Abstract
The objective of the current study was to delineate the pathway of complement activation that is crucial for the induction of experimental autoimmune anterior uveitis (EAAU). We studied the development of EAAU in melanin-associated antigen (MAA)-sensitized Lewis rats treated with antibody against C4 or factor B. Control animals received isotype IgG control. Antibody against C4 had no effect on EAAU, and all of the animals developed EAAU similar to those injected with control IgG. In contrast, EAAU was completely inhibited in all MAA-sensitized Lewis rats injected with factor B antibody. Treatment with anti-factor B antibody resulted in suppression of ocular complement activation. Adoptive transfer of T lymphocytes harvested from draining lymph nodes of donor animals treated with anti-factor B did not transfer EAAU to naïve syngenic rats. Anti-factor B antibody inhibited the ability of MAA-specific CD4+ T cells to proliferate (in vitro) in response to MAA in a dose-dependent manner. Level of TNF-α and IFN-γ decreased in the presence of anti-factor B. Collectively, our results provide the novel finding that complement activation via the alternative pathway contributes to intraocular inflammation in EAAU, and anti-factor B-mediated inhibition of EAAU is due to diminished antigen-specific CD4+ T cell responses to MAA. Our findings explain the interactions between the complement system and T cells that are critical for the induction of EAAU and may lead to the development of therapy for idiopathic anterior uveitis based on selective blockade of the alternative pathway.
Keywords: Complement, Cytokine, Immunology, Inflammation, Vision, Complement Alternative Pathway, Cytokines, Ocular Autoimmunity, T Cells, Uveitis
Introduction
Uveitis, a vision-threatening intraocular inflammatory disease is one of the leading causes of vision loss worldwide. Anterior uveitis (AU)2 refers to the inflammation of the anterior segment of the eye, which includes the iris and the ciliary body (CB). Idiopathic AU is the most common form of intraocular inflammation in humans (1–3). Experimental autoimmune anterior uveitis (EAAU) is an organ-specific autoimmune disease of the eye that serves as an animal model of noninfectious autoimmune idiopathic AU (4–15). The EAAU model has been extensively used in our laboratory to understand the underlying mechanisms involved in the pathogenesis of idiopathic AU (5–15). In this experimental model, inbred Lewis rats are subcutaneously immunized in the footpad with melanin-associated antigen (MAA) isolated from the iris and the CB, and EAAU is induced in these animals by an antigen-specific CD4+ T cell response to MAA (5–15). The hallmark pathologic features of EAAU include lymphocytic infiltration in the iris, the CB, and the anterior segment of the eye with no inflammation of the retina (5–15). Antigen-specific CD4+ T cells can adoptively transfer disease into naïve syngenic recipients (7, 8, 13) and are the predominant inflammatory cells within the uvea (5–15).
Previous reports from our laboratory have established a critical role of complement in EAAU (9, 10). Complement has been recognized as a key innate immune defense system that plays an important role in fighting infections and modulating various immune and inflammatory responses by bridging the innate immunity with adaptive immune responses (16–21). The complement systems consist of more than 35 proteins circulating in blood and expressed on cell membranes. These proteins interact with one another in three distinctive activation cascades: the classical, the lectin, and the alternative, which can be triggered by different stimuli. Complement component 4 (C4) is a component of both the classical and lectin pathways, whereas factor B is the integral component of the alternative pathway (19–21). Complement activation via these pathways is under strict control of complement regulatory proteins; disruption of the balance between complement activation and regulation can result in deleterious effects on the host and contributes to several diseases (22–36). Recently we have reported that suppression of complement activation by recombinant complement regulatory protein inhibits EAAU (15). However, the contribution of each complement activation pathway in EAAU has not yet been described. In this study we focused on identifying which complement activation pathway is involved in the induction of EAAU. Furthermore, we investigated the possible mechanisms by which EAAU is modulated in the animals where complement activation pathways are blocked in vivo.
EXPERIMENTAL PROCEDURES
Animals
Pathogen-free male Lewis rats (5–6 weeks old) were obtained from Harlan Sprague-Dawley. This study was approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences (Little Rock, AR).
Induction and Evaluation of EAAU
MAA was purified from bovine iris and CB as previously described by us (5, 8). Male Lewis rats were immunized with 100 μl of stable emulsion containing 75 μg of MAA emulsified (1:1) in complete Freund's adjuvant (Sigma-Aldrich) using a single-dose induction protocol in the hind footpad as previously described (5, 8). The animals were examined daily between days 7 and 30 post-injection for the clinical signs of uveitis using slit lamp biomicroscopy. EAAU was scored by an observer unaware of the experimental design, and the intensity of uveitis was scored on the arbitrary scale of 0 to 4 (5, 8). Eyes were also harvested at various time points for histological analysis to assess the course and severity of inflammation using the criteria previously reported (5, 8).
Antibodies
Purified IgG fractions of goat anti-human C4 (Immunology Consultants Laboratory, Newberg, OR) and goat anti-human factor B (R & D Systems Inc., Minneapolis, MN) were used. Purified normal goat IgG purchased from R & D Systems Inc. served as isotype control.
Histology
Freshly enucleated rat eyes were fixed in neutral buffered 10% formalin solution (Sigma-Aldrich) for 24 h at room temperature, dehydrated in ethanol through ascending series of ethanol concentrations, and embedded in paraffin. Five-micron-thick sections were stained with hematoxylin and eosin purchased from Fisher. The sections were examined using a light microscope (Olympus, Center Valley, PA).
Sample Collection
The rats were perfused through the heart using sterile pyrogen-free saline after being anesthetized, and the eyes were immediately enucleated. Intraocular tissue, which consisted of uvea, retina, lens, aqueous humor, and vitreous was prepared as previously described (10, 11, 37) and was used for RNA and protein extraction for RT-PCR and Western blot analysis, respectively. Rat blood (anti-coagulant-free) was collected by cardiac puncture, and the serum was isolated.
