Abstract
Although capsular polysaccharide (CPS) is critical for meningococcal virulence, the molecular basis of alternative complement pathway (AP) regulation by meningococcal CPSs remains unclear. Using serum with only the AP active, the ability of strains to generate C3a (a measure of C3 activation) and subsequently deposit C3 fragments on bacteria was studied in encapsulated group A, B, C, W-135, and Y strains and their isogenic unencapsulated mutants. To eliminate confounding AP regulation by membrane-bound factor H (fH; AP inhibitor) and lipooligosaccharide sialic acid, the meningococcal fH ligands (fHbp and NspA) and lipooligosaccharide sialylation were deleted in all strains. Group A CPS expression did not affect C3a generation or C3 deposition. C3a generated by encapsulated and unencapsulated group B and C strains was similar, but CPS expression was associated with reduced C3 deposition, suggesting that these CPSs blocked C3 deposition on membrane targets. Paradoxically, encapsulated W-135 and Y strains (including the wild-type parent strains) enhanced C3 activation and showed marked C3 deposition as early as 10 min; at this time point C3 was barely activated by the unencapsulated mutants. W-135 and Y CPSs themselves served as a site for C3 deposition; this observation was confirmed using immobilized purified CPSs. Purified CPSs bound to unencapsulated meningococci, simulated findings with naturally encapsulated strains. These data highlight the heterogeneity of AP activation on the various meningococcal serogroups that may contribute to differences in their pathogenic mechanisms.
Keywords: Bacteria, Carbohydrate, Complement, Immunology, Polysaccharide, Neisseria meningitidis
Introduction
Neisseria meningitidis is an important cause of bacterial meningitis and sepsis worldwide. Based on the antigenic composition of its capsular polysaccharide, N. meningitidis can be divided into various serogroups. Of the 13 serogroups described thus far (1), five (A, B, C, W-135, and Y) are responsible for most cases of invasive meningococcal disease worldwide (2). With very few exceptions (3–5), almost every case of invasive meningococcal disease is caused by a strain that expresses capsular polysaccharide.
The complement system constitutes an important arm of innate immune defenses against invasive meningococcal infection. Persons deficient in terminal complement components (C5 through C9) or components of the alternative pathway (such as properdin and factor D) are highly predisposed to meningococcal disease (6–20). Deficiencies of inhibitors of the complement system such as factor H and factor I also predispose individuals to meningococcal disease because lack of complement inhibition in plasma leads to uncontrolled C3 activation and consumption (12, 21). Epidemiologic data suggest that persons with deficiencies of properdin or terminal complement components may be more predisposed to disease caused by groups W-135 and Y and other rare serogroups (13–14). Why certain serogroups of N. meningitidis are overrepresented in persons with complement deficiencies is not fully understood.
Several microbes express capsular polysaccharides as part of their virulence armamentarium. In general, capsules are believed to limit complement activation on the microbial surface and are anti-opsonophagocytic (22). Prior studies have shown that expression of group B and C meningococcal capsules limit alternative pathway-mediated C3 deposition on the bacterial surface (23, 24). The capsules of both of these serogroups are homopolymers of sialic acid. The group B capsule is composed of α(2,8)-, whereas the group C capsule is composed of α(2,9)-linked polysialic acid. Other sialic acid containing capsules such as the capsular polysaccharide of type III group B streptococci have also been shown to limit alternative pathway activation (25). In the case of group B streptococci, the tertiary structure of the capsule, which is dependent upon the negative charge of the carboxylate residue of sialic acid, limits activation of the alternative pathway. However, the molecular basis of alternative pathway regulation by meningococcal group B and C capsules has not been elucidated.
To our knowledge, there are no published data on regulation of the alternative pathway by the other clinically important meningococcal capsular polysaccharides (A, W-135, and Y). The serogroup A polysaccharide is a homopolymer of N-acetylmannosamine 1-phosphate, and serogroup W-135 and Y capsules comprise disaccharide repeating units of 6-d-Galα(1,4)-NANAα(2,6)- and 6-d-Glcα(1,4)-NANAα(2,6)-, respectively (1). The polysaccharides of serogroups A, C, W-135, and Y are also variably O-acetylated (26, 27). The purpose of this study was to examine how capsular polysaccharides from each the five major meningococcal serogroups interact with the alternative pathway of complement to better understand differences in the pathophysiology among the various serogroups.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Mutants
N. meningitidis strains and their isogenic mutants used in this study are listed in Table 1. The mutant strain pairs differ in capsular polysaccharide expression; all mutant strains lacked lipooligosaccharide (LOS)2 sialic acid (group A meningococcal strains do not endogenously sialylate their LOS) and fHbp and NspA expression; these outer membrane components regulate the alternative pathway of complement and were excluded to avoid confounding of results that examined alternative pathway regulation by encapsulated bacteria (28–31). Construction of lst (32), fHbp (30), and nspA (29) deletion mutants have been described previously. For simplicity, encapsulated and unencapsulated mutants have been referred to as “Cap+” and “Cap−,” respectively.
TABLE 1.
Bacterial strains used in this study and their relevant characteristics
| Straina | Relevant characteristics |
|---|---|
| A2594 | A:4:P1.9b:ST-5; invasive isolate (Germany) (Ref. 32); encapsulated; LOS not sialylated |
| A2594 fHbp NspA | Encapsulated; LOS not sialylated; fHbp::ermR; nspA::spcR |
| A2594 mynB fHbp NspA | Unencapsulated (mynB::cmR) isogenic mutant of A2594 fHbp NspA |
| H44/76 | B:15:P1.7,16: ST-32 (Ref. 65); invasive isolate; encapsulated; LOS sialylated |
| H44/76 lst fHbp NspA | Encapsulated; insertional inactivation of lipooligosaccharide (LOS) sialyltransferase (lst::kanR); LOS not sialylated; fHbp::ermR; nspA::spcR |
| H44/76 siaD lst fHbp NspA | Unencapsulated (siaD::cmR) isogenic mutant of H44/76 lst fHbp NspA |
| C2120 | C:NT:P1.5,2:ST-11 (Ref. 66); invasive isolate; encapsulated; LOS sialylated |
| C2120 lst fHbp NspA | Encapsulated; insertional inactivation of LOS sialyltransferase (lst::kanR); LOS not sialylated; fHbp::ermR; nspA::spcR |
| C2120 siaD lst fHbp NspA | Unencapsulated (siaD::cmR) isogenic mutant of C2120 lst fHbp NspA |
| W171c | W135:NT:P1.10:ST-11 (Ref. 26); invasive isolate; encapsulated; LOS sialylated |
| W171 lst fHbp NspA | Encapsulated; insertional inactivation of LOS sialyltransferase (lst::kanR); LOS not sialylated; fHbp::ermR; nspA::spcR |
| W171 siaD lst fHbp NspA | Unencapsulated (siaD::cmR) isogenic mutant of W171 lst fHbp NspA |
| Y2220 | Y:21:P1.15 (ST-172) (Ref. 26); carrier isolate; encapsulated; LOS sialylated |
| Y2220 lst fHbp NspA | Encapsulated; insertional inactivation of LOS sialyltransferase (lst::kanR); LOS not sialylated; fHbp::ermR; nspA::spcR |
| Y2220 siaD lst fHbp NspA | Unencapsulated (siaD::cmR) isogenic mutant of Y2220 lst fHbp NspA |
| LNP19995 lst fHbp NspA | W135:2a:P1.5:ST-11; invasive isolate; encapsulated; insertional inactivation of LOS sialyltransferase (lst::kanR); LOS not sialylated; fHbp::ermR; nspA::spcR |
| LNP19995 siaD lst fHbp NspA | Unencapsulated (siaD::cmR) isogenic mutant of LNP19995 lst fHbp NspA |
| Y2225 lst fHbp NspA | Y:NT:P1.2:ST-11 (Ref. 26); encapsulated; insertional inactivation of LOS sialyltransferase (lst::KanR); LOS not sialylated; fHbp::ermR; nspA::spcR |
| Y2225 siaD lst fHbp NspA | Unencapsulated (siaD::cmR) isogenic mutant of Y2225 lst fHbp NspA |
a All mutant strains were constructed for this study.
b Denotes that PorA VR typing (67) was used to define the serosubtype.
c W171 is also known as ATCC 35559, or M-603.
