Abstract
Pathogenesis by Bacillus anthracis requires coordination between two distinct activities: plasmid-encoded virulence factor expression (which protects vegetative cells from immune surveillance during outgrowth and replication) and chromosomally encoded sporulation (required only during the final stages of infection). Sporulation is regulated by at least five sensor histidine kinases that are activated in response to various environmental cues. One of these kinases, BA2291, harbors a sensor domain that has ∼35% sequence identity with two plasmid proteins, pXO1-118 and pXO2-61. Because overexpression of pXO2-61 (or pXO1-118) inhibits sporulation of B. anthracis in a BA2291-dependent manner, and pXO2-61 expression is strongly up-regulated by the major virulence gene regulator, AtxA, it was suggested that their function is to titrate out an environmental signal that would otherwise promote untimely sporulation. To explore this hypothesis, we determined crystal structures of both plasmid-encoded proteins. We found that they adopt a dimeric globin fold but, most unusually, do not bind heme. Instead, they house a hydrophobic tunnel and hydrophilic chamber that are occupied by fatty acid, which engages a conserved arginine and chloride ion via its carboxyl head group. In vivo, these domains may therefore recognize changes in fatty acid synthesis, chloride ion concentration, and/or pH. Structure-based comparisons with BA2291 suggest that it binds ligand and dimerizes in an analogous fashion, consistent with the titration hypothesis. Analysis of newly sequenced bacterial genomes points to the existence of a much broader family of non-heme, globin-based sensor domains, with related but distinct functionalities, that may have evolved from an ancestral heme-linked globin.
Keywords: Anion Transport, Bacterial Protein Kinases, Bacterial Signal Transduction, Bacterial Toxins, Calorimetry, Crystal Structure, Evolution, Fatty Acid-binding Protein, Hemoglobin, Histidine Kinases
Introduction
Fully virulent Bacillus anthracis carries two large plasmids, pXO1 and pXO2, that are responsible for the production of its major virulence factors, anthrax toxin and the poly-γ-d-glutamic acid capsule (1–3). Following host-triggered germination of B. anthracis spores, toxin and capsule enable the bacilli in their vegetative form to evade the host's immune system and replicate rapidly in the lymphatic system and bloodstream. If the infection is not treated at an early stage, toxemia and septicemia leading to host death may rapidly follow (4–7).
Sporulation is required for long term survival of B. anthracis following host death, but the process must be carefully coordinated with toxin expression and the progress of the infection because sporulating cells (in contrast to encapsulated vegetative cells) are susceptible to host defenses. Given that sporulation is directed primarily by chromosomal genes, close coordination with the regulatory elements encoded by plasmid genes is therefore required for effective pathogenesis.
There is much evidence for such chromosome-plasmid “cross-talk,” although the overall picture is far from clear (for review, see Ref. 8). For example, the pXO1-encoded protein, AtxA, regulates synthesis of toxin (also pXO1-encoded) as well as capsule (pXO2-encoded) (9–12). AtxA is also part of a regulatory network for S-layer synthesis (a further defensive layer distinct from the capsule and peptidoglycan cell wall), a task performed by chromosomal proteins (13). In turn, the synthesis of AtxA is regulated by the chromosomally encoded transcription factor Spo0A, the master regulator of sporulation (14, 15), which completes a regulatory link between sporulation and virulence factor expression.
Spo0A is activated by phosphorylation to induce or repress transcription of genes required or not required for sporulation, respectively (16). The regulatory pathway controlling Spo0A is more complex than most two-component signal transduction systems. In B. anthracis, this pathway includes at least five (chromosomally encoded) sensor histidine kinases that are capable of inducing sporulation (17) as well as several aspartyl phosphatases, one of them encoded by the pXO1 virulence plasmid, that inhibit sporulation (18, 19).
Sensor histidine kinases are the primary sensors of environmental cues and form a large family of signaling proteins in both Gram-positive and Gram-negative bacteria. They have a modular architecture comprising at least a “sensor” domain and a catalytic domain (which includes the phosphorylatable domain, DHp, and the ATP-binding domain) that autophosphorylates on a histidine residue in response to sensor domain activation. Phosphohistidine is a high energy species that transfers its phosphoryl group to an aspartic acid residue on the downstream effector (for review, see Ref. 20).
BA2291 is one of the most active kinases in promoting sporulation in B. anthracis (17). It also appears to be unique in using GTP rather than ATP as its energy source for phosphorylation (21), and orthologs are found in most members of the Bacillus cereus group (a subfamily of the genus Bacillae, which includes B. anthracis and B. thuringiensis (an insect pathogen), but not Bacillus subtilis). Two plasmid-encoded proteins, pXO1-118 and pXO2-61, express “sensor-only” domains that share ∼35% sequence identity with BA2291 (22) and are only found in B. anthracis and certain strains of B. cereus that harbor similar plasmids. The pXO1-118 gene lies next to and is divergently transcribed from the atxA gene, whereas pXO2-61 lies within the region directing capsule synthesis. A microarray study found that transcription of pXO2-61 was strongly up-regulated when the atxA gene was present (12); and overexpression of pXO2-61 was found to reduce sporulation of B. anthracis in a BA2291-dependent manner (22). Expression of BA2291 in a B. subtilis model induced sporulation when expressed at lower copy levels, and this was repressed by co-expression of either pXO1-118 or pXO2-61. However, higher levels of BA2291 expression led to repression of sporulation, suggesting that when the activating signal is in limited supply, the kinase activity is reversed, and BA2291 acts as a phosphatase. These observations led to a model in which the plasmid-encoded sensor domains modulate BA2291 activity by titrating the sporulation signal, thereby preventing premature sporulation (22).