RT-PCR Analysis
Total RNA was extracted using the RNeasy kit (Qiagen), and 0.1 μg of total RNA was used to detect the mRNA levels of β-actin, C4, and factor B by RT-PCR. The primers for rat proteins were synthesized at Integrated DNA Technologies (Coralville, IA). Primer sequences and the predicted sizes of the amplified cDNA are as follows: β-actin, 5′-GTTTGAGACCTTCAACACC-3′ (forward) and 5′-GTGGCCATCTCTTGCTCGAAGTC-3′ (reverse) (318 bp); C4, 5′-AGGCTGCCCTGGGTAAAGTGAATA-3′ (forward) and 5′-TTTCGAGTCGGACCCAGACAACAA-3′ (reverse) (290 bp); and factor B, 5′-TCCGAGACTTGCTGGATATTGGCA-3′ (forward) and 5′-TCCACGACCTTGATGGAGTGT-3′ (reverse) (412 bp). Polymerase chain reaction was performed using 25, 30, and 35 cycles, and all three cycles gave similar results. The negative controls consisted of the omission of RNA template or reverse transcriptase from the reaction mixture. PCR products were analyzed on a 2% agarose gel and visualized using GelDocXR and Quantity One 4.2.0 program (Bio-Rad). The experiment was repeated three times.
Western Blot Analysis
Pooled intraocular tissue was homogenized in 500 μl of ice-cold PBS containing 1% protease inhibitors and 1% Nonidet P-40 (37). The homogenate was centrifuged at 14,000 × g at 4 °C for 15 min, the supernatant was subjected to SDS-PAGE on 12% linear slab gel, and the separated proteins were transferred to a polyvinylidene fluoride membrane. Human complement serum (Sigma) was also used in the Western blot analysis. The blots were blocked in 5% BSA for 1 h at room temperature and were probed with purified IgG fraction of antibodies against C4 or factor B for 1 h at room temperature or at 4 °C overnight. The blots were also probed with monoclonal β-actin antibody. Control blots were treated with the same dilution of isotype IgG control. After washing and incubation with HRP-conjugated secondary antibody (1:5000 dilution), the blots were developed using the ECL Western blot analysis detection system (ECL Plus; Amersham Biosciences). The experiment was repeated three times.
Effect of Anti-human C4 and Anti-human factor B Antibody on Rat Serum Complement Activity
MAA-sensitized Lewis rats were sacrificed at day 19 post-immunization (peak of EAAU), and blood was collected. Rat serum (n = 10 rats/antibody) was incubated with different concentrations (0, 0.5, 1, and 2 μg) of isotype IgG control, anti-C4, or anti-factor B antibody at 37 °C for 30 min. Antibody-treated serum samples were used in the classical pathway hemolytic assay and the alternative pathway activity assay (both described below) to confirm inhibition of the classical pathway and the alternative pathway activity, respectively. The experiments were repeated three times.
Classical Pathway Hemolytic Assay
Total hemolytic complement activity in serum was determined using sensitized sheep erythrocytes (Diamedix, Miami, FL) according to the manufacturer's directions with some modifications. Briefly, 150 μl of antibody-sensitized sheep erythrocytes were incubated with sequentially diluted rat serum (treated with anti-C4 or anti-factor B antibody) to give a total volume of 200 μl at 37 °C for 60 min. Serum treated with PBS was used to determine the 100% value for complement-dependent serum hemolytic activity.
Alternative Pathway Activity Assay
Alternative pathway activity in rat serum was measured using a modification of the zymosan assay that measures C3 deposition on zymosan particles (38, 39). Briefly, 10 μl of anti-factor B-treated or isotype IgG control-treated rat serum was incubated with 106 (5 μl) activated zymosan particles (Complement Technology, Tyler, Texas) at 37 °C for 20 min in PBS containing 1% BSA, 10 mm EGTA, and 5 mm MgCl2. The reaction was stopped by adding EDTA to the final concentration of 10 mm. The particles were then washed with cold PBS containing 1% BSA. The washed particles were treated with FITC-conjugated goat anti-rat C3 antibody (MP Biomedicals, Solon, OH) at 4 °C for 20 min, and surface C3 was analyzed by flow cytometry. Flow cytometric analysis was performed on FACSCalibur (BD Biosciences, San Jose, CA), and data were analyzed in cytometry software (Win MDI 2.8; Windows Multiple Document Interface for Flow Cytometry). Alternative pathway activity was calculated as the percentage of C3 deposition = (mean percentage of particle fluorescence of the sample reaction) − (mean percentage of particle fluorescence of the background).
In Vivo Antibody Administration
MAA-sensitized Lewis rats received eight injections (0.5 mg/kg) at 24-h intervals of polyclonal anti-human C4 (n = 3 rats) or anti-human factor B (n = 3 rats) antibody via the intraperitoneal route at days 4–11 post-immunization. Control animals (n = 3 rats) received a similar treatment with appropriate isotype IgG control. The onset of clinical disease, as well as its daily progression, in these animals was monitored from day 7 following immunization until day 30. The animals were sacrificed on various days post-immunization, and the severity of ocular inflammation was determined by histology. The experiment was repeated three times.
Adoptive Transfer of EAAU
MAA-sensitized Lewis rats injected with anti-factor B antibody (group 1) or isotype IgG control (group 2) described above were sacrificed at day 12 post-immunization, and popliteal lymph nodes (LNs) were harvested separately from donor rats in each group (7, 8, 13). A single-cell suspension of popliteal lymph node cells (LNCs) was made in complete RPMI 1640 culture medium containing 10% FBS, and LNCs harvested from animals in groups 1 and 2 were cultured with MAA (20 μg/ml) for 3 days. Nonadherent cells were collected, and the lymphocytes were purified by Histopaque gradient (Sigma-Aldrich). Lymphocytes (10 × 106) were separately injected into naïve Lewis rats via the tail vein. In some experiments T lymphocytes were purified as described previously (7, 8, 13). These experiments were repeated three times.
