Abstract
Stargardt disease, also known as juvenile macular degeneration, occurs in approximately one in 10,000 people and results from genetic defects in the ABCA4 gene. The disease is characterized by premature accumulation of lipofuscin in the retinal pigment epithelium (RPE) of the eye and by vision loss. No cure or treatment is available. Although lipofuscin is considered a hallmark of Stargardt disease, its mechanism of formation and its role in disease pathogenesis are poorly understood. In this work we investigated the effects of long-term administration of deuterium-enriched vitamin A, C20-D3-vitamin A, on RPE lipofuscin deposition and eye function in a mouse model of Stargardt's disease. Results support the notion that lipofuscin forms partly as a result of the aberrant reactivity of vitamin A through the formation of vitamin A dimers, provide evidence that preventing vitamin A dimerization may slow disease related, retinal physiological changes and perhaps vision loss and suggest that administration of C20-D3-vitamin A may be a potential clinical strategy to ameliorate clinical symptoms resulting from ABCA4 genetic defects.
Keywords: Eye, Lipid Chemistry, Retina, Vision, Vitamin A, ABCA4
Introduction
Autosomal recessive Stargardt disease (STGD1) is a macular dystrophy resulting from mutations in the ABCA4 (ABCR) gene. The age of onset of Stargardt disease is typically 10–20 years of age and leads in almost all cases to blindness by age 50 (1, 2). A hallmark of the disease is premature lipofuscin accumulation in the retinal pigment epithelium (RPE)2 of the eye. The RPE is critical for the neurosensory retina homeostasis; it acts as a transport exchange system with blood capillaries and is critical for regeneration and phagocytosis of photoreceptor outer segments. It is hypothesized that when RPE lipofuscin reaches a critical level, it contributes to a decline in cell function (3–8) resulting in the degeneration of the macular region of the neurosensory retina leading to loss of central vision (9–11).
The defective gene in Stargardt, ABCA4, encodes for an outer segment rim protein (RmP). The function of RmP is to transport the all-trans-retinaldehyde-phosphatidylethanolamine (retinaldehyde-PE) Schiff base from the luminal side of the disk membrane to the cytosolic face, where retinaldehyde can then be converted back to retinol (3). In the absence of its proper transporter, the retinaldehyde-PE conjugates may react to form vitamin A dimers (A2E and ATR-dimer among others), which are then deposited in the RPE after phagocytosis of the photoreceptors outer segments. Numerous studies have demonstrated the toxicity of vitamin A dimers to cultured RPE cells and they are hypothesized to play a key role in lipofuscin formation and subsequent retinal degeneration (12, 13). Nevertheless, the exact mechanisms that lead to lipofuscin accumulation or to vision loss as a result of the impaired transport of the retinaldehyde-PE conjugates remain unclear.
In the accompanying article, we have shown that the rate-determining step in vitamin A dimerization is the cleavage of a C20 carbon-hydrogen bond of the retinaldehyde-PE Schiff base (14). Replacing the C20 hydrogen atoms of vitamin A with deuterium atoms (i.e. C20-D3-vitamin A) makes this bond harder to cleave and impedes vitamin A dimerization (14). In this study we sought to determine whether retarding the intrinsic reactivity of vitamin A to dimerize could slow lipofuscin formation in the RPE and delay changes associated with human Stargardt disease. To accomplish this we raised ABCA4−/− mutant albino mice (the mouse model of human Stargardt's disease) on diets containing either C20-D3-vitamin A (the treated group) or vitamin A at its natural isotopic abundance (the control group) and measured the concentration of vitamin A dimers, lipofuscin and other biological markers indicative of ocular health in both groups. Treated mice exhibited an 80% reduction in A2E, a 95% reduction in ATR dimer and a 70% decrease in fundus autofluorescence at three months of age. After six months, the treated group showed fewer lipofuscin granules as visualized qualitatively by electron microscopy, and at 12 months they showed improved eye function as measured by electroretinogram (ERG). These results suggest that pathological phenotypes that arise from defects in the ABCA4 gene may result from the dimerization of vitamin A and may be ameliorated by impeding the ability of vitamin A to dimerize. These results further indicate that administration of C20-D3-vitamin A may be a viable therapeutic approach to prevent vitamin A dimerization and slow the progression of associated retinal diseases.
EXPERIMENTAL PROCEDURES
Compounds
C20-D3-retinyl acetate (the acetate form of vitamin A) was prepared according to the literature (15) and contained 5% D0, 15% D2, and 80% D3 at the C20 position, as measured using APCI (atmospheric pressure chemical ionization) mass spectrometry (MS) by calculating the ratio of peaks at the corresponding values of m/z. A2E was prepared according to the literature (16). Retinol (17) and retinaldehyde (18) were prepared from retinyl acetate according to literature procedures. The structures of all synthesized compounds were verified using 1H,13C NMR spectroscopy and/or MS.
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee of Columbia University and complied with guidelines set forth by the Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals). All animals were housed under 12-h light/dark cycles in environmentally controlled rooms. The ABCA4-null mutant mice (129/Sv × C57BL/6J) used in this study were homozygous for the Rpe65 Leu450 variant and were obtained from a colony housed in the Columbia University Medical Center; they have been described in the literature (19).
Long Term Treatment
For the treated group, C20-D3-retinyl acetate was blended with sucrose and added to a purified diet (at 20,000 IU/kg diet) that was deficient in vitamin A. For the control group, the same amount of retinyl acetate was added to the same base purified diet. Harlan Teklad (Madison, WI) prepared the final diets. ABCA4−/− breeding pairs were divided into two groups and fed the diets, through pregnancy and lactation. We evaluated only the offspring of mice that had been on the above diets for a minimum of six months.
Dietary Exchange of Vitamin A in Mature Mice
As above, C20-D3-retinyl acetate was blended with sucrose and added to a purified diet (at 100,000 IU/kg diet). 2-Month-old ABCA4−/− animals raised on standard rodent chow were then placed on the C20-D3-vitamin A diet for one additional month.
A2E and ATR-dimer Quantification
Tissues were processed and A2E was measured by HPLC as previously described (14). ATR-dimer was simultaneously analyzed at 515 nm by measuring the total area of the peaks that displayed the characteristic UV-Vis spectroscopic absorbance profile of ATR-dimer (20, 21).
Retinoid Quantification
Ocular retinol, retinyl palmitate, and retinaldehyde were quantified using standard HPLC techniques by comparing the HPLC retention times and peak areas of unknown samples with those of known standards. Retinol and retinyl palmitate were detected at 325 nm and retinaldehyde at 380 nm.
Liver Vitamin A Isotopic Ratio
For the quantification of retinyl ester isotopic ratios from liver, 10 mg of liver was homogenized in butanol (100 μl). The homogenates were clarified through centrifugation and then analyzed directly using MS.