To explore the molecular mechanisms of BA2291 regulation and the nature of the sporulation signal, we determined the crystal structures of pXO1-118 and pXO2-61 at high resolution. We show that they adopt a dimeric globin fold but, most unusually, do not bind heme. We demonstrate that they bind fatty acid and a halide ion (most likely chloride) in a central cavity, and we show that the key structural features of ligand recognition and dimerization are conserved in a large family of kinases found in bacilli and related species. This suggests that they recognize the same environmental cue(s) and support the titration mechanism for the sensor-only domains. Recognizable homologs are also found in a number of distinct bacterial phyla, and our analysis points to an evolution of the globin family into a versatile group of “heme-free” environmental sensors.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification
The plasmid for Escherichia coli overexpression of B. anthracis ORF118 was obtained by cloning the PCR-amplified coding sequence using oligonucleotides BaORF1185′Nde (5′-GAGTGGACATATGGAAGCAACAAAACG-3′) and BaORF1183′Bam (5′-CTATAGGATCCAAAAATTTCAAGGTG-3′) into plasmid pET28a (Stratagene) digested with NdeI and BamHI. The coding sequence for residues 1–146 of BA2291 was amplified using oligonucleotides BaKin5′Nde (5′-TATTCGTCATATGGAAATGGAGGGAATG-3′) and BaKinXho2 (5′-TTTCCCTCGAGTTTTATAATATAATTTCCGAGTAT-3′), and the fragment cloned into pET28a digested with NdeI and XhoI. For pXO2-61, a synthetic gene was purchased from GenScript Co. and subcloned into pET28 as described for pXO1-118. Expression was obtained in E. coli BL21(DE3) cells grown in LB medium after induction with 0.1 mm isopropyl β-d-1-thiogalactopyranoside for 4 h at 32 °C. All proteins included a His6 tag and were purified by nickel affinity chromatography on a His-trap chelating column (Pharmacia), followed by His tag removal by thrombin and size exclusion on Superdex 75 or Superdex 200 columns (Amersham Biosciences), the apparent molecular mass in each case was consistent with a dimer. pXO1-118 was stored at −20 °C in 20 mm Tris-HCl, pH 7.4, 1 m NaCl, 50 μm KCl, 5 mm DTT. pXO2-61 and BA2291 sensor domains required 500 mm NaCl to keep the proteins stable for long term storage. For pXO1-118, selenomethionine-labeled protein was purified using a similar protocol, except that cells were grown in minimal medium supplemented with selenomethionine (23). The molecular mass of all proteins was confirmed by SDS-PAGE and MALDI-TOF mass spectrometry.
Crystallization, Data Collection, and Structure Solution
Native and selenomethionine-labeled pXO1-118 were crystallized by sitting or hanging drop vapor diffusion at room temperature by mixing 3 μl of precipitant solution (40% (v/v) PEG 300, 100 mm Tris-HCl, pH 5.4, 5% (w/v) PEG 1000), and 3 μl of protein solution at 14 mg/ml. Rod-shaped crystals grew within 3 days in space group P3221 with cell dimensions a = 89.9 Å, c = 35.3 Å. One native and one selenomethionine data set at the selenium absorption edge were collected at the Stanford Synchrotron Research Lightsource beamline 9-2, and the Brookhaven National Synchrotron Light Source beamline X26C, respectively, at 100 K. Diffraction images were processed and scaled with the HKL package (24). SOLVE (25) located four selenium sites, leading to initial phases with a figure of merit of 0.32. Density modification increased the figure of merit to 0.60; and automatic model building in RESOLVE (26) generated a model that was 77% complete. Manual model building was carried out in O (27), and the structure refined with REFMAC5 (28) and simulated-annealing using CNS (29). The final model for pXO1-118 contains a single domain (residues 1–150), plus 3 nonnative N-terminal residues, 1 molecule of undecanoic acid, 95 water molecules, and 1 Cl− ion; and has Rwork = 0.181 and Rfree = 0.225 for data from 80 to 1.76 Å resolution. A dimer is generated by rotation of the monomer about a crystallographic dyad.
pXO2-61 was crystallized by the microbatch method under paraffin oil. Crystals were obtained in 2 days from a buffer containing 1 m NaI, 20% (v/v) PEG 3350, 100 mm Tris-HCl, pH 7.5. Crystals belong to space group P212121 with unit cell dimensions a = 44.1, b = 62.6 and c = 124.7 Å. Data were collected at the Stanford Synchrotron Research Lightsource to 1.49 Å resolution and processed with the HKL package. The structure was solved by molecular replacement using the refined pXO1-118 structure as the search model. Model building and refinement were carried out in O and REFMAC5. The final model has Rwork = 0.177 and Rfree = 0.209 for data from 60 to 1.49 Å resolution. The asymmetric unit contains 2 molecules forming a dimer (residues 5–136 for each molecule), 364 water molecules, 26 I− ions, and 8 Na+ ions. The solvent content is 56.7%. Data collection and refinement statistics are summarized in Table 1. The stereochemical quality of both models was assessed using PROCHECK (30).
TABLE 1.
X-ray data collection, phasing and refinement
| Parameter | Phasing λpeak | Model refinement |
|
|---|---|---|---|
| pXO1-118 | pXO2-61 | ||
| Space group | P3221 | P212121 | |
| Cell dimensions (Å) | a = 89.9 | a = 44.1 | |
| c = 35.3 | b = 62.6 | ||
| c = 124.7 | |||
| Wavelength (Å) | 0.9781 | 0.97923 | 0.97923 |
| Resolution range (Å) | 50–2.5 | 80–1.76 | 60–1.49 |
| Observations | 70,890 | 173,637 | 408,633 |
| Unique reflections | 5,888 | 16,461 | 56,717 |
| Completenessa (%) | 99.8 (100) | 99.5 (95.0) | 98.9 (94.5) |
| Rsyma,b (%) | 6.9 (25) | 5.7 (46) | 6.8 (32) |
| Rworkc/Rfreed (%) | 0.185/0.241 | 0.177/0.209 | |
| Protein atoms | 1,408 | 2,628 | |
| Water molecules | 95 | 364 | |
| Other ions | 1 | 34 | |
| Ligand | 1 | ||
| Root mean square deviation | |||
| Bonds (Å) | 0.027 | 0.012 | |
| Angles (°) | 1.70 | 1.37 | |
| Average B-factor (Å2) | |||
| Main chain | 25.0 | 18.9 | |
| Side chain | 32.7 | 24.0 | |
| Water | 34.6 | 35.9 | |
| Ligands/Ions | 37.4 | 39.7 | |
a Numbers in parentheses are for highest resolution shell.
b Rsym = Σ|Ih − <Ih>|/ΣIh, where <Ih> is the average intensity over symmetry equivalent reflection.
c R-factor = Σ|Fobs − Fcalc| − Fobs, where summation is over the data used for refinement.
d Rfree was calculated using 5% of data excluded from refinement.
GC-MS
200 μl of chloroform was added to 0.1–1 ml of 10 mg/ml sensor domain. The resulting two-phase system was sonicated for 10 min, incubated at 70 °C for 1 h, and then centrifuged. The organic phase was separated by syringe. For pXO2-61, the carboxylic acid group of the fatty acid was verified by using 20 μl of bis-trimethylsyliltrifluoroacetamide and 20 μl of pyridine incubated for 1.5 h at 65 °C. Samples were evaporated to dryness under a stream of N2 and reconstituted in 100 μl of methylene chloride, prior to analysis by GC-MS (Scripps Center for Mass Spectrometry).