Sera and Complement Reagents
To selectively study the alternative pathway we used either C2-depleted serum (lot number 16; Complement Technology, Inc.) or normal human serum (NHS) that contained MgCl2 and EGTA (both to a final concentration of 10 mm; Mg/EGTA-NHS). Preliminary experiments showed that C3 fragment deposition on bacteria using C2-depleted serum or Mg/EGTA-NHS were similar. When necessary, all complement pathways were inactivated by heating serum at 56 °C for 30 min. For some experiments, IgG and IgM were depleted from serum as follows. NHS that contained EDTA (final concentration 10 mm) was passed over protein G-Sepharose and anti-human IgM-agarose (both from Sigma) in tandem at 4 °C. The fall-through was spin concentrated and dialyzed against PBS containing 0.1 mm EDTA to its original volume. Depletion of IgG and IgM was confirmed by Western blotting and hemolytic activity of the serum that was reconstituted with 1 mm Ca2+ and 1 mm Mg2+ was confirmed with the Total Hemolytic Complement assay (Binding Site, Birmingham, United Kingdom).
Antibodies
mAb G-3E that recognizes the 68-kDa α1′ chain of iC3b (33, 34) was used to detect iC3b targets on bacteria in Western blotting assays and to measure iC3b deposited on immobilized capsular polysaccharide. Factor H bound to bacteria was detected by flow cytometry with sheep polyclonal anti-human factor H (Lifespan Biosciences). Anti-group A mAb JW-A-1 (IgG2a), anti-group C mAb KS-C-1 (IgG3), anti-group W-135 mAb JW-W1b (IgG2b), and anti-group Y mAb JW-Y2a (IgM) were provided by Drs. Dan M. Granoff (Childrens Hospital Oakland Research Institute, Oakland, CA) and Jo Anne Welsch (Novartis Vaccines), whereas anti-group B mAb 2-2-B (IgM) was obtained from the National Institute for Biological Standards and Control (Potters Bar, Hertfordshire, UK). Chicken polyclonal anti-human C3d that recognizes the α chain of C3 (∼115 kDa), α′ chain (∼106 kDa) of C3b, α1′ chain of iC3b (∼68 kDa), C3d and C3dg was from Quidel (San Diego; catalogue number A800). mAb 755 is a directed epitope spanned by amino acids 1499 and 1519 of the C3 amino acid sequence (SwissProt data base, accession no. P01024) and therefore recognizes the α′ chain (∼106 kDa) of C3b and the α2′ fragment (∼40 kDa) of iC3b (35). All secondary antibodies (alkaline phosphatase-conjugated anti-mouse IgG, anti-mouse IgM and anti-chicken IgY, and anti-sheep IgG-FITC) were from Sigma.
Purified Polysaccharides and Methylated Human Albumin
Purified serogroup A, C, W-135, and Y meningococcal capsular polysaccharides were obtained from the National Institutes Biological Standards and Controls (NIBSC, Porton Down, Salisbury, UK). Serogroup B meningococcal capsular polysaccharide was the kind gift of Dr. Jo Anne Welsch (Novartis Vaccines). Methylated human albumin (NIBSC) was used to bind capsular polysaccharides to microtiter wells (see below).
Flow Cytometry
Flow cytometry to detect factor H bound to bacteria was performed as described previously (29). Briefly, bacteria grown overnight on chocolate agar plates were washed with Hanks' balanced salt solution (HBSS) containing 1 mm Ca2+ and 1 mm Mg2+ (HBSS2+) and suspended to a final concentration of 3 × 108 cells/ml; 108 organisms were centrifuged and incubated with factor H purified from human plasma (Complement Technology, Inc.) at 20 μg/ml. Bound factor H was detected using affinity-isolated sheep anti-human factor H (Lifespan Biosciences, Seattle, WA) followed by FITC-labeled anti-sheep IgG (Sigma).
To detect binding of purified capsular polysaccharide to unencapsulated bacteria, bacteria as prepared above were suspended in HBSS2+ and incubated separately with each purified capsular polysaccharide to a final concentration of 125 μg/ml for 10 min at 37 °C. Capsule bound to bacteria was detected by FACS using the respective mAb against capsule at a concentration of 10 μg/ml (with the exception of anti-group B capsular mAb 2-2-B that was provided as lyophilized ascites and used at a 1:100 dilution), followed by the appropriate FITC-labeled secondary antibody at a concentration of 1:100 (Sigma).
Western Blotting
Western blotting to detect C3 fragments deposited on meningococci was performed as described previously (36). Briefly, 1 × 108 bacteria suspended in HBSS2+ were incubated with C2-depleted serum (final concentration 25% (v/v)) in a final reaction volume of 80 μl for 10 or 30 min at 37 °C. Bacteria were washed twice in HBSS2+ and lysed in NuPAGE® LDS Sample Buffer (4X) (Invitrogen) containing 10% 2-mercaptoethanol. In some experiments, the bacteria were divided into two aliquots treated either with buffer alone (C3 fragments bound to its targets through ester and amide linkages are left intact) or methylamine, pH 11, at a concentration of 1 m (to selectively disrupt ester bonds, but leave amide bonds intact), for 1 h at 37 °C. Direct determination of the specific amide-bound C3b/iC3b is not possible, because it cannot be separated intact from acceptor surfaces without altering its primary structure. Proteins were separated on NuPAGE Novex 4–12% BisTris gradient gels using NuPAGE MOPS running buffer (Invitrogen). Proteins were transferred to a PVDF membrane (Millipore, Billerica, MA) by Western blotting. iC3b was detected using mAb G-3E (tissue culture supernatants containing ∼20 μg/ml of mAb diluted 1:4 in TBS) that recognizes a neoepitope in the α1′ chain of iC3b, followed by goat anti-mouse IgG conjugated to alkaline phosphatase. To reduce viscosity of bacterial lysates caused by DNA, in some experiments serum-coated bacteria were osmotically lysed by incubation for 15 min in ddH2O at 37 °C, followed by treatment with DNase I (50 units; Invitrogen) for 30 min at 37 °C. Addition of NuPAGE® LDS Sample Buffer (4X) and electrophoresis was performed as described above.
C3a ELISA
Activation of C3 is accompanied by release of the C3a fragment from the N terminus of the α chain of C3 into the fluid phase. 1 × 108 bacteria were suspended in 60 μl HBSS2+, followed by the addition of 20 μl of C2-depleted serum (final serum concentration in the reaction mixture was 25%). Aliquots were obtained at sequential time points and reactions were stopped by adding EDTA to a final concentration of 20 mm. C3a released by activation of C3 in the reaction mixture was measured in serial dilutions of the EDTA-inactivated reaction mixtures using the MicroVue C3a Plus EIA kit (Quidel Corp., San Diego, CA). The concentration of C3a in the reaction mixtures was calculated using OD values that fell within the linear range of the standard curve of the EIA kit.
Capsular Polysaccharide Binding of C3 by ELISA
Methylated human albumin was used to bind capsular polysaccharide to microtiter wells as described previously (37). Briefly, each capsular polysaccharide (10 μg/ml in 10 mm PBS) was mixed with methylated human albumin (10 μg/ml in 10 mm PBS) at a 1:1 ratio and mixtures were used to coat individual microtiter wells for 15 h at 22 °C. Wells were blocked with PBS, 2% BSA for 1 h at 37 °C, followed by addition of 20% Mg/EGTA-NHS (alternative pathway specific) for 1 h at 37 °C. Heat inactivated Mg/EGTA-NHS (20%) was used as a control. Factor H and factor I present in serum convert bound C3b to iC3b. iC3b was detected using tissue culture supernatant that contained anti-iC3b mAb G-3E (33) followed by anti-mouse IgG conjugated to alkaline phosphatase.