Immunohistochemistry
Five-micron-thick paraffin-embedded tissue sections of eyes were immunostained for membrane attack complex (MAC) and C3 using polyclonal antibody (raised in rabbit) reactive with rat/mouse C9 (1:1000) and IgG fraction of goat antiserum to rat C3 (1:500; MP Biomedicals), respectively. Rabbit polyclonal antibody reactive with rat/mouse C9 was kindly provided by Prof. B. P. Morgan (University of Wales College of Medicine, Cardiff, UK). Cy3-labeled goat anti-rabbit IgG (Sigma) and Cy3-labeled rabbit anti-goat IgG (Zymed Laboratories Inc., San Francisco, CA) were used as the secondary antibodies for MAC and C3 staining, respectively. Paraffin sections of rat eye were also immunostained for C4 and factor B using goat anti-human C4 (Immunology Consultants Laboratory) and goat anti-human factor B (R & D Systems Inc.) respectively. Cy3-labeled rabbit anti-goat (Sigma) was used as the secondary antibody for both C4 and factor B staining. Control stains were performed with irrelevant antibodies of the same Ig subclass at concentrations similar to those of the primary antibodies. Additional controls consisted of staining by omission of the primary or secondary antibody. The sections were covered with mounting medium with DAPI (ProLong Gold Mounting Medium; Invitrogen) and were examined under fluorescence microscope (Olympus, Center Valley, PA). The experiment was repeated three times.
T Cell Proliferation Assay
At day 7 post-immunization, popliteal LNs were harvested from MAA-immunized Lewis rats (n = 3 rats), and a single cell suspension of LNCs (purified by Histopaque gradient) was prepared in complete RPMI 1640 culture medium. The culture medium did not contain fetal bovine serum. Non-heat-inactivated normal rat serum (5%) was added to the medium and was used as the source of factor B and complement system. LNCs were stimulated with MAA (20 μg/ml) in 96-well plates (BD Biosciences). LNCs were cultured in the presence of different concentration (0.5, 1.0 and 2.0 μg/ml) of anti-factor B antibody or isotype IgG control (2.0 μg/ml). Proliferation was assessed by dilution of carboxyfluorescein diacetate succinimidyl ester (CFSE) using a Cell Trace CFSE cell proliferation kit according to the manufacturer's protocol (Molecular Probes and Invitrogen). For flow analysis CFSE-labeled nonadherent cells were collected and labeled with anti-CD3-APC and anti-CD4-PE (both from BD Biosciences). The percentage of proliferating cells in response to MAA was assessed by the dilution of CFSE using the ModFit Proliferation Wizard program (Verity Software, Topsham, ME). The experiment was repeated three times.
Cytokine ELISA
Total lymphocytes (purified by Histopaque gradient) harvested from popliteal LNs of MAA-sensitized Lewis rats (n = 3 rats) sacrificed at day 7 post-immunization were cultured with MAA (20 μg/ml) in the presence of anti-factor B antibody (2.0 μg/ml) or isotype IgG control (2.0 μg/ml) for 48 h. The cells were cultured in complete RPMI 1640 culture medium containing non-heat-inactivated normal rat serum (5%) instead of fetal bovine serum (described above). Supernatants were collected after 48 h for quantitative ELISA for TNF-α and IFN-γ (both from BD PharMingen, San Diego, CA). ELISA was performed according to the manufacturer's recommendations. The concentration of each cytokine was calculated by computer software using the standard curves obtained from known concentrations (ELISA kit). The experiment was repeated three times.
Statistical Analysis
The data are expressed as the means ± S.D. The data were analyzed and compared using Student's t test, and the differences were considered statistically significant at p < 0.05.
RESULTS
Presence of C4 and Factor B in the Naïve Rat Eye
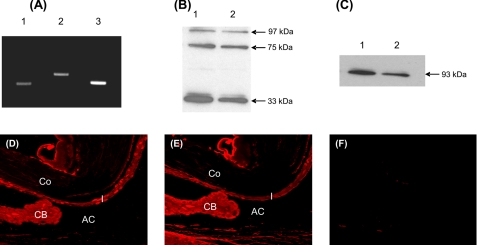
Intraocular tissue harvested from naïve rat eye was used to detect the mRNA (n = 9 eyes) and protein (n = 9 eyes) for C4 and factor B by RT-PCR and Western blot analysis, respectively. RT-PCR analysis revealed the presence of C4 (290 bp) and factor B (412 bp) transcripts in the naive rat eye (Fig. 1A). Intraocular tissue and serum samples were used in Western blot analysis. Human complement serum was used as the positive control and was purchased from Sigma. Under reducing conditions, polyclonal anti-human C4 identified three protein bands at 97, 75, and 33 kDa in human complement serum (Fig. 1B, lane 1) and naïve rat eye (Fig. 1B, lane 2). Factor B was detected at a molecular mass of 93 kDa in human complement serum (Fig. 1C, lane 1) or naive rat eye (Fig. 1C, lane 2) under nonreducing conditions. Immunohistochemistry using anti-human C4 and anti-human factor B antibody and paraffin sections of normal rat eye (n = 9 eyes) was utilized to localize C4 and factor B within the eye. The iris, the CB, and the anterior chamber strongly stained for C4 (Fig. 1D) and factor B (Fig. 1E). Weak staining was observed in the cornea and retina using anti-C4 and anti-factor B antibody (data not shown). Control sections treated with an irrelevant antibody showed no staining (Fig. 1F).
FIGURE 1.
Expression of complement components C4 and factor B in naïve rat eye. A, C4 and factor B mRNA were analyzed by RT-PCR. Representative ethidium bromide-stained agarose gel (2%) shows C4 (290 bp, lane 1) and factor B (412 bp, lane 2) transcripts in the eye of naïve Lewis rats. β-Actin (318 bp, lane 3) was used as the positive control. B and C, total protein from serum and rat intraocular tissue was separated on 12% SDS-PAGE. B and C, representative Western blots shown were probed with polyclonal antibody against human C4 (B) and human factor B (C). B, Western blot probed with anti-human C4 antibody revealed three bands with molecular masses of 97, 75, and 33 kDa in human serum (lane 1, positive control) and naive rat eye (lane 2) under reducing condition. C, under nonreducing conditions, factor B is visualized at the expected molecular mass of 93 kDa in human serum (lane 1, positive control) and naïve rat eye (lane 2). D and E, immunofluorescence micrographs show staining for C4 (D) and factor B (E) within the eyes of naïve Lewis rats. F, no staining was observed in the control sections. Co, cornea; I, iris; AC, anterior chamber. Objective magnification, ×40. The results shown are representative of three independent experiments.
These results of Western blot analysis and immunohistochemistry demonstrate that C4 and factor B proteins are present in the eye of naïve Lewis rats and revealed the reactivity of polyclonal antibodies against human C4 and against human factor B with rat proteins. Furthermore, our results that both mRNA and protein for C4 and factor B are present in the eye of naïve Lewis rats suggest that these proteins are produced locally in the eye.
