Abstract
The structure and intrinsic activities of conserved STAS domains of the ubiquitous SulP/SLC26 anion transporter superfamily have until recently remained unknown. Here we report the heteronuclear, multidimensional NMR spectroscopy solution structure of the STAS domain from the SulP/SLC26 putative anion transporter Rv1739c of Mycobacterium tuberculosis. The 0.87-Å root mean square deviation structure revealed a four-stranded β-sheet with five interspersed α-helices, resembling the anti-σ factor antagonist fold. Rv1739c STAS was shown to be a guanine nucleotide-binding protein, as revealed by nucleotide-dependent quench of intrinsic STAS fluorescence and photoaffinity labeling. NMR chemical shift perturbation analysis partnered with in silico docking calculations identified solvent-exposed STAS residues involved in nucleotide binding. Rv1739c STAS was not an in vitro substrate of mycobacterial kinases or anti-σ factors. These results demonstrate that Rv1739c STAS binds guanine nucleotides at physiological concentrations and undergoes a ligand-induced conformational change but, unlike anti-σ factor antagonists, may not mediate signals via phosphorylation.
Keywords: Anion Transport, Fluorescence, NMR, Nucleotide, Photoaffinity Labeling, GDP, GTP, SpoIIAA, Anti-σ Factor Antagonist, Solute Carrier 26
Introduction
The sulP/SLC26 gene superfamily of anion transporters is conserved throughout phylogeny (1–3). Mutations reported among the 11 human SLC26 genes underlie autosomal recessive congenital chondrodysplasia (4), diarrhea (5), goiter (6), and deafness (6, 7). Engineered null mutations in mouse Slc26 genes with human orthologs as yet unassociated with human disease have generated phenotypes of nephrolithiasis (8, 9), distal renal tubular acidosis (10), gastric achlorhydria (10, 11), and altered regulation of bicarbonate secretion by duodenum (12) and pancreatic duct (13, 14). In yeast, plants, and worms, the sulp gene products mediate transport of SO42−, whereas in mammals the related SLC26 gene products transport a wide range of monovalent anions in addition to divalent SO42− and oxalate. Although sulp genes are present in genomes throughout the eubacteriae and archeae, indirect evidence of anion transport function by bacterial SulP polypeptides has to date been presented only for Mycobacterium tuberculosis Rv1739c (15), Synechococcus BicA (16, 17), and Escherichia coli YchM (18).
The majority of bacterial SulP proteins and all known eukaryotic SulP/SLC26 proteins carry a C-terminal cytoplasmic “sulfate transporter anti-σ factor antagonist” (STAS)4 domain that shares distant sequence homology with bacterial anti-σ factor antagonist proteins (19, 20). Bacterial σ factors control specific transcriptional programs through activation of core RNA polymerase. All σ factors are negatively regulated by anti-σ factors. In Bacillus subtilis, σ F (σF) initiates and controls the sporulation program. The anti-σ factor for σF is SpoIIAB, a Ser/Thr kinase under the inhibitory control of the anti-σ antagonist SpoIIAA. ATP-liganded SpoII AB binds and inhibits σF. SpoIIAA activates σF through sequestration of SpoIIAB (ATP), which displaces σF from its complex with SpoIIAB. SpoIIAA phosphorylation (at Ser58) by bound SpoIIAB leads to its dissociation from the ADP form of SpoIIAB. Phosphorylated SpoIIAA can then be dephosphorylated by phosphatase SpoIIE (summarized in Ref. 21). However, the GTP-binding and hydrolase activities of SpoIIAA (22) have unclear physiological functions, and no known relationship to either sporulation or the growth-promoting functions of the ribosome-associated GTPase, Obg (23).
The structure of SpoIIAA has been solved by heteronuclear NMR in solution (24), and x-ray crystallography (25) in phosphorylated and unphosphorylated forms, and in complex with SpoIIAB bound to either ADP or ATP (21). Published preliminary structures of additional STAS domain proteins include the NMR solution structure of Thermotoga maritima putative anti-σ antagonist TM1442 in phosphorylated and unphosphorylated states (26), the NMR and crystal structures of T. maritima putative anti-σ antagonist TM1081 (27), and the crystal structure of the putative stressosome component RsbS from Moorella thermoacetica (28). The SpoIIAA structure has been used to model the eukaryotic SLC26 STAS domain, including congenital chloride diarrhea missense mutations in the human SLC26A3 domain that result in subtle chemical shift changes as detected by 1H-15N two-dimensional HSQC NMR (29). However, the amino acid assignments of this structure have not been reported and, until recently, no other structural information had been released for STAS domains of SulP/SLC26 anion transporters.
We have overexpressed and purified the 560-aa putative SO42− transporter Rv1739c from M. tuberculosis, comprising an N-terminal trans-membrane domain (aa 1–414) and a C-terminal cytoplasmic STAS domain (aa 442–560) (15). Previously, we reported the secondary structural features present in the C-terminal STAS domain using heteronuclear NMR spectroscopy (30). Here we report the three-dimensional NMR structure of the Rv1739c STAS domain (aa 437–560). Using intrinsic fluorescence quench data and 1H-15N chemical shift perturbation (CSP) experiments, we demonstrate that the monomeric STAS domain binds guanine nucleotides at physiological concentrations. The purified STAS domain is also associated with detectable GTPase activity. CSP analysis reveals that STAS binding of GDP elicits a more extensive conformational perturbation than does GTP. In silico docking calculations support these CSP data, suggesting that GDP binds preferentially, whereas GTP binding involves a subset of the GDP-interacting residues. The overexpressed Rv1739c STAS domain is not a phosphoprotein as isolated from E. coli or Mycobacterium smegmatis, and is not phosphorylated in vitro by recombinant Ser-Thr kinases or anti-σ factors of M. tuberculosis. While this work was in editorial review, reports appeared presenting crystal and solution structures of a centrally deleted core portion of the STAS domain from rat Slc26a5/prestin (31) and a crystal structure of the full-length STAS domain from E. coli SulP protein YchM (18).
EXPERIMENTAL PROCEDURES
Purification of Rv1739c STAS Domain
A polypeptide encompassing aa 437–560 of Rv1739c was overexpressed for study of the STAS domain, based on alignment with STAS domains from E. coli ychM and Sultr1.2, and with structurally characterized STAS domains from anti-σ factor antagonists of B. subtilis, Bacillus sphericus, and T. maritima 1442. For clarity, Rv1739c 437–560 are referred to as Rv1739c STAS aa 1–124 in this study. The Rv1739c STAS domain containing a C-terminal His6 tag was overexpressed in the E. coli strain Tuner DE4pLacI (Novagen) as previously described (30). The protein purified from the nickel-nitrilotriacetic acid column was >96% pure as detected by Coomassie Blue staining of SDS-PAGE or PFO-PAGE gels. Subsequent gel filtration (Superdex 75 FPLC) yielded a homogeneous single peak eluting at a retention time that corresponds to monomeric STAS (∼15 kDa), and is observed as a single band on overloaded SDS-PAGE (supplemental Fig. S1). Protein identity was confirmed by mass spectrometry. Unlabeled and uniformly 13C/15N- or 15N-labeled STAS-His6 NMR samples of 0.7–1.0 mm concentration in 50 mm sodium phosphate, 275 mm NaCl, pH 7.2, were prepared and subjected to NMR spectroscopy (supplemental “Methods” and Ref. 30). Rv1739c STAS expression in M. smegmatis is described under supplemental “Methods”.