Retina Vitamin A Isotopic Ratio
Animals were dark-adapted overnight and four retinas were collected under dark conditions using night vision goggles and a dissecting microscope fitted with a night vision viewer. The retinas were homogenized in butanol (100 μl), the homogenates were clarified through centrifugation and then analyzed directly using MS.
RPE Autofluorescence
Quantification of RPE autofluresence was performed as essentially described by Katz et al. (22). Briefly, under a 10× stereomicroscope with standard illumination, eyecups were dissected out, separated from the retina, and slits were made along the periphery so that they could lay flat. The eyecups were then positioned with the RPE cells up in a 35 × 10 mm round, biomedical-grade polystyrene Petri dish and covered with a 22 × 22 glass coverslip and a phosphate buffered saline solution containing calcium and magnesium (Dulbecco's). All autofluorescence measurements were made under identical conditions over the span of 2 days, using a 10× objective attached to a confocal laser-scanning microscope (Zeiss LSM 510; excitation 488; emission >520 LP). For autofluorescence quantification, two-three images were recorded at different locations that displayed a uniform layer of RPE cells for each retina. Then for each image, mean pixel intensity was measured at four different locations, using the program ImageJ (National Institutes of Health, Bethesda, MD).
Electron Microscopy
For each diet, the right eyes of two 6-month-old animals were evaluated. The eyes were fixed overnight in 4% formaldehyde and 1% glutaraldehyde in 0.1 m phosphate buffer (pH 7.4). They were then immersed in 8% (0.2 m) sucrose in 0.1 m phosphate buffer (3 × 15 min) and post-fixed in 1% osmium tetroxide in 0.1 m phosphate buffer (1 h). The eyes were dehydrated (50% ethanol, 15 min; 70% ethanol, 15 min; 95% ethanol, 15 min; 100% ethanol, 2 × 15 min), embedded (100% propylene oxide, 2 × 15 min; 1:1 EMBed 812:propylene oxide, 1–2 h; 2:1 EMBed 812:propylene oxide, overnight; embedded in beam capsules; baked in an oven at 60 °C, 48 h). The eyes were sectioned (by first cutting thick sections (0.5–1.0 μm), observing the sections under a microscope to determine the precise location of the cuts, and then ultrathin sectioning at a thickness of 60–90 nm) and next the sections were placed onto grids, stained (uranyl acetate, 15 min; lead citrate, 5 min) and observed under an electron microscope. Total area and size of dense electron bodies were measured using imageJ.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
For statistical power, three biological replicates were used for each data point. RNA was isolated in triplicate from 10-pooled eyecups together with the retinas (for a total of 15 animals, or 30 eyes per group) using a Qiagen RNeasy minikit and QIA shredder columns, according to the manufacturer's instructions. One microgram of RNA was reverse transcribed using an RT2 First Strand Kit and applied to PCR array plates (PAMM-077: Mouse Inflammatory Response and Autoimmunity PCR Array from SABiosciences). Plates were processed in an Applied Biosystems 7500 Fast Real-Time PCR System, using automated baseline and threshold cycle detection. RT-PCR results were calculated using the Delta-Delta CT method; data were interpreted using SABioscience web-based PCR array data analysis tool.
ERG Recordings
The light stimulus was delivered from a desktop ganzfeld stimulator (Color Dome, Diagnosys, Littleton, MA) and responses were recorded on the Espion console. Prior to ERG analysis, ophthalmoscopic examination confirmed that all eyes were free of opacities or other gross anomalies. Animals were dark-adapted overnight and all subsequent procedures were performed under a dim, 660-nm LED light source. Pupils were dilated with phenylephrine hydrochloride (2.5%) and cyclopentolate hydrochloride (0.5%) applied topically to the cornea. After 10 min, mice were anesthetized with an intraperitoneal injection of a mixture of ketamine (80 mg kg−1) and xylazine (5 mg kg−1) while corneal hydration was maintained through topical application of methyl cellulose (1 drop, ∼50 mg; topical to cornea; Methocel; Dow Chemical, Zürich, Switzerland). Corneal anesthesia was achieved with tetracaine hydrochloride (0.5%, one drop; Bausch and Lomb). To maintain a body temperature of 37 °C, animals were rested on a homeothermic blanket connected to a temperature control unit (Harvard Apparatus). The active electrodes were fashioned from a 0.005 inch platinum-iridium wire; they were formed in loops and attached to 1.5-mm, female, DIN connectors and placed on the right and left corneas. Electrical contact between the corneas and electrodes was achieved using a drop of 1% methylcellulose. A saline-soaked cotton-wick electrode was placed in the mouth and a needle electrode was placed subcutaneously in the scalp, which served as reference and ground leads, respectively. Recordings were taken simultaneously from both eyes.
Flicker ERG
Flashes were provided by a red (635 ± 25 nm) LED at 0.041μmol m−2 s−1. Animals were presented with three flashes for each frequency. All curves were captured and viewed; curves judged to be excessively noisy were excluded. For each animal, the peak-to-trough amplitude of each harmonic was measured and averaged to obtain the final amplitude for each frequency. Final ERG values for each frequency and age group were obtained by averaging the average amplitude for each frequency measured for each animal in the group. The p values were calculated by using the distribution of the average amplitude for each frequency in the respective group.
ERG a- and b-waves
we presented single flashes of white light of nine intensities from a xenon lamp. For each intensity, we presented five flashes. We measured a-wave amplitudes from baseline-to-trough and b-wave amplitudes from the a-wave trough to the b-wave peak. Final values of a- and b-amplitudes were calculated by averaging the average a- and b-wave amplitudes respectively, for each animal.
Statistical Analysis
Comparisons between groups were made using two-tailed, unpaired t-tests. A p value of less than 0.05 was considered statistically significant. Data are reported as means along with standard deviations. The “n” value denotes the number of animals used in each group for a particular outcome measure.