Isothermal Titration Calorimetry
ITC5 was performed using a VP-ITC calorimeter from Microcal (Northampton, MA). 8 μl of fatty acid solution (1.6–2.6 mm) was injected into cells containing 100 μm protein (pXO1-118 or pXO2-61) in 20 mm Tris, pH 7.4, and either 500 mm or 1 m NaCl, respectively. All titrations were performed at 23 °C, and each experiment involved 37 injections. Myristic acid (n-C14:0) and palmitic acid (n-C16:0) were purchased from Sigma-Aldrich. 12-Methyltetradecanoic acid (anteiso-C15:0) and 13-methyltetradecanoic acid (iso-C15:0) were purchased from Indofine Chemical Co. Palmitoleic acid was purchased from Fluka. Data were analyzed using Microcal Origin software provided by the manufacturer.
Analytical Ultracentrifugation (AUC)
Sedimentation equilibrium experiments were performed in a ProteomeLab XL-I (BeckmanCoulter) AUC. Protein samples (pXO2-61 or BA2291 sensor domain) in 20 mm Pipes, pH 7.5, 500 mm NaCl, were loaded at concentrations of 0.5, 0.167, and 0.056 mg/ml in 6-channel equilibrium cells and spun in an An-50 Ti 8-place rotor at 25,000 rpm for 24 h at 20 °C. Data were analyzed with HeteroAnalysis software (J. L. Cole and J. W. Lary, University of Connecticut). In each case, an ideal equilibrium model gave a convincing fit for a monomer-dimer equilibrium in solution, with dimer molecular mass values of 32.5 kDa (pXO2-61) and 33.8 kDa (BA2291 sensor domain) and no evidence for higher oligomers.
Yeast Two-hybrid Analysis
The yeast two-hybrid system (Clontech) was used to explore interactions between pXO1-118 and AtxA. Coding genes were singly cloned into the bait plasmid (pGBT9) and prey plasmid (pGAD424). Assays were performed in the yeast strain AH109. We detected interaction when pXO1-118 was present on both pGBT9 and pGAD424, consistent with homodimer formation in yeast cells. There was no evidence for AtxA-pXO1-118 interactions (data not shown).
Bioinformatics Analysis
Homologs of sensor domains were found using CS-BLAST (31).
RESULTS
Crystal and Solution Structures of pXO1-118 and pXO2-61
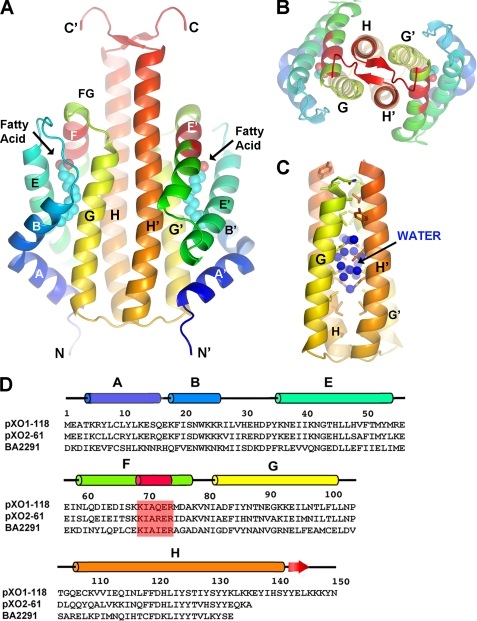
We solved the crystal structures of pXO1-118 and pXO2-61 at 1.76 Å and 1.49 Å resolution, respectively (see “Experimental Procedures,” Table 1, and Figs. 1 and 2). The asymmetric unit of pXO1-118 contains a single sensor domain that adopts the globin fold. Although it does not bind heme, we follow the standard nomenclature for hemoglobins: helices A, B, E, F, G, and H are present in pXO1-118, whereas helices C and D are replaced by an ordered loop (BE). A dimer is formed across a crystallographic dyad, mediated by the packing of the G and H helices from apposing monomers, forming an antiparallel, left-handed four-helix bundle (Fig. 1) that buries a large interface (∼3,500 Å2).
FIGURE 1.
Structure of the B. anthracis pXO1-118 sensor domain. A, ribbon representation of the dimer (side view), with termini and helices labeled (according to standard globin nomenclature). Helices are colored in spectral order (blue → orange) for each monomer. Labels for second monomer are primed. The KIAXER motif within helix F is colored red. Fatty acid is shown as cyan (methylene carbons) and red (carboxyl oxygens) spheres. B, same as in A, but orthogonal view looking down the 2-fold axis of the dimer. C, same view as in A, highlighting the dimer interface, with water molecules found only in a central region. D, sequence alignments of pXO1-118, pXO2-61, and the sensor domain of the B. anthracis sporulation kinase, BA2291. Secondary structure elements for pXO1-118 are indicated, as is the KIAXER motif.
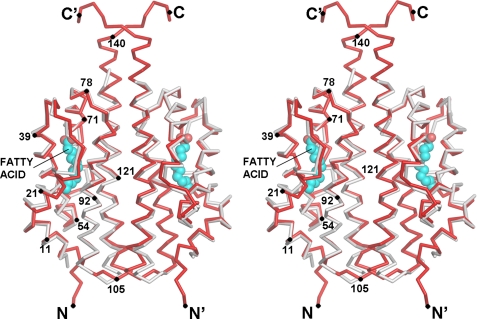
FIGURE 2.
Stereo Cα overlay of pXO1-118 (red) and pXO2-61 (gray) dimers, with N and C termini labeled. Occasional residue numbering is given for guidance. The fatty acid in crystals of pXO1-118 is shown in cyan (methylene carbons) and red (carboxyl oxygens).
As expected, the structure of pXO2-61 (Fig. 2) is very similar to that of pXO1-118 in both tertiary fold and dimer organization. The asymmetric unit in this case contains a dimer, and the monomers superpose with a root mean square deviation of 0.43 Å for Cα carbons. The most obvious external difference arises from a shorter C-terminal helix, leading to a more compact shape and a decrease in the dimerization interface, to 2,200 Å2. The dimers of pXO1-118 and pXO2-61 superpose with a root mean square difference of 1.2 Å for 264 Cα carbons (0.85 Å for pairwise comparisons of single domains).
By AUC, we found that pXO2-61 forms dimers in solution at concentrations greater than or equal to micromolar (dimer Kd = 0.6 μm). We also purified the sensor domain from BA2291 and found that it also forms dimers in solution, with a similar Kd = 0.8 μm (supplemental Fig. 1). In addition, we demonstrated that pXO1-118 forms dimers in cells, as evidenced in a yeast two-hybrid system (see “Experimental Procedures”).