NMR Spectroscopy
NMR experiments were performed at 298 K on a Bruker Avance 600 MHz spectrometer equipped with a 5-mm triple resonance PFG (z axis) probe. All NMR data were acquired in gradient-selected, sensitivity-enhanced mode. NMR data were processed using NMRPipe/NMRDraw processing software (32), and analyzed using ANSIG (33). Protein backbone and side chain resonances of 1H, 13C, and 15N were assigned using standard two-dimensional, three-dimensional, and triple resonance experiments (34–36) as detailed under supplemental “Methods”.
Restraint Generation and Structure Determination of Rv1739c STAS Domain
A set of correlated NOE cross-peaks were generated by manual and automated NOE assignment using CYANA-2.1 (37) in two-dimensional [1H,1H]-NOESY (τm = 90, 120, and 150 ms), three-dimensional 15N-resolved [1H,1H]-NOESY (τm = 120 ms), and 13C-resolved [1H,1H]-NOESY (τm = 120 ms) spectra. Distance restraints were derived from NOE cross-peak intensities and from hydrogen bond measurements. Dihedral angles were determined using standard protocols (37–39) as detailed under supplemental “Methods”. Three-dimensional structures were calculated with CYANA-2.1 using the torsion-angle dynamics protocol (37) (supplemental “Methods”). An ensemble of 30 structures selected for stereochemical quality assessment and the lowest Cyana target function was subjected to molecular dynamics simulation in explicit water for refinement using CNS (40).
Structure quality was assessed with PROCHECK-NMR (41). The 25 structures of the highest refinement represented the solution structure of Rv1739c STAS. Figures were generated with MOLMOL (42) and VMD (43), and sequences were aligned with ClustalW and LALIGN.
Heteronuclear NMR Investigation of Rv1739c STAS-Nucleotide Interaction
The Rv1739c STAS interaction with nucleotides was measured by collecting a series of two-dimensional 1H-15N HSQC experiments. Rv1739c STAS was titrated with increasing concentrations of nucleotides to a saturating concentration of 20 mm. CSP data were determined using weighted-average chemical shifts (Δδweighted) for each amino acid residue; Δδweighted = [(Δ1H)2 + (Δ15N/5)2]1/2 (see supplemental “Methods”).
In Silico Docking of STAS-Nucleotide Complexes
In silico docking calculations were carried out using AutoDock Vina (44). An exhaustive search was carried out to find and cluster the best docked poses of the GDP and GTP complexes with Rv1739c STAS. The average structure of the STAS domain was used as a starting target molecule for these docking studies (see supplemental “Methods”).
Photoaffinity Labeling of Rv1739c STAS Domain
Rv1739c STAS was incubated with biotinylated azido-GTP in the presence or absence of 2 mm GTP, and briefly irradiated. An aliquot of the terminated reaction was electrophoresed, blotted, developed, and visualized as detailed (supplemental “Methods”).
Intrinsic Fluorescence Quench of Rv1739C STAS
Gel filtration-purified Rv1739c STAS in buffer A (25 mm Tris, 100 mm NaCl, pH 7.2) was centrifuged before use. Free acid forms of ATP, GTP, and GDP (Sigma), GTPγS, and GDPβS (Jena Biosciences) of the highest purity available were prepared as stock solutions in buffer A. Rv1739c STAS (12–20 μm; initial volume 200 μl) was preincubated in 48-well plates at 24 °C with nucleotides in the absence or presence of 1 mm added MgCl2 (final volume: 217 μl). Steady-state intrinsic fluorescence intensity of RV1739c STAS was then recorded at λem 290–400 nm at 2-nm intervals with fixed λex = 280 nm (SpectraMax M5, Molecular Devices, Sunnyvale, CA) in the presence of sequentially increasing nucleotide concentrations or titrated with buffer A alone. Rv1739c STAS fluorescence at each nucleotide concentration was corrected for dilution and for inner filter effect contributions of added nucleotide, as described (45). The effects of identical sequential increases in added nucleotide were assessed on the intrinsic fluorescence of a mixture of free tryptophan plus 3 molar eq of free tyrosine (the 1:3 Trp:Tyr molar ratio reflected in the native Rv1739c STAS amino acid sequence), with identical data acquisition parameters and instrument settings. The inner filter effect correction factor was determined as the ratio of fluorescence intensity of the Trp:Tyr solution in the absence of nucleotide to that in the presence of nucleotide at each emission wavelength. The inner filter effect correction factor was ≤2 at all nucleotide concentrations.
Inner filter effect-corrected, normalized fluorescence intensity data were plotted as a function of nucleotide concentration and fit (SigmaPlot, Systat) to a single site ligand-binding model,
 |
where F0 − F is the change in fluorescence intensity at 316 nm (λmax) following the addition of nucleotides at concentration [S], ΔFmax is the maximum change in fluorescence intensity, and Kd is the STAS-nucleotide dissociation constant.
Assay of Nucleotidase Activity
Rv1739c STAS or human RhoA-GST (as a positive control) were mixed with [γ-32P]GTP or [γ-32P]ATP. Reactions were stopped with SDS, and fractionated by thin layer chromatography (TLC). Excised spots containing inorganic [32P]phosphate were counted (Packard Tri-Carb 2200CA) in Ecoscint A (National Diagnostics, Atlanta, GA) with 97% efficiency (supplemental “Methods”).
Protein Phosphorylation Assays
See supplemental “Methods” for kinase overexpression and purification. In vitro phosphotransfer assays were performed in reaction buffer (300 mm NaCl, 50 mm Tris, pH 8.0, 0.5 mm Tris(2-carboxyethyl) phosphine hydrochloride, 10% glycerol). Each reaction contained 15 μg of STAS Rv1739c substrate and 1.5 μg of Ser/Thr kinase or anti-σ factor kinase. Reactions were initiated by simultaneous addition of 1 μCi of [γ-32P]ATP (800 Ci/mmol and 10 mCi/ml; MP Biomedicals), 50 μm ATP (Sigma), and 0.5 mm MnCl2. Reactions proceeded for 30 min at room temperature, then subjected to SDS-PAGE and imaged as described (46).
RESULTS
NMR Spectroscopy of Rv1739c STAS Domain
The 124-aa Rv1739c STAS domain (Rv1739c aa 437–560) with a C-terminal His6 tag (supplemental Fig. S1) was expressed and purified for NMR spectroscopy and biophysical studies. Supplemental Fig. S2 shows sequential 13Cα, and 13Cβ assignments for the first α-helix (supplemental Fig. S2A) and third β-strand in Rv1739c STAS (supplemental Fig. S2B). The 1H-15N two-dimensional HSQC spectrum of the STAS domain (30) exhibited well dispersed resonances for nearly all backbone and side chain proton-nitrogen pairs (supplemental Fig. S2C), with previously reported 1H-, 15N-, and 13C-chemical shifts (BMRB accession number 16052) (30). Xaa-Pro peptide bond conformations were predicted from chemical shift values of 15N, 1HΝ, 1Hα, 13Cα, 13Cβ, and 13CO resonances using PROMEGA (spin.niddk.nih.gov/bax/software) revealed a trans-configuration for each of the 5 Pro residues of Rv1739c STAS.