RESULTS
Ocular A2E and ATR-dimer
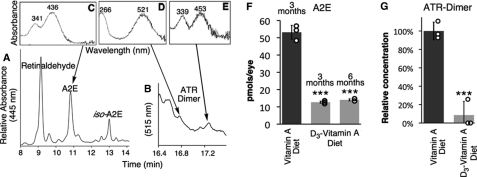
We raised two groups of ABCA4−/− mice on diets containing either C20-D3-all-trans-retinyl acetate (the treated group) or all-trans-retinyl acetate (the control group). At three months of age we measured the concentration of vitamin A dimers contained in the RPE (eyecup) and retina by HPLC (Fig. 1, A–E). In the treated group we measured 13 ± 0.6 pmol of A2E/eye corresponding to 80% less (p < 0.001) A2E (Fig. 1F) than in the control group, which averaged 53 ± 4 pmol of A2E/eye (n = 15 animals for each group). Similarly, we measured 95% less ATR-dimer (Fig. 1G) in the treated animals relative to the controls (n = 15 animals per group, p < 0.001). The concentrations of ATR-dimer in the treated group approached the limit of detection resulting in more deviation in this measurement. At six months of age, we took a second measurement of A2E (Fig. 1F), which showed an unchanged (p > 0.05) amount of A2E in the treated animals between 3 and 6 months. For comparison, 3-month-old albino ICR wild-type mice raised on a standard rodent chow had 15 ± 1 pmol of A2E per eyecup (14). Mice in the treated group therefore resulted in levels of vitamin A dimers comparable to levels seen in healthy wild-type mice raised on standard diets.
FIGURE 1.
C20-D3-vitamin A impedes A2E- and ATR-dimer biosynthesis in a mouse model of Stargardt disease. A and B, representative HPLC traces of retinoids and vitamin A dimers extracted from the eyes of ABCA4−/− mice raised on vitamin A. C–E, UV-Vis spectra of the represented peaks. In this report, A2E refers to both A2E and its geometric isomer, iso-A2E, and ATR-dimer refers to peaks between 16.8 and 17.4 min, those with characteristic absorbance spectra of ATR dimer. Relative concentrations of A2E at 3 and 6 months (F) and ATR-dimer at 3 months (G) in control (vitamin A) and treated (C20-D3-vitamin A) animals. Each smaller open circle represents a measurement for 10-pooled eyecups for a total of n = 15 animals per bar. ***: p < 0.001.
Ocular C20-D3-vitamin A Incorporation
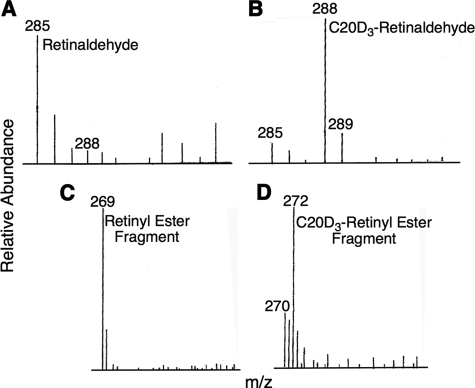
In the retina, vitamin A exists primarily as retinaldehyde. We used MS to confirm the presence of C20-D3-retinaldehyde in the retina of the treated animals. Under MS conditions retinaldehyde was visualized at m/z (mass-to-charge ratios) of 285 in the untreated group (Fig. 2A). In the treated group, in addition to a smaller baseline peak at 285 m/z there was a larger peak at 288 m/z corresponding to C20-D3-retinaldehyde ion (Fig. 2B), suggesting that vitamin A at natural abundance had been substantially replaced with C20-D3-vitamin A in the retina of the treated animals.
FIGURE 2.
Vitamin A is swapped for C20-D3-vitamin A in treated animals. Representative MS of retina (top) and liver (bottom) extracts from animals raised on naturally occurring vitamin A (control group, panels A and C) or on C20-D3-vitamin A (treated group, panels B and D). Numbers above lines are m/z ratios.
Liver C20-D3-vitamin A Incorporation
In mammals, 80–90% of the body's vitamin A is stored in the liver as retinyl esters. Thus to investigate the extent to which body vitamin A had been replaced with C20-D3-vitamin A, we measured the hepatic ratio of deuterated vitamin A to native vitamin A using MS. Under the MS conditions in place, liver retinyl esters from untreated animals fragmented to yield a base peak at m/z 269 (23) (Fig. 2C). We did not observe a base peak at m/z 269 in the animals raised on C20-D3-vitamin A, but instead, we observed a base peak shifted by three mass units to m/z 272 and corresponding to the C20-D3-retinyl ester fragment (Fig. 2D). This result confirms that these animals mainly stored C20-D3-vitamin A and that hydrogen-deuterium exchange was negligable during the use and storage of C20-D3-vitamin A. Additionally, through HPLC analysis we found no statistical difference in the concentrations of liver and ocular retinoids in both groups except that in the treated group the retinoids were enriched with deuterium.
RPE Autofluorescence
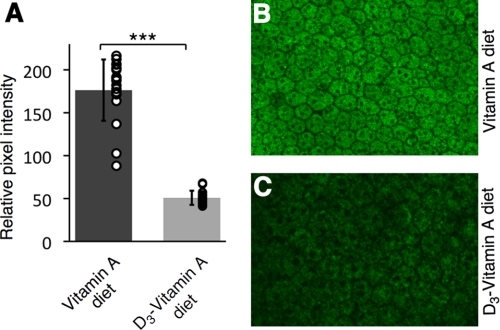
In human Stargardt disease, areas of hyper autofluorescence are frequently observed throughout the retina (24). Fundus autofluorescence, which arises primarily from the RPE, has been used as a clinical marker of disease progression related to age and/or inherited macular degenerations. Thus we compared the RPE autofluorescence of the two groups by examining the flat mounted eyecups using a confocal scanning laser microscope. The treated group exhibited a 70% decrease in mean autofluorescence intensity relative to that of the control (n = 5, p < 0.001) (Fig. 3).
FIGURE 3.
C20-D3-vitamin A reduces RPE autofluorescence in the Stargardt mouse. A, average retinaldehyde autofluorescence from RPE cells (eyecups), from 5 three-month-old mice administered vitamin A (control, left bar) and 5 three-month-old mice administered C20-D3-vitamin A (treatment, right bar). Circles represent individual pixel intensity measurements for each flat mount image. Standard deviations are shown. ***: p < 0.001. B and C, representative confocal fluorescence microscopy images taken under identical conditions of an eyecup from a treated (C) and control animal (B).
Visualization of Lipofuscin Granules
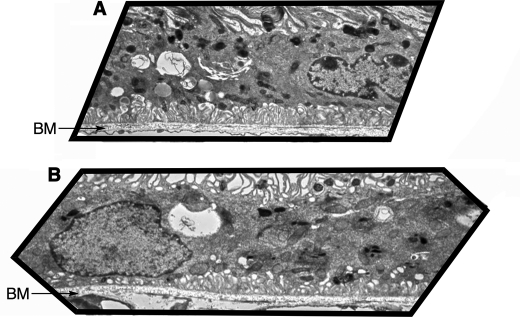
ABCA4−/− mice exhibit a similar ultrastructural pattern to humans with early stage STGD (25). Thus, as a first approximation of retinal health, we examined the number and location of dense lysosomal bodies at 6 months using electron microscopy (Fig. 4). The treated group clearly had fewer-electron-dense lysosomal bodies than the control animals. The accumulation of lysosomal bodies in the control animals was most notable in the apical portions of the cytoplasm, a typical feature of the RPE of aged (24-month) wild-type mice (Fig. 4A) (26). On the other hand, only a few lysosomal bodies, sparsely distributed throughout the cytoplasm, were visible in the RPE of the treated animals (Fig. 4B). This finding is in accord with observations of the RPE layer of younger wild-type animals (26).