Serum Bactericidal Assay
Bactericidal assays were performed as described previously (29, 38). Bacteria from an overnight culture on chocolate agar plates were inoculated onto fresh chocolate agar and allowed to grow for ∼6 h at 37 °C in 5% CO2. C2-depleted serum was used at a final concentration of 50% (v/v) in bactericidal assays. In some instances, purified C2 was added to C2-depleted serum to a final concentration of 40 μg/ml as a control. Briefly, 2000 colony forming units of meningococci were incubated with serum (concentrations specified for each experiment) in a final reaction volume of 150 μl. Aliquots of 25 μl were plated in duplicate at the start of the assay (t0) and after incubating the reaction mixture at 37 °C for 30 min (t30). Survival was calculated as the number of viable colonies at t30 relative to baseline colony counts at t0.
Statistical Analysis
Differences between groups of observations were determined using a paired two-tailed t test.
RESULTS
Encapsulated W-135 and Y Strains Generate High Levels of C3a at 10 Min
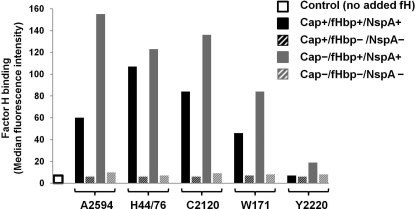
To identify the specific role of capsular polysaccharide in modulating alternative pathway activation it was first necessary to eliminate variables known to regulate alternative pathway activation on Neisseriae. These include LOS sialic acid (28, 39, 40) and the ligands for factor H, fHbp (30, 41), and NspA (29). Strains representing capsular groups A, B, C, W-135, and Y used in this study lacked LOS sialic acid expression (group A strains do not sialylate LOS and in all other strains the sialyltransferase, lst, was insertionally inactivated) and were also mutated to abrogate fHbp and NspA expression. Fig. 1 confirms lack of factor H binding by the fHbp/NspA double mutants in each of the five encapsulated (Cap+) groups and their unencapsulated (Cap−) mutant counterparts (data with H44/76 and A2594 have been shown previously (29)). Wild-type strain Y2220 expresses very low levels of fHbp and binds barely detectable levels of factor H by flow cytometry, as we have described previously (29, 30).
FIGURE 1.
Deletion of fHbp and NspA abrogates factor H binding to meningococcal mutants used in this study. Meningococcal strains A2594 (group A), H44/76 (group B), C2120 (group C), W171 (group W-135), and Y2220 (group Y) that lacked LOS sialic acid, but expressed capsule (Cap+/fHbp+/NspA+; solid black bars) and their isogenic mutants that were deficient in capsule production (Cap−/fHbp+/NspA+; solid gray bars) were further mutated to delete both known meningococcal factor H-binding proteins, fHbp (30, 41) and NspA (29). The Cap+ and Cap− strains that lacked fHbp and NspA are shown by the black and gray hatched bars, respectively. The five sets of isogenic mutant strains were incubated with factor H (20 μg/ml) and bacteria-bound factor H was detected using sheep polyclonal anti-human factor H followed by anti-sheep IgG-FITC. The y axis represents the median fluorescence intensity of the entire bacterial population. A representative control (factor H omitted from the reaction mixture) with group A strain 2594 is shown; all strains yielded similar control fluorescence values.
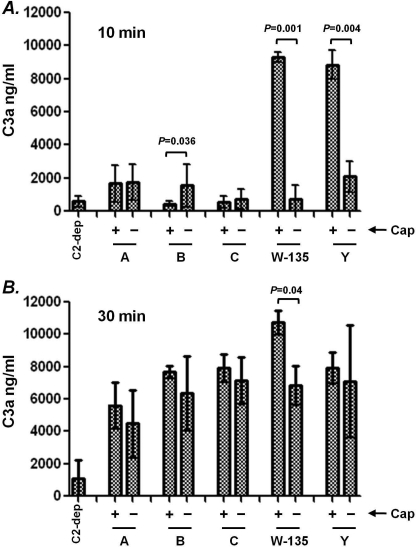
The C3 molecule comprises a 115-kDa α chain that is linked to a 75-kDa β chain through a disulfide bond. Deposition of C3 fragments on the bacterial surface is preceded by activation of C3, during which the ∼9-kDa C3a fragment is cleaved from the N terminus of the 115-kDa α chain of C3 (reviewed in Ref. 42). To determine whether capsular polysaccharide expression affected C3 activation by the alternative pathway, we compared C3a generation at 10 and 30 min in reaction mixtures containing C2-depleted serum and either encapsulated organisms (representing each major capsule group) or their isogenic unencapsulated mutant counterparts. Rather surprisingly, at 10 min, Cap+ group W-135 and Y strains generated high amounts of C3a compared with their unencapsulated counterparts (Fig. 2A). At this time point, only small amounts of C3a were generated by all other strains tested. Although the Cap− group B strain generated statistically higher amounts of C3a at 10 min compared with its Cap+ counterpart, the total amount of C3a produced by this strain was ∼5-fold less compared with C3a generated by Cap+ W-135 and Y strains. At 30 min, all strains produced high amounts of C3a and differences between Cap+ and Cap− mutant pairs, with the exception of the group W-135 mutant pair, were not significant (Fig. 2B). Thus, expression of group W-135 or Y capsular polysaccharides was associated with rapid alternative pathway activation, evidenced by high C3a generation at 10 min. However, all strains were activated by C3 through the alternative pathway by 30 min.
FIGURE 2.
C3a generation by Cap+ and Cap− isogenic meningococcal mutants. Five Cap+ and Cap− isogenic mutant pairs of meningococcal strains (one representative of each meningococcal serogroup) that lacked LOS sialic acid, fHbp, and NspA were incubated with C2-depleted human serum (final concentration 25% (v/v)). Aliquots were collected at 10 and 30 min and C3a generated in the reaction mixture was measured by ELISA. Baseline levels of C3a generation were measured in control reaction mixtures that contained C2-depleted serum in HBSS2+ (no bacteria added) and is shown by the bar labeled C2-dep. Each data point represents the mean ± S.D. of 3 separate experiments.
Rapid C3 Fragment Deposition Occurs on Encapsulated Group W-135 and Y N. meningitidis
Cleavage of the C3a fragment from the N terminus of the α chain of C3 is accompanied by exposure of a labile internal thioester bond in the resulting ∼106-kDa α′ chain. The α′ chain remains linked to the 75-kDa β chain by a disulfide bond and this metastable C3b binds to surfaces through the reactive thioester in the α′ chain (43). The calculated half-life of the activated thioester is ∼60 μs (43, 44) and within this short period the metastable C3b molecule must bind to a surface target (in this instance, the meningococcal surface) though a covalent ester or amide bond; failure of the metastable C3b to react with a target molecule results in its reaction with a H2O molecule and the resulting hydrolyzed C3b molecule remains in solution. iC3b is formed by cleavage of the α′ chain into α1′ (∼68 kDa), α2′ (∼40 kDa), and C3f (∼2 kDa) fragments by factor H and factor I (43). In the unreduced state, the α1′ and α2′ fragments remain united through a second disulfide bond; the α1′ fragment is covalently attached to the target molecule. Electrophoresis under reducing conditions results in migration of the β chain (present in both C3b and iC3b) at its calculated mass of ∼75 kDa, whereas the 106-kDa α′ chain of C3b and the 68-kDa α1′ chain of iC3b migrate covalently bound to their targets; the free α2′ chain of iC3b migrates independently at 40 kDa.