Distance and Dihedral Restraints of Rv1739c STAS Domain
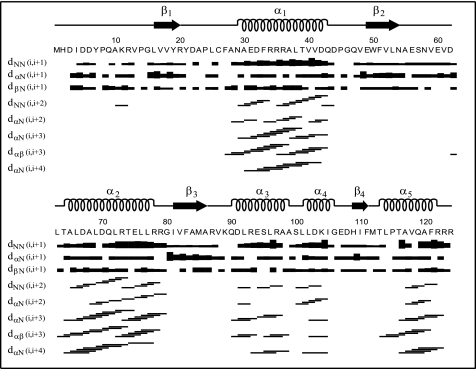
NMR-derived experimental restraints used in determining the three-dimensional structure of Rv1739c STAS consisted of 1692 unique NOEs, 88 hydrogen bond restraints, and 163 (ϕ, ψ)-backbone angle restraints (Table 1). Sequential and medium range NOEs are summarized in Fig. 1. Of 311 long range NOEs assigned, 68 are from 1Hα and 1HΝ backbone-side chain interactions, 200 are from side chain-side chain interactions, and 43 represent backbone-backbone interactions. Hydrophobic and aromatic residues contribute 62 and 34% of the long range NOEs identified in Rv1739c STAS.
TABLE 1.
Structural statistics for Rv1739c STAS domain of M. tuberculosis
Statistics for the ensemble of 25 energy-minimized structures.
| Restraints | |||
|---|---|---|---|
| NOEs | |||
| Total | 1692 | ||
| Intraresidue (|i-j| = 0) | 447 | ||
| Sequential (|i-j| = 1) | 538 | ||
| Medium range (1 < (|i-j|) ≤ 4) | 396 | ||
| Long range (|i-j|) > 4) | 311 | ||
| Hydrogen bonds | 88 | ||
| Average distance restraints per residue | 14.4 | ||
| Dihederal angles | Φ | ϕ | |
| 13Cα secondary shifts based | 67 | 67 | |
| Generated using Talosa | 96 | 96 | |
| Structural quality | |||
| Ramachandran map of residues 1–124 | (in %) | ||
| Most favored regions | 66.2 | ||
| Additionally allowed regions | 26.5 | ||
| Generously allowed regions | 5.3 | ||
| Disallowed regions | 1.9 | ||
| Average root mean square deviation frommmean coordinatesb (in Å) | |||
| Backbone atoms (N, Cα, C′) | 0.87 ± 0.17 | ||
| All heavy atoms | 1.36 ± 0.24 | ||
| Restraint violation | |||
| Upper distance (>0.5 Å) | ∼1.57 | ||
| Dihedral angle (>5°) | 0 | ||
a Dihedral angles generated using chemical shifts of 13Cα, 13Cβ, 1HΝ, 1Hα, 13C′.
b Root mean square deviation value determined for residue range 15–121.
FIGURE 1.
Plot of sequential and medium range NOEs for Rv1739c STAS. NOEs are assigned in three-dimensional 13C- and 15N-edited NOESY-HSQC, and two-dimensional 1H-1H NOESY spectra of Rv1739c STAS. Bar thickness is proportional to NOE intensity. α-Helices are depicted as spirals. β-Sheets are depicted as arrows.
Three-dimensional Structure of Rv1739c STAS
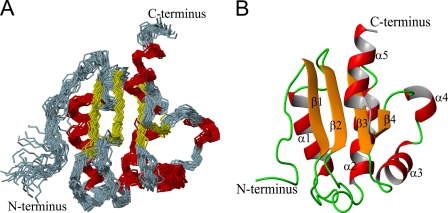
Fig. 2A illustrates an ensemble of the 25 best NMR conformers of the Rv1739c STAS domain superimposed on their backbone N, Cα, and C′ atoms. These structures were selected using standard structural criteria including: low target function, minimal upper distance NOE violations >0.5 Å, and absence of dihedral angle violations >5°. The low root mean square deviation values represented by the superimposed ensemble and the identification in allowed regions of the Ramachandran map of 98.1% of amino acid residues support the precision and stereochemical quality of the calculated structures (Table 1).
FIGURE 2.
Solution structure of Rv1739c STAS. A, backbone representation (superimposed on N, Cα, and C′ atoms) of an ensemble of the 25 lowest energy conformers of Rv1739c STAS. B, representative Rv1739c STAS conformer comprises a four-stranded β-sheet with five α-helices. The positions of the backbone and all heavy atoms exhibit root mean square deviation values of 0.87 ± 0.17 and 1.36 ± 0.24 Å, respectively, relative to the mean structure (Table 1). 98.1% of all residues occupy favorable or allowed regions within the Ramachandran map.
Fig. 2B presents the mean solution structure of Rv1739c STAS. The structure includes five well defined helices (α1, 30–42; α2, 65–77; α3, 91–99; α4, 102–105; and α5, 114–121), and four β-strands (β1, 16–19; β2, 50–53; β3, 82–86; and β4, 110–111) in a parallel orientation to form a β-sheet structure. Packing of helices α1 and α2 is stabilized by NOEs between adjacent regions within the fold. Supplemental Fig. S3A shows the calculated electrostatic potential surface of Rv1739c STAS. The net surface charge of −3 reflects 20 negatively charged and 17 positively charged surface residues that are distributed non-uniformly across the Rv1739c STAS surface.
The structural order/rigidity of Rv1739c was evaluated using heteronuclear {1H}-15N NOE data obtained for 108 of 119 non-proline amino acids, and plotted as a function of amino acid sequence (supplemental “Methods” and Fig. S3B). The mean {1H}-15N NOE value for all residues was 0.79 ± 0.02, and was higher in more structured regions (0.84 ± 0.02) than in loop regions (0.71 ± 0.02). NOE values suggest the β2 (0.92 ± 0.02) and α4 (0.78 ± 0.05) regions as having higher and lower structural order/rigidity. The lower NOE value for the N-terminal 15 aa residues (0.55 ± 0.02) supports N-terminal fraying and conformational flexibility often associated with the presence of multiple prolines.
Structural Comparison of Rv1739c STAS with Other STAS Domains
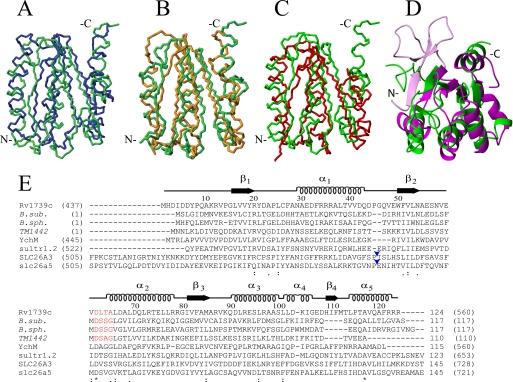
Fig. 3, A–D, superimpose the backbone atoms of Rv1739c STAS with the crystal structure of non-phosphorylated B. sphaericus SpoIIAA, the NMR structure of B. subtilis SpoIIAA, the NMR structure of T. maritima TM1442, and the crystal structure of Rattus norvegicus Slc26a5/prestin. The structures are quite similar despite divergent primary sequence identities of <18%.
FIGURE 3.