The dimer interfaces of pXO1-118 and pXO2-61 include upper and lower hydrophobic regions that are more closely packed; whereas in the central section the helices diverge, and the interface is chiefly hydrophilic, comprising a large number of direct and water-mediated hydrophilic interactions (Fig. 1C). We found a total of 34 (pXO1-118) and 20 (pXO2-61) solvent molecules buried at distinct locations in this space. pXO1-118 has an additional interface formed by the ends of the longer C-terminal helices, which form a short antiparallel cross-over β-sheet with clusters of Tyr and Lys residues. Given the high homology between the BA2291 sensor domain and the plasmid domains (35% identity, 59% similarity) and the existence of a dimer in all cases, it is reasonable to assume that BA2291 will share a very similar tertiary and quaternary organization.
pXO1-118 and pXO2-61 Are Non-heme Globins
Three-dimensional comparisons of the plasmid-encoded sensor domains using the DALI server (32) identified many structures with the globin fold that superpose with root mean square differences in the 2.3–2.7 Å range for ∼150 Cαs (supplemental Table I). However, the Protein Data Bank contains no close sequence homologs (identities range from 8 to 14%). Moreover, most proteins with the globin fold have an Fe-heme co-factor sandwiched between the E and F helices. The most similar structures (in both tertiary and quaternary organization) are two bacterial (B. subtilis and Geobacter) heme-containing oxygen sensor domains, which form dimers via a similar pairing of the G and H helices. There is one globin structure that lacks co-factor altogether: the B. subtilis stress response regulator RsbR (33); in this case, the dimerization helices (G and H) bend inward, eliminating the co-factor cavity. Mammalian hemoglobins have closely related tertiary folds, but only one, the recently discovered cytoglobin, has a related dimeric organization (34).
The cyanobacter phycocyanins also adopt a globin-like fold (35); they are electron transfer proteins involved in photosynthesis and bind porphyrin-like moieties via cysteine-mediated thioether bonds. However, they have large N-terminal extensions, and their quaternary organization is quite distinct from any of the hemoglobins.
pXO1-118 and pXO2-61 Contain a Central Cavity
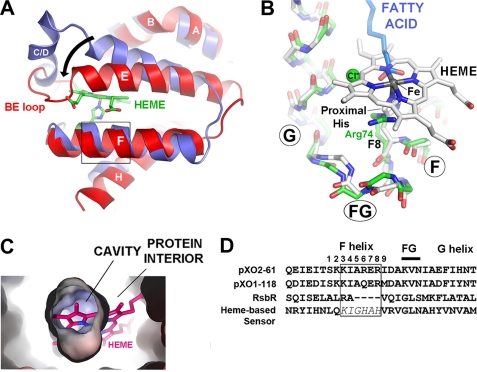
An overlay of pXO1-118 and the B. subtilis oxygen-sensor domain (36) illustrates the overall similarity in secondary and tertiary folds (Fig. 3A) and shows how helix E of pXO1-118 rotates and bends to pack more closely against helix F, partially filling the space that is occupied by heme in the oxygen sensor. The short rigid BE loop (which replaces the CD helix/turn) packs against the FG turn, stabilizing this conformation (see below). Oxygen-binding hemoglobins are linked to the heme via a “proximal histidine” at the eighth position of helix F (F8). In pXO1-118 (and pXO2-61), there is no similarly located histidine, and, as expected, attempts to reconstitute the proteins with hemin were unsuccessful (data not shown). Remarkably, DALI-based structural alignment places pXO1-118 residue Arg-74 at the F8 heme location (Fig. 3B). A cross-section through the central cavity (Fig. 3C) further demonstrates the steric mismatch between heme and the pXO1-118 cavity. As discussed below, Arg-74 is an invariant residue that appears to play a key role in binding an alternative co-factor (fatty acid; see Fig. 3B) and thus may be considered a structural and functional analog of the proximal histidine. Note that in RsbR, DALI-based structural alignment indicates that helix F is truncated such that there is no analog of F8 (Fig. 3D), consistent with its being co-factor-free.
FIGURE 3.
pXO1-118 has a globin fold but does not bind heme. A, overlay of pXO1-118 monomer (red) with the heme-based sensor from Geobacter sulfurreduccens (blue). Heme and heme-linked proximal histidine are shown for the latter. Helices A, B, F, and the FG turn, G (not visible) and H overlap closely, whereas for pXO1-118, helix E tilts and bends toward helix F, occluding the heme pocket (fatty acid has been omitted for clarity). B, same overlay as in A (rotated ∼90° about a vertical axis), showing the FG region in greater detail. Note how well the main chain aligns through the FG turn. The Cα of residue F8 is labeled. In the heme-based sensor, this residue is the proximal histidine, which engages the heme-linked iron atom (gray sphere). In pXO1-118, this residue is Arg-74, which engages the head group of the fatty acid (carboxyl oxygens are in red), which nearly coincides with the position of the heme-linked iron. The chloride ion (green) also lies nearby. C, cut-away view of the cavity at the center of pXO1-118 (view is similar to B), showing that its shape is incompatible with heme binding. Note that the hydrophilic propionate side chains (on the right side of the heme) are confined within the protein (in this hypothetical model) because of the close packing of helices E and F. D, structure-based sequence comparisons of the F and G helices of the sensor domains and RsbR, which does not bind co-factor, illustrating the truncated F helix that lacks an F8 residue (the standard globin numbering scheme is given for the F helix). The conserved KIAXER motif is boxed. The heme-based sensor is also aligned to illustrate its related motif.
Crystals of pXO1-118 Contain a Buried Fatty Acid
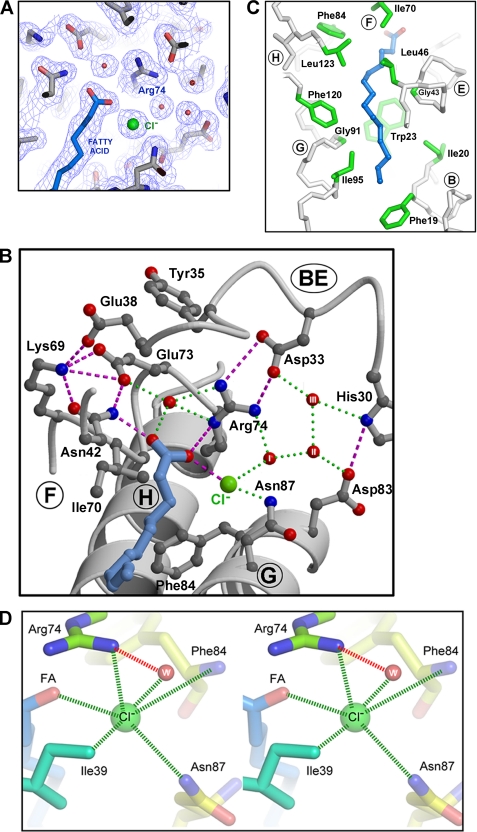
The central cavity runs roughly parallel with helices E and G, with a contour length of ∼20 Å (Fig. 4). It comprises a narrow (∼6-Å diameter) tunnel lined with hydrophobic and aromatic residues, capped at one end by residue Phe-19, which appears to act as a gate, adopting conformations that either open or close the hydrophobic entrance to the tunnel. The other end of the tunnel opens into a hydrophilic chamber, which is sealed from bulk solvent by a “canopy” created by the packing of the BE and FG turns. It includes water molecules as well as a heavier anion, which we believe to be chloride (see below).