Previous reports have shown that almost all C3b deposited on the meningococcal surface is converted to iC3b (35, 45) as early as 10 min. The mutants used in this study were constructed to minimize alternative pathway inhibition by LOS sialic acid, fHbp, and NspA. We sought to determine the extent of processing of C3b deposited on the bacterial surface to iC3b in these strains. All 10 mutant strains were incubated with Mg/EGTA-NHS for 30 min and samples were treated with methylamine to release C3 fragments linked to their targets through ester linkages. We confirmed that iC3b was also the predominant C3 fragment present on all mutant strains using a polyclonal antibody directed against C3d as well as mAb 755, which is directed against the α′ chain of C3b (∼106 kDa) and the α2′ chain of iC3b (∼40 kDa) (supplemental Fig. S1). The data in supplemental Fig. S1 reveal quantitative differences in the amount of C3 fragments deposited on the mutant pairs and a more detailed characterization of bacteria-bound iC3b was carried out next. For simplicity, henceforth we have used “iC3b deposition” to imply initial C3b deposition with subsequent conversion to iC3b. In subsequent experiments a well characterized anti-iC3b mAb, mAb G-3E, that specifically recognizes a neoepitope on the 68-kDa α1′ chain of iC3b (33, 34) was used.
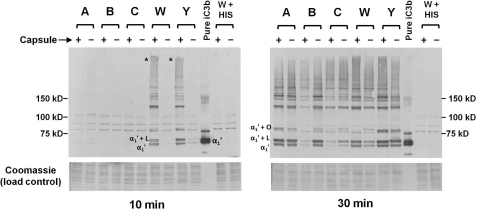
Consistent with C3a generation (Fig. 2), at 10 min almost no iC3b was deposited on the derivatives of the group A, B, and C strains (Fig. 3, left blot). However, the Cap+ W-135 and Y strains showed markedly increased iC3b deposition compared with their Cap− counterparts. A high molecular mass anti-iC3b-reactive “smear” (indicated by asterisks at the point of entry of samples into the gel) was seen in the Cap+ W-135 and Y lanes. Note that the iC3b α1′ chain in the lane containing purified iC3b migrates as a doublet (∼68- and ∼70-kDa bands) probably because a fraction of the α′ chain of C3b was not cleaved at the second site by factor I to release the 2-kDa C3f fragment. As expected, more iC3b deposition on all strains was seen at 30 min (Fig. 3, right blot). LOS (∼4 kDa) and the phase variable opacity proteins (∼25 kDa) have previously been characterized as Neisserial ligands for C3 fragments (36, 46, 47) and the locations of adducts of the α1′ chain of iC3b linked covalently to LOS and opacity proteins have been indicated in Fig. 3 as α1′ + L and α1′ + O, respectively. Expression of the group A capsule did not limit iC3b deposition, whereas expression of the group B capsule markedly decreased iC3b deposition. Expression of group C capsule expression was associated with slightly less iC3b deposition; the difference was most evident in the band indicated by “α1′ + L” (the 68-kDa α1′ chain of iC3b linked covalently to LOS). The Cap+ group W-135 strain showed greater iC3b deposition than its corresponding Cap− mutant. Although more subtle than observations with the group W-135 mutant pair, the Cap+ group Y strain also showed more iC3b than its Cap− isogenic mutant. Differences in the amount of C3 fragments deposited on the mutant strain pairs are also evident in supplemental Fig. S1. A portion of iC3b detected in lanes containing bacteria treated with serum migrates as free (or released) α1′ fragment despite the lack of treatment with a nucleophile such as methylamine. Spontaneous release of C3b previously covalently linked by ester bonds to surfaces such as sheep RBCs (48), glycerol (48, 49), IgG aggregates or tyrosine (50) has been reported. This spontaneous release of covalently bound C3 has been attributed to hydrolytic attack by the His residue (that corresponds to the His residue at position 1106 in the ester bond-forming C4B isoform of C4) 113 amino acids C-terminal to the ester-forming Gln residue on the ester bond (43).
FIGURE 3.
iC3b deposition on isogenic Cap+ and Cap− meningococci. The five isogenic Cap+ and Cap− pairs of N. meningitidis were incubated with C2-depleted human serum for either 10 or 30 min and bacteria-bound iC3b was analyzed by Western blotting with anti-iC3b mAb G-3E. The position of the α1′ (68 kDa) fragment of C3 is indicated in the control lane that contains purified iC3b (see text for an explanation of the α1′ “doublet”). Both blots (10 min (left) and 30 min (right) incubations) were exposed to the alkaline phosphatase substrate for the same duration. “α1′ + L” and “α1′ + O” indicate the positions of adducts of LOS or opacity-associated protein covalently linked to the α1′ iC3b fragment, respectively (36, 46, 47). The asterisks in the “10-min blot” indicate high-molecular mass complexes that contain iC3b in the lanes containing Cap+ W and Y strains. Proteins on the membrane migrating below ∼40 kDa were stained with Coomassie Blue and served as a loading control. Cap+ and Cap− derivatives of strain W171 were incubated with heat-inactivated C2-depleted serum (lanes marked W + HIS) and served as controls to validate the specificity of covalently bound C3 fragments to bacteria.
Capsule-specific antibodies may enhance alternative pathway-mediated C3 deposition and bacterial killing (23, 51, 52). To exclude the possibility that antibodies in the C2-depleted serum contributed to increased C3 activation and C3 fragment deposition on the Cap+ W-135 and Y strains, we incubated bacteria with serum depleted of IgG and IgM, which simulated results with C2-depleted serum (data not shown).
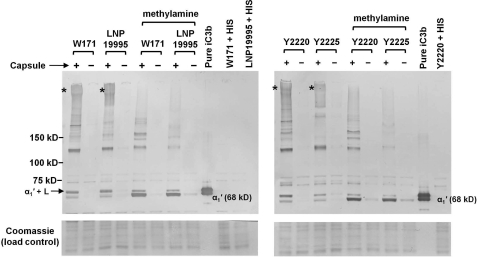
The rather unexpected finding of increased alternative pathway activation on encapsulated group W-135 and Y strains prompted us to examine an additional strain from each of these two serogroups to ensure that these results were not unique to strains W171 and Y2220. Strains LNP19995 (group W-135) and Y2225 (group Y), lacking the ability to sialylate their LOS or bind to fH via fHbp or NspA, and their isogenic Cap− mutants were incubated with 25% C2-depleted serum for 10 min and bacterial lysates were examined for iC3b deposition. The Cap+ and Cap− mutants of strains W171 and Y2220 were included as controls for comparison. As seen in Fig. 4, more iC3b was deposited on each Cap+ strain compared with its Cap− counterpart. C3 fragments bind covalently to their targets on Neisseria predominantly through ester linkages (36). To define the linkage specificity between the iC3b and its targets on the Cap+ and Cap-W-135 and Y strains, samples were treated with 1 m methylamine, pH 11, which disrupts ester linkages but leaves amide bonds intact. Also seen in Fig. 4, methylamine treatment was associated with a decrease in intensity of almost every band and the diffuse high molecular mass anti-iC3b reactive material. As expected, a concomitant increase in the intensity of free 68-kDa α1′ chain of iC3b released from its targets was noted.
FIGURE 4.
Demonstration of rapid and enhanced complement activation on additional Cap+ group W and Y strains of N. meningitidis. Group W strain LNP19995 and group Y strain Y2225 that lacked LOS sialic acid, fHbp, and NspA were incubated with C2-depleted human serum (25% (v/v)) for 10 min and iC3b deposited on bacteria was analyzed by Western blotting. Strains W171 and Y2220 that had been used in previous experiments were used as controls to facilitate comparison. Following incubation with serum, half the samples were treated with 1 m methylamine, pH 11, to disrupt ester linkages between the α1′ chain of iC3b and its targets; amide linkages between iC3b and its targets remain unaffected with methylamine treatment. The position of the free 68-kDa α1′ chain of iC3b is indicated. “α1′ + L” indicates the position of the LOS-iC3b adduct (36). The asterisks indicate the anti-iC3b-reactive high molecular mass complexes that are seen only in the encapsulated strains and are decreased in intensity following methylamine treatment. Loading controls (Coomassie stain) and controls where bacteria are incubated with heat-inactivated serum (HIS) are as described in the legend to Fig. 3.