Structural and sequence alignment of Rv1739c STAS domain. A–D, average backbone structure of the Rv1739c STAS domain (green) aligned with: in A, crystal structure of non-phosphorylated SPOIIAA from B. sphaericus (blue, PDB code 1H4Z, root mean square deviation 3.6 Å); in B, solution NMR structure of B. subtilis SPOIIAA (orange, PDB code 1AUZ, root mean square deviation 3.9 Å); in C, solution NMR structure of T. maritima TM1442 (red, PDB 1SBO, root mean square deviation 3.3 Å); in D, overlay of a ribbon representation of Rv1739c STAS (green) with the crystal structure of an engineered core STAS domain from rat Slc26A5/prestin (magenta, PDB code 3LLO, root mean square deviation 3.3 Å). The region shown in light magenta comprises the N-terminal 15 aa of the prestin STAS (light magenta) extending beyond the Rv1739c STAS N-terminal residues. Backbone N, Cα, and C′ atoms were superposed onto secondary structured regions. (Overlays were obtained by superposition of backbone atoms as follows: in A, Rv1739c STAS domain residues 16–20, 30–42, 49–54, 65–77, 81–85, 91–98, and 114–120 were superposed on B. sphaericus SPOIIAA residues 12–16, 23–35, 44–49, 57–69, 76–80, 84–91, and 104–110. In B, STAS domain residues 16–20, 30–42, 49–54, 65–77, 81–85, and 91–97 were superposed on B. subtilis SPOIIAA residues 12–16, 25–37, 44–49, 60–72, 76–80, and 87–93. In C, STAS domain residues 16–20, 30–42, 50–54, 65–77, and 82–86 were superposed on TM1442 residues 14–18, 30–42, 46–50, 59–71, and 78–82. In D, Rv1739c STAS domain residues 16–20, 49–54, 65–77, 81–85, 91–98, and 110–111 were superposed on rat prestin STAS residues 536–540, 640–645, 655–667, 673–677, 681–688, and 702–703.) E, amino acid sequence alignment (ClustalW with manual adjustments) of Rv1739c STAS with STAS domains and structurally characterized anti-σ factor antagonists (italicized) from other organisms, including SpoIIAA from B. subtilis (B. sub.); SpoIIAA from B. sphericus (B. sph.), and TM1442 (T. maritima). Above the aligned sequences are the tertiary structural elements and aa numbers of Rv1739c STAS. Numbers in parentheses are the aa residues encompassing each STAS domain or each (full-length) anti-σ factor antagonist. The conserved DSSG motif of B. subtilis SpoIIAA (red) and its phosphorylated residue Ser58 correspond to DLTA and Thr64 of Rv1739c STAS (red). The STAS domains from E. coli YchM and Sultr1.2 from A. thaliana are of lengths comparable with that of Rv1739c. The STAS domain of 764 aa human SLC26A3/DRA encompasses aa 505–728, and that of rat slc26a5/prestin encompasses aa 505–721. Both are shown with excision of the IVS region of ∼75 aa between helix α1 and strand β2 (blue arrowheads, Rv1739c STAS-based nomenclature (28)). Numbers without parentheses at alignment C termini show lengths of the depicted sequences. In parentheses are the number of aa residues in each full-length polypeptide. Asterisks under the sequences mark positions of complete sequence conservation.
The overall tertiary fold of Rv1739c STAS resembles that of SpoIIAA from B. sphaericus and unphosphorylated SpoIIAA from B. subtilis, with one face of the central β-sheet juxtaposed to helices α1 and α2, and the other face exposed to the aqueous environment. The flexible structure of the N-terminal 14 aa residues of Rv1739c STAS contrasts with the structured N-terminal regions of SpoIIAA from B. sphaericus and B. subtilis. These include single β strands of 8 or 3 residues, respectively, packed in antiparallel orientation to the subsequent β strands arrayed in parallel, which together form a central β sheet. Helices α1 and α2 of Rv1739c STAS and B. subtilis SpoIIAA are similar in length, but shorter than the corresponding helices in B. sphaericus SpoIIAA. Helix α4 of Rv1739c STAS is oriented away from the protein core as in B. sphaericus, but closely packed to the main fold in B. subtilis and TM1442. Rv1739c STAS helix α3 extends parallel to the central β-sheet, in contrast to a perpendicular orientation of α3 in B. subtilis SpoIIAA. β1 and β2 strands of Rv1739c STAS and TM1442 are of similar length, but shorter than those of SpoIIAA. The β3-strands in Rv1739c (aa 80–86) and B. sphaericus SpoIIAA are similar in length. However, this region is shorter in length in TM1442 and unstructured in B. subtilis SpoIIAA.
The topological fold of Rv1739c STAS closely resembles that of the rat prestin construct truncated at its C terminus and devoid of its “intervening sequence” (IVS) region aa 564–636. A notable exception to this similarity is the N-terminal 15-aa extension present in prestin STAS, which includes a “β1 strand” at its extreme N terminus and a “β0 strand” in the immediately juxtaposed loop (31). Structural comparison among SulP/SLC26 polypeptides reveals that the presence of a common C-terminal β4 strand (aa 110–111 of Rv1739c STAS) is often absent from anti-σ factor antagonist structures. In addition, whereas helices α1 and α2 of anti-σ factor antagonists are arrayed in parallel orientation, the corresponding α1 and α2 helices of SulP/SLC26 STAS domains are oriented along divergent axes.
The conserved 56DSSG motif of SpoIIAA constitutes a canonical phosphorylation site at Ser58 (Fig. 3E, in red). Sequence alignment suggests that Thr64 of Rv1739c, 62DLTA (also in red), provides a homologous phosphorylation site of Rv1739c. Superimposed tertiary structures further support the alignment of Ser58 of SPOIIAA (PDB code 1AUZ) with the partially surface exposed Thr64 of Rv1739c STAS (PDB code 2KLN). These phosphorylation motifs in both SpoIIAA and Rv1739c are bracketed by hydrophobic residues. In addition to Thr64, many conserved residues of Rv1739c STAS reside within a localized patch comprising the β2-α2 region (Fig. 3E).
Guanine Nucleotide Binding by Rv1739c STAS
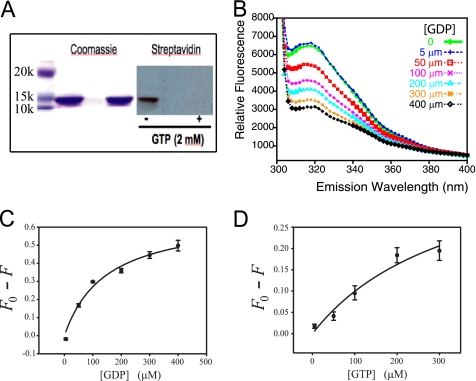
The reported nucleotide binding properties of the anti-σ factor antagonist SpoIIAA (22) and the STAS domain of the blue light receptor YtvA from B. subtilis (47) prompted us to evaluate the nucleotide binding potential of Rv1739c STAS. As illustrated in Fig. 4A, Rv1739c STAS was photolabeled by a γ-biotinylated derivative of 8-N3-GTP, and this photolabeling was prevented in the presence of excess unlabeled GTP. The presence of a unique Trp residue, Trp50 (aa 486 of Rv1739c holoprotein), in Rv1739c STAS and Tyr residues at STAS positions 7, 19, and 21 (Fig. 3E and supplemental Fig. S4A) allowed assessment of nucleotide binding using intrinsic fluorescence quench methods.
FIGURE 4.