FIGURE 4.
Sensor domain interactions with fatty acid and chloride. A, slice through the 2Fo − Fc electron density map of pXO1-118 showing fatty acid (carbon in blue, carboxyl oxygens in red, labeled O-I and O-II (see “Results”)), chloride ion (green), and water molecules (red balls) in the context of the hydrophilic chamber. B, model of the same region as in A, showing the salt bridge and H-bonding network comprising the canopy that encloses the hydrophilic chamber. Note the network of water molecules (circled and labeled I–III) that trace a potential exit route for the chloride ion. Helices and turns are labeled (and circled) for guidance. The orientation of the amide side chain of Asn-87, which plays a key role in chloride and fatty acid binding, is unambiguous, as it is defined by two tandem H-bonds to the main chain of Leu-28. C, conserved interactions between the methylene carbons of the fatty acid and the hydrophobic/aromatic side chains lining the walls of the tunnel. Tetradecanoic acid has been modeled. The side chains of conserved residues are shown in green. The Cα atoms of two conserved glycines (Gly-43 and Gly-91) that delimit the cavity width in that region are also labeled. Trp-23 at the back of the pocket is invariant in all Bacillus sensor domains. Phe-19 is the gatekeeper, shown in the open configuration. D, stereo view of the chloride ion coordination site, showing H-bonding, Coulombic and van der Waals interactions with protein and fatty acid (the O-II carboxyl oxygen is labeled). Bond distances are provided in Table 3 and compared with an E. coli GadB regulatory site.
In pXO1-118, the cavity is also occupied by continuous worm-like electron density that ends in a symmetric bifurcation, consistent with the presence of a fatty acid (Fig. 4A). Using mass spectrometry (GC-MS), we determined that the major chloroform-soluble nonprotein component in the crystals is palmitic (hexadecanoic) acid, presumably derived from the cell wall of E. coli (37) during expression or cell lysis (supplemental Fig. 2). The tunnel is long enough to completely bury tetradecanoic acid (with the Phe-19 gate in the open position), and additional methylene groups would presumably extrude into solvent. We searched for an authentic Bacillus co-factor by incubating E. coli-purified pXO1-118 with crude B. cereus cell extract. The protein was then repurified and its crystal structure determined. However, we observed no significant difference in the co-factor density (data not shown), suggesting that a similar molecule binds in vivo.
GC-MS analysis demonstrated the presence of fatty acid in E. coli-purified pXO2-61 as well as the BA2291 sensor domain (supplemental Fig. 2). However, it is not present in the crystals of pXO2-61 (BA2291 failed to crystallize), most likely due to the different crystallization conditions. Thus, pXO1-118 crystallized in sodium chloride, whereas pXO2-61 required the presence of 1.0 m sodium iodide. Iodide is a strongly chaotropic agent that disfavors complex formation and increases the solubility of hydrophobic moieties (38), consistent with the expulsion of fatty acid from the protein. The higher pH required for crystal growth of pXO2-61 (pH 7.5 versus 5.4) may also disfavor binding (see below). Notwithstanding, pXO2-61 binds fatty acids in solution (in the absence of iodide) with an affinity similar to that of pXO1-118 (see below). Despite the absence of fatty acid, a nearly identical tunnel and canopy are observed in crystals of pXO2-61. Weak density observed within the tunnel has been modeled as solvent/buffer; it is quite distinct from that in pXO1-118, and the density stops well short of the Phe-19 gatekeeper, which adopts an ordered, closed conformation.
Conserved Hydrophilic Chamber Engages the Fatty Acid Head Group and a Halide Ion
The BE and FG turns pack closely together at the top of the domain, forming an extended salt-bridge/H-bonded network (canopy) that seals the chamber from bulk solvent, and engages the fatty acid carboxyl head group and a tightly bound halide ion (Fig. 4). A prominent feature of the canopy is a motif, 69KIAXER74 (where X is any amino acid), at the end of the F helix, which is invariant among the sensor domain orthologs within the B. cereus group. Thus, Lys-69 and Glu-73 form part of a salt bridge/H-bonding network that engages a fatty acid carboxyl oxygen (labeled O-I in Fig. 4) via the side chain amide nitrogen of Asn-42 (E helix). Ile-70 lies at the beginning of the hydrophobic tunnel, making close contact with the first two methylenes of the fatty acid. Ala-71 points away from the binding pocket, but its short side chain allows the F helix to pack closely against the H helix, which likely explains its conservation. The residue at position 72 points out into solution and, accordingly, is not conserved. Arg-74 makes a bifurcated salt bridge to Asp-33 (BE turn) and engages both fatty acid carboxyl oxygens (O-I and O-II) via its positively charged Nδ, forming a direct H-bond to O-II and a water-mediated bond to O-I.
A second prominent feature within the hydrophilic chamber of both pXO1-118 and pXO2-61 is a halide ion. In the case of pXO2-61, a very strong electron density peak is observed. An anomalous Difference Fourier identifies the peak as an iodide ion (peak height ∼10 σ) because it is the only species present in the protein or crystallization liquor with significant anomalous scattering at the wavelength employed, 0.98 Å (supplemental Fig. 3). It is the only iodide ion visible within the tunnel or indeed within any buried region of the dimer. We have therefore assigned a strong peak in the analogous position in crystals of pXO1-118 as chloride because iodide is absent from that crystallization liquor whereas chloride is abundant, and it has been established in other systems (see below) that chloride and iodide typically bind competitively to the same sites. Moreover, crystallographic refinement with the appropriate halide ions yielded B values comparable with those of the surrounding protein, consistent with full occupancy of the sites.
The environment of the chloride ion is shown in Fig. 4D. Its closest contact (2.6 Å) is made with the second carboxyl oxygen of the fatty acid, O-II (suggesting that this oxygen is protonated and forms a H-bond); further contacts are made with the side chain of Arg-74, as well as the side chain amide nitrogen of Asn-87. Contacts with the hydrophobic side chain of Ile-39, and one directly bound water molecule, complete the coordination sphere. The structure of this site is remarkably well preserved in pXO2-61, except that a second water molecule takes the place of the fatty acid, and the larger but more polarizable iodide ion has similar but distinct bond lengths (Table 2). It should also be noted that there is an identical chain of well defined H-bonded water molecules tracing a narrow hydrophilic channel through the top of the canopy into bulk solvent, suggesting a pathway for halide exchange that does not require release of the fatty acid (Fig. 4B).
TABLE 2.
