eldred G. E., Lasky M. R. (1993) Nature 361, 724–726 [DOI] [PubMed] [Google Scholar]
- 2. Parish C. A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14609–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bridges C. D., Alvarez R. A., Fong S. L. (1982) Invest. Ophthalmol. Vis. Sci. 22, 706–714 [PubMed] [Google Scholar]
- 4. Ben-Shabat S., Parish C. A., Hashimoto M., Liu J., Nakanishi K., Sparrow J. R. (2001) Bioorg. Med. Chem. Lett. 11, 1533–1540 [DOI] [PubMed] [Google Scholar]
- 5. Sparrow J. R., Parish C. A., Hashimoto M., Nakanishi K. (1999) Invest. Ophthamol. Vis. Sci. 40, 2988–2995 [PubMed] [Google Scholar]
- 6. Avalle L. B., Wang Z., Dillon J. P., Gaillard E. R. (2004) Exp. Eye. Res. 78, 895–898 [DOI] [PubMed] [Google Scholar]
- 7. Bergmann M., Schütt F., Holz F. G., Kopitz J. (2004) FASEB J. 18, 562–564 [DOI] [PubMed] [Google Scholar]
- 8. Finnemann S. C., Leung L. W., Rodriguez-Boulan E. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3842–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holz F. G., Schütt F., Kopitz J., Eldred G. E., Kruse F. E., Völcker H. E., Cantz M. (1999) Invest. Ophthalmol. Vis. Sci. 40, 737–743 [PubMed] [Google Scholar]
- 10. Lakkaraju A., Finnemann S. C., Rodriguez-Boulan E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11026–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rózanowski B., Cuenco J., Davies S., Shamsi F. A., Zadlo A., Dayhaw-Barker P., Rózanowska M., Sarna T., Boulton M. E. (2008) Photochem. Photobiol. 84, 650–657 [DOI] [PubMed] [Google Scholar]
- 12. Schütt F., Bergmann M., Kopitz J., Holz F. G. (2001) Ophthalmologe. 98, 721–724 [DOI] [PubMed] [Google Scholar]
- 13. Schütt F., Bergmann M., Kopitz J., Holz F. G. (2002) Ophthalmologe. 99, 861–865 [DOI] [PubMed] [Google Scholar]
- 14. Shamsi F. A., Boulton M. (2001) Invest. Ophthalmol. Vis. Sci. 42, 3041–3046 [PubMed] [Google Scholar]
- 15. Sparrow J. R., Nakanishi K., Parish C. A. (2000) Invest. Ophthalmol. Vis. Sci. 41, 1981–1989 [PubMed] [Google Scholar]
- 16. Washington I., Turro N. J., Nakanishi K. (2006) Photochem. Photobiol. 82, 1394–1397 [DOI] [PubMed] [Google Scholar]
- 17. Washington I., Jockusch S., Itagaki Y., Turro N. J., Nakanishi K. (2005) Angew. Chem. Int. Ed. Engl. 44, 7097–7100 [DOI] [PubMed] [Google Scholar]
- 18. Ben-Shabat S., Itagaki Y., Jockusch S., Sparrow J. R., Turro N. J., Nakanishi K. (2002) Angew. Chem. Int. Ed. Engl. 41, 814–817 [DOI] [PubMed] [Google Scholar]
- 19. Friedman D. S., O'Colmain B. J., Muñoz B., Tomany S. C., McCarty C., de Jong P. T., Nemesure B., Mitchell P., Kempen J. (2004) Arch. Ophthalmol. 122, 564–572 [DOI] [PubMed] [Google Scholar]
- 20. Golczak M., Maeda A., Bereta G., Maeda T., Kiser P. D., Hunzelmann S., Lintig J., Blaner W. S., Palczewski K. (2008) J. Biol. Chem. 283, 9543–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maiti P., Kong J., Kim S. R., Sparrow J. R., Allikmets R., Rando R. R. (2006) Biochemistry 45, 852–860 [DOI] [PubMed] [Google Scholar]
- 22. Radu R. A., Mata N. L., Nusinowitz S., Liu X., Sieving P. A., Travis G. H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4742–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radu R. A., Han Y., Bui T. V., Nusinowitz S., Bok D., Lichter J., Widder K., Travis G. H., Mata N. L. (2005) Invest. Ophthalmol. Vis. Sci. 46, 4393–4401 [DOI] [PubMed] [Google Scholar]
- 24. Baehr W., Wu S. M., Bird A. C., Palczewski K. (2003) Vision Res. 43, 2957–2958 [DOI] [PubMed] [Google Scholar]
- 25. Maeda A., Maeda T., Golczak M., Imanishi Y., Leahy P., Kubota R., Palczewski K. (2006) Mol. Pharmacol. 70, 1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radding C. M., Wald G. (1956) J. Gen. Physiol. 39, 923–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pardoen J. A., Mulder P. P., Van den Berg E. M. (1985) Can. J. Chem. 63, 1431–1435 [Google Scholar]