Rv1739c STAS binds guanine nucleotides. A, photoaffinity labeling of Rv1739c STAS with 8-N3-GTP γ-biotinyl-LC-PEO-amine, in the absence and presence of 2 mm GTP. A representative of three similar experiments is shown. B, quench of intrinsic fluorescence of Rv1739c STAS (12–20 μm) by the indicated concentrations of GDP. C, concentration dependence for quench of the Rv1739c STAS peak intrinsic fluorescence by GDP (K½ = 146 ± 38 μm; r2 = 0.98). D, concentration dependence for quench of Rv1739c STAS peak fluorescence by GTP (K½ = 347 ± 227 μm; r2 = 0.96). λex was 280 nm; λem was scanned between 300 and 400 nm. Values in C and D are mean ± S.E. (n ≥ 3).
Rv1739c STAS fluorescence emission λmax was observed at 316 nm. Fig. 4B illustrates the gradual quench of intrinsic STAS fluorescence with progressively increasing concentrations of GDP. Supplemental Fig. S4 shows comparable quench by increasing concentrations of GTP, of non-hydrolyzable guanine nucleotides, and ATP. The observed quench isotherms are compatible with a single nucleotide binding site with dissociation constants (K½, in μm) of 146 ± 38 for GDP, 347 ± 227 for GTP (Fig. 4, C and D), 152 ± 53 for GDPβS (supplemental Fig. S4, E and F), and 326 ± 131 for GTPγS (supplemental Fig. S4, C and D and Table 2). K½ values (in μm) were higher in the presence of 1 mm MgCl2, 587 ± 337 for GDP and 727 ± 251 for GTP (Table 2). K½ for ATP in the absence of MgCl2 was 756 ± 232 μm (supplemental Fig. S4, G and H). Thus, the nucleotide affinity rank-order profile for Rv1739c STAS appeared to be GDP > GTP > ATP. Maximum STAS fluorescence quench was observed at ≤400 μm nucleotide except in the case of ATP, which required 2 mm nucleotide. The percent quench varied from 19 (for GTP) and 49% for GDP (Table 2). These data suggest a nucleotide interaction surface in Rv1739C STAS that directly or indirectly perturbs Trp50 and/or the three Tyr residues through conformational effects on adjacent or interposed residues.
TABLE 2.
Binding affinities of nucleotide-Rv1739c STAS interaction
K½ values from fit of Rv1739c STAS fluorescence intensity quench by the indicated nucleotides at λem 316 nm.
| Nucleotide | K½ ± S.E. | r2 |
|---|---|---|
| μm | ||
| GTP | 347 ± 227 | 0.96 |
| GTPγS | 326 ± 131 | 0.98 |
| GTP + 1 mm MgCl2 | 727 ± 251 | 0.99 |
| GDP | 146 ± 38 | 0.98 |
| GDPβS | 152 ± 53 | 0.98 |
| GDP + 1 mm MgCl2 | 587 ± 337 | 0.97 |
| ATP | 756 ± 232 | 0.94 |
GTPase Activity Associated with Rv1739c STAS
Intrinsic signaling or enzymatic activities of bacterial SulP-associated STAS domains remain undefined. B. subtilis SpoIIAA exhibits GTPase activity (22), but the GTP-binding protein YtvA lacks detectable GTPase activity (48). We therefore evaluated Rv1739c for the presence of intrinsic nucleotidase activity. As shown in supplemental Fig. S5A, the FPLC-purified Rv1739c STAS preparation exhibited modest GTPase activity that was STAS-dependent, temperature-sensitive, inactivated by SDS (supplemental Fig. S5B), and time-dependent. STAS-associated ATPase activity was not detected (not shown).
Residues Influenced by Nucleotide Binding in Rv1739c STAS
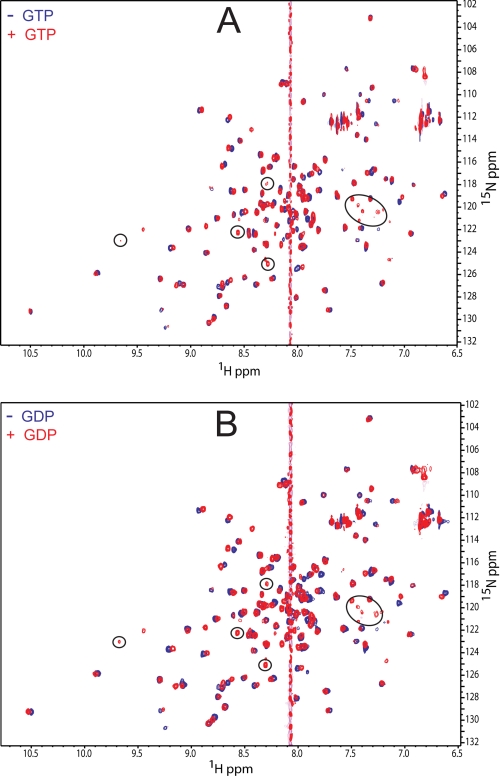
Fig. 5 shows two-dimensional 1H-15N HSQC overlays of Rv1739c STAS domain resonances in the absence (blue contours) and presence (red contours) of saturating concentrations of GTP (A) or GDP (B). Perturbations with weighted CSPs (Δδweighted) ≥ 0.05 were considered significant (supplemental Fig. S6). CSP analysis revealed that the GDP-induced conformational change in Rv1739c STAS (Fig. 5B) affected a larger number of residues with greater extent of perturbation than those altered by the addition of GTP (Fig. 5A). GDP significantly perturbed 16 residues (red in Fig. 6, C and D), including Ala10, Arg12, Val13, Gly15, Val41, Asp44, Gln47, Val48, Arg79, Gly106, Glu107, Asp108, His109, Ile110, Arg122, and Arg124. GTP perturbed a subset of these residues (red in Fig. 6, A and B), including Ala10, Arg12, Val13, Gly15, Val48, Val61, Arg79, His109, and Arg124. The results are consistent with the lower K½ value for GDP than for GTP (Fig. 4 and Table 2). The amino acids exhibiting the largest GDP-induced CSPs are illustrated at higher resolution in supplemental Fig. S6, A and B.
FIGURE 5.
NMR detection of guanine nucleotide interactions with Rv1739 STAS. A, two-dimensional 1H-15N HSQC spectra of Rv1739c STAS in the absence (blue contours) and presence of 20 mm GTP (red contours). B, two-dimensional 1H-15N HSQC spectra of Rv1739c STAS in the absence (blue contours) and presence of 20 mm GDP (red contours). Circled resonances indicate residues detected only in the presence of nucleotide. The guanine proton peak is at ∼8.07 ppm.
FIGURE 6.
Guanine nucleotide-shifted residues highlighted in the Rv1739c STAS structure. A and C, STAS backbone representation is in blue; residues perturbed by ≥0.05 ppm following addition of 20 mm GTP (A) or GDP (C) are in red. B and D, the van der Waal surface representation of STAS structure (shown in two orientations) is in blue; residues were perturbed by ≥0.05 ppm following addition of 20 mm GTP (B) or GDP (D) are in red and annotated. Side chains of annotated residues Trp50 and Thr64 are in cyan.
The observed nucleotide-induced conformational perturbations were predominantly localized within the solvent-exposed loops and immediately adjacent residues, as evidenced by the conformational flux of Val41 at the end of helix α1, of Ile110 localized at the end of strand β4, and Arg122 near the end of the C-terminal helix α5. Many nucleotide-perturbed residues localize to a single face of Rv1739c STAS. Val61, located near the semi-conserved, potential hydrolase nucleophile Thr64, is the only residue perturbed >0.05 ppm by GTP alone (Fig. 6A and supplemental Fig. S6C).