Effect of Anti-human C4 and Anti-human Factor B Antibody on Rat Serum Complement Activity
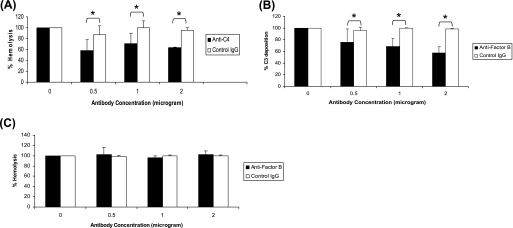
After establishing that anti-human C4 and anti-human factor B can be used to detect corresponding proteins in rat eyes, we determined the inhibitory activity of these antibodies against rat complement using in vitro methods. The ability of anti-human C4 to inhibit rat serum complement was tested using the classical pathway hemolytic assay. Inhibition of alternative pathway activity in rat serum by anti-human factor B antibody was measured using the in vitro zymosan-dependent alternative pathway activity assay. Our results presented in Fig. 2A demonstrated inhibition of the classical pathway activity in vitro by anti-human C4 antibody relative to control IgG. The percentage of inhibition of rat serum classical pathway activity was ∼25, 29, and 32% with 0.5, 1.0, and 2.0 μg of anti-human C4, respectively. Anti-human factor B inhibited the functional activity of the alternative pathway, and the percentage of inhibition was dependent on the dose of the antibody (Fig. 2B). The percentage of inhibition of rat serum alternative pathway activity was ∼18, 31, and 41% with 0.5, 1.0, and 2.0 μg of anti-human factor B, respectively. Importantly, anti-factor B antibody had no effect on the functional activity of the classical pathway (Fig. 2C). Taken together, our above mentioned data clearly demonstrate that polyclonal antibodies against human C4 and human factor B identify corresponding rat proteins and inhibit the functional activity of complement system in rat serum.
FIGURE 2.
Assessment of inhibitory activity of anti-human C4 and anti-human factor B on rat serum complement activity in vitro. MAA-sensitized Lewis rats were sacrificed at day 19 post-immunization (peak of EAAU), and blood was collected. Rat serum was treated with different concentrations (0, 0.5, 1, and 2 μg) of anti-C4 (A) or anti-factor B antibody (B and C). Anti-C4-treated (■) serum samples were used in the classical pathway hemolytic assay (A). Anti-factor B-treated (■) serum samples were used in the alternative pathway activity assay (B) and the classical pathway hemolytic assay (C). The rat serum treated with the same concentrations (0, 0.5, 1, and 2 μg) of isotype IgG (□) was used as control in all of the experiments. *, p < 0.05.
Complement Activation Pathways in the Induction of EAAU
To determine that activation of the classical and/or the alternative and/or the lectin pathway of the complement system is critical for the development of EAAU, we studied the development of EAAU in Lewis rats treated with polyclonal antibodies against C4 (n = 9 rats) or factor B (n = 9 rats) or isotype IgG control (n = 9 rats). C4 is the component of both the classical and lectin pathways; thus anti-C4 antibody will block the activation of the complement system via both the classical and lectin pathways. Factor B is the key component of the alternative pathway, and anti-factor B antibody will specifically block the alternative pathway.
Effect of Anti-C4 on EAAU
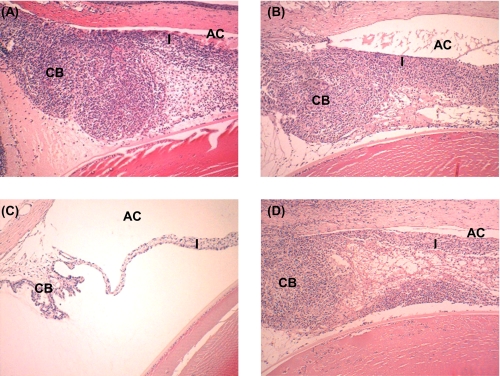
We first explored whether the induction of EAAU required activation via the classical and/or the lectin pathway. MAA-sensitized animals received polyclonal antibody directed against C4 (n = 9 rats) or isotype IgG control (n = 9 rats) separately via intraperitoneal route on days 4–11 post-immunization. Our data demonstrate that anti-C4-treated animals developed severe disease that followed the normal pattern of EAAU both clinically and histologically (Table 1 and Fig. 3A). All nine MAA-sensitized animals treated with control IgG also developed EAAU in both eyes (Table 1 and Fig. 3B). Clinical and histopathological examination revealed that the disease observed in control IgG-treated rats was similar to that in animals treated with anti-C4. These results demonstrate that treatment with anti-C4 antibody had no effect on EAAU, thus suggesting that the classical and/or lectin pathways are not critical for the development of EAAU.
TABLE 1.
Effect of anti-C4 and anti-factor B antibodies on EAAU
The incidence of EAAU is given as positive/total eyes following clinical examination.
| Treatment in vivo | Eyes with EAAU |
Day of onseta | Duration of diseasea | |||
|---|---|---|---|---|---|---|
| Incidence | Mild | Moderate | Severe | |||
| days | ||||||
| Anti-C4 | 18/18 | – | – | 18/18 | 13.8 ± 0.5 | 12.8 ± 0.5 |
| Anti-factor B | 0/18 | – | – | – | – | – |
| Isotype IgG control | 18/18 | – | – | 18/18 | 14.2 ± 0.7 | 12.0 ± 2 |
a The values are the means ± standard deviation. The severity of inflammation on histopathologic examination was grouped as mild (1+), moderate (2+ to 3+), or severe (4+).
FIGURE 3.
Effect of anti-C4 and anti-factor B on EAAU. MAA-sensitized Lewis rats treated intraperitoneally with anti-human C4 (A), anti-human factor B (C), or isotype IgG control (B and D) were sacrificed at day 19 post-immunization (peak of EAAU), and the eyes were subjected to histopathological examination after hematoxylin and eosin staining. Representative histologic sections of rat eyes from different experimental groups stained with hematoxylin and eosin are shown. Severe EAAU developed in animals injected intraperitoneally with anti-C4 antibody (A). The iris (I), the CB, and the anterior chamber (AC) were infiltrated with inflammatory cells. In contrast, no inflammation was observed in the eyes of Lewis rats treated similarly with anti-factor B antibody (C). B and D represent histopathology of eyes from MAA-sensitized Lewis rats treated with isotype IgG control. Objective magnification, ×10.