Environments of the halide ions and comparison with an allosteric site in E. coli GadB
Interatomic bond distances are given in Å; see Fig. 4D. s/c, side chain.
| 118/61 | Cl- | I- | E. coli GadB | I- |
|---|---|---|---|---|
| Å | Å | Å | ||
| Arg-74 Cγ/Nδ | 4.6/4.2 | 4.4/4.1 | Arg-17 NH/Cϵ | 3.5/3.7 |
| 84NH | 4.1 | 4.6 | 17NH | 4.0 |
| Asn-87 Nδ2 | 3.2 | 3.7 | 18NH | 4.3 |
| Water | 3.1 | 3.5, 3.6 | Water | 3.7 |
| Ile-39 s/c | 4.3 | 4.1 | Trp s/c | 4.1 |
| Fatty acid –CO2H | 2.8 | 3.4 | Ser-OH | 3.2 |
The site fits the general characteristics of a buried chloride-binding pocket, as described (37). Such sites require sufficient positive potential to neutralize the anion, through Coulombic and/or H-bonding interactions, but the coordination geometry is less stringent than for a typical metal-binding site. Notwithstanding, the site in our sensor domains resembles a well characterized buried site in the E. coli protein, GadB, where chloride plays an allosteric, pH-dependent role (38). Furthermore, the authors in that case showed that bromide and iodide could readily substitute for chloride (see Table 2).
The binding pocket has similarities with other fatty acid-binding proteins (but created from a different protein architecture); e.g. the nuclear receptor HNF4α, which creates a hydrophobic tunnel and buried arginine to engage the fatty acid (39). The Protein Data Bank also contains an example of a bacterial PAS domain (from the signaling protein, RV1364C, from Mycobacterium tuberculosis; Protein Data Bank code, 3K3C) which houses a palmitic acid in a broader pocket. In this case, both an Arg and an Asp (O-O distance = 2.6 Å) coordinate the carboxylic acid, implying that the fatty acid is protonated in this case also. However, no other ions were identified in either pocket.
pXO1-118 and pXO2-61 Bind Reversibly to Fatty Acids in Solution
The fatty acid-binding pocket as well as the dimerization interfaces, are well conserved structurally between pXO1-118 and pXO2-61, and sequence comparisons suggest that the key binding/dimerization determinants of the sensor domains are conserved in BA2291 and indeed throughout the B. cereus family of BA2291-related histidine kinases (as well as relatives such as Geobacillus) (Fig. 5). This suggests that they will all bind the same or a similar ligand and function in a similar way.
FIGURE 5.
Conservation of structure and function in the sensor domain family. A, structure-based sequence alignment and conservation of functional surfaces: fatty acid-binding pocket (hydrophobic tunnel), highlighted in green; canopy creating fatty acid/chloride-binding site (blue); dimer interface (yellow); and other conserved residues important for structural integrity (cyan). Sequences are grouped into four classes (I–IV) as defined under “Results.” Full names of the organisms and genes are as follows. Group I: B. anthracis pXO1-118 (NP_052814), B. anthracis pXO2-61 (NP_653040), B. cereus G2941 (ZP_00236329), B. cellulosilyticus DSM 2522 hypothetical protein BcellDRAFT_3961 (ZP_06365458). Group II: B. anthracis BA2291 (NP_844676.1), B. cereus sensor histidine kinase (YP_083662.1), B. thuringiensis sensor histidine kinase (YP_036402.1). Group III: B. coahuilensis m4-4 sensor histidine kinase (ZP_03225478), Bacillus sp. SG-1 histidine protein kinase (ZP_01860026), Brevibacillus brevis NBRC 100599 probable two-component sensor histidine kinase (YP_002774563), Geobacillus thermoglucosidasius C56-YS93 histidine kinase (ZP_06810042), Geobacillus sp. C56-T31 histidine kinase (YP_003671552), Bacillus sp. NRRL B-14911 sensor histidine kinase (ZP_01170643), Bacillus sp. SG-1 (ZP_01860665); Group IV: Anaeromyxobacter dehalogens 2CP-C conserved hypothetical protein (ACL66915.1), Chlorobium luteolum DSM 273 hypothetical protein Plut_0146 (ABB23036.1), Chloroherpeton thalassium ATCC 35110 conserved hypothetical protein (ACF14428.1), Syntrophus aciditrophicus SB hypothetical cytosolic protein (ABC79005.1), Candidatus Solibacter usitatus Ellin6076 hypothetical protein Acid_3380 (ABJ84353.1). B, stereo image of a pXO1-118 monomer, together with the dimer interface of the second monomer. Main chain (N-Cα-C) is shown as ribbon. The Cα positions of residues highlighted in A are shown as spheres, using the same color scheme. Fatty acid is in cyan and red, chloride ion in magenta.
The tunnel appears to be optimal for a C14 fatty acid, and there is also a distinct bend in the tunnel around methylene 8, raising the possibility of specificity for a cis-monounsaturated fatty acid (bacteria possess few polyunsaturated fatty acids). We therefore measured the binding of 5 different Bacillus fatty acids by ITC. We found that both pXO1-118 and pXO2-61 bound fatty acid reversibly, and we measured binding to two saturated, unbranched (myristic and palmitic acids), two saturated branched (12-methyltetradecanoic and 13-methyltetradecanoic acids), and one monounsaturated (palmitoleic acid). We found that all bound exothermically with a stoichiometry ∼1:1. However, we did not detect significant selectivity among the fatty acids tested (binding affinities were in the range of 10–40 μm (Table 3 and supplemental Fig. 4).
TABLE 3.
Binding affinities (Kd) of pXO2-61 and pXO1-118 for selected fatty acids
| Fatty acid | pXO2-61 | pXO1-118 |
|---|---|---|
| μm | ||
| Myristic acid | 40 ± 12 | 25 ± 9 |
| Palmitic acid | 41 ± 17 | 24 ± 7 |
| 12-Methyltetradecanoic acid | 41 ± 19 | 14 ± 8 |
| 13-Methyltetradecanoic acid | 46 ± 15 | 13 ± 6 |
| Palmitoleic acid | 20 ± 10 | 30 ± 8 |
New Globin Functionality
Our crystal structures and sequence comparisons illustrate a novel ligand-binding modality for the globin fold, one that does not involve heme or any related porphyrin-like attachment. Structural alignments point to a remarkable equivalence between the proximal histidine of heme proteins and the key arginine residue within the KIAXER motif, strongly suggesting an evolutionary relationship. Indeed the heme-based oxygen sensor has the related sequence, KIGHAH, in which its Ile packs against the hydrophobic moiety of the co-factor (heme), just as the analogous Ile of pXO1-118 does against fatty acid. The alanine/glycine residue serves a similar purpose, ensuring tight packing between the F and H helices (and providing a potential link between ligand recognition and dimerization).