- 28. Bergen H. R., Furr H. C., Olson J. A. (1988) J. Label. Com. Radiopharm. 25, 11–21 [Google Scholar]
- 29. Isler O., Ronco A., Guex W., Hindley N. C., Huber W., Dialer K., Kofler M. (1949) Helv. Chim. Acta 32, 489–505 [DOI] [PubMed] [Google Scholar]
- 30. Um S. J., Kwon Y. J., Han H. S., Park S. H., Park M. S., Rho Y. S., Sin H. S. (2004) Chem. Pharm. Bull. 52, 501–506 [DOI] [PubMed] [Google Scholar]
- 31. Bench B. J., Liu C., Evett C. R., Watanabe C. M. (2006) J. Org. Chem. 71, 9458–9463 [DOI] [PubMed] [Google Scholar]
- 32. Wingerath T., Kirsch D., Spengler B., Kaufmann R., Stahl W. (1997) Anal. Chem. 69, 3855–3860 [DOI] [PubMed] [Google Scholar]
- 33. Sakai N., Decatur J., Nakanishi K., Eldred G. E. (1996) J. Am. Chem. Soc. 118, 1559–1560 [Google Scholar]
- 34. Katz M. L., Gao C. L., Rice L. M. (1996) Mech. Ageing. Dev. 92, 159–174 [DOI] [PubMed] [Google Scholar]
- 35. Saettel N. J., Wiest O. (2000) J. Org. Chem. 65, 2331–2336 [DOI] [PubMed] [Google Scholar]
- 36. Rodríguez-Otero J. (1999) J. Org. Chem. 64, 6842–6848 [DOI] [PubMed] [Google Scholar]
- 37. Roberts R. D., Ferran H. F., Gula M. J., Spencer T. A. (1980) J. Am. Chem. Soc. 102, 7054–7058 [Google Scholar]
- 38. Harkness J. E., Wagner J. E. (1989) The Biology and Medicine of Rabbits and Rodents, Lea and Febiger, Philadelpha [Google Scholar]
- 39. Radu R. A., Yuan Q., Hu J., Peng J. H., Lloyd M., Nusinowitz S., Bok D., Travis G. H. (2008) Invest. Ophthalmol. Vis. Sci. 49, 3821–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vogel R., Slakter J., McLeod K. (2009) A Phase II, Double-Masked, Placebo-Controlled, Dose-Comparison Study of the Safety and Efficacy of Fenretinide in the Treatment of Geographic Atrophy in Subjects with Age-Related Macular Degeneration: Baseline Lesion Size, Characteristics, and Preliminary Progression Data Presentation, in A Presentation during the annual meeting for the Association for Research in Vision and Ophthalmology, Fort Lauderdale, FL [Google Scholar]
- 41. Gronemeyer H., Miturski R. (2001) Cell. Mol. Biol. Lett. 6, 3–52 [PubMed] [Google Scholar]
- 42. (1996) J. Cell. Biochem. Suppl. 26, 269–307 [PubMed] [Google Scholar]
- 43. Ervin R. B., Wright J. D., Wang C. Y., Kennedy-Stephenson J. (2004) Adv. Data. 339, 1–4 [PubMed] [Google Scholar]
- 44. Bendich A., Langseth L. (1989) Am. J. Clin. Nutr. 49, 358–371 [DOI] [PubMed] [Google Scholar]
- 45. (1995) Nutrient Requirements of Laboratory Animals, Fourth Revised Ed., National Academy Press, Washington, D. C: [PubMed] [Google Scholar]
- 46. Szweda L. I. (1994) J. Biol. Chem. 269, 8712–8715 [PubMed] [Google Scholar]
- 47. Underwood B. A., Siegel H., Weisell R. C., Dolinski M. (1970) Am. J. Clin. Nutr. 23, 1037–1042 [DOI] [PubMed] [Google Scholar]
- 48. Ribaya-Mercado J. D., Solon F. S., Dallal G. E., Solomons N. W., Fermin L. S., Mazariegos M., Dolnikowski G. G., Russell R. M. (2003) Am. J. Clin. Nutr. 77, 694–699 [DOI] [PubMed] [Google Scholar]
- 49. Kong J., Kim S. R., Binley K., Pata I., Doi K., Mannik J., Zernant-Rajang J., Kan O., Iqball S., Naylor S., Sparrow J. R., Gouras P., Allikmets R. (2008) Gene. Ther. 15, 1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Karan G., Lillo C., Yang Z., Cameron D. J., Locke K. G., Zhao Y., Thirumalaichary S., Li C., Birch D. G., Vollmer-Snarr H. R., Williams D. S., Zhang K. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4164–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kim S. R., Fishkin N., Kong J., Nakanishi K., Allikmets R., Sparrow J. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11668–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mata N. L., Weng J., Travis G. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bakall B., Radu R. A., Stanton J. B., Burke J. M., McKay B. S., Wadelius C., Mullins R. F., Stone E. M., Travis G. H., Marmorstein A. D. (2007) Exp. Eye. Res. 85, 34–43 [DOI] [PubMed] [Google Scholar]