FIGURE 4.
C20-D3-vitamin A reduces lipofuscin deposits. Representative electron micrographs of RPE layer from 6-month-old ABCA4−/− mice raised on a diet containing either vitamin A (panel A), revealing electron-dense bodies (dark spots, total relative area of 8147; average relative size 24, area fraction 5.1); and C20-D3-vitamin A (panel B), revealing ∼50% fewer electron-dense bodies, distributed throughout the cytoplasm (total relative area of 4164, average relative size 13, area fraction 2.6). BM: Bruch's membrane. Same magnification level between the two micrographs.
Ocular Inflammation
It is believed that RPE cell debris (lipofuscin) serves as a chronic inflammatory stimulus for complement activation and a potential nucleation site for drusen formation (27–29). Thus at 12 months, we used quantitative RT-PCR to determine whether the decreased ocular lipofuscin in response to treatment altered the expression of inflammatory cytokines and chemokines as well as their receptors. We measured a statistically significant decrease in C4b (by 1.51-fold; p = 0.02) and IL-7 (by 1.46-fold; p = 0.04) expression and an increased expression of CCr1 (by 2.57-fold; p = 0.04), Lt-α (by 2.14-fold; p = 0.04) and Ly96 (1.1-fold; p = 0.01) for treated mice compared with controls (n = 15 animals or 30 eyes per group, Fig. 5). The remaining 77 genes of the PAMM-077 Mouse Inflammatory Response and Autoimmunity PCR Array showed no statistically significant difference between the two groups (p > 0.05).
FIGURE 5.
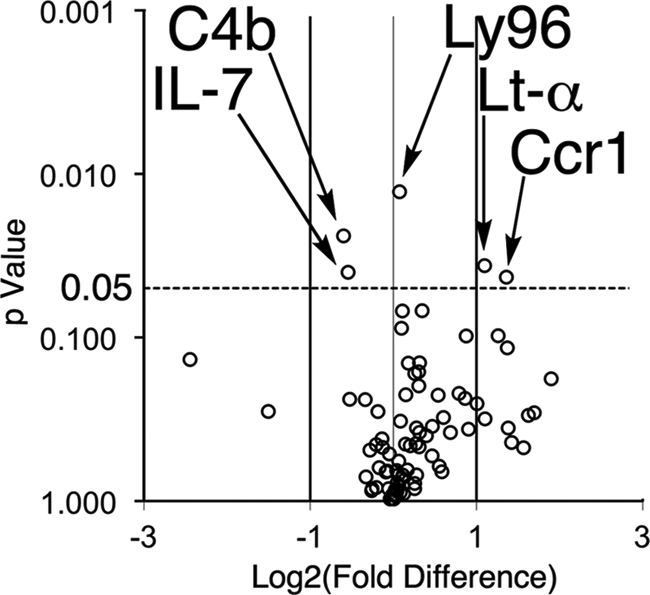
Animals raised on C20-D3-vitamin A have altered ocular gene expression. Volcano plot of p values correlated to fold change in gene expression in 1-year-old treated animals relative to controls, as measured using qRT-PCR. Experiments were done in triplicate with a sample size of n = 15 animals per group or 30 eyes.
Electrophysiology
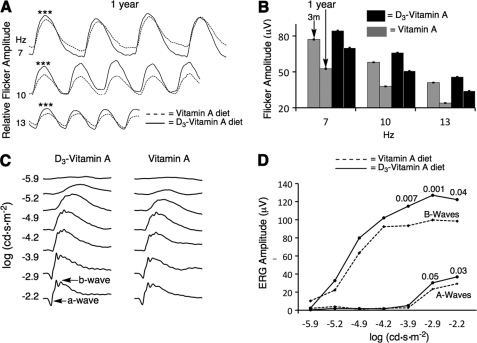
To evaluate eye health, we measured electroretinogram (ERG) amplitudes in dark-adapted mice in response to red light flickering at various frequencies. We chose to use longer-wavelength red light, which is poorly absorbed by pre-retina tissue, to minimize potential differences in pre-retina filtering between the two groups of animals. Fig. 6A summarizes the ERG amplitudes in response to a red light flickering at 7, 10, and 13 flashes per second (Hz) for treated and control mice at one year of treatment. The treated animals had statistically larger flicker ERG responses relative to those of the control group for all three of the frequencies tested (n = 10 for the control and n = 15 for the treated group, p < 0.001). Fig. 6C summarizes the flicker recording measured at one year (data from panel 6A) and three months. In three months animals, there was no statistical significance in the ERG amplitudes of the two groups. However, upon aging, the flicker response decreased. This decrease in amplitude was more pronounced for the control animals: the 7-, 10-, and 13-Hz flicker responses decreased by 31, 34, and 42%, respectively upon aging. On the other hand, for the treated animals, the 7-, 10-, and 13-Hz flicker responses decreased by only 16, 23, and 26%, respectively, showing a significant (p < 0.001) delay in the age-related decline in ERG signal.
FIGURE 6.
C20-D3-vitamin A delays electrophysiological retinal deterioration in a mouse model of Stargardt disease. A, average flicker ERG curves in response to 635 ± 25 nm light flickering at different frequencies, at one year. n = 10 animals were used for the vitamin A group (control) and n = 15 for the C20-D3-vitamin A group (treatment). ***: p < 0.001. B, change in flicker ERG amplitude upon aging for control and treated mice. The first bar of each grouping represents 3-month-olds and the second bar 1-year-olds (data from panel A). At 3 months, the difference between the control and treated groups were not statistically significant. Standard errors are shown. C, average ERG curves in response to a single flash of white light for 1-year-old animals raised on the two diets. D, ERG intensity response curves for the a- (lower curves) and b-waves (top curves) corresponding to ERG curves shown in panel C. The numbers above the curves are p values.
We also measured the dark-adapted ERG response to a single flash of white light ranging in intensity over five log units after one year of treatment (Fig. 6C). From intensity response curves (Fig. 6D) we calculated a- and b-wave maximum amplitudes. Treated animals had significantly larger maximum amplitudes (7 μV larger for the a-wave; 30 μV larger for the b-wave) compared with the untreated animals.