The studies above that indicate increased complement activation by, and increased iC3b deposition, on group W-135 and Y strains were performed with isogenic mutant strains that lack several important surface molecules (fHbp, NspA, and LOS sialic acid) that may serve to enhance virulence. To determine whether these observations were relevant to wild-type meningococcal isolates expressing fHbp, NspA, and LOS sialic acid, the five encapsulated wild-type parent strains were incubated with C2-depleted serum and C3a generation and iC3b deposition were measured. Consistent with the data obtained with the mutant strains thus far, wild-type parent group W-135 and Y strains activated the alternative pathway rapidly (evidenced by greater C3a generation at 10 min; supplemental Fig. S2A) and also bound more iC3b (supplemental Fig. S2B) than strains that belong to the other three groups at this time point. Again, iC3b was associated with high molecular mass targets on the W-135 and Y strains (indicated by the asterisk in supplemental Fig. S2B).
Collectively, the data presented thus far show that expression of group W-135 and Y capsular polysaccharides is associated with increased alternative pathway activation at 10 min (evidenced by increased C3a generation) and also with increased C3 fragment deposition compared with their Cap− isogenic mutants. These observations also extended to the corresponding wild-type strains.
W-135 and Y Capsules Serve as Targets for C3 Fragments on Intact Bacteria
Increased C3 activation and C3 fragment deposition on Cap+ group W-135 and Y strains compared with Cap− strains raised the possibility that capsular polysaccharide itself may serve to facilitate alternative pathway activation. Capsular polysaccharides of N. meningitidis are high molecular mass glycans that migrate as “diffuse” high molecular mass material (>100 kDa) when analyzed by Western blot using group specific antisera. The observation of diffuse anti-iC3b-reactive material above ∼200 kDa in the Cap+ W-135 and Y lanes indicated by the asterisks in Figs. 3 and 4 (10-min time points) raised the possibility that the W-135 and Y capsules themselves may serve as targets for C3 fragment deposition and facilitate amplification of the alternative pathway.
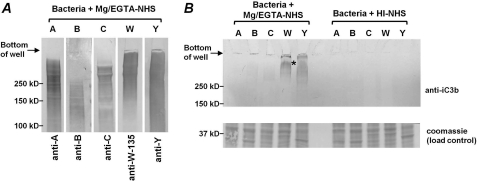
To reduce the sample viscosity, attributed to bacterial DNA, bacteria were treated with DNase I following incubation with Mg/EGTA-treated serum prior to electrophoresis and Western blotting. To determine the approximate molecular masses and migration of the capsular polysaccharide of the strains used in this study, electrophoresed and Western blotted DNase I-treated samples of bacteria were probed with the respective anti-capsular mAbs. As expected, all capsules migrated as diffuse, high molecular mass smears (Fig. 5A). Thus, any capsule covalently linked to the 68-kDa α1′ chain of iC3b would be expected to migrate at molecular masses above 150 kDa. As seen in Fig. 5B, iC3b-reactive material was seen in lanes that contained Cap+ group W-135 and Y strains but not in lanes containing other capsular groups. No reactivity was seen in any lane that contained bacteria incubated with heat-inactivated NHS. These data provide evidence for alternative pathway-mediated iC3b deposition on the capsular polysaccharides of group W-135 and Y strains.
FIGURE 5.
iC3b is deposited on group W-135 and Y capsular polysaccharides. A, Western blotting to detect capsular polysaccharide on Cap+ strains. The five Cap+ strains that were incubated with 25% (v/v) Mg/EGTA-NHS were Western blotted and probed with a mAb against the respective capsular polysaccharide. No reactivity was seen in control blots (anti-capsular mAb excluded; data not shown). B, iC3b is deposited selectively on group W-135 and Y capsular polysaccharides. The Cap+ mutant strains were incubated with 25% (v/v) Mg/EGTA-treated serum for 30 min. Bacteria incubated with heat-inactivated normal human serum (HI-NHS) served as negative controls. Following washing of bacteria, the pellets were treated with DNase I to decrease sample viscosity. Western blotting for iC3b detection and Coomassie staining to confirm similar loading across strains was performed as described in the legends to Figs. 3 and 4.
Deposition of C3 Fragments Occurs Preferentially on Immobilized Group W-135 and Y Capsular Polysaccharides
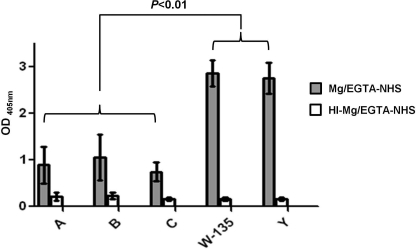
To further demonstrate the specificity of iC3b deposition on group W-135 and Y capsular polysaccharides, we immobilized purified group A, B, C, W-135, and Y capsules on microtiter wells. Mg/EGTA-NHS was added to the wells to a final concentration of 20% and bound iC3b was detected with anti-iC3b mAb G-3E. High levels of iC3b bound to wells coated with group W-135 and Y polysaccharides; ∼3-fold lower levels of deposition were observed in wells coated with group A, B, and C polysaccharides (Fig. 6, gray shaded bars). The differences in iC3b deposition on the W-135 and Y capsules were significantly higher than iC3b deposition on group A, B, or C capsules (p < 0.01). Controls included: (a) polysaccharide-coated wells incubated with heat-inactivated Mg/EGTA-treated serum (HI-Mg/EGTA-NHS) (Fig. 6, open bars) and (b) wells that had been coated with methylated human albumin with Mg/EGTA-NHS added (not shown). All controls yielded similar A405 nm readings (<0.2). Serum depleted of IgG and IgM yielded similar results as Mg/EGTA-NHS (data not shown), which provided evidence that antibodies in serum were not responsible for increased iC3b deposition on group W-135 and Y capsular polysaccharides.
FIGURE 6.
Deposition of iC3b on immobilized purified capsular polysaccharides. Microtiter wells were coated with purified group A, B, C, W-135, and Y capsular polysaccharides followed by addition of 20% (v/v) Mg/EGTA-treated normal human serum (Mg/EGTA-NHS; gray bars). Deposited iC3b was detected with an anti-iC3b mAb. Control wells were incubated with heat-inactivated Mg/EGTA-NHS (HI-Mg/EGTA-NHS; open bars). Additional controls, where Mg/EGTA-NHS was added to wells coated with human serum albumin alone yielded signals less than that seen with HI-Mg/EGTA-treated wells. Each bar shows the mean ± S.D. of 4 separate experiments.
Surface-bound, but Not Soluble, Group W-135 and Y Capsular Polysaccharides Activate the Alternative Pathway
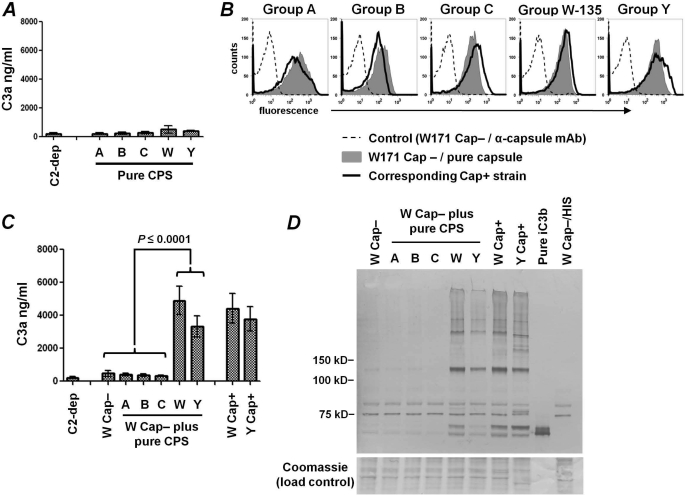
Having shown that intact Cap+ W-135 and Y meningococci rapidly activate the alternative pathway and that immobilized purified W-135 and Y polysaccharides bind higher amounts of C3b that is converted to iC3b, we questioned whether purified capsular group W-135 and Y polysaccharides in solution could activate C3 in alternative pathway-specific serum. Each of the purified capsular polysaccharides was added separately to 25% (v/v) C2-depleted serum at a final concentration of 125 μg/ml and the amount of C3a generated was measured by ELISA. C2-depleted serum in HBSS2+ (no added polysaccharide) served as a control. Purified soluble capsular polysaccharide did not activate C3 above baseline control levels (Fig. 7A).