The fold family containing pXO2-61 and pXO1-118 has been classified by PFAM as “Family: HisK_N (PF09385).” The PFAM data base currently contains more than 100 sequences, very few of which have been functionally characterized. Many are the sensor domains of B. cereus family kinases, which are identical (or nearly so) to BA2291. However, there are also many distinct sequences, which we have subclassified into four groups in light of our structural analysis (see Fig. 5).
Group I comprises the sensor-only domains from Bacillus. In addition to pXO1-118 and pX02-61, a number of B. cereus species have acquired a pXO1-like plasmid, and these contain genes that are highly homologous to pXO1-118. The only other sequence is from the recently annotated genome of B. cellulosilyticus, an alkaliphilic, salt-tolerant, spore-forming bacterium (40). Its sequence is more divergent but still demonstrates all of the key structural features associated with the other Group I members. It is not reported whether it is plasmid-encoded.
Group II comprises the sensor histidine kinases of the BA2291 family. As noted, these are found in a large number of B. cereus/B. anthracis strains/isolates, as well as the related B. thuringiensis, an insect pathogen. Their sensor domains are nearly invariant (>90–100% identity), so we assume that they are sensing the same or similar signal. Note that only a small fraction of these species/strains harbor a plasmid-borne sensor-only domain.
Group III comprises sensor domains from a more disparate collection of sensor histidine kinases. They are all from the class Bacilli, but from different genera within the family Bacillaceae, including Geobacillus and Brevibacillus. These typically show approximately 30–45% identity to the BA2291 class, but again display the key sequence characteristics, suggesting identical or similar functionality.
Group IV comprises sensor-only domains from bacteria from distinct phyla, for which no cognate kinases have been identified. Most of the organisms bearing these genes occupy distinct or unique environmental niches and utilize a wide variety of energy sources. For example, the phylum Chlorobi (green sulfur bacteria) is well represented. These are Gram-negative bacteria that can degrade a wide variety of chloroaromatic compounds as well as toxic metals (41, 42). The sequences are, not surprisingly, more divergent (∼20–25% identity) from those of the Groups I and II, but they still include most of the hallmark residues involved in structural integrity, and, to a lesser extent, co-factor binding and dimerization. They all have Gly or Ala at the 3rd position of the motif, consistent with a conserved packing between helices F and H. They typically have a longer BE turn, suggesting a structure more closely related to heme-binding globins. Nothing is known about the function of these domains, but for some members of this group there is divergence of the KIAXER motif, suggesting related but distinct functions. For example, in an Anaeromyxobacter sensor, a histidine replaces the inward-facing Ile, whereas in an Acidobacter sensor, the Arg at position 6 is replaced by cysteine. Both changes introduce side chains with the potential to generate novel functionalities within the central cavity.
DISCUSSION
We have identified two ligands that bind to pXO1-118 and pXO2-61 sensor domains in vitro, fatty acid and halide (chloride), and our data strongly suggest that the chromosomally encoded kinase, BA2291, behaves similarly and will therefore sense the same environmental cue in vivo, thus supporting the proposal that the plasmid-encoded sensor domains inhibit untimely sporulation by titrating the signal (22). Our studies point to roles for fatty acid (or similar molecule), chloride ion, and possibly pH (see below), as signaling cues, which should inform the next set of experiments to define their activity in vivo.
Fatty acid synthesis is up-regulated in preparation for sporulation, both in quantity and type, with a shift toward monounsaturated species. Thus, the sensor domains might recognize a fatty acid that is synthesized in the build-up to sporulation. Our short survey of saturated and unsaturated fatty acids did not offer any clues in this regard, as they all bound with similar affinity. Nevertheless, the binding pocket we have described does have a distinct shape that could be optimized for a specific unsaturated species. Specificity of this kind has been observed for an unrelated sensor kinase, DesK, from B. subtilis (43), which recognizes C16 fatty acids with a double bond at the Δ5 position.
Chloride ion concentrations differ by 10–20-fold between the different host tissues that B. anthracis must encounter during pathogenesis, from the intracellular milieu of the macrophage (∼5 mm) to the extracellular fluids (lymphatic and plasma >100 mm) (44), which could reasonably offer a trigger for sporulation (that needs to be suppressed). We have demonstrated the existence of a specific chloride ion-binding site that is intimately involved in the binding of fatty acid, raising the possibility that chloride (or some other small anion) is the “ligand” and fatty acid the “co-factor,” by analogy with oxygen binding to heme in the hemoglobins. We note that the ligand binding geometry is consistent with the fatty acid being protonated, enabling one carboxyl oxygen (O-II) to form a favorable hydrogen bond with the chloride ion. Thus, fatty acid binding might be both chloride- and pH-dependent within the physiological range (cf. the Bohr/chloride effects in hemoglobin (45)) and the combinatorial effects of chloride and pH on the activity of E. coli decarboxylase, GadB (38)). Furthermore, significant changes in environmental pH are associated with different steps in B. anthracis pathogenesis, any of which could, in principle, offer a trigger for sporulation. However, further work is clearly required to test this hypothesis.
The broader significance of our work lies in the definition of a new family of globin-based sensor domains that utilize an alternative (non-heme) co-factor, but which nevertheless appear to be closely linked evolutionarily to the heme-bearing globins. The majority of sensor domains within this family (that have been sequenced so far) come from genomes within the family Bacillaceae and are contained within a histidine kinase architecture that is very similar structurally and most likely functionally to the B. anthracis BA2291 sporulation kinase. By contrast, recent genome sequencing efforts have uncovered a subfamily of sensor-only domains that are found in a range of bacteria from unrelated phyla. In general, rather little is known about these bacteria, other than a shared propensity for utilizing unusual energy sources such as the hydrolysis of chloroaromatics. Nothing is currently known about the function of their putative sensor domains, but it will be most interesting to see whether they play a role in regulating these unusual metabolic functions.
Supplementary Material
Acknowledgments
We thank Annie Heroux for collecting diffraction data at the National Synchrotron Light Source, Brookhaven National Laboratory. We thank the staff of the Stanford Synchrotron Research Lightsource Structural Molecular Biology Program, part of a national user facility operated by Stanford University on behalf of the United States Department of Energy, Office of Basic Energy Sciences, and by the National Institutes of Health (NCRR, Biomedical Technology Program, and NIGMS).
This work was supported, in whole or in part, by National Institutes of Health Grants AI055789 and AI055860. This work was also supported by Department of Defense Grant W81XWH-10-1-0093. The Brookhaven National Synchrotron Light Source is supported by the United States Department of Energy, Division of Materials Sciences and Division of Chemical Sciences Contract DE-AC02-98CH10886.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table I, Figs. 1–4, and an additional reference.
The atomic coordinates and structure factors (codes 3PMC and 3PMC) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- ITC
- isothermal titration calorimetry
- AUC
- analytical ultracentrifugation.