Addition of GTP or GDP to Rv1739c resulted in emergence in the 1H-15N two-dimensional HSQC spectra of additional resonances (circled in Fig. 5, A and B) of near-identical (1H, 15N) coordinates. Line widths were broadened to a greater degree by GDP than by GTP. These ligand-induced resonances may belong to previously unassigned loop residues and/or to Ser95 in helix α3 (30). Alternatively, these resonances may reflect slow conformational exchange of select residues.
In Silico Docking of the STAS-Nucleotide Complex
Docking of GDP and GTP into the three-dimensional structure of Rv1739c STAS was performed using AutoDock Vina (44). These in silico experiments identified two potential nucleotide binding sites in Rv1739c STAS (supplemental Fig. S7). One site involves residues localized primarily near the STAS N terminus, whereas the other includes predominantly C-terminal residues.
STAS-GDP Docking
A flexible grid-based GDP-STAS docking protocol defined 9 clustered poses for the STAS domain bound to GDP, as illustrated under supplemental Fig. S7. In the lowest energy pose, the GDP-interacting amino acid residues include Gln9, Gly15, Tyr19, Leu77, Arg78, Arg79, Gly80, Lys104, Ile105, Glu107, Asp108, Pro115, Val118, Gln119, and Arg122. Many of these residues are, or are adjacent to, those exhibiting above-threshold CSP in NMR titration studies. GDP binding is preferentially clustered to C-terminal STAS residues, with 7/9 poses supporting this preferred docked complex.
STAS-GTP Docking
Several interacting amino acids identified in the STAS-GDP complex were also identified in the GTP-STAS complex. However, GTP exhibited a modest preference for the STAS N terminus over its C terminus. The GTP-interacting residues of the clustered poses included Gln9, Gly15, Leu77, Arg78, Arg79, Gly80, Lys104, Ile105, Glu107, Asp108, Val118, Gln119, and Arg122 (supplemental Fig. S7).
Rv1739c STAS Appears Not to Be a Phosphoprotein
Mass spectrometric analysis of the Rv1739c STAS domain isolated from E. coli revealed no phosphate. Because the Rv1739c STAS polypeptide may have undergone dephosphorylation during purification, we evaluated its ability to serve as an in vitro phosphorylation substrate. Supplemental Fig. S8 shows, however, that none of the 11 recombinant, purified M. tuberculosis Ser/Thr kinases stably phosphorylated the Rv1739c STAS domain in vitro. Moreover, Rv1739c STAS did not serve as the phosphorylation substrate for any of the four purified, recombinant M. tuberculosis anti-σ factors.
Given that the isolated Rv1739c STAS may be a poor phosphorylation substrate in vitro, we assessed the phosphorylation status of FLAG-tagged Rv1739c STAS overexpressed in M. smegmatis. Importantly the Rv1739c overexpressed in M. smegmatis lacked an immunodetectable phosphothreonine (not shown), further supporting the conclusion that Rv1739c is not a substrate for mycobacterial Ser/Thr or anti-σ factor kinases.
DISCUSSION
We have reported the three-dimensional NMR structure of the STAS domain from the SulP/SLC26 polypeptide Rv1739c of M. tuberculosis. We have compared this structure with previously solved structures of anti-σ factor antagonists, as well as with structures of STAS domain structures from putative anion transporters reported during review of this manuscript. Rv1739c STAS exhibited binding of nucleotides with affinities at the upper end of the physiological range, and with rank order of GDP > GTP ≫ ATP. GDP and GTP elicited distinct conformational changes in the Rv1739c STAS structure. Low rates of GTP hydrolysis were associated with nominally pure Rv1739c STAS. Rv1739c STAS underwent no detectable phosphorylation in vitro, or when overexpressed in E. coli or M. smegmatis.
Solution Structure of Rv1739c STAS
Despite its very limited amino acid sequence similarity to other STAS domains, the Rv1739c STAS solution structure superimposes closely with previously reported NMR and x-ray crystallography structures of anti-σ factor antagonists. However, in its absence of a β strand within the most N-terminal residues 1–14, Rv1739c STAS differs from the structured N-terminal regions observed in the other anti-σ factor antagonist structures (see above). The lack of N-terminal structure may be intrinsic to Rv1739c STAS, perhaps due in part to the presence of two proline residues: Pro8 and Pro14, which may contribute to the conformational heterogeneity and general disruption of short β strand formation in this region. Alternatively, the lack of N-terminal structure may reflect the absence of particular additional residues in this construct (i.e. ∼420–426) that link the reported STAS domain structure to the C-terminal end of the transmembrane domain, or the absence of a physiological interacting protein or ligand. With the exception of this unstructured N-terminal region, the Rv1739c backbone is a well defined, rigid/ordered structure, as shown by heteronuclear NOE data (supplemental Fig. S3B). The short intervening loop connecting regions of the secondary structure in Rv1739c resemble those of other anti-σ factor antagonists and E. coli SulP ychM (18), but contrast with the longer intervening loops in STAS domains of mammalian SLC26 proteins (Fig. 3, D and E).
The Rv1739c STAS domain construct studied here lacks the 15-aa N-terminal extension present in the crystal structure of the C-terminal truncated, IVS-deleted rat prestin STAS structure (Fig. 3E). This 15-aa extension includes the first β-strand (“β1”) observed in rat prestin STAS (31). Deletion from the prestin STAS domain of its 73-aa IVS, of yet unknown function, was crucial for its crystallization, as was truncation from the rat prestin construct of its C-terminal 9 aa (31). Indeed, the secondary structure prediction program Psipred also predicts the presence of this β1 strand in rat prestin STAS containing the IVS, but considerably shortened compared with β1 in the crystal structure. Moreover, Psipred predicts the presence of the β1 strand in all human SLC26 STAS domains modified to include comparable IVS deletions. However, without computational deletion of this IVS, only SLC26A1, SLC26A2, and SLC26A7 are predicted to retain the β1 strand, whereas SLC26A3, SLC26A4, SLC26A6, SLC26A8, SLC26A9, and SLC26A11 are predicted to lack the β1 strand.
In contrast, the secondary structure prediction program Yaspin suggests that the presence of this STAS β1 strand of prestin is IVS-independent in rat prestin and in some other human SLC26 polypeptide paralogs. However, Yaspin also predicts the absence of the β1 strand structure from the STAS domains of human SLC26A1, SLC26A3, SLC26A4, and SLC26A11, as well as from the STAS domains of Arabidopsis thaliana sulfate transporters Sultr2.1 and Sultr2.2. Thus, the β1 strand may not be an essential part of all STAS domain structures, and its presence in some SLC26 gene products may be influenced by the presence or absence of the IVS sequence.
Residues Pro543, Tyr545, Tyr546, and Val655 of the rat prestin STAS domain have been proposed to constitute a juxtamembrane binding surface. The corresponding Rv1739c STAS residues Pro24, Cys26, Phe27, and Thr64 are similarly oriented. Such an interaction could be with the lipid bilayer or, as proposed for Synechococcus BicA, with a cytoplasmic loop of the SulP transmembrane domain.