Effect of Anti-factor B on EAAU
MAA-sensitized Lewis rats were treated with polyclonal antibody against human factor B to explore the contribution of the alternative pathway in the development of EAAU. EAAU was completely inhibited in all MAA-injected Lewis rats (n = 9 rats) by treatment with anti-factor B antibody (Table 1 and Fig. 3C). Clinical and histopathological examination revealed that EAAU did not develop in MAA-sensitized animals injected with anti-factor B antibody (Table 1 and Fig. 3C). In contrast, treatment with isotype IgG control had no effect on EAAU, and all of the animals (n = 9 rats) developed EAAU in both eyes (Table 1 and Fig. 3D).
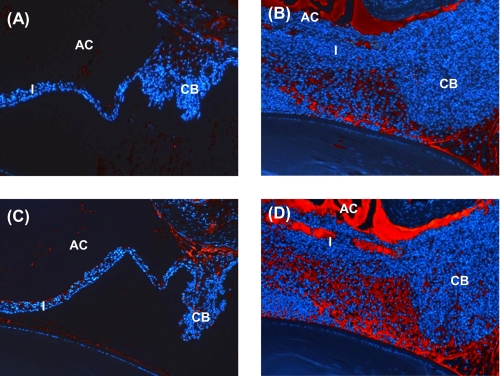
Next we determined whether the ocular complement system is affected by systemic administration of anti-factor B antibody in MAA-sensitized Lewis rats. Formation of C3 split products and MAC in the eye was used as a measure of local complement activation. The effect of anti-factor B treatment on the formation of C3 split products and MAC was investigated by immunofluorescent staining of paraffin sections of the rat eyes. MAA-sensitized Lewis rats received intraperitoneal injections of anti-factor B or isotype IgG control separately on days 4–11 post-immunization (described above). The rats were sacrificed at day 19 post-immunization, and the harvested eyes were stained for C3 and MAC using goat anti-rat C3 and rabbit anti-rat C9 respectively. Goat anti-rat C3 recognizes C3 split products (C3b and iC3b) as well as intact C3 (9, 37). Immunofluorescence analysis of the eyes revealed intense deposition of both C3 (including C3 split products) and MAC in control IgG-injected animals sacrificed at day 19 post-immunization (Fig. 4, B and D, respectively). In contrast, C3 (Fig. 4A) and MAC (Fig. 4C) deposition was markedly reduced in anti-factor B-injected rats at this time point. Collectively, our results suggest that complement activation via the alternative pathway is necessary for the induction of EAAU because the inhibition of factor B, a key component of the alternative pathway, significantly inhibited EAAU.
FIGURE 4.
Effect of alternative pathway inhibition on ocular complement activation. MAA-sensitized Lewis rats treated intraperitoneally with anti-factor B (A and C) or isotype IgG control (B and D) were sacrificed 19 days after EAAU induction. Paraffin sections of rat eyes were subjected to immunohistochemical staining with antibodies to rat C3 (A and B) and rat C9 (C and D). Immunofluorescence analysis revealed very faint staining for C3 (A) and MAC (C) in the eye of animals treated with anti-factor B antibody. At this time point, high levels of C3 (B) and MAC (D) were detected in the eyes of Lewis rats injected with isotype IgG control.
Effect of Factor B on MAA-specific T cells
Because EAAU is a T cell-mediated autoimmune disease (5–15), experiments were carried out to determine whether the inhibition of the alternative pathway affects the activity of MAA-specific T cells. To address this question, the effect of anti-factor B antibody on adoptive transfer EAAU, T cell proliferation, and cytokine profile was explored.
Adoptive Transfer EAAU
Total cells from the popliteal lymph nodes of anti-factor B and isotype IgG control-treated animals were cultured separately in the presence of MAA (20 μg/ml) for 3 days. Nonadherent cells were collected, and lymphocytes purified by Histopaque gradient were transferred to naïve Lewis rats via the tail vein. Adoptive transfer of 10 million in vitro primed lymphocytes isolated from the popliteal lymph nodes of anti-factor B-treated MAA-sensitized Lewis rats did not induce EAAU (both clinically and histologically) in naïve syngenic rats (Table 2). In contrast, 10 million lymphocytes from the popliteal lymph nodes of MAA-sensitized Lewis rats treated with isotype IgG control transferred EAAU to naive syngenic rats (Table 2). Similar results were obtained when purified T lymphocytes from anti-factor B-treated and isotype IgG control animals were used in adoptive transfer experiments (Table 2).
TABLE 2.
Effect of anti-factor B antibody on adoptive transfer EAAU
The incidence of EAAU given as positive/total eyes following clinical examination. The cells were transferred intravenously via the tail vein.
| Donor treatment (in vivo) | Cells transferred to recipients |
EAAU in recipients |
|||
|---|---|---|---|---|---|
| Number of cells (106) | Cell population | Incidence | Score | Day of onseta | |
| Anti-factor B | 10 | Lymphocytes | 0/18 | – | – |
| Isotype IgG control | 10 | Lymphocytes | 18/18 | Severe | 6.5 ± 0.5 |
| Anti-factor B | 10 | T Lymphocytes | 0/12 | – | – |
| Isotype IgG control | 10 | T Lymphocytes | 12/12 | Severe | 7 ± 0 |
a The values are the means ± standard deviation. The severity of inflammation on histopathologic examination was grouped as mild (1+), moderate (2+ to 3+), or severe (4+).