FIGURE 7.
Only group W-135 and Y capsular polysaccharides bound to bacteria, but not free soluble polysaccharide, enhance alternative pathway activation. A, purified capsular polysaccharides in solution do not generate C3a. C2-depleted serum (25% (v/v)) was incubated with soluble purified capsular polysaccharides (each at a concentration of 125 μg/ml) for 10 min at 37 °C and C3a generated was measured by ELISA. Each bar represents the mean ± S.D. of 2 separate experiments. B, binding of purified capsular polysaccharides to Cap− N. meningitidis. W171 Cap−/fHbp−/NspA− (referred to as W Cap−) was incubated separately with purified polysaccharides (125 μg/ml) representing each of the 5 major meningococcal serogroups. Bound polysaccharide was detected using a mAb specific for each of the serogroups (gray shaded histograms). Binding of the mAb to the corresponding Cap+ mutant strain used in this study (i.e. A2594, H44/76, C2120, W171, and Y2220) is shown by the solid black histogram. Controls where the Cap− W171 mutant without any added polysaccharide was incubated with the anti-capsule mAb and secondary conjugate are shown by the broken lines. One of two reproducible experiments is shown. C, group W-135 and Y polysaccharides induce rapid C3a generation when bound to bacteria. Strain W171 Cap− was incubated with soluble purified capsular polysaccharides (each at a concentration of 125 μg/ml), followed by the addition of C2-depleted serum (25% (v/v)) for 10 min at 37 °C; C3a generated in the reaction mixture was measured by ELISA. Encapsulated strains W171 and Y2220 served as positive controls, whereas W171 Cap− plus C2-depleted serum (no added capsular polysaccharide) and C2-depleted serum alone were used as negative controls. Each bar represents the mean ± S.D. of 3 separate experiments. D, deposition of iC3b on W171 Cap− coated with purified capsular polysaccharides. The bacterial strains incubated with C2-depleted serum as described in C were washed, lysed, and Western blotted to analyze iC3b binding.
In light of the lack of complement activation by soluble capsular polysaccharides, but the ability of group W-135 and Y capsules to augment C3 activation and bind iC3b either in the context of whole bacteria (Figs. 3–5) or when immobilized to microtiter wells (Fig. 6), we hypothesized that purified W-135 and Y capsules, if bound to the surface of a Cap− strain, would result in enhanced complement activation. We mixed each group-specific polysaccharide capsule separately with an unencapsulated W-135 strain and examined: (a) binding of each of the capsules to the Cap− group W-135 strain; (b) C3a generation by the alternative pathway of each group-specific capsule surface-bound to an otherwise Cap− W-135 strain; and (c) iC3b (the result of C3b cleavage) deposition by the alternative pathway onto each group-specific capsule bound to the bacterial surface. Shown in Fig. 7B, each of the 5 purified capsular polysaccharides bound to the representative Cap− W-135 strain, W171 Cap−. The amount of purified capsule that bound to W171 Cap− was similar to the amount of capsule elaborated by the corresponding wild-type strain that expressed capsule intrinsically. Fig. 7C shows C3a generation that resulted from activation of the alternative pathway by Cap- strain W171 displaying each of the group-specific polysaccharides. Adding purified W-135 or Y capsule to W171 Cap− resulted in increased C3a generation at 10 min and parallel results of C3a generation by intrinsically encapsulated W-135 and Y strains (labeled W171 Cap+ and Y2220 Cap+, respectively). As hypothesized, minimal C3 activation occurred with W171 Cap− lacking added polysaccharide or when the group A, B, or C capsules were added to this strain. Consistent with C3 activation by bacteria-bound purified W-135 and Y capsular polysaccharides, Western blotting revealed increased iC3b deposition on these “reconstituted” mutants (Fig. 7D) to an extent similar to that seen with W171 Cap+ and Y2220 Cap+. No iC3b was detected on W171 Cap− alone or W171 Cap− in the presence of group A, B, or C capsular polysaccharides. The sites of iC3b deposition were similar to those seen on intrinsically encapsulated W-135 and Y strains (labeled W Cap+ and Y Cap+, respectively) (Fig. 7D). Taken together, these data provide strong evidence that group W-135 and Y capsular polysaccharides on the bacterial surface facilitate alternative pathway activation and that these two capsular groups themselves are targets for C3 fragment deposition and may serve as a foci for alternative pathway amplification.
The Alternative Pathway Alone Cannot Support Complement-dependent Killing of N. meningitidis
Having demonstrated rapid and high levels of alternative pathway-mediated C3 fragment deposition on encapsulated (Cap+) W-135 and Y mutants, we questioned whether these mutants would be more susceptible to killing by the alternative pathway of complement alone. Each mutant was incubated with C2-depleted serum to a final concentration of 50%. All 5 Cap+ and Cap− mutant pairs showed 100% survival in C2-depleted serum. Reconstituting the C2-depleted serum with physiologic concentrations (∼40 μg/ml) of purified C2 to restore the classical and lectin pathways of complement resulted in complete (100%) killing of Y Cap− that was used as a control (data not shown). These data indicate that the alternative pathway alone cannot support complement-dependent killing of these strains of N. meningitidis even when regulation by direct binding of factor H to bacteria is absent.
DISCUSSION
Bacterial polysaccharide capsules are important virulence factors of several human pathogens including N. meningitidis, Haemophilus influenzae, Streptococcus pneumonia, Escherichia coli K1, and group B streptococci. Capsules are believed to function by inhibiting complement activation and enhancing resistance to opsonophagocytosis (22). Sialic acid-containing capsules have received particular attention because of the well defined role of sialic acid residues on erythrocytes in regulating the alternative pathway (53, 54). Several studies have shown that sialic acid residues on bacteria, as on host cells, regulate the alternative pathway of complement (55, 56). The alternative pathway is regulated by sialic acid expressing capsules of type III group B streptococci and E. coli K1 (25). Jarvis and Vedros (23) showed that desialylation of group B meningococci (E. coli K1 and group B meningococcal capsules are structurally similar) enhanced alternative pathway activation. Uria et al. (24) showed that group C meningococcal strains that express very high levels of capsular polysaccharide because of the presence of an IS1301 insertion element between the capsule biosynthesis (sia) and capsule transport (ctr) operons activated the alternative pathway less than an isogenic mutant that lacked the IS1301 element and expressed normal amounts of capsule. Consistent with these studies, our data show that group B and C capsule expression decreased C3 fragment deposition on bacteria. Taken together with the observation that Cap+ group B and C strains did not limit the amount of C3 activated through the alternative pathway compared with their Cap− isogenic mutants (measured by C3a generation in Fig. 2), we speculate that group B and C capsule expression may interfere with the ability of activated C3 to form covalent linkages with its membrane targets on these strains. Expression of the group A capsule also did not impact the rate or amount of alternative pathway activation by bacteria. However, unlike the group B and C capsules, group A capsule expression did not interfere with C3b deposition on membrane targets. Unexpectedly, our studies showed that the W-135 and Y capsular polysaccharides enhanced activation of the alternative pathway.