Rv1739c STAS Is a Nucleotide-binding Protein
Photoactivated 8-N3-biotinyl-GTP labeled Rv1739c STAS, and labeling was blocked in the presence of excess GTP (Fig. 4A). We monitored the intrinsic fluorescence of Rv1739c STAS as a function of added nucleotide rather than directly monitoring fluorophor-labeled nucleotides, because these latter experiments often exhibit unique, fluorophor-related changes in ligand binding affinity and/or hydrolytic activity that are specific to individual GTP-binding proteins (49). Both GTP and GDP quenched the intrinsic fluorescence of Rv1739c STAS (Fig. 4), consistent with nucleotide binding directly or indirectly altering the electrostatic environments of residues Trp50 and/or Tyr7, Tyr19, or Tyr21. The K½ for GDP binding was 146 μm, about half the value for GTP. K½ values for both GDP and GTP were indistinguishable from those of their respective thioester analogs. The K½ for binding of ATP was ∼2-fold higher than for GTP (supplemental Fig. S4F), a property shared with SpoIIAA (22). The presence of 1 mm Mg2+ reduced binding affinity 2-fold for GTP and ∼4-fold for GDP (Table 2), as noted for the STAS domain of B. subtilis photosensor YtvA (48). These data are the first to demonstrate guanine nucleotide binding to a SulP transporter STAS domain. The low guanine nucleotide binding affinities of Rv1739c STAS are within the range of previously reported GTP binding constants as high as 1.1 mm (supplemental Table S1), but contrast with the GTP K½ of 0.25 μm reported for B. subtilis SpoIIAA (22).
Rv1739c STAS Nucleotide Binding Site(s)
NMR CSP studies (Fig. 5 and supplemental Fig. S6) suggest a semi-contiguous surface in Rv1739c STAS involved in binding GDP and/or GTP (Fig. 6). Among the Rv1739c STAS residues perturbed by >0.05 ppm in the presence of nucleotides, Val41 and Val61 (and Val48 to a lesser degree) are hydrophobic residues conserved in other anti-σ factor antagonists and in STAS domains (Fig. 3D). In silico docking of STAS with GDP and GTP (supplemental Fig. S7) supported involvement of residues perturbed in the CSP studies (Figs. 5 and 6, and supplemental Fig. S6). Similar to the NMR CSP data, 7 of the 9 calculated poses in the presence of GDP support binding to an interface that involves residues localized within the C-terminal region of the STAS domain, including aa Gly106–Ile110 (Fig. 6C and supplemental S6D). The CSP data suggest (with support from the docked poses) that the C-terminal binding site is perturbed to a greater degree by GDP than by GTP (Figs. 5 and 6, and supplemental Fig. S6, C and D). GTP docking involved only a subset of residues at this interface, as well as additional residues of the STAS N-terminal region (supplemental Fig. S7A). Some of the nucleotide-perturbed residues, including those of the N-terminal region, Val48 and Val61, correspond to regions in anti-σ factor antagonist TM1081, which exhibit millisecond scale conformational changes in solution but not in the crystal, suggesting their involvement in binding or catalytic activity (27).
The CSP data and docking calculations suggest two possible nucleotide binding sites in the presence of 20 mm nucleotide, whereas intrinsic fluorescence quench data at 30–100-fold lower nucleotide concentrations are compatible with a single binding site (Table 2). The single binding site predicted from fluorescence quench measurements may be one of the two sites suggested by CSP and docking calculations, with recruitment of the second site only at much higher ligand concentrations. Alternatively, both sites may bind nucleotide in the physiological concentration range, with only one of the sites influencing intrinsic fluorescence. The positioning of the Tyr and Trp residues in Rv1739c STAS suggests the N-terminal site could be that site.
GTPase Activity Associated with Rv1739c STAS
Anti-σ factor antagonists differ in their nucleotide hydrolase activities. Whereas SpoIIAA hydrolyzed GTP (22), YtvA had undetectable GTP hydrolase activity (47, 50). Rv1739c STAS-associated GTPase activity was detectable as slow inorganic [32P]phosphate release from [γ-32P]GTP (supplemental Fig. S5) in the nominal absence of Mg2+. The hydrolysis rate was not increased upon addition of 1 mm Mg2+. Rv1739c STAS-associated GTPase activity was only ∼8-fold lower than that of RhoA-GST in the same conditions. The low rates thus likely reflect low input substrate concentrations, because measured Rv1739c STAS GTPase activity was orders of magnitude slower than that reported for SpoIIAA in the presence of the ratio [GTP]/K½(GTP) = 30 (22). However, Rv1739c STAS-associated subsaturated GTPase activity was only ∼20–50-fold lower than the GTP-saturated GTPase rates of Saccharomyces cerevisiae Ypt6 (51, 52) and human Rheb (49). Thus, Rv1739c STAS-associated GTPase activity was near the low-end of the reported GTPase rates. Nonetheless, the presence of copurifying contaminant GTPase(s) in our Rv1739c STAS preparation remains a potential explanation.
Possible Physiological Roles of GTP Binding by Rv1739c STAS
The GTP K½ value within the intracellular range of [GTP] suggests a physiological role for nucleotide binding by Rv1739c STAS. Nucleotide binding might regulate Rv1739c localization or anion transport that is mediated by or associated with Rv1739c function, directly or through alteration of protein-protein interaction.
Rv1739c STAS, unlike SpoIIAA, was not detectably phosphorylated (supplemental Fig. S8). The mechanistic role of SpoIIAA phosphorylation remains unclear, because substitution of this serine residue by acidic residues did not phenocopy the activity of serine phosphorylation (24, 25, 27). The STAS domains of human SLC26A3 (29) and human SLC26A9 (53) interact in vitro with the phosphorylated R domain of CFTR, but the reported regulatory interactions of SLC26 polypeptides and CFTR in vitro and in vivo have varied. PKC phosphorylation of the human SLC26A6 STAS domain has been suggested to regulate Cl−/HCO3− exchange activity through CAII binding (54), but this phosphorylation did not regulate oxalate transport (55), and its relationship to CFTR is unknown. Although the R domain of CFTR is not shared among other ABC proteins, Rv1739c might function as a regulator of the M. tuberculosis ABC family sulfate permease CysTWA (15). E. coli ychM STAS domain has been shown to interact with subunits of other ABC family permeases (18).
M. bovis cysA mutants lack sulfate uptake in resting cultures (56), yet cysA and subI sulfate uptake mutants of Mycobacterium bovis survive in mice as well as wild type M. bovis (57). Thus, under the stressful conditions inside acidifying compartments of cells, mycobacteria might rely on stress-regulated expression of SulP Rv1739c. Indeed, Rv1739c is among the sulfate transport and metabolism genes up-regulated in the dormant phase of infection in cultured macrophages and in granulomas of intact mice (58). Similarly, the STAS domain-containing SulP protein ychM of E. coli is also up-regulated in stress conditions of RpoN (σ54) deletion (59) and nitrofurantoin treatment (60), and severely down-regulated in a virulence-impaired E. coli O157:H7 strain with deletion of the conserved, alternative σ factor and stress regulator RpoS (61). The recent discoveries that E. coli ychM binds to acyl carrier protein, an abundant, small polypeptide with many additional interaction partners (62), and that its deletion influences multiple gene products that regulate fatty acid biosynthesis (18), add new complexity to these questions. Furthermore, stress regulation (as nutrient supply and redox potential) has been proposed to regulate, on a cyclical basis, the direct interaction of A. thaliana SulP sulfate transporter Sultr1;2 with the cysteine synthase, O-acetylserine (thiol) lyase (63). A STAS-related or STAS-mediated guanine nucleotide cycle could potentially regulate the proposed cycle of binding and unbinding. Thus, the STAS domain of SulP anion transporters may sense environmental or metabolic stress through nucleotide binding, exchange, and/or hydrolysis to modulate anion transport and/or scaffolding of signaling proteins or enzymes as part of the regulation of cell metabolism and other functions.