T Cell Proliferation
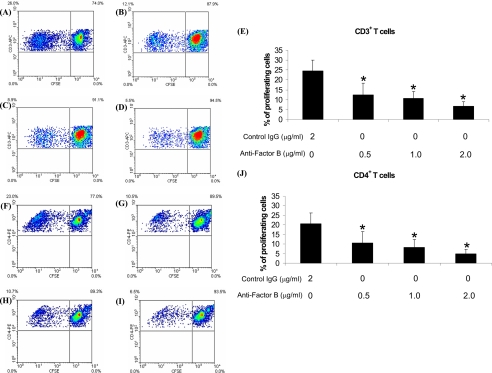
We next investigated whether the proliferation of MAA-specific T cells requires the presence of intact alternative pathway. We compared the proliferation of T cells in the presence and absence of factor B using anti-factor B antibody and an in vitro T cells proliferation assay. Total lymphocytes purified from popliteal LNs of MAA-sensitized animals sacrificed at day 7 post-immunization were labeled with CFSE using the cell trace CFSE cell proliferation kit and were cultured with MAA (20 μg/ml) for 6 days. Non-heat-inactivated normal rat serum (5%) was added to the culture wells and was used as the source of factor B. LNCs were cultured in the presence of different concentration (0.5, 1.0, and 2.0 μg/ml) of anti-factor B antibody or control IgG (2.0 μg/ml). On day 6, these in vitro cultured CFSE-labeled lymphocytes were labeled with anti-rat CD3-APC and anti-rat CD4-PE and analyzed for in vitro proliferation using flow cytometry to investigate the effect of anti-factor B antibody on T cell proliferation. Our data show that the presence of anti-factor B antibody in the cell culture significantly (p < 0.05) inhibited the proliferation of MAA-specific CD3+ T cells in vitro compared with control IgG isotype in a dose-dependent manner (Fig. 5, A–E). Similarly, CD4+ T cells cultured in the presence of anti-factor B antibody proliferated at a significantly (p < 0.05) lower rate compared with the CD4+ T cells cultured in the presence of control IgG (Fig. 5, F–J). Inhibition of CD4+ T cell proliferation was also dependent on the dose of anti-factor B antibody (Fig. 5, F–J).
FIGURE 5.
Effect of anti-factor B antibody on the proliferation of MAA-specific T cells. Cells from lymph nodes at day 7 post-immunization were used. Density plots (A–D and F–I) show representative flow cytometric data, and E and J represent cumulative data from three separate experiments. Treatment with 0.5 μg/ml (B and E), 1 μg/ml (C and E), and 2 μg/ml (D and E) anti-factor B antibody inhibited the proliferation of CD3+ T cells compared with the treatment with isotype control (2 μg/ml; A and E) in a dose-dependent manner. A similar effect was observed on CD4+ T cells when treated with 0.5 μg/ml (G and J), 1 μg/ml (H and J), and 2 μg/ml (I and J) anti-factor B antibody compared with isotype control (2 μg/ml; F and J). *, p < 0.05.
Cytokine Levels
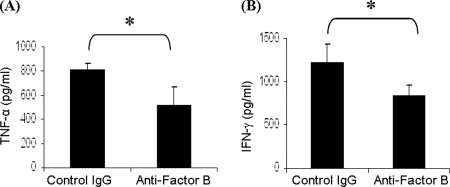
ELISA was used to determine whether there is a difference in cytokine expression by LNCs in the presence and the absence of an intact alternative pathway. We analyzed the expression of TNF-α and IFN-γ. MAA-immunized animals were sacrificed at day 7 post-immunization, and popliteal LNCs were harvested. Total lymphocytes were cultured with MAA (20 μg/ml) for 48 h in the presence of anti-factor B or isotype IgG control, and the supernatant was collected for ELISA. ELISA results revealed that the expression of TNF-α (Fig. 6A) and IFN-γ (Fig. 6B) proteins was significantly (p < 0.05) decreased in the presence of anti-factor B compared with cells cultured in the presence of control IgG. Together, our data demonstrate that the presence of fully intact alternative pathway is critical for MAA-specific T cell responses in EAAU.
FIGURE 6.
Effect of anti-factor B antibody on cytokine expression. Popliteal lymph node cells from MAA-sensitized Lewis rats produce/secrete less TNF-α (A) and IFN-γ (B) in the presence of anti-factor B antibody compared with when cultured in the presence of isotype IgG control as determined by ELISA. *, p < 0.05.
DISCUSSION
EAAU, a model of human idiopathic autoimmune anterior uveitis, is a CD4+ T cell-mediated organ-specific autoimmune disease of the eye (4–15). We have previously reported that the complement system is central for the induction of EAAU in Lewis rats (9). We have further shown a direct in vivo role for endogenous ocular complement regulatory proteins in down-regulating complement activation during EAAU (10). A recent report from our laboratory demonstrated that the suppression of complement activation by the recombinant complement regulatory protein Crry-Ig inhibited the induction of EAAU (15). We further demonstrated that Crry-Ig was effective in suppressing EAAU in Lewis rats after the disease had already developed (15). Overall, our findings provide strong evidence that the presence as well as activation of complement system is critical for the development of EAAU. However, the studies on the role of complement activation pathways in the pathogenesis of EAAU have not been performed. The current study examined which pathway of complement activation influences autoimmune uveitis. We believe that this is the first study to link the alternative pathway of complement activation to MAA-specific T cell responses that are critical to the development of pathology associated with EAAU.
We first determined the expression of complement components C4 and factor B mRNA and proteins in naïve rat eye and observed that C4 and factor B were constitutively expressed in the eyes of naïve Lewis rats. Polyclonal antibodies against human C4 and factor B were utilized to investigate the contribution of complement activation pathways in the induction of EAAU. The ability of these antibodies to recognize rat proteins and to inhibit complement activity in rat serum was proven by immunohistochemistry, by Western blot analysis, and by showing that polyclonal antibodies against human C4 and human factor B inhibit the functional activity of rat complement. We observed that EAAU was completely inhibited by intraperitoneal injections of polyclonal antibodies against factor B but not when anti-human C4 was administered similarly. Treatment with anti-factor B antibody inhibited ocular complement activation as well. Thus, the results of our present study suggest that alternative pathway of complement activation is crucial for the induction of EAAU, because in the current study we established that treatment with anti-factor B specifically inhibits the alternative pathway activity. Complement activation via the alternative pathway has been shown to be critical in various human and animal models of human diseases (34, 40–43).
A combination of both in vivo and in vitro methods was used to dissect the molecular mechanism(s) accounting for the role of the alternative pathway in EAAU. Specific experiments were carried out to determine whether the presence of factor B, an essential element of the alternative pathway, is crucial for the stimulation and/or function of T cells that are present in the draining (popliteal) LNs of MAA-sensitized Lewis rats. EAAU is a T cell-mediated autoimmune disease of the eye where a CD4+ T cell-mediated immune response is initiated in the popliteal LNs of Lewis rats after footpad injection of MAA (5–15).