Activation of the alternative pathway occurs in three phases: a lag phase, an amplification phase, and a plateau phase (57). The lag phase on alternative pathway activator surfaces ranges from about 1 to 2 min for rabbit erythrocytes to 6–8 min for zymosan and cryptococci (57, 58); maximal C3 deposition occurs by about 10 min. The duration of the lag phase varies on different strains of E. coli, and data reported with E. coli O4 revealed a lag phase of about 10 min (57). Similarly, our data showed that the rate of C3 activation varied among meningococcal strains. The novel and rather unexpected finding in this study was that the group W-135 and Y capsular polysaccharides, both of which contain sialic acid, showed the shortest lag phase and facilitated alternative pathway activation. The linkage specificity and substitutions of the sialic acid moiety are crucial factors that determine whether a sialoglycan inhibits the alternative pathway. For example, 9-O-acetylation of sialic acid on erythrocytes enhances activation of the alternative pathway of complement (59). The capsular sialic acids of certain group W-135 and Y strains may be O-acetylated at the 7 or 9 positions (enzymatic O-acetylation likely occurs at the 7 position; subsequent non-enzymatic isomerization results in migration of the acetyl group to the 9 position) (26). However, we do not believe that acetylation is responsible for increased activation of the alternative pathway in this case because neither strain W171 nor Y2225 express the O-acetyltransferase (the result of deletion of part (Y2225) or the entire (W171) oatWY gene) (26). We speculate that the Gal and Glc residues in group W-135 and Y capsular polysaccharides, respectively, may provide the electron-donating -OH group that forms a covalent ester bond with C3. Why the group A capsule, which is devoid of sialic acid, does not rapidly activate the alternative pathway is not clear. We speculate that the N-acetyl and phosphate substitutions of the mannose residues of the group A capsule may prevent C3b deposition and render it a relatively poor activator of complement.
In accordance with prior work by Kozel (60), who showed that purified cryptococcal capsular polysaccharide bound to unencapsulated Cryptoocccus neoformans, we observed binding of purified meningococcal capsular polysaccharides to Cap− bacteria. Interestingly, only immobilized or bacteria-bound W-135 and Y capsular polysaccharides activated the alternative pathway; the same capsules in solution did not activate C3. These data are consistent with previous studies, which showed that soluble yeast β-glucans do not activate the alternative pathway, but turbid β-glucan suspensions that contain the same glycosidic linkages are potent activators of C3 in Mg/EGTA-treated serum (61). A likely explanation is that high local concentrations of polysaccharide are achieved when capsule is associated with a bacterial surface, which would provide abundant −OH groups that can participate in forming ester linkages with the short-lived activated C3 molecule (half-life <100 μs (43, 62)) and therefore permit efficient amplification of the alternative pathway. In contrast, the density of −OH groups that can serve as electron donors to react with metastable C3 when capsules are in the fluid phase is probably too low to permit efficient perpetuation of the positive feedback loop of the alternative pathway at the concentrations that we have tested.
The goal of the current study was limited to defining the role of capsular polysaccharides, independent of other variables that can limit alternative pathway activation, such as LOS sialylation (28) and expression of the meningococcal ligands for factor H, fHbp (30, 31, 41), and NspA (29). It is likely that one or more of these variables may act in concert with capsular polysaccharide to confer the high levels of serum resistance that often are characteristic of wild-type Cap+ strains. Although beyond the scope of this study, an examination of the interactions between these variables in determining how meningococci limit complement activation is merited.
None of the mutants tested were killed by C2-depleted serum despite lacking fHbp, NspA, capsule, and LOS sialic acid. These data reiterate the importance of activation of the classical pathway in mediating direct complement-dependent killing of Neisseriae (63, 64). C3 deposition through the classical pathway can also recruit the alternative pathway to amplify complement activation. The differences in the rates of alternative pathway activation and the sites of C3 fragment deposition on the various meningococcal serogroups that we have described may translate to differences in their pathogenic mechanisms, for example, their ability to interact with professional phagocytes, and merit further investigation.
Supplementary Material
Acknowledgments
We thank Dr. Peter A. Rice (University of Massachusetts) for insightful discussions and critical reading of the manuscript. We thank Dr. Dan M. Granoff and Dr. Jo Anne Welsch for anti-group A, C, W-135, and Y mAbs and for strain LNP19995. We thank Dr. Ulrich Vogel (Universität Würzburg, Würzburg, Germany) for providing all other strains used in this study and their siaD, myn B, or lst mutants.
This work was supported, in whole or in part, by National Institutes of Health Grants AI054544, AI 084048, and AI32725.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- LOS
- lipooligosaccharide
- HBSS
- Hanks' balanced salt solution
- Cap
- capsule
- NHS
- normal human serum
- fHbp
- factor H-binding protein
- NspA
- Neisserial surface protein A
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
REFERENCES
- 1. Frosch M., Vogel U. (2006) in Handbook of Meningococcal Disease (Frosch M., Maiden M. C. J., eds) pp. 145–162, Wiley-VCH, Weinheim, Germany [Google Scholar]
- 2. van Deuren M., Brandtzaeg P., van der Meer J. W. (2000) Clin Microbiol. Rev. 13, 144–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Findlow H., Vogel U., Mueller J. E., Curry A., Njanpop-Lafourcade B. M., Claus H., Gray S. J., Yaro S., Traoré Y., Sangaré L., Nicolas P., Gessner B. D., Borrow R. (2007) J. Infect. Dis. 195, 1071–1077 [DOI] [PubMed] [Google Scholar]
- 4. Hoang L. M., Thomas E., Tyler S., Pollard A. J., Stephens G., Gustafson L., McNabb A., Pocock I., Tsang R., Tan R. (2005) Clin. Infect. Dis. 40, e38–42 [DOI] [PubMed] [Google Scholar]
- 5. Vogel U., Claus H., von Müller L., Bunjes D., Elias J., Frosch M. (2004) J. Clin. Microbiol. 42, 2898–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alper C. A., Abramson N., Johnston R. B., Jr., Jandl J. H., Rosen F. S. (1970) N. Engl. J. Med. 282, 350–354 [DOI] [PubMed] [Google Scholar]
- 7. Barba G. M., Kaufmann T. J., Schneider P. M., Rittner C., Brai M. (1994) Clin. Immunol. Immunopathol. 72, 83–89 [DOI] [PubMed] [Google Scholar]
- 8. Biesma D. H., Hannema A. J., van Velzen-Blad H., Mulder L., van Zwieten R., Kluijt I., Roos D. (2001) J. Clin. Invest. 108, 233–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Densen P., Weiler J. M., Griffiss J. M., Hoffmann L. G. (1987) N. Engl. J. Med. 316, 922–926 [DOI] [PubMed] [Google Scholar]
- 10. Ellison R. T., 3rd, Kohler P. F., Curd J. G., Judson F. N., Reller L. B. (1983) N. Engl. J. Med. 308, 913–916 [DOI] [PubMed] [Google Scholar]
- 11. Figueroa J., Andreoni J., Densen P. (1993) Immunol. Res. 12, 295–311 [DOI] [PubMed] [Google Scholar]
- 12. Figueroa J. E., Densen P. (1991) Clin. Microbiol. Rev. 4, 359–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fijen C. A., Kuijper E. J., Hannema A. J., Sjöholm A. G., van Putten J. P. (1989) Lancet 2, 585–588 [DOI] [PubMed] [Google Scholar]