Supplementary Material
Acknowledgments
We thank Dr. John F. Heneghan (Beth Israel Deaconess Medical Center) and Drs. James Sudmeier and Gillian Henry (Tufts University Biological NMR Center) for helpful discussion. All NMR experiments were performed at the Tufts University Biological NMR Center.
This work was supported by National Institutes of Health Grants R01 DK43495 (to S. L. A.), P30 DK34854 (Harvard Digestive Diseases Center to S. L. A.), and P01 AI68135 (TB Structural Genomics Consortium to T. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods,” Table S1, and Figs. S1–S8.
The atomic coordinates and structure factors (code 2KLN) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- STAS
- sulfate transporter and anti-σ factor antagonist
- SLC26
- solute carrier family 26
- SulP
- sulfate permease
- HSQC
- heteronuclear single quantum correlation
- CSP
- chemical shift perturbation
- IVS
- intrinsic variable sequence
- aa
- amino acid
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- GDPβS
- guanosine 5′-O-2-(thio)diphosphate
- PDB
- Protein Data Bank
- CFTR
- cystic fibrosis transmembrane conductance regulator.
REFERENCES
- 1. Sherman T., Chernova M. N., Clark J. S., Jiang L., Alper S. L., Nehrke K. (2005) Am. J. Physiol. Cell Physiol. 289, C341–C351 [DOI] [PubMed] [Google Scholar]
- 2. Felce J., Saier M. H., Jr. (2004) J. Mol. Microbiol. Biotechnol. 8, 169–176 [DOI] [PubMed] [Google Scholar]
- 3. Dorwart M. R., Shcheynikov N., Yang D., Muallem S. (2008) Physiology 23, 104–114 [DOI] [PubMed] [Google Scholar]
- 4. Hästbacka J., de la Chapelle A., Mahtani M. M., Clines G., Reeve-Daly M. P., Daly M., Hamilton B. A., Kusumi K., Trivedi B., Weaver A. (1994) Cell 78, 1073–1087 [DOI] [PubMed] [Google Scholar]
- 5. Höglund P., Haila S., Socha J., Tomaszewski L., Saarialho-Kere U., Karjalainen-Lindsberg M. L., Airola K., Holmberg C., de la Chapelle A., Kere J. (1996) Nat. Genet. 14, 316–319 [DOI] [PubMed] [Google Scholar]
- 6. Everett L. A., Glaser B., Beck J. C., Idol J. R., Buchs A., Heyman M., Adawi F., Hazani E., Nassir E., Baxevanis A. D., Sheffield V. C., Green E. D. (1997) Nat. Genet. 17, 411–422 [DOI] [PubMed] [Google Scholar]
- 7. Liu X. Z., Ouyang X. M., Xia X. J., Zheng J., Pandya A., Li F., Du L. L., Welch K. O., Petit C., Smith R. J., Webb B. T., Yan D., Arnos K. S., Corey D., Dallos P., Nance W. E., Chen Z. Y. (2003) Hum. Mol. Genet. 12, 1155–1162 [DOI] [PubMed] [Google Scholar]
- 8. Jiang Z., Asplin J. R., Evan A. P., Rajendran V. M., Velazquez H., Nottoli T. P., Binder H. J., Aronson P. S. (2006) Nat. Genet. 38, 474–478 [DOI] [PubMed] [Google Scholar]
- 9. Dawson P. A., Russell C. S., Lee S., McLeay S. C., van Dongen J. M., Cowley D. M., Clarke L. A., Markovich D. (2010) J. Clin. Invest. 120, 706–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu J., Song P., Nakamura S., Miller M., Barone S., Alper S. L., Riederer B., Bonhagen J., Arend L. J., Amlal H., Seidler U., Soleimani M. (2009) J. Biol. Chem. 284, 29470–29479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu J., Song P., Miller M. L., Borgese F., Barone S., Riederer B., Wang Z., Alper S. L., Forte J. G., Shull G. E., Ehrenfeld J., Seidler U., Soleimani M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17955–17960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tuo B., Riederer B., Wang Z., Colledge W. H., Soleimani M., Seidler U. (2006) Gastroenterology 130, 349–358 [DOI] [PubMed] [Google Scholar]
- 13. Wang Y., Soyombo A. A., Shcheynikov N., Zeng W., Dorwart M., Marino C. R., Thomas P. J., Muallem S. (2006) EMBO J. 25, 5049–5057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishiguro H., Namkung W., Yamamoto A., Wang Z., Worrell R. T., Xu J., Lee M. G., Soleimani M. (2007) Am. J. Physiol. Gastrointest. Liver Physiol. 292, G447–455 [DOI] [PubMed] [Google Scholar]
- 15. Zolotarev A. S., Unnikrishnan M., Shmukler B. E., Clark J. S., Vandorpe D. H., Grigorieff N., Rubin E. J., Alper S. L. (2008) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 149, 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Price G. D., Woodger F. J., Badger M. R., Howitt S. M., Tucker L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 18228–18233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shelden M. C., Howitt S. M., Price G. D. (2010) Mol. Membr. Biol. 27, 12–23 [DOI] [PubMed] [Google Scholar]
- 18. Babu M., Greenblatt J. F., Emili A., Strynadka N. C., Reithmeier R. A., Moraes T. F. (2010) Structure 18, 1450–1462 [DOI] [PubMed] [Google Scholar]
- 19. Aravind L., Koonin E. V. (2000) Curr. Biol. 10, R53–55 [DOI] [PubMed] [Google Scholar]
- 20. Clarkson J., Campbell I. D., Yudkin M. D. (2003) Biochem. J. 372, 113–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masuda S., Murakami K. S., Wang S., Anders Olson C., Donigian J., Leon F., Darst S. A., Campbell E. A. (2004) J. Mol. Biol. 340, 941–956 [DOI] [PubMed] [Google Scholar]
- 22. Najafi S. M., Harris D. A., Yudkin M. D. (1996) J. Bacteriol. 178, 6632–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kuo S., Demeler B., Haldenwang W. G. (2008) J. Bacteriol. 190, 6625–6635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kovacs H., Comfort D., Lord M., Campbell I. D., Yudkin M. D. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 5067–5071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Seavers P. R., Lewis R. J., Brannigan J. A., Verschueren K. H., Murshudov G. N., Wilkinson A. J. (2001) Structure 9, 605–614 [DOI] [PubMed] [Google Scholar]
- 26. Etezady-Esfarjani T., Placzek W. J., Herrmann T., Wüthrich K. (2006) Magn. Reson. Chem. 44, S61–70 [DOI] [PubMed] [Google Scholar]
- 27. Serrano P., Pedrini B., Geralt M., Jaudzems K., Mohanty B., Horst R., Herrmann T., Elsliger M. A., Wilson I. A., Wüthrich K. (2010) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 66, 1393–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quin M., Newman J., Firbank S., Lewis R. J., Marles-Wright J. (2008) Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 64, 196–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dorwart M. R., Shcheynikov N., Baker J. M., Forman-Kay J. D., Muallem S., Thomas P. J. (2008) J. Biol. Chem. 283, 8711–8722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma A. K., Ye L., Zolotarev A. S., Alper S. L., Rigby A. C. (2009) Biomol. NMR Assign. 3, 99–102 [DOI] [PubMed] [Google Scholar]
- 31. Pasqualetto