First, our results of adoptive transfer experiments outlined here demonstrated that T lymphocytes harvested from donor animals in which the alternative pathway was blocked did not transfer EAAU to naïve syngenic rats when administered intravenously. These results suggest that MAA-specific T cells in the animals in which the alternative pathway was blocked were functionally impaired. We have established that EAAU is CD4+ T cell-mediated disease and cannot be induced by the adoptive transfer of primed CD8+ T cells or immune sera (6–8). Next, the proliferative capacity of MAA-specific popliteal LN derived T cells in the presence or absence of anti-factor B antibody was compared. We found that the ability of MAA-specific CD3+ and CD4+ T cells to proliferate in response to the antigen was significantly reduced by blocking factor B. Finally, the experiments were carried out to determine the effect of factor B on cytokine expression. Our data suggest that the lack of factor B had an inhibitory effect on the secretion/production of TNF-α and IFN-γ by draining LNCs. These cytokines are produced by activated T cells (44, 45).
It is worthwhile to note that in the current study lymphocytes were purified from the draining lymph nodes of MAA-sensitized Lewis rats on the seventh day after immunization for the experiments exploring the effect of alternative pathway on T cell proliferation and cytokine profile. Thus, it is possible that in EAAU, complement affects MAA-specific T cell responses through a combination of two mechanisms: 1) through a direct effect on T cells and 2) through alteration of APC function, which will modulate the ability of APCs to prime T cells. The specific contribution of complement to these mechanisms in EAAU is under investigation in our laboratory. It has become apparent that the stimulation and activation of T cells depend not only on the presentation of the peptide-MHC complex by APCs in lymph nodes or secondary lymphoid organs but also include various other factors (46). Studies in the literature suggest that the complement plays an important role in antigen presentation by APCs as well as antigen-specific T cell responses (20, 21, 47–49).
In conclusion, we report a novel finding that the alternative pathway plays a key role in the immunopathology of autoimmune uveitis and explain how blocking the alternative pathway inhibits EAAU. Our results provide evidence that blocking of the alternative pathway affects the function of MAA-specific T cells, which results in diminished autoimmune responses in EAAU. Thus, our study provides new insights into the underlying pathogenic mechanisms in autoimmune uveitis. Understanding these mechanisms is critical so that effective therapies based on selective blockade of the alternative pathway could be developed for the treatment of human idiopathic AU. This approach may represent a better strategy because inhibiting the alternative pathway will not affect the classical and lectin pathways of complement activation, which will continue to protect the host from pathogens.
Acknowledgments
We thank Ruslana G. Tytarenko for help with histologic studies and Andrea Harris for help with the use of the flow cytometer.
This work was supported, in whole or in part, by National Institutes of Health Grants EY018812 and EY014623. This work was also supported by grants from the Pat and Willard Walker Eye Research Center (Jones Eye Institute, University of Arkansas for Medical Sciences, Little Rock, AR).
- AU
- anterior uveitis
- EAAU
- experimental autoimmune anterior uveitis
- MAA
- melanin-associated antigen
- CFSE
- carboxyfluorescein diacetate succinimidyl ester
- LN
- lymph node
- LNC
- LN cell
- MAC
- membrane attack complex
- CB
- ciliary body
- C4
- complement component 4
- APC
- antigen-presenting cell.
REFERENCES
- 1. Gritz D. C., Wong I. G. (2004) Ophthalmology 111, 491–500 [DOI] [PubMed] [Google Scholar]
- 2. Bora N. S., Kaplan H. J. (2007) Chem. Immunol. Allergy 92, 213–220 [DOI] [PubMed] [Google Scholar]
- 3. Bloch-Michel E., Nussenblatt R. B. (1987) Am. J. Ophthalmol. 103, 234–235 [DOI] [PubMed] [Google Scholar]
- 4. Broekhuyse R. M., Kuhlmann E. D., Winkens H. J., Van Vugt A. H. (1991) Exp. Eye Res. 52, 465–474 [DOI] [PubMed] [Google Scholar]
- 5. Bora N. S., Kim M. C., Kabeer N. H., Simpson S. C., Tandhasetti M. T., Cirrito T. P., Kaplan A. D., Kaplan H. J. (1995) Invest. Ophthalmol. Vis. Sci. 36, 1056–1066 [PubMed] [Google Scholar]
- 6. Kim M. C., Kabeer N. H., Tandhasetti M. T., Kaplan H. J., Bora N. S. (1995) Curr. Eye Res. 14, 703–710 [DOI] [PubMed] [Google Scholar]
- 7. Bora N. S., Woon M. D., Tandhasetti M. T., Cirrito T. P., Kaplan H. J. (1997) Invest. Ophthalmol. Vis. Sci. 38, 2171–2175 [PubMed] [Google Scholar]
- 8. Bora N. S., Sohn J. H., Kang S. G., Cruz J. M., Nishihori H., Suk H. J., Wang Y., Kaplan H. J., Bora P. S. (2004) J. Immunol. 172, 7086–7094 [DOI] [PubMed] [Google Scholar]
- 9. Jha P., Sohn J. H., Xu Q., Nishihori H., Wang Y., Nishihori S., Manickam B., Kaplan H. J., Bora P. S., Bora N. S. (2006) Invest. Ophthalmol. Vis. Sci. 47, 1030–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jha P., Sohn J. H., Xu Q., Wang Y., Kaplan H. J., Bora P. S., Bora N. S. (2006) J. Immunol. 176, 7221–7231 [DOI] [PubMed] [Google Scholar]