Dietary Exchange of Vitamin A in Mature Mice
In the above series of experiments, we used animals that had been raised from birth on diets containing either native or C20-D3-vitamin A. To replicate more realistic conditions, we measured whether a change in dietary vitamin A from the standard form to C20-D3-vitamin A in adult mice could also slow A2E-biosynthesis. Thus we placed two-month-old ABCA4−/− animals raised on a standard commercial diet on a new diet containing 100,000 IU of deuterated vitamin A/kg feed, approximately five times the amount of vitamin A contained in most commercial diets. After one month on this diet, we compared retinal A2E levels to age-matched animals raised on a standard rodent diet. The treated animals had ∼60% less (p < 0.001) A2E relative to animals raised on the standard rodent diet (20 ± 5 pmol/eye in the treated group versus 53 ± 4 pmol/eye for the untreated group), this level was only slightly higher (20%) than the level of A2E in three-months-old animals raised exclusively on C20-D3-vitamin A from birth. By use of MS we determined that after one month of treatment, 35% of liver vitamin A was the deuterated form.
DISCUSSION
The ABCA4−/− mouse was created as an animal model of Stargardt disease (25). In this work, we raised two groups of ABCA4−/− mice on diets containing vitamin A as either C20-D3-all-trans-retinyl acetate (the treated group) or all-trans-retinyl acetate at its natural isotopic abundance (the control group). While both groups received the same amount of their respective vitamin A, deuteration of vitamin A at the C20 position impeded vitamin A dimerization as measured by a significant decrease in A2E and ATR-dimer, and was consistent with previously reported data for wild-type mice (14). The decrease in RPE autofluorescence in the treated group, as measured by confocal laser scanning microscopy, suggested a decrease in RPE lipofuscin granule concentration, a conclusion that was further supported by a decrease in electron dense bodies observed in electron micrographs of RPE cells. As these mice are albino, melanolysosomes were most likely not a significant contributor to these electron dense deposits. Autofluorescence data seemed to indicate that deuterium incorporation at carbon C20 of vitamin A hindered the formation of lipofuscin deposits. Vitamin A dimers themselves comprise only a small fraction of lipofuscin granules (0.019–0.024%)(30,31), have different fluorescence quantum yields, lifetimes and profiles relative to those of lipofuscin granules, and unlike the granules, their autofluorescence diminishes upon exposure to light (32).
The C4b gene encodes the basic form of complement factor 4. The observed decrease in C4b expression measured by qPCR in the treated animals was also in accord with decreased vitamin A dimers and lipofuscin. Indeed, prior work has suggested that vitamin A dimers can activate the complement cascade (33) and that C4b expression is increased in aged mouse retinas (34, 35). Although the exact roles of C4b and of the previously mentioned inflammatory genes in macular degeneration and retinal heath are not fully understood, it could be suggested that there was an effect of long-term C20-D3-vitamin A administration on ocular inflammation when compared with control animals raised on vitamin A at natural abundance. Future work elucidating the role of these genes will be needed to further interpret these results.
The ERG records the summation of electrical responses by the eye in response to light stimuli and is widely used as a measure of retinal health. The a-wave is thought to reflect photoreceptor function while the b-wave and the flicker response are thought to originate from neurons in the retina that are postsynaptic to the photoreceptors and reflect ON-bipolar cell function, but can also be used as sensitive indicators of photoreceptor function (36). ABCA4−/− animals have been reported to exhibit slow, ongoing photoreceptor degeneration as measured by the ERG a-wave (25) and outer nuclear layer (ONL) thickness (37) at twelve months of age. This age related decline in ERG amplitude may be greater (38) or similar in magnitude (39, 40) when compared with wild-type animals depending on the reference study. Indeed, direct comparisons with a “wild-type” control can be complicated by the fact that phenotypic variability may be caused by alleles, which are not per se linked to the target locus (41, 42). For example, there have been conflicting reports on whether ABCA4−/− mice exhibit slower (4, 25) or faster (43) ERG dark-adaptation times depending on the wild-type animal used as controls. For this study, in the absence of a congenic ABCA4−/− strain, we compared ERG changes between two groups of genetically matched animals. Because the most pronounced difference between the control and treated animals was the amount of ocular vitamin A dimers and lipofuscin, we propose that the larger ERG responses from the treated animals provide evidence that retinal function in this animal model of Stargardt disease could be preserved through the slowing of vitamin A dimerization and presumably lipofuscin accumulation.
C20-D3-vitamin A is attractive considering that deuterium incorporation at the C20 position is not expected to interfere with the endogenous role of vitamin A in the body, as the C20 carbon hydrogen bond does not seem to be cleaved during normal vitamin A metabolism (44). Our observation that the C20 deuteriums were not exchanged for hydrogens in vitamin A isolated from the livers of animals raised exclusively on C20-D3-vitamin A, supports the notion that this bond is not involved in vitamin A processing except in the synthesis of vitamin A dimers. We have now raised several generations of animals exclusively on C20-D3-vitamin A and have not noticed any abnormalities (i.e. clean, sleek, well groomed fur, and good skin and mucosal color, alert, socially active, and tended to explore the cage perimeter). Furthermore, deuterium-enriched vitamin A has been used safely in humans for over 20 years to study the biological role of vitamin A, identify its metabolites and establish its pharmacokinetics and pharmacodynamics (45). Thus, we do not expect long-term consumption of C20-D3-vitamin A to produce any side effects outside of those developed from normal vitamin A consumed under widely recognized safe doses. The fact that we also observed a 60% decrease in A2E in the study involving adult mice fed with C20-D3-vitamin A for 1 month suggests that lipofuscin pigment biosynthesis can be slowed soon after administration of C20-D3-vitamin A, before liver reserves are completely swapped to the deuterated form of the vitamin. This may be the result of faster exchange of new ingested dietary vitamin A with the RPE versus liver reserves and is consistent with prior work on wild-type mice (14). A similar effect in humans would make C20-D3-vitamin A an attractive clinical approach to delay vitamin A dimerization.
In humans (16, 46–48) and rodent models (25, 49–53) of macular degeneration, high levels of vitamin A dimers correlate with poor retina and RPE health. Using in vitro models, vitamin A dimers have been shown to destabilize lipid membranes (54), act as a retinoid X receptor (RXR) agonist (55), act as RPE65 antagonist, activate the complement cascade (56), inhibit cholesterol metabolism (57), deactivate mitochondria, inhibit phagocytosis (58), act as pro-oxidants (59), associate with DNA (60, 61) and form peroxides (62, 63). Nevertheless, whether vitamin A dimers contribute to, are merely a symptom of, or even protect against retinal degeneration is still controversial (64, 65). This work suggests that slowing down vitamin A dimerization may delay age-related ocular changes and supports the notion that these dimers do indeed contribute to retinal degeneration.
Notably, our data suggest that dimerization of retinaldehyde may contribute to the formation of ocular lipofuscin. This conclusion is consistent with observations noted in animals raised on vitamin A deficient diets (22), in animals (67) and humans (68) with RPE65 genetic defects, and in animals administered vitamin A cycle antagonists (TDH, acutane, Ret-NH2) (69–71) or with impeded delivery of ocular vitamin A (Fenretinide) (72). In all of the above conditions, the concentration of vitamin A in the retina was decreased along with the amount of RPE lipofuscin. However, vitamin A is a potent physiological modulator (73), and vitamin A deficiency or visual cycle inhibition could in theory result in a decrease in lipofuscin by a variety of mechanisms. In this work, we show that vitamin A dimerization and lipofuscin formation can both be slowed by decreasing the reactivity of vitamin A while keeping its concentration and presumably its processing by the visual cycle the same.
To the best of our knowledge, this is the first report of a slowing of electrophysiological retinal degeneration, as measured by ERG, in a mouse model of Stargardt and suggests that reducing the ability of vitamin A to dimerize may slow age-related retinal physiological changes. Prior physiological evidence demonstrating the toxicity of vitamin A dimers and lipofuscin has been elusive. In this report we present evidence that is in accord with the general hypothesis that lipofuscin is not benign but can impair cellular function and contribute to retinal degeneration. Psychophysical measurements of visual function are of future interest.
Data presented here also indicate that hampering the activity of retinaldehyde through deuterium incorporation may be a promising strategy for slowing vitamin A dimerization and lipofuscin formation resulting from ABCA4 mutations. The overall strategy is outlined in Fig. 7. It has been estimated that one in 20 people carry a disease-associated ABCA4 allele (74–76), and ABCA4 mutations are believed to be responsible for a large variety of retinal degenerations. Although these mutations most commonly result in Stargardt disease and fundus flavimaculatus, some forms of cone-rod degeneration, retinitis pigmentosa and an increased risk of developing age-related macular degeneration (AMD) have been linked to ABCA4 mutations (77, 78). In all of these diseases, the concentrations of vitamin A dimers are above normal. Other retinal diseases are also marked by excessive accumulation of vitamin A dimers, such as Best disease (47) and potentially others.
FIGURE 7.
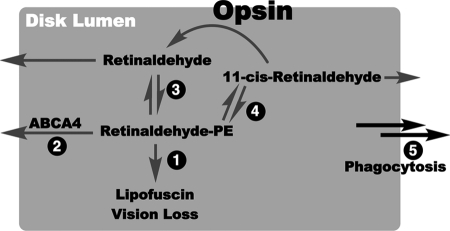
Hypothesized model of lipofuscin prevention by C20-D3-vitamin A. Pathways for retinaldehyde/phosphatidylethanolamine Schiff base (Retinaldehyde-PE) clearance from the disk lumen. The shaded area represents the disk lumen. Clearance pathways are marked 1–5. Arrows going outside the gray area represent movement out of the disk lumen. In theory, the retinaldehyde-PE Schiff base that forms in the rod outer segment after photon isomerization may follow several pathways: 1) it can react to form A2E leading to lipofuscin; 2) it may be actively (ABCA4 mediated) or passively (diffusion) transported out of the disk lumen; 3) it may dissociate back to retinaldehyde and PE and retinaldehyde may then be actively or passively transported out of the disk lumen (79); 4) it may isomerize, dissociate and the generated 11-cis-retinaldehyde may recombine with opsin to form rhodopsin (66) or 11-cis-retinaldehyde and its other isomers may be actively or passively transported out of the disk lumen; and lastly 5) it may be delivered to the RPE directly when the outer segment is phagocytosed. In pathways 2–5 retinaldehyde can presumably be reincorporated back into the visual cycle. Once outside the disk lumen retinaldehyde can be reduced to retinol. In the case of ABCA4 defects leading to Stargardt disease pathway two is slowed resulting in, presumably, an increase in pathway one leading initially to vitamin A dimerization, lipofuscin formation and ultimately vision loss. By the incorporation of deuterium atoms at the C20 position of vitamin A, we have shown in prior work that pathway one becomes higher in energy, and as a result is slowed down. This should allow more time for retinaldehyde to be re-incorporated into the visual cycle via pathways 2–5. Through this mechanism, the use of C20-deuterated vitamin A may be an effective methodology toward mitigating the clinical consequences of alterations in the ABCA4 gene.
In inherited or age related macular degenerations and dystrophies, the mechanism by which vitamin A dimerizes requires the breaking of a C20-H bond of vitamin A. As such, deuterium incorporation at the C20 position should slow vitamin A dimerization in all of these pathologies. Although the exact role of vitamin A dimers such as A2E in the formation of lipofuscin and in the pathogenesis of macular degeneration is still yet to be fully elucidated, the concentration of ocular vitamin A dimers increase with age in all people, early during the pathogenesis of Stargardt disease and in other inherited macular dystrophies and are widely believed to be detrimental. As a result there is a rationale for clinically preventing vitamin A dimerization as a means of preventing vision loss.
Acknowledgments
We thank Theresa Swayne for technical assistance and Rando Allikmets and Leonide Saad for comments.
This work was supported by the International Retinal Research Foundation.
- RPE
- retinal pigment epithelium
- A2E
- N-retinylidene-N-retinylethanolamine
- ATR-dimer
- all-trans-retinaldehyde dimer.
REFERENCES
- 1. Fishman G. A., Farber M., Patel B. S., Derlacki D. J. (1987) Ophthalmology 94, 809–814 [DOI] [PubMed] [Google Scholar]
- 2. Rotenstreich Y., Fishman G. A., Anderson R. J. (2003) Ophthalmology. 110, 1151–1158 [DOI] [PubMed] [Google Scholar]
- 3. Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. (1999) Cell 98, 13–23 [DOI] [PubMed] [Google Scholar]
- 4. Mata N. L., Tzekov R. T., Liu X., Weng J., Birch D. G., Travis G. H. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1685–1690 [PubMed] [Google Scholar]
- 5. Gouras P., Ivert L., Mattison J. A., Ingram D. K., Neuringer M. (2008) Graefes. Arch. Clin. Exp. Ophthalmol. 246, 1403–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burns R. P., Feeney-Burns L. (1980) Trans. Am. Ophthalmol. Soc. 78, 206–225 [PMC free article] [PubMed] [Google Scholar]
- 7. Ishibashi T., Sorgente N., Patterson R., Ryan S. J. (1986) Invest. Ophthalmol. Vis. Sci. 27, 184–193 [PubMed] [Google Scholar]
- 8. Spencer W. H. (1965) Trans. Am. Acad. Ophthalmol. Otolaryngol. 69, 662–667 [PubMed] [Google Scholar]
- 9. Kennedy C. J., Rakoczy P. E., Constable I. J. (1995) Eye. 9, 763–771 [DOI] [PubMed] [Google Scholar]
- 10. Sparrow J. R., Boulton M. (2005) Exp. Eye Res. 80, 595–606 [DOI] [PubMed] [Google Scholar]
- 11. de Jong P. T. (2006) N. Engl. J. Med. 355, 1474–1485 [DOI] [PubMed] [Google Scholar]
- 12. Sparrow J. R., Fishkin N., Zhou J., Cai B., Jang Y. P., Krane S., Itagaki Y., Nakanishi K. (2003) Vision Res. 43, 2983–2990 [DOI] [PubMed] [Google Scholar]
- 13. Lamb L. E., Simon J. D. (2004) Photochem. Photobiol. 79, 127–136 [DOI] [PubMed] [Google Scholar]
- 14. Kaufman Y., Ma L., Washington I. (2011) J. Biol. Chem. 286, 7858–7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergen H. R., Furr H. C., Olson J. A. (1998) J. Labelled Compd. Radiopharm. 25, 11–21 [Google Scholar]
- 16. Parish C. A., Hashimoto M., Nakanishi K., Dillon J., Sparrow J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14609–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Isler O., Ronco A., Guex W., Hindley N. C., Huber W., Dialer K., Kofler M. (1949) Helv. Chim. Acta. 32, 489–505 [DOI] [PubMed] [Google Scholar]
- 18. Henbest H. B., Jones E. R., Owen T. C. (1957) J. Chem. Soc. 4909–4912 [Google Scholar]
- 19. Kim S. R., Fishkin N., Kong J., Nakanishi K., Allikmets R., Sparrow J. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 11668–11672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fishkin N. E., Sparrow J. R., Allikements R., Nakanishi K. (2005) Proc. Natl. Acad. Sci. U.S.A. 100, 7091–7096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim S. R., Jang Y. P., Jockusch S., Fishkin N. E., Turro N. J., Sparrow J. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19273–19278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Katz M. L., Drea C. M., Robison W. G., Jr. (1986) Mech. Ageing. Dev. 35, 291–305 [DOI] [PubMed] [Google Scholar]
- 23. Wingerath T., Kirsch D., Spengler B., Kaufmann R., Stahl W. (1997) Anal. Chem. 69, 3855–3860 [DOI] [PubMed] [Google Scholar]
- 24. Gomes N. L., Greenstein V. C., Carlson J. N., Tsang S. H., Smith R. T., Carr R. E., Hood D. C., Chang S. (2009) Invest. Ophthalmol. Vis. Sci. 50, 3953–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weng J., Mata N. L., Azarian S. M., Tzekov R. T., Birch D. G., Travis G. H. (1999) Cell 9, 13–23 [DOI] [PubMed] [Google Scholar]
- 26. Mishima H., Kondo K. (1981) Albrecht. Von. Graefes. Arch. Klin. Exp. Ophthalmol. 216, 209–217 [DOI] [PubMed] [Google Scholar]
- 27. Donoso L. A., Kim D., Frost A., Callahan A., Hageman G. (2006) Surv. Ophthalmol. 51, 137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anderson D. H., Radeke M. J., Gallo N. B., Chapin E. A., Johnson P. T., Curletti C. R., Hancox L. S., Hu J., Ebright J. N., Malek G., Hauser M. A., Bowes Rickman C., Bok D., Hageman G. S., Johnson L. V. (2010) Prog. Retin. Eye. Res. 29, 95–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sivaprasad S., Chong N. V. (2006) Eye 20, 867–872 [DOI] [PubMed] [Google Scholar]
- 30. Rózanowska M., Pawlak A., Rózanowski B., Skumatz C., Zareba M., Boulton M. E., Burke J. M., Sarna T., Simon J. D. (2004) Invest. Ophthalmol. Vis. Sci. 45, 1052–1060 [DOI] [PubMed] [Google Scholar]
- 31. Davies S., Elliott M. H., Floor E., Truscott T. G., Zareba M., Sarna T., Shamsi F. A., Boulton M. E. (2001) Free. Radic. Biol. Med. 31, 256–265 [DOI] [PubMed] [Google Scholar]
- 32. Gaillard E. R., Atherton S. J., Eldred G., Dillon J. (1995) Photochem. Photobiol. 61, 448–453 [DOI] [PubMed] [Google Scholar]
- 33. Mullins R. F., Russell S. R., Anderson D. H., Hageman G. S. (2000) FASEB J. 14, 835–846 [PubMed] [Google Scholar]
- 34. Chen M., Muckersie E., Forrester J. V., Xu H. (2010) Invest. Ophthalmol. Vis. Sci. 51, 5888–5898 [DOI] [PubMed] [Google Scholar]
- 35. Chen H., Liu B., Lukas T. J., Neufeld A. H. (2008) PLoS One 3, e2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lei B., Chang B. (2003) Invest. Ophthalmol. Vis. Sci. 44, U304–U304 [Google Scholar]
- 37. Wu L., Nagasaki T., Sparrow J. R. (2010) Adv. Exp. Med. Biol. 664, 533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bayer A. U., Neuhardt T., May A. C., Martus P., Maag K. P., Brodie S., Lütjen-Drecoll E., Podos S. M., Mittag T. (2001) Invest. Ophthalmol. Vis. Sci. 42, 1258–1265 [PubMed] [Google Scholar]
- 39. Li C., Cheng M., Yang H., Peachey N. S., Naash M. I. (2001) Optom. Vis. Sci. 78, 425–430 [DOI] [PubMed] [Google Scholar]
- 40. Gresh J., Goletz P. W., Crouch R. K., Rohrer B. (2003) Vis. Neurosci. 20, 211–220 [DOI] [PubMed] [Google Scholar]
- 41. Olson E. N., Arnold H. H., Rigby P. W., Wold B. J. (1996) Cell 85, 1–4 [DOI] [PubMed] [Google Scholar]
- 42. Sigmund C. D. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1425–1429 [DOI] [PubMed] [Google Scholar]
- 43. Pawar A. S., Qtaishat N. M., Little D. M., Pepperberg D. R. (2008) Invest. Ophthalmol. Vis. Sci 49, 2743–2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. (1996) J. Cell. Biochem. Suppl. 26, 269–307 [PubMed] [Google Scholar]
- 45. Furr H. C., Green M. H., Haskell M., Mokhtar N., Nestel P., Newton S., Ribaya-Mercado J. D., Tang G., Tanumihardjo S., Wasantwisut E. (2005) Public Health Nutr. 8, 596–607 [DOI] [PubMed] [Google Scholar]
- 46. Eldred G. E., Lasky M. R. (1993) Nature 361, 724–726 [DOI] [PubMed] [Google Scholar]
- 47. Bakall B., Radu R. A., Stanton J. B., Burke J. M., McKay B. S., Wadelius C., Mullins R. F., Stone E. M., Travis G. H., Marmorstein A. D. (2007) Exp. Eye. Res. 85, 34–43 [DOI] [PubMed] [Google Scholar]
- 48. Mata N. L., Weng J., Travis G. H. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 7154–7159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Karan G., Lillo C., Yang Z., Cameron D. J., Locke K. G., Zhao Y., Thirumalaichary S., Li C., Birch D. G., Vollmer-Snarr H. R., Williams D. S., Zhang K. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 4164–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ambati J., Anand A., Fernandez S., Sakurai E., Lynn B. C., Kuziel W. A., Rollins B. J., Ambati B. K. (2003) Nat. Med. 9, 1390–1397 [DOI] [PubMed] [Google Scholar]
- 51. Sparrow J. R., Yoon K. D., Wu Y., Yamamoto K. (2010) Invest. Ophthalmol. Vis. Sci. 51, 4351–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chan C. C., Ross R. J., Shen D., Ding X., Majumdar Z., Bojanowski C. M., Zhou M., Salem N., Jr., Bonner R., Tuo J. (2008) Ophthalmic. Res. 40, 124–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maeda A., Golczak M., Maeda T., Palczewski K. (2009) Invest. Ophthalmol. Vis. Sci. 50, 5435–5443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De S., Sakmar T. P. (2002) J. Gen. Physiol. 120, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Iriyama A., Fujiki R., Inoue Y., Takahashi H., Tamaki Y., Takezawa S., Takeyama K., Jang W. D., Kato S., Yanagi Y. (2008) J. Biol. Chem. 283, 11947–11953 [DOI] [PubMed] [Google Scholar]
- 56. Zhou J., Jang Y. P., Kim S. R., Sparrow J. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16182–16187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lakkaraju A., Finnemann S. C., Rodriguez-Boulan E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11026–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Finnemann S. C., Leung L. W., Rodriguez-Boulan E. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3842–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dontsov A. E., Sakina N. L., Bilinska B., Krzyzanowski L., Feldman T. B., Ostrovsky M. A. (2005) Dokl. Biochem. Biophys. 405, 458–460 [DOI] [PubMed] [Google Scholar]
- 60. Sparrow J. R., Zhou J., Cai B. (2003) Invest. Ophthalmol. Vis. Sci. 44, 2245–2251 [DOI] [PubMed] [Google Scholar]
- 61. Sparrow J. R., Vollmer-Snarr H. R., Zhou J., Jang Y. P., Jockusch S., Itagaki Y., Nakanishi K. (2003) J. Biol. Chem. 278, 18207–18213 [DOI] [PubMed] [Google Scholar]
- 62. Ben-Shabat S., Itagaki Y., Jockusch S., Sparrow J. R., Turro N. J., Nakanishi K. (2002) Angew. Chem. Int. Ed. Engl. 41, 814–817 [DOI] [PubMed] [Google Scholar]
- 63. Washington I., Jockusch S., Itagaki Y., Turro N. J., Nakanishi K. (2005) Angew. Chem. Int. Ed. Engl. 44, 7097–7100 [DOI] [PubMed] [Google Scholar]
- 64. Maeda A., Maeda T., Golczak M., Chou S., Desai A., Hoppel C. L., Matsuyama S., Palczewski K. (2009) J. Biol. Chem. 284, 15173–15183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wielgus A. R., Chignell C. F., Ceger P., Roberts J. E. (2010) Photochem. Photobiol. 86, 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Groenendijk G. W., Jacobs C. W., Bonting S. L., Daemen F. J. (1980) Eur. J. Biochem. 106, 119–128 [DOI] [PubMed] [Google Scholar]
- 67. Katz M. L., Redmond T. M. (2001) Invest. Ophthalmol. Vis. Sci. 42, 3023–3030 [PubMed] [Google Scholar]
- 68. Lorenz B., Wabbels B., Wegscheider E., Hamel C. P., Drexler W., Preising M. N. (2004) Ophthalmology 111, 1585–1594 [DOI] [PubMed] [Google Scholar]
- 69. Maiti P., Kong J., Kim S. R., Sparrow J. R., Allikmets R., Rando R. R. (2006) Biochemistry 45, 852–860 [DOI] [PubMed] [Google Scholar]
- 70. Radu R. A., Mata N. L., Nusinowitz S., Liu X., Sieving P. A., Travis G. H. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4742–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Golczak M., Maeda A., Bereta G., Maeda T., Kiser P. D., Hunzelmann S., von Lintig J., Blaner W. S., Palczewski K. (2008) J. Biol. Chem. 283, 9543–9554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Radu R. A., Han Y., Bui T. V., Nusinowitz S., Bok D., Lichter J., Widder K., Travis G. H., Mata N. L. (2005) Invest. Ophthalmol. Vis. Sci. 46, 4393–4401 [DOI] [PubMed] [Google Scholar]
- 73. Katz M. L., Eldred G. E., Robison W. G., Jr. (1987) Mech. Ageing. Dev. 39, 81–90 [DOI] [PubMed] [Google Scholar]
- 74. Yatsenko A. N., Shroyer N. F., Lewis R. A., Lupski J. R. (2001) Hum. Genet. 108, 346–355 [DOI] [PubMed] [Google Scholar]
- 75. Jaakson K., Zernant J., Kulm M., Hutchinson A., Tonisson N., Glavac D., Ravnik-Glavac M., Hawlina M., Meltzer M. R., Caruso R. C., Testa F., Maugeri A., Hoyng C. B., Gouras P., Simonelli F., Lewis R. A., Lupski J. R., Cremers F. P., Allikmets R. (2003) Hum. Mutat. 22, 395–403 [DOI] [PubMed] [Google Scholar]
- 76. Maugeri A., van Driel M. A., van de Pol D. J., Klevering B. J., van Haren F. J., Tijmes N., Bergen A. A., Rohrschneider K., Blankenagel A., Pinckers A. J., Dahl N., Brunner H. G., Deutman A. F., Hoyng C. B., Cremers F. P. (1999) Am. J. Hum. Genet. 64, 1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Klevering B. J., Deutman A. F., Maugeri A., Cremers F. P., Hoyng C. B. (2005) Graefes. Arch. Clin. Exp. Ophthalmol. 243, 90–100 [DOI] [PubMed] [Google Scholar]
- 78. Koenekoop R. K. (2003) Ophthalmic. Genet. 24, 75–80 [DOI] [PubMed] [Google Scholar]
- 79. Rando R. R., Bangerter F. W. (1982) Biochem. Biophys. Res. Commun. 104, 430–436 [DOI] [PubMed] [Google Scholar]