REFERENCES
- 1. Okinaka R. T., Cloud K., Hampton O., Hoffmaster A. R., Hill K. K., Keim P., Koehler T. M., Lamke G., Kumano S., Mahillon J., Manter D., Martinez Y., Ricke D., Svensson R., Jackson P. J. (1999) J. Bacteriol. 181, 6509–6515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ezzell J. W., Welkos S. L. (1999) J. Appl. Microbiol. 87, 250. [DOI] [PubMed] [Google Scholar]
- 3. Candela T., Mock M., Fouet A. (2005) J. Bacteriol. 187, 7765–7772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mock M., Fouet A. (2001) Annu. Rev. Microbiol. 55, 647–671 [DOI] [PubMed] [Google Scholar]
- 5. Kang T. J., Fenton M. J., Weiner M. A., Hibbs S., Basu S., Baillie L., Cross A. S. (2005) Infect. Immun. 73, 7495–7501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drysdale M., Heninger S., Hutt J., Chen Y., Lyons C. R., Koehler T. M. (2005) EMBO J. 24, 221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu H., Sa Q., Koehler T. M., Aronson A. I., Zhou D. (2006) Cell. Microbiol. 8, 1634–1642 [DOI] [PubMed] [Google Scholar]
- 8. Perego M., Hoch J. A. (2008) Trends Microbiol. 16, 215–221 [DOI] [PubMed] [Google Scholar]
- 9. Dai Z., Sirard J. C., Mock M., Koehler T. M. (1995) Mol. Microbiol. 16, 1171–1181 [DOI] [PubMed] [Google Scholar]
- 10. Uchida I., Hornung J. M., Thorne C. B., Klimpel K. R., Leppla S. H. (1993) J. Bacteriol. 175, 5329–5338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drysdale M., Bourgogne A., Hilsenbeck S. G., Koehler T. M. (2004) J. Bacteriol. 186, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bourgogne A., Drysdale M., Hilsenbeck S. G., Peterson S. N., Koehler T. M. (2003) Infect. Immun. 71, 2736–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mignot T., Mock M., Fouet A. (2003) Mol. Microbiol. 47, 917–927 [DOI] [PubMed] [Google Scholar]
- 14. Hadjifrangiskou M., Chen Y., Koehler T. M. (2007) J. Bacteriol. 189, 1874–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saile E., Koehler T. M. (2002) J. Bacteriol. 184, 370–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stephenson K., Hoch J. A. (2002) Mol. Microbiol. 46, 297–304 [DOI] [PubMed] [Google Scholar]
- 17. Brunsing R. L., La Clair C., Tang S., Chiang C., Hancock L. E., Perego M., Hoch J. A. (2005) J. Bacteriol. 187, 6972–6981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bongiorni C., Stoessel R., Shoemaker D., Perego M. (2006) J. Bacteriol. 188, 487–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bongiorni C., Stoessel R., Perego M. (2007) J. Bacteriol. 189, 2637–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szurmant H., Hoch J. A. (2010) Curr. Opin. Microbiol. 13, 190–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scaramozzino F., White A., Perego M., Hoch J. A. (2009) J. Bacteriol. 191, 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. White A. K., Hoch J. A., Grynberg M., Godzik A., Perego M. (2006) J. Bacteriol. 188, 6354–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harrison C. J., Bohm A. A., Nelson H. C. (1994) Science 263, 224–227 [DOI] [PubMed] [Google Scholar]
- 24. Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 25. Terwilliger T. C., Berendzen J. (1999) Acta Crystallogr. D Biol. Crystallogr. 55, 849–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Terwilliger T. C. (2001) Acta Crystallogr. D Biol Crystallogr. 57, 1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kleywegt G. J., Zou J. Y., Kjeldgaard M., Jones T. A. (2001) in International Tables for Crystallography: Crystallography of Biological Macromolecules (Rossmann M. G., Arnold E. eds) pp. 353–356, Kluwer Academic Publishers, Dordrecht, The Netherlands [Google Scholar]
- 28. Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 29. Brünger A. T., Adams P. D., Clore G. M., DeLano W. L., Gros P., Grosse-Kunstleve R. W., Jiang J. S., Kuszewski J., Nilges M., Pannu N. S., Read R. J., Rice L. M., Simonson T., Warren G. L. (1998) Acta Crystallogr. D Biol. Crystallogr. 54, 905–921 [DOI] [PubMed] [Google Scholar]
- 30. Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 31. Biegert A., Söding J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3770–3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holm L., Sander C. (1993) J. Mol. Biol. 233, 123–138 [DOI] [PubMed] [Google Scholar]
- 33. Murray J. W., Delumeau O., Lewis R. J. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17320–17325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Sanctis D., Dewilde S., Pesce A., Moens L., Ascenzi P., Hankeln T., Burmester T., Bolognesi M. (2004) J. Mol. Biol. 336, 917–927 [DOI] [PubMed] [Google Scholar]
- 35. Contreras-Martel C., Matamala A., Bruna C., Poo-Caamaño G., Almonacid D., Figueroa M., Martínez-Oyanedel J., Bunster M. (2007) Biophys. Chem. 125, 388–396 [DOI] [PubMed] [Google Scholar]
- 36. Zhang W., Phillips G. N., Jr. (2003) Structure 11, 1097–1110 [DOI] [PubMed] [Google Scholar]
- 37. Song Y., Gunner M. R. (2009) J. Mol. Biol. 387, 840–856 [DOI] [PubMed] [Google Scholar]
- 38. Gut H., Pennacchietti E., John R. A., Bossa F., Capitani G., De Biase D., Grütter M. G. (2006) EMBO J. 25, 2643–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dhe-Paganon S., Duda K., Iwamoto M., Chi Y. I., Shoelson S. E. (2002) J. Biol. Chem. 277, 37973–37976 [DOI] [PubMed] [Google Scholar]
- 40. Nogi Y., Takami H., Horikoshi K. (2005) Int. J. Syst. Evol. Microbiol. 55, 2309–2315 [DOI] [PubMed] [Google Scholar]
- 41. Sakurai H., Ogawa T., Shiga M., Inoue K. (2010) Photosynth. Res. 104, 163–176 [DOI] [PubMed] [Google Scholar]
- 42. Pérez-Pantoja D., De la Iglesia R., Pieper D. H., González B. (2008) FEMS Microbiol. Rev. 32, 736–794 [DOI] [PubMed] [Google Scholar]
- 43. Aguilar P. S., Hernandez-Arriaga A. M., Cybulski L. E., Erazo A. C., de Mendoza D. (2001) EMBO J. 20, 1681–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lodish H., Baltimore D., Berk A., Zipursky L. S., Matsudaira P., Darnell J. (2000) Molecular Cell Biology, 4th Ed., pp. 640–641, W. H. Freeman and Company, New York [Google Scholar]
- 45. Giardina B., Mosca D., De Rosa M. C. (2004) Acta Physiol. Scand. 182, 229–244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.