- 11. Jha P., Matta B., Lyzogubov V., Tytarenko R., Bora P. S., Bora N. S. (2007) Invest. Ophthalmol. Vis. Sci. 48, 5091–5100 [DOI] [PubMed] [Google Scholar]
- 12. Matta B., Jha P., Bora P. S., Bora N. S. (2008) Am. J. Pathol. 173, 1440–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jha P., Manickam B., Matta B., Bora P. S., Bora N. S. (2009) J. Biol. Chem. 284, 31401–31411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matta B., Jha P., Bora P. S., Bora N. S. (2010) Immunol. Cell Biol. 88, 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manickam B., Jha P., Hepburn N. J., Morgan B. P., Harris C. L., Bora P. S., Bora N. S. (2010) Mol. Immunol. 48, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morgan B. P. (1995) Crit. Rev. Clin. Lab. Sci. 32, 265–298 [DOI] [PubMed] [Google Scholar]
- 17. Sohn J. H., Bora P. S., Suk H. J., Molina H., Kaplan H. J., Bora N. S. (2003) Nat. Med. 9, 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Carroll M. C. (2004) Nat. Immunol. 5, 981–986 [DOI] [PubMed] [Google Scholar]
- 19. Morgan B. P., Harris C. L. (eds) (1999) Complement Regulatory Proteins, Academic Press, San Diego, CA [Google Scholar]
- 20. Kemper C., Atkinson J. P. (2007) Nat. Rev. Immunol. 7, 9–18 [DOI] [PubMed] [Google Scholar]
- 21. Dunkelberger J. R., Song W. C. (2010) Mol. Immunol. 47, 2176–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pasinetti G. M. (1996) Neurobiol. Aging 17, 707–716 [DOI] [PubMed] [Google Scholar]
- 23. Jha P., Bora P. S., Bora N. S. (2007) Mol. Immunol. 44, 3901–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bora N. S., Jha P., Bora P. S. (2008) Semin. Immunopathol. 30, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singhrao S. K., Neal J. W., Morgan B. P., Gasque P. (1999) Exp. Neurol. 159, 362–376 [DOI] [PubMed] [Google Scholar]
- 26. Tsokos G. C. (ed) (2004) Complement in Autoimmunity, Karger, Basel [Google Scholar]
- 27. Gaede K. I., Baumeister E., Heesemann J. (1995) Infect. Immun. 63, 3697–3701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hietala M. A., Jonsson I. M., Tarkowski A., Kleinau S., Pekna M. (2002) J. Immunol. 169, 454–459 [DOI] [PubMed] [Google Scholar]
- 29. Tran G. T., Hodgkinson S. J., Carter N., Killingsworth M., Spicer S. T., Hall B. M. (2002) J. Immunol. 168, 4293–4300 [DOI] [PubMed] [Google Scholar]
- 30. Vriesendorp F. J., Flynn R. E., Malone M. R., Pappolla M. A. (1998) Acta Neuropathol. 95, 297–301 [DOI] [PubMed] [Google Scholar]
- 31. An F., Li Q., Tu Z., Bu H., Chan C. C., Caspi R. R., Lin F. (2009) Invest. Ophthalmol. Vis. Sci. 50, 3778–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kaya Z., Afanasyeva M., Wang Y., Dohmen K. M., Schlichting J., Tretter T., Fairweather D., Holers V. M., Rose N. R. (2001) Nat. Immunol. 2, 739–745 [DOI] [PubMed] [Google Scholar]
- 33. Bora P. S., Sohn J. H., Cruz J. M., Jha P., Nishihori H., Wang Y., Kaliappan S., Kaplan H. J., Bora N. S. (2005) J. Immunol. 174, 491–497 [DOI] [PubMed] [Google Scholar]
- 34. Bora N. S., Kaliappan S., Jha P., Xu Q., Sohn J. H., Dhaulakhandi D. B., Kaplan H. J., Bora P. S. (2006) J. Immunol. 177, 1872–1878 [DOI] [PubMed] [Google Scholar]
- 35. Copland D. A., Hussain K., Baalasubramanian S., Hughes T. R., Morgan B. P., Xu H., Dick A. D., Nicholson L. B. (2010) Clin. Exp. Immunol. 159, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen M., Daha M. R., Kallenberg C. G. (2010) J. Autoimmun. 34, J276–J286 [DOI] [PubMed] [Google Scholar]
- 37. Sohn J. H., Kaplan H. J., Suk H. J., Bora P. S., Bora N. S. (2000) Invest. Ophthalmol. Vis. Sci. 41, 3492–3502 [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Y., Qiao F., Atkinson C., Holers V. M., Tomlinson S. (2008) J. Immunol. 181, 8068–8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark A., Weymann A., Hartman E., Turmelle Y., Carroll M., Thurman J. M., Holers V. M., Hourcade D. E., Rudnick D. A. (2008) Mol. Immunol. 45, 3125–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thurman J. M., Royer P. A., Ljubanovic D., Dursun B., Lenderink A. M., Edelstein C. L., Holers V. M. (2006) J. Am. Soc. Nephrol. 17, 707–715 [DOI] [PubMed] [Google Scholar]
- 41. Banda N. K., Thurman J. M., Kraus D., Wood A., Carroll M. C., Arend W. P., Holers V. M. (2006) J. Immunol. 177, 1904–1912 [DOI] [PubMed] [Google Scholar]
- 42. Mihai S., Chiriac M. T., Takahashi K., Thurman J. M., Holers V. M., Zillikens D., Botto M., Sitaru. C. (2007) J. Immunol. 178, 6514–6521 [DOI] [PubMed] [Google Scholar]
- 43. Banda N. K., Levitt B., Glogowska M. J., Thurman J. M., Takahashi K., Stahl G. L., Tomlinson S., Arend W. P., Holers V. M. (2009) J. Immunol. 183, 5928–5937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abbas A. K., Lichtman A. D. (2003) Cellular and Molecular Immunology, Saunders, Philadelphia, PA [Google Scholar]
- 45. Romagnani S. (2000) Ann. Allergy Asthma Immunol. 85, 9–8; quiz 18, 21 [DOI] [PubMed] [Google Scholar]
- 46. Unanue E. R. (2002) Immunol. Rev. 185, 86–102 [DOI] [PubMed] [Google Scholar]
- 47. Kerekes K., Prechl J., Bajtay Z., Józsi M., Erdei A. (1998) Int. Immunol. 10, 1923–1930 [DOI] [PubMed] [Google Scholar]
- 48. Yalcindag A., He R., Laouini D., Alenius H., Carroll M., Oettgen H. C., Geha R. S. (2006) J. Allergy Clin. Immunol. 117, 1455–1461 [DOI] [PubMed] [Google Scholar]
- 49. Jacquier-Sarlin M. R., Gabert F. M., Villiers M. B., Colomb M. G. (1995) Immunology 84, 164–170 [PMC free article] [PubMed] [Google Scholar]