- 14. Fijen C. A., Kuijper E. J., te Bulte M. T., Daha M. R., Dankert J. (1999) Clin. Infect. Dis. 28, 98–105 [DOI] [PubMed] [Google Scholar]
- 15. Lehner P. J., Davies K. A., Walport M. J., Cope A. P., Würzner R., Orren A., Morgan B. P., Cohen J. (1992) Lancet 340, 1379–1381 [DOI] [PubMed] [Google Scholar]
- 16. Lim D., Gewurz A., Lint T. F., Ghaze M., Sepheri B., Gewurz H. (1976) J. Pediatr. 89, 42–47 [DOI] [PubMed] [Google Scholar]
- 17. Morris J. T., Kelly W. J. (1992) South Med. J. 85, 1030–1031 [DOI] [PubMed] [Google Scholar]
- 18. Petersen B. H., Graham J. A., Brooks G. F. (1976) J. Clin. Invest. 57, 283–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rosen M. S., Lorber B., Myers A. R. (1988) Arch. Intern. Med. 148, 1441–1442 [DOI] [PubMed] [Google Scholar]
- 20. Söderström C., Sjöholm A. G., Svensson R., Ostenson S. (1989) Scand. J. Infect. Dis. 21, 259–265 [DOI] [PubMed] [Google Scholar]
- 21. Reis E. S., Falcão D. A., Isaac L. (2006) Scand. J. Immunol. 63, 155–168 [DOI] [PubMed] [Google Scholar]
- 22. Taylor C. M., Roberts I. S. (2005) Contrib. Microbiol. 12, 55–66 [DOI] [PubMed] [Google Scholar]
- 23. Jarvis G. A., Vedros N. A. (1987) Infect. Immun. 55, 174–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uria M. J., Zhang Q., Li Y., Chan A., Exley R. M., Gollan B., Chan H., Feavers I., Yarwood A., Abad R., Borrow R., Fleck R. A., Mulloy B., Vazquez J. A., Tang C. M. (2008) J. Exp. Med. 205, 1423–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. (1982) J. Immunol. 128, 1278–1283 [PubMed] [Google Scholar]
- 26. Claus H., Borrow R., Achtman M., Morelli G., Kantelberg C., Longworth E., Frosch M., Vogel U. (2004) Mol. Microbiol. 51, 227–239 [DOI] [PubMed] [Google Scholar]
- 27. Gudlavalleti S. K., Datta A. K., Tzeng Y. L., Noble C., Carlson R. W., Stephens D. S. (2004) J. Biol. Chem. 279, 42765–42773 [DOI] [PubMed] [Google Scholar]
- 28. Estabrook M. M., Griffiss J. M., Jarvis G. A. (1997) Infect. Immun. 65, 4436–4444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lewis L. A., Ngampasutadol J., Wallace R., Reid J. E., Vogel U., Ram S. (2010) PLoS Pathog. 6, e1001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madico G., Welsch J. A., Lewis L. A., McNaughton A., Perlman D. H., Costello C. E., Ngampasutadol J., Vogel U., Granoff D. M., Ram S. (2006) J. Immunol. 177, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schneider M. C., Exley R. M., Chan H., Feavers I., Kang Y. H., Sim R. B., Tang C. M. (2006) J. Immunol. 176, 7566–7575 [DOI] [PubMed] [Google Scholar]
- 32. Madico G., Ngampasutadol J., Gulati S., Vogel U., Rice P. A., Ram S. (2007) J. Immunol. 178, 4489–4497 [DOI] [PubMed] [Google Scholar]
- 33. Iida K., Mitomo K., Fujita T., Tamura N. (1987) Immunology 62, 413–417 [PMC free article] [PubMed] [Google Scholar]
- 34. McQuillen D. P., Gulati S., Ram S., Turner A. K., Jani D. B., Heeren T. C., Rice P. A. (1999) J. Infect. Dis. 179, 124–135 [DOI] [PubMed] [Google Scholar]
- 35. Vogel U., Weinberger A., Frank R., Müller A., Köhl J., Atkinson J. P., Frosch M. (1997) Infect. Immun. 65, 4022–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lewis L. A., Ram S., Prasad A., Gulati S., Getzlaff S., Blom A. M., Vogel U., Rice P. A. (2008) Infect. Immun. 76, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carlone G. M., Frasch C. E., Siber G. R., Quataert S., Gheesling L. L., Turner S. H., Plikaytis B. D., Helsel L. O., DeWitt W. E., Bibb W. F., et al. (1992) J. Clin. Microbiol. 30, 154–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shaughnessy J., Lewis L. A., Jarva H., Ram S. (2009) Infect. Immun. 77, 2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vogel U., Claus H., Heinze G., Frosch M. (1999) Infect. Immun. 67, 954–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vogel U., Hammerschmidt S., Frosch M. (1996) Med. Microbiol. Immunol. 185, 81–87 [DOI] [PubMed] [Google Scholar]
- 41. Schneider M. C., Prosser B. E., Caesar J. J., Kugelberg E., Li S., Zhang Q., Quoraishi S., Lovett J. E., Deane J. E., Sim R. B., Roversi P., Johnson S., Tang C. M., Lea S. M. (2009) Nature 458, 890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walport M. J. (2001) N. Engl. J. Med. 344, 1058–1066 [DOI] [PubMed] [Google Scholar]
- 43. Law S. K., Dodds A. W. (1997) Protein Sci. 6, 263–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sim R. B., Twose T. M., Paterson D. S., Sim E. (1981) Biochem. J. 193, 115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jarvis G. A. (1994) Infect. Immun. 62, 1755–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Edwards J. L., Apicella M. A. (2002) Cell. Microbiol. 4, 585–598 [DOI] [PubMed] [Google Scholar]
- 47. Ram S., Cox A. D., Wright J. C., Vogel U., Getzlaff S., Boden R., Li J., Plested J. S., Meri S., Gulati S., Stein D. C., Richards J. C., Moxon E. R., Rice P. A. (2003) J. Biol. Chem. 278, 50853–50862 [DOI] [PubMed] [Google Scholar]
- 48. Venkatesh Y. P., Minich T. M., Law S. K., Levine R. P. (1984) J. Immunol. 132, 1435–1439 [PubMed] [Google Scholar]
- 49. Venkatesh Y. P., Levine R. P. (1988) Mol. Immunol. 25, 821–828 [DOI] [PubMed] [Google Scholar]
- 50. Sahu A., Pangburn M. K. (1995) Mol. Immunol. 32, 711–716 [DOI] [PubMed] [Google Scholar]
- 51. Edwards M. S., Nicholson-Weller A., Baker C. J., Kasper D. L. (1980) J. Exp. Med. 151, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Selander B., Käyhty H., Wedege E., Holmström E., Truedsson L., Söderström C., Sjöholm A. G. (2000) J. Clin Immunol. 20, 138–149 [DOI] [PubMed] [Google Scholar]
- 53. Fearon D. T. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 1971–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pangburn M. K., Müller-Eberhard H. J. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 2416–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Austen K. F., Fearon D. T. (1979) Adv. Exp. Med. Biol. 120B, 3–17 [PubMed] [Google Scholar]
- 56. Severi E., Hood D. W., Thomas G. H. (2007) Microbiology 153, 2817–2822 [DOI] [PubMed] [Google Scholar]
- 57. Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. (1983) J. Immunol. 131, 1930–1935 [PubMed] [Google Scholar]
- 58. Kozel T. R., Wilson M. A., Murphy J. W. (1991) Infect. Immun. 59, 3101–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Varki A., Kornfeld S. (1980) J. Exp. Med. 152, 532–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kozel T. R. (1977) Infect. Immun. 16, 99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Czop J. K., Austen K. F. (1985) J. Immunol. 135, 3388–3393 [PubMed] [Google Scholar]
- 62. Sim E., Wood A. B., Hsiung L. M., Sim R. B. (1981) FEBS Lett. 132, 55–60 [DOI] [PubMed] [Google Scholar]
- 63. Ingwer I., Petersen B. H., Brooks G. (1978) J. Lab. Clin Med. 92, 211–220 [PubMed] [Google Scholar]
- 64. Lewis L. A., Choudhury B., Balthazar J. T., Martin L. E., Ram S., Rice P. A., Stephens D. S., Carlson R., Shafer W. M. (2009) Infect. Immun. 77, 1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Frasch C. E., Zollinger W. D., Poolman J. T. (1985) Rev. Infect. Dis. 7, 504–510 [DOI] [PubMed] [Google Scholar]
- 66. Vogel U., Morelli G., Zurth K., Claus H., Kriener E., Achtman M., Frosch M. (1998) J. Clin. Microbiol. 36, 2465–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sacchi C. T., Lemos A. P., Brandt M. E., Whitney A. M., Melles C. E., Solari C. A., Frasch C. E., Mayer L. W. (1998) Clin. Diagn. Lab. Immunol. 5, 845–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.