Abstract
l-Galactose (l-Gal), a monosaccharide involved in l-ascorbate and rhamnogalacturonan II (RG-II) biosynthesis in plants, is produced in the cytosol by a GDP-d-mannose 3,5-epimerase (GME). It has been recently reported that the partial inactivation of GME induced growth defects affecting both cell division and cell expansion (Gilbert, L., Alhagdow, M., Nunes-Nesi, A., Quemener, B., Guillon, F., Bouchet, B., Faurobert, M., Gouble, B., Page, D., Garcia, V., Petit, J., Stevens, R., Causse, M., Fernie, A. R., Lahaye, M., Rothan, C., and Baldet, P. (2009) Plant J. 60, 499–508). In the present study, we show that the silencing of the two GME genes in tomato leaves resulted in approximately a 60% decrease in terminal l-Gal content in the side chain A of RG-II as well as in a lower capacity of RG-II to perform in muro cross-linking. In addition, we show that unlike supplementation with l-Gal or ascorbate, supplementation of GME-silenced lines with boric acid was able to restore both the wild-type growth phenotype of tomato seedlings and an efficient in muro boron-mediated cross-linking of RG-II. Our findings suggest that developmental phenotypes in GME-deficient lines are due to the structural alteration of RG-II and further underline the crucial role of the cross-linking of RG-II in the formation of the pectic network required for normal plant growth and development.
Keywords: Carbohydrate Biosynthesis; Carbohydrate Function; Carbohydrate Structure; Cell Wall; Galactose; GDP-d-mannose 3,5-Epimerase; l-Galactose; Rhamnogalacturonan II
Introduction
In plant cells, l-galactose (l-Gal) is a monosaccharide that is synthesized by epimerization of GDP-d-mannose into GDP-l-galactose (GDP-l-Gal) via the action of a cytosolic GDP-d-mannose 3,5-epimerase (GME,2 EC 5.1.3.18) (1). GDP-l-Gal is used for the biosynthesis of l-ascorbate and cell wall polysaccharides. It can be converted into free l-Gal in the cytosol by a specific phosphorylase and phosphatase, and then into l-ascorbate (2, 3). Alternatively, GDP-l-Gal can be transferred into the Golgi apparatus and incorporated into cell wall polymers by the action of l-galactosyltransferases. So far, l-Gal has mainly been identified in rhamnogalacturonan II (RG-II) (4).
Much attention has been paid to the biosynthesis of l-ascorbate to investigate the function of this vitamin as an antioxidant and as a regulator of plant development. No viable ascorbate-less plants have ever been reported, thereby suggesting that vitamin C may be important for plant growth. Recently, it has been demonstrated that the partial inactivation of the GME in RNAi-silenced tomato (Solanum lycopersicum) lines resulted in a 40–60% decrease of l-ascorbate content as well as growth defects affecting both cell division and cell expansion (5). These transgenic tomato lines also exhibited increased fragility and loss of fruit firmness. Furthermore, supplementation of RNAi-silenced lines with l-Gal was able to restore the wild-type levels of l-ascorbate but did not rescue the growth defects (5). Together, these data suggest that growth phenotypes in GME-deficient lines are most likely related to the alteration of l-Gal-containing polysaccharides such as RG-II rather than to l-ascorbate deficiency.
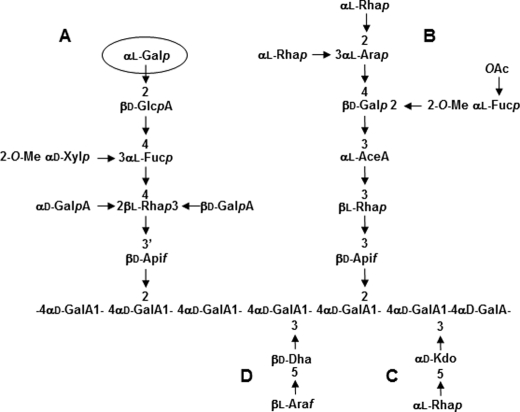
Pectins are complex acidic polysaccharides of the primary cell wall containing three distinct domains: homogalacturonan, rhamnogalacturonan I (RG-I), and RG-II. RG-II is the most structurally complex pectic polysaccharide and is composed of an α-1,4-linked homogalacturonan backbone substituted with four structurally different oligosaccharide side chains (A–D) (Fig. 1) (6). Twelve different glycosyl residues are present in RG-II, including 3-deoxy-d-manno-octulosonic acid (Kdo), aceric acid, apiose, and 3-deoxy-d-lyxo-heptulosonic acid (7, 8). In addition, l-arabinose exists in both pyranose and furanose forms. Furthermore, the hexose residue located at the nonreducing end of the side chain A and originally reported as a d-galactose, has been more recently shown to be in the l-configuration (Fig. 1) (4). Despite its high complexity, RG-II is evolutionary conserved as it is present in the primary cell wall of all vascular plants (6). In the primary cell wall, RG-II exists predominantly as a dimer that is cross-linked by a borate diester between two apiosyl residues of side chain A (7–10). This boron-mediated cross-linking of RG-II induces the in planta formation of a three-dimensional pectic network that is believed to regulate cell wall properties and plant growth (11, 12). To date, it has been shown that any mutation that affects the RG-II structure modifies RG-II dimerization and plant development (13, 14). The link between RG-II dimerization and plant development has been deduced mainly from the study of mutants affected in the biosynthesis of RG-II monomers. For example, the analysis of mur1, an Arabidopsis mutant deficient in the synthesis of l-fucose, was the first study demonstrating the relationship between the RG-II cross-linking in the primary cell wall and plant growth (13). In that report, the authors demonstrated that both the dwarf phenotype and RG-II cross-linking deficiency can be reversed by supplying a high concentration of boric acid to the mutant. In addition, a study of the depletion of UDP-d-apiose/UDP-d-xylose synthases confirmed the importance of RG-II structural integrity for normal growth in vascular plants (14). Moreover, the inactivation of Kdo synthesis resulted in nonviable null mutants. Pollen tubes from mutants impaired in the cytosolic d-arabinose-5-P isomerase or Kdo-8-P synthase were unable to elongate properly and accomplish fertilization (15, 16). Altogether, these data demonstrate that RG-II is important for normal plant development.
FIGURE 1.
Glycosyl sequence of RG-II. RG-II is composed of an α-1,4-linked homogalacturonan backbone that is substituted with four side chains A–D. RG-II contains a terminal α(1,2)-l-Gal (circled) on side chain A.
In the present study, we report that GME-silenced tomato lines display an alteration of the RG-II structure and a reduced in muro dimerization. In addition, we show that supplementation of these lines with boric acid is able to restore both normal growth in tomato seedlings and normal in muro cross-linking of RG-II.
EXPERIMENTAL PROCEDURES
Plant Material and Culture
Wild-type cherry tomato plants (S. lycopersicum L. cv. West Virginia 106) and l-66 and l-108 GME-silenced lines plants were grown for 30 days in a greenhouse as described previously (17). For in vitro culture, seeds were treated by 4% calcium hypochlorite for 20 min and rinsed three times with deionized water. Seeds were sown on one-fourth strength Murashige and Skoog (MS) medium (Kalys-Duchefa) containing 3% sucrose and 0.15% Phytagel (Sigma) under a 16/8-h (light/dark) photoperiod at 400 μmol · m−2 · s−1 and 25°C. For borate supplementation assays, the MS medium was complemented with increasing concentrations of boric acid ranging from 0.05 to 2.4 mm. Optimal conditions for restoration of a wild-type phenotype were obtained with 1.2 mm boric acid.
Isolation of RG-II
One g of freeze-dried 30-day-old leaves was suspended in 50 ml of aqueous 70% (v/v) ethanol, heated at 70 °C for 15 min, and centrifuged at 5,000 rpm for 10 min. These treatments were repeated three times. The insoluble residue was incubated in chloroform/methanol (v/v) and then with acetone. The residue was treated with 0.1 m NaOH for 4 h at 4 °C to saponify the methyl and acetyl esters. The suspension was adjusted to pH 5 with 10% (v/v) acetic acid and then digested with an endopolygalacturonase from Aspergillus niger (30 units; Megazyme) for 16 h at 30 °C. The suspension was centrifuged, and the insoluble residue was washed with water. The endopolygalacturonase-soluble fraction was incubated successively with an α-amylase from Aspergillus oryzae (100 units; Megazyme) for 16 h at 25 °C and with endoxylanase from A. niger (50 units; Megazyme) for 16 h at 30 °C. The solution was dialyzed (3.5-kDa cutoff dialysis tubing) against deionized water and freeze-dried. RG-I and RG-II polysaccharides were then separated from the endopolygalacturonase-solubilized material by size-exclusion chromatography on a Superdex-75 column HR 10/30 column (Amersham Biosciences) in 50 mm ammonium formate, pH 5, at a flow rate of 0.1 ml · min−1 and detected with a refractive index detector (Shodex-RI71). For all experiments, RG-II fractions were collected and run once again under the same conditions used for the quantification of RG-II dimer and monomer. For borate supplementation assays, isolation of RG-II and quantification of RG-II dimer/monomer ratio in tomato seedlings were carried out on 20 8-day-old seedlings grown on MS medium either complemented or not with 1.2 mm boric acid.
Mild Acid Hydrolysis of RG-II
Mild acid hydrolysis was carried out as reported previously (18). One mg of purified RG-II was hydrolyzed in 200 μl of 0.1 m trifluoroacetic acid for 16 h at 40 °C.
Gas-Liquid Chromatography (GLC)
The samples were submitted to a 16-h methanolysis at 80 °C with 500 μl of dried 1 m methanolic-HCl (Supelco). After evaporation of the methanol, the methyl glycosides were converted into their trimethylsilyl derivatives at 80 °C for 20 min with 200 μl of the silylation reagent (HMDS:TMCS:pyridine, 3:1:9; Supelco) and then analyzed by GLC. The gas chromatograph (Varian CP-3800, Varian) is equipped with a flame ionization detector, a WCOT fused silica capillary column (length 25 m, inner diameter 0.25 mm) with CP-Sil 5 CP as stationary phase and helium as gas vector. The oven temperature program was 2 min at 120 °C, 10 °C/min to 160 °C, and 1.5 °C/min to 220 °C and then 20 °C/min to 280 °C. The quantification of sugar was carried out by the integration of peaks and the determination of the corresponding molar values using response factors established with standard monosaccharides. Absolute configuration (d or l) of galactose residues of RG-II was determined by GLC analysis of their trimethylsilyl (+)-butylglycosides (4, 19). RG-II samples were submitted to butanolysis using 1 m (+)-butanol-HCl (Sigma). The resulting (+)-butylglycosides were then converted into their trimethylsilyl derivatives as described before. Absolute configuration of galactose derivatives was determined by comparison of retention times with trimethylsilyl (+)-butylglycoside derivatives l- and d-galactose standards. Quantification of l-Gal was achieved by calculating the ratio between trimethylsilyl (+)-butylglycoside derivatives of l-Gal and Kdo.
Mass Spectrometry
Electrospray ionization mass spectrometry (ESI-MS) was performed on a Q-TRAP mass spectrometer (Applied Biosystems). Samples in 95% water/5% acetonitrile and 0.02% formic acid (v/v) were infused through Proxeon nanospray capillaries (Proxeon Biosystems). The ion source conditions were adjusted for optimal sample ionization. Infusion needle potential values were adapted between 0.9 and 1.5 kV. Spectra were acquired in positive mode with a scan rate of 1,000 atomic mass units · s−1 and accumulated until a satisfactory signal to noise ratio was obtained. Other voltages were as recommended by the manufacturer.
RESULTS
GME Silencing in Tomato Results in a Severe Reduction of l-Galactose Incorporation in RG-II Side Chain A
In a previous study, it was demonstrated that two RNAi GME-silenced tomato lines (l-66 and l-108) exhibited a severe reduction of GME transcripts, a reduction of l-ascorbate content (40–60% in leaves), and an altered growth phenotype (5). We analyzed these two lines to determine whether the alteration of GME expression in tomato affects RG-II structure. We isolated RG-II from leaves of 30-day-old wild-type plants and the two GME-silenced tomato lines. Pectins were extracted from leaf material, and RG-II was separated from two populations of RG-I and short oligogalacturonides (Fig. 2a). RG-II fractions were collected, and their sugar composition was determined (Table 1). Table 1 shows the sugar composition of RG-II isolated from wild-type and the two GME-silenced lines. The monosaccharide composition of RG-II from wild-type plants is similar to that reported previously for tomato plants (20). However, high galactose, arabinose, and GalA contents suggest the presence of contaminating polysaccharides such as RG-I and/or homogalacturonans. Specific RG-II monosaccharides, such as Kdo, aceric acid, apiose, 3-deoxy-d-lyxo-heptulosonic acid, 2-O-methyl xylose, and 2-O-methyl fucose, were clearly detected. In comparison, transgenic lines contained less Gal, suggesting that GME silencing may affect galactose incorporation in RG-II.
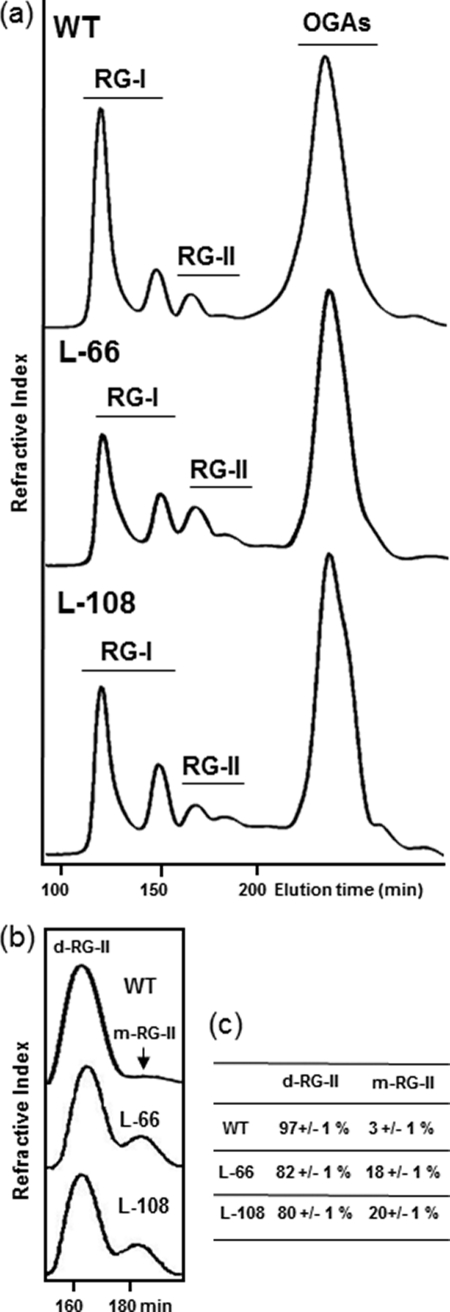
FIGURE 2.
In muro cross-linking of RG-II in tomato wild-type (WT) plants and GME-silenced lines. a, pectins from leaves of WT, l-66, and l-108 plants were treated with endopolygalacturonase, and the resulting RG-I, RG-II, and oligogalacturonide (OGA) fractions were separated by size-exclusion chromatography. b, RG-II fractions from a were collected and separated on the same column for accurate relative quantification of RG-II dimer (d-RG-II) and monomer (m-RG-II). c, relative percentage of RG-II dimer and monomer in wild-type plants and GME-silenced lines. Data represent the mean of three experiments ± S.D.
TABLE 1.
Glycosyl residue composition of RG-II isolated from leaves of wild-type and the l-66 and l-108 GME-silenced tomato lines
| Monosaccharidea | Wild-type | l-66 | l-108 |
|---|---|---|---|
| mol % | mol % | mol % | |
| Ara | 9.6 ± 0.2 | 8.2 ± 0.5 | 9.6 ± 0.2 |
| Rha | 14.5 ± 1.5 | 12.7 ± 3.0 | 15.6 ± 1.2 |
| Xyl | 1.5 ± 1.0 | 1.7 ± 1.5 | 2.0 ± 0.1 |
| Fuc | 2.3 ± 0.1 | 3.1 ± 0.2 | 3.0 ± 0.1 |
| GalA | 36.5 ± 1.4 | 45.6 ± 4.0 | 36.1 ± 3.2 |
| GlcA | 6.9 ± 0.8 | 8.0 ± 0.3 | 7.9 ± 1.3 |
| Glc | 1.1 ± 0.6 | 0.9 ± 0.6 | 1.4 ± 0.6 |
| Man | 1.8 ± 0.7 | 1.1 ± 0.5 | 2.1 ± 0.1 |
| 2-O-Me Xyl | 2.0 ± 0.2 | 1.7 ± 0.1 | 2.2 ± 0.1 |
| 2-O-Me Fuc | 1.9 ± 0.2 | 1.7 ± 0.5 | 2.3 ± 0.2 |
| AceA | 1.9 ± 0.8 | 2.0 ± 0.6 | 3.2 ± 0.7 |
| Dha | Detectedb | Detectedb | Detectedb |
| Api | 2.0 ± 0.2 | 1.9 ± 0.3 | 2.0 ± 0.2 |
| Kdo | 2.4 ± 0.5 | 2.5 ± 0.5 | 2.8 ± 0.3 |
| Gal | 17.3 ± 2.7 | 9.3 ± 0.1 | 10.4 ± 0.7 |
| l-Gal/Kdoc | 1.02 ± 0.16 | 0.34 ± 0.10 | 0.33 ± 0.09 |
a AceA, aceric acid; Api, apiose; Dha, 3-deoxy-d-lyxo-heptulosonic acid.
b Due to overlapping of GLC peaks, the quantification of Dha was not achieved.
c Molar ratio between trimethylsilyl (+)-butylglycosides derivatives of l-galactose and Kdo. Data represent the mean of three experiments ± S.D.
A decrease of the Gal content was reported previously in cell wall polysaccharides of the GME-silenced tomato lines (5). Because RG-II contains both d- and l-galactosyl residues, we then focused our study on the specific quantification of l-Gal, the galactose enantiomer related to GME activity. The absolute configuration of galactose residues of RG-II fractions was determined by GLC analysis of their trimethylsilyl (+)-butylglycosides by comparison with l-Gal and d-Gal standards (19, 20). As a consequence, we paid attention to the specific quantification of the l-Gal content between wild-type and transgenic leaves. In agreement with previous data on Arabidopsis and wine RG-II (4), both configurations were detected in tomato RG-II fractions. Assuming the presence of one Kdo residue/RG-II molecule, we were able to quantify unambiguously the l-Gal content in RG-II by integrating its corresponding GLC peaks relatively to trimethylsilyl (+)-butylglycoside derivatives of Kdo, independently of the amount of d-Gal originating from either the RG II side chain B or contaminating RG-I. The l-Gal/Kdo ratio in wild-type leaves (Table 1) is consistent with the presence of one l-Gal residue on the side chain A in tomato RG-II. In contrast, both GME-silenced lines exhibited a reduction of >60% in RG-II l-Gal content. These data demonstrated that the reduction of GME transcripts in l-66 and l-108 GME-silenced tomato lines resulted in a severe decrease of l-Gal incorporation into RG-II.
To characterize their structure more precisely, RG-II fractions isolated from leaves of both wild-type and GME-silenced lines were submitted to a mild acidic hydrolysis and the resulting oligosaccharides analyzed by ESI-MS (Fig. 3). Due to the presence of an acidic-labile apiose residue at their reducing ends (Fig. 1), the side chains A and B can be specifically released by hydrolysis with 0.1 m trifluoracetic acid at 40 °C (18). As shown in Fig. 3a, numerous oligosaccharides were generated from RG-II of leaves of wild-type tomato. The structural identification of these RG-II oligosaccharide fragments was deduced on the basis of their molecular mass measured by EI-MS and fragmentation patterns obtained by tandem ESI-MS-MS according to Séveno et al. (18, 21). EI-MS of oligosaccharides released by mild acidic hydrolysis of wild-type tomato RG-II showed M+Na+ ions at m/z 1225 and 1267, corresponding to the partially acetylated side chain B (Figs. 1 and 3a). Ions at m/z 933 and 1,079, and their corresponding acetylated forms at m/z 975 and 1121, were assigned to the side chain B fragments lacking one- or two-terminal rhamnosyl residues (Fig. 1). Additional molecular ions at m/z 1301, 1155, and 993 were assigned to the sodium adduct of the side chain A and fragments. As reported previously, the detection of oligosaccharides of various lengths is due to the partial degradation of the side chains during the acidic treatment rather than to the presence of glycoforms in the native RG-II (18). Oligogalacturonides GalA5 and GalA6 originating from the homogalacturonan backbone were also generated during the mild acidic hydrolysis. Because l-Gal was reported to be located on the nonreducing end of the side chain A (4), a more detailed analysis of this oligosaccharide sequence was carried out (Fig. 4). The structures of the main molecular ions at m/z 1301 and 1155 of the side chain A were confirmed by ESI-MS/MS fragmentation (Fig. 4, a and b). The fragmentation patterns were in full agreement with MS/MS data previously reported for the side chain A of Arabidopsis RG-II (18). This is the first reported fine structure of tomato RG-II, and the similarity with the Arabidopsis data further underlines the high level of RG II structural conservation in plants.
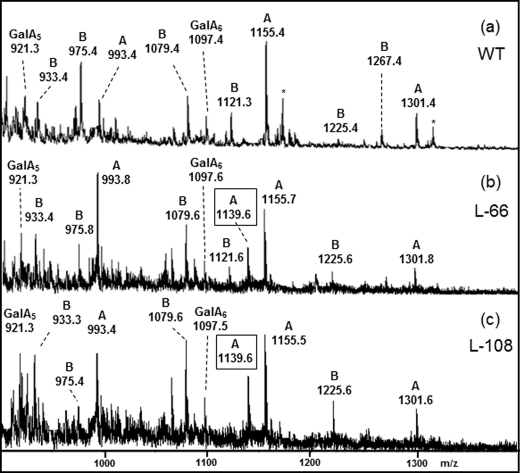
FIGURE 3.
Structural analysis of the RG-II side chains A and B by ESI-MS. ESI mass spectra of oligosaccharide fragments released by mild acidic hydrolysis of RG-II isolated from leaves of tomato wild-type plants (a), GME-silenced l-66 (b), and l-108 lines (c) are shown. Ions were assigned to M+Na+ adducts of either side chain A and B, as well as to fragments lacking terminal residues. GalA5 and GalA6 refer to the galacturonic acid backbone fragments. *, potassium adduct.
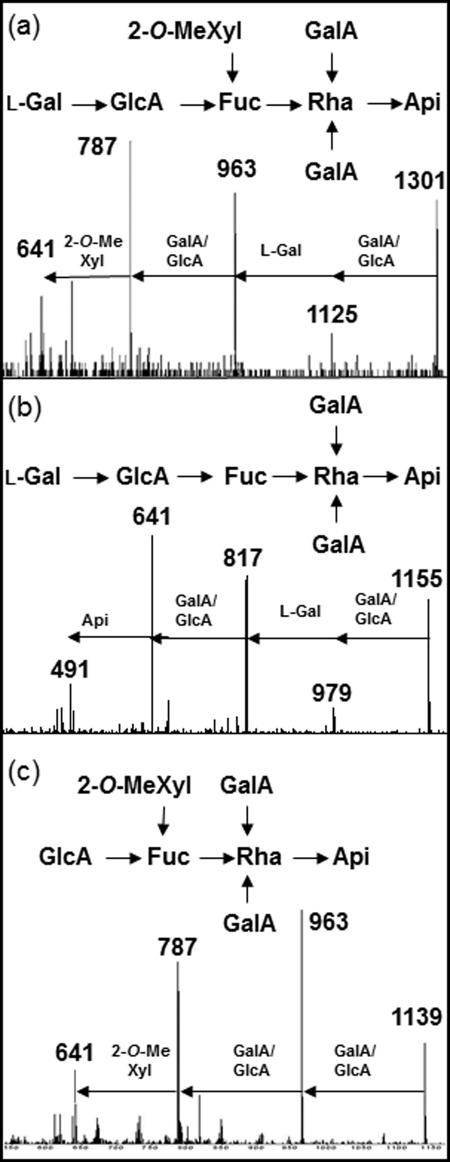
FIGURE 4.
Structural analysis of RG-II side chain A by ESI-MS/MS. a and b, fragmentation patterns obtained by ESI-MS/MS of ions at m/z 1301 (a) and 1155 (b) detected in the ESI source spectrum of wild-type tomato RG-II from Fig. 3a. c, ESI-MS/MS spectrum of ion at m/z 1139 detected in the ESI-MS spectrum of RG-II from the l-66 line. This ion was specifically detected in the ESI-MS of RG-II of the GME-silenced l-66 and l-108 lines (Fig. 3, b and c).
Similar analyses (Fig. 3, b and c) of l-66 and l-108 GME-silenced lines indicated the presence of the same main ions assigned to the side chains A and B of wild-type samples. In addition, a major ion at m/z 1139 was clearly observed in both transgenic lines. This ion was not detected in the MS spectrum of the wild-type RG-II, indicating that it did not result from the acidic hydrolysis of the side chain A in transgenic lines. This additional ion at m/z 1139 differs by 162 mass units (anhydrohexose) from the M+Na+ molecular ion of side chain A, suggesting that it lacks an l-galactosyl residue (the unique hexose residue located on this chain; Fig. 1). The ESI-MS/MS fragmentation pattern of this ion was in agreement with the structure of side chain A lacking the nonreducing end l-Gal (Fig. 4c). In conclusion, and together with the RG-II sugar composition, the MS analyses demonstrate that the silencing of GME in tomato leaves resulted in a reduced incorporation of terminal l-Gal residues into the side chain A of this pectic molecule.
GME Silencing in Tomato Alters Borate Cross-linking of RG-II and Plant Growth
A previous study of GME-silenced tomato lines revealed a 5-fold increase in stem fragility compared with wild-type tomato plants (5). Such a cell wall phenotype was described previously for plants exhibiting a reduced borate cross-linking of RG-II, either in boron-deficient plants or in plants with mutations affecting their RG-II structure (10–13). We therefore decided to investigate the capacity of RG-II to cross-link in muro via a borate diester in the two GME-silenced tomato lines. RG-II fractions from the two lines were collected, and RG-II monomer and dimer were separated by size-exclusion chromatography and quantified. As illustrated (Fig. 2, b and c), the borate ester cross-linked RG-II dimer accounts for about 97% of wild-type RG-II from tomato leaves but only for 80 and 82% of the RG-II in l-66 and l-108 GME-silenced lines, respectively. This suggests that the decrease of l-Gal incorporation in transgenic tomato lines resulted in a reduced capacity of its RG-II to perform an efficient in muro cross-linking.
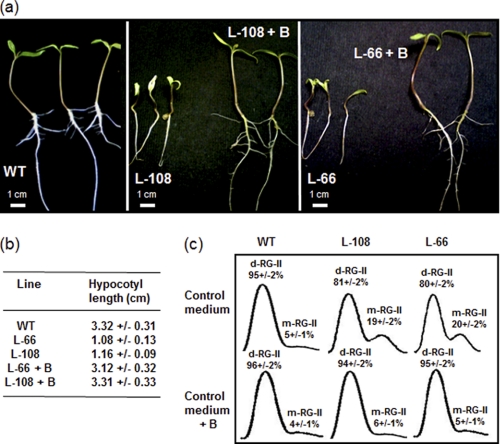
Previous work has shown that supplementation of RNAi-silenced tomato lines by l-Gal was able to rescue wild-type levels of l-ascorbate but has no effect on growth defects (5). To investigate whether the growth phenotype of these GME-silenced lines was a consequence of the observed reduced cross-linking of RG-II, we grew them in the presence of high concentrations of boric acid (Fig. 5). As shown in Fig. 5a, 8-day-old seedlings of l-66 and l-108 GME-silenced lines exhibited dwarf phenotypes compared with wild-type seedlings. Supplementation with boric acid of these two lines restored a wild-type phenotype of tomato seedlings (Fig. 5, a and b).
FIGURE 5.
Normal growth phenotype of seedlings and RG-II dimerization are restored in tomato GME-silenced lines by supplementation with boric acid. a, seedlings at 8 days after sowing of wild-type (WT) tomato and GME-silenced lines (l-66 and l-108) after growth in MS medium alone or in MS medium supplemented with 1.2 mm boric acid. (l-66 + B and l-108 + B). b, length of the hypocotyls of 8-day-old seedlings of WT tomato and transgenic lines with and without boric acid supplementation. Data are means ± S.D. of 9 individual plants. c, chromatogram and quantification of RG-II dimer (d-RG-II) and monomer (m-RG-II) in l-106 and l-66 GME-silenced lines grown in MS medium alone (control) or in the MS medium supplemented with 1.2 mm boric acid (+ B). Data represent the mean of three experiments ± S.D.
The effects of boron supplementation on RG-II dimerization were then investigated in seedlings of l-66 and l-108 GME-silenced lines. As shown in Fig. 5c, RG-II from the silenced lines exhibited a reduced in muro cross-linking compared with wild-type seedlings as found for RG-II isolated from mature leaves. In contrast, this deficiency was no longer observed in seedlings grown in the boron-supplemented medium. Together, these data suggest that the growth defects of GME-silenced tomato lines are likely to result from the reduced borate cross-linking of RG-II within the primary cell wall.
DISCUSSION
Two lines of evidence demonstrate that the silencing of GME in tomato affects l-Gal incorporation into the pectic polysaccharide RG-II of the primary cell wall. First, l-Gal content in RG-II of the two transgenic lines is only one-third of that from wild-type plants. Second, the incorporation of a terminal nonreducing end hexosyl residue in the RG-II side chain A is reduced in these lines. Because l-Gal is the unique hexose residue located in the side chain A of Arabidopsis RG-II (6), our results suggest that l-galactosylation of this oligosaccharide chain is reduced in GME-silenced tomato lines. Together, these results indicate that the severe reduction of GME transcripts in transgenic tomato lines affects the incorporation of l-Gal into RG-II, most likely by limiting the availability of the corresponding GDP-l-Gal for the RG-II-specific Golgi l-galactosyltransferase.
Supplementation of tomato GME-silenced lines with l-Gal was previously shown to rescue wild-type levels of l-ascorbate but did not rescue a wild-type phenotype (5). Presumably, the observed growth defect is not due to vitamin deficiency because l-Gal is the precursor of l-ascorbate, and supplementation restores normal vitamin synthesis. In contrast, it is possible that the phenotype is related to impaired cell wall biosynthesis. Because l-Gal cannot be converted in the cytosol into its GDP-activated form (1) the supplementation of GME-silenced lines with l-Gal cannot restore the cytosolic wild-type content of GDP-l-Gal and thus normal cell wall biosynthesis. This failure to restore normal growth by l-Gal supplementation, as well as the results reported in this paper, strongly suggest that the developmental phenotypes observed in tomato transgenic lines are related to the inability of RG-II to perform its central role during primary cell wall formation rather than to l-ascorbate deficiency. Interestingly, no GME mutants have been isolated to date. The inactivation of l-Gal synthesis in GME mutants might result in nonviable plants as recently demonstrated for mutants affected in Kdo synthesis, another RG-II-specific monosaccharide (15, 16). However, the absence of l-ascorbate may also be critical for plant viability, and it would therefore be interesting to analyze mutants affected in the last steps (post-GME activity) of vitamin C biosynthesis to discriminate between the effects of l-ascorbate deficiency and alteration of primary cell wall formation.
Analyses of the fucose-deficient Arabidopsis mur1 mutant (13) and of the boron-deficient bor1 mutant (22) demonstrated the relationship between borate cross-linking of RG-II in the primary cell wall and normal plant growth. Because GME silencing was demonstrated to result in growth defects (5) and in the alteration of the RG-II structure (this study) in transgenic tomato lines, we investigated in muro cross-linking of RG-II and the effects of borate supplementation in silenced lines. We showed that the decrease of l-Gal incorporation in RG-II of transgenic tomato lines led to a reduced capacity of this pectic molecule to cross-link in muro. Although the dimerization defect in GME-silenced lines is less severe (20%) than observed in the Arabidopsis mur1 mutant (50%), it should be noticed that, in contrast to this fucose-deficient mutant, l-Gal content in RG-II of GME-silenced tomato lines is only reduced to 35–40% of that from wild-type plants. The deficiency in RG-II cross-linking was observed repeatedly in three independent preparations of both mature leaves and seedlings of the two tomato lines. A similar alteration of RG-II dimerization was also observed in pectins isolated from fruits of these lines (data not shown). It is noteworthy that the two GME-silenced lines analyzed in this study exhibited comparable developmental defects and increased fragility in comparison with control plants (5) as well as comparable decreases in l-Gal incorporation and reduced RG-II dimerization. This further supports a direct link between RG-II alteration and proper primary cell wall organization. Furthermore, as demonstrated for mur1 (13), the supplementation of transgenic tomato lines with boric acid restored both a wild-type phenotype in seedlings and efficient cross-linking of RG-II. Therefore, we hypothesize that the developmental phenotype of l-66 and l-108 transgenic lines results from the reduced cross-linking capacity of the modified RG-II.
The Arabidopsis mur1 mutant is devoid of l-Fuc in its aerial parts and accumulates l-Gal and 2-O-methyl l-Gal in place of l-Fuc and 2-O-Me Fuc in the RG-II side chains A and B, respectively (4, 13). In addition, mur1 RG-II contains only 50% of the amount of 2-O-Me Xyl present in wild-type plants. In vitro studies showed that mur1 RG-II formed a dimer less readily and that this dimer is less stable than the wild-type RG-II counterpart (13). Because 2-O-Me Xyl is located on side chain A, the oligosaccharide chain involved in the RG-II dimerization through a borate diester, it is temping to speculate that the decrease of the boron-mediated cross-linking capacity in mur1 results from the partial lack of 2-O-Me Xyl in side chain A rather than the replacement of l-Fuc by l-Gal in side chains A and B. Similarly, the partial deficiency in cross-linking of RG-II in GME-silenced tomato lines may also be caused by structural changes in RG-II and as a consequence a decreased affinity for boron because the l-Gal deficiency also affects the side chain A structural integrity. At the molecular level, RG-II monomer was proposed from high resolution nuclear magnetic resonance and molecular modeling to adopt a general shape of a disk where the OH-2 and OH-3 of the apiosyl residue in side chain A that are involved in the formation of the borate diester in RG-II dimers, are free from steric interactions and exposed at the surface (8). Although l-Gal and 2-O-Me Xyl are remote from the apiosyl residue, their absence could lead to a slight reorganization of neighboring residues of the side chain A, including the apiosyl residue involved in the RG-II cross-linking. Such a reorganization would in turn affect the affinity of the apiosyl residue for boron thereby reducing its cross-linking capacity. Addition of exogenous boric acid allows the increase of the local cell wall borate concentration up to the optimal condition required for the recovery of the wild-type dimerization level.
In mur1 Arabidopsis mutant, the two l-fucose residues of RG-II are replaced by l-Gal (4, 13). Such a substitution was also observed in N-linked glycans of endogenous glycoproteins (23) and suggested that fucosyltransferases involved in RG-II and N-glycan biosynthesis are able to use GDP-l-Gal as substrate in the absence of the appropriate nucleotide sugar. In contrast, neither an increase in fucose content nor the detection of side chain A containing a terminal fucosyl residue was observed in RG-II isolated from the GME-silenced tomato lines. This suggests that the l-galactosyltransferase involved in side chain A biosynthesis is highly specific for l-Gal and requires a C-6 hydroxylated group for nucleotide sugar binding to its catalytic site.
In conclusion, this study further underlines the crucial role of the boron-mediated cross-linking of RG-II in the formation of the three-dimensional pectic network required for normal plant growth and development. This absolute requirement for boron in the primary cell wall may be one of the main selection pressures that have maintained the structure of RG-II unchanged in the plant kingdom during evolution. The mechanism by which reduced RG-II dimerization affects plant growth remains to be elucidated. The study of tomato plants showing altered RG-II structures represents an original way to decipher the function of RG-II during cell wall biogenesis, particularly in the context of fruit development and ripening (5). The importance of ascorbic acid and cell wall biosynthesis in the establishment of nutritional (antioxidant), and sensory (texture) quality traits of fruit, as well as the existence of interconnections between these pathways represent key issues of agronomic importance that can be studied using tomato as a model.
Acknowledgments
We thank the proteomic analysis platform at the University of Rouen for access to the mass spectrometry facilities and Professors Jean Claude Mollet and Simon Hawkins for critical reading of the manuscript.
This work was supported by the University of Rouen.
- GME
- GDP-d-mannose 3,5-epimerase
- ESI-MS
- electrospray ionization mass spectrometry
- Kdo
- 3-deoxy-d-manno-octulosonic acid
- MS medium
- Murashige and Skoog medium
- RG-I
- rhamnogalacturonan I
- RG-II
- rhamnogalacturonan II.
REFERENCES
- 1. Reiter W. D., Vanzin G. F. (2001) Plant Mol. Biol. 47, 95–113 [PubMed] [Google Scholar]
- 2. Laing W. A., Wright M. A., Cooney J., Bulley S. M. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 9534–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolucka B. A., Van Montagu M. (2007) Phytochemistry 68, 2602–2613 [DOI] [PubMed] [Google Scholar]
- 4. Reuhs B. L., Glenn J., Stephens S. B., Kim J. S., Christie D. B., Glushka J. G., Zablackis E., Albersheim P., Darvill A. G., O'Neill M. A. (2004) Planta 219, 147–157 [DOI] [PubMed] [Google Scholar]
- 5. Gilbert L., Alhagdow M., Nunes-Nesi A., Quemener B., Guillon F., Bouchet B., Faurobert M., Gouble B., Page D., Garcia V., Petit J., Stevens R., Causse M., Fernie A. R., Lahaye M., Rothan C., Baldet P. (2009) Plant J. 60, 499–508 [DOI] [PubMed] [Google Scholar]
- 6. O'Neill M. A., Ishii T., Albersheim P., Darvill A. G. (2004) Annu. Rev. Plant Biol. 55, 109–139 [DOI] [PubMed] [Google Scholar]
- 7. O'Neill M. A., Warrenfeltz D., Kates K., Pellerin P., Doco T., Darvill A. G., Albersheim P. (1996) J. Biol. Chem. 271, 22923–22930 [DOI] [PubMed] [Google Scholar]
- 8. Pérez S., Rodríguez-Carvajal M. A., Doco T. (2003) Biochimie 85, 109–121 [DOI] [PubMed] [Google Scholar]
- 9. Kobayashi M., Matoh T., Azuma J. (1996) Plant Physiol. 110, 1017–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ishii T., Matsunaga T., Pellerin P., O'Neill M. A., Darvill A., Albersheim P. (1999) J. Biol. Chem. 274, 13098–13104 [DOI] [PubMed] [Google Scholar]
- 11. Fleischer A., O'Neill M. A., Ehwald R. (1999) Plant Physiol. 121, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ishii T., Matsunaga T., Hayashi N. (2001) Plant Physiol. 126, 1698–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Neill M. A., Eberhard S., Albersheim P., Darvill A. G. (2001) Science 294, 846–849 [DOI] [PubMed] [Google Scholar]
- 14. Ahn J. W., Verma R., Kim M., Lee J. Y., Kim Y. K., Bang J. W., Reiter W. D., Pai H. S. (2006) J. Biol. Chem. 281, 13708–13716 [DOI] [PubMed] [Google Scholar]
- 15. Johnson M. A., von Besser K., Zhou Q., Smith E., Aux G., Patton D., Levin J. Z., Preuss D. (2004) Genetics 168, 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Delmas F., Séveno M., Northey J. G., Hernould M., Lerouge P., McCourt P., Chevalier C. (2008) J. Exp. Bot. 59, 2639–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alhagdow M., Mounet F., Gilbert L., Nunes-Nesi A., Garcia V., Just D., Petit J., Beauvoit B., Fernie A. R., Rothan C., Baldet P. (2007) Plant Physiol. 145, 1408–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Séveno M., Voxeur A., Rihouey C., Wu A. M., Ishii T., Chevalier C., Ralet M. C., Driouich A., Marchant A., Lerouge P. (2009) Planta 230, 947–957 [DOI] [PubMed] [Google Scholar]
- 19. Gerwig G. J., Kamerling J. P., Vliegenthart J. F. (1979) Carbohydr. Res. 77, 10–17 [DOI] [PubMed] [Google Scholar]
- 20. Doco T., Williams P., Vidal S., Pellerin P. (1997) Carbohydr. Res. 297, 181–186 [DOI] [PubMed] [Google Scholar]
- 21. Spellman M. W., McNeil M., Darvill A. G., Albersheim P. (1983) Carbohydr. Res. 122, 131–153 [Google Scholar]
- 22. Noguchi K., Yasumori M., Imai T., Naito S., Matsunaga T., Oda H., Hayashi H., Chino M., Fujiwara T. (1997) Plant Physiol. 115, 901–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rayon C., Cabanes-Macheteau M., Loutelier-Bourhis C., Salliot-Maire I., Lemoine J., Reiter W. D., Lerouge P., Faye L. (1999) Plant Physiol. 119, 725–734 [DOI] [PMC free article] [PubMed] [Google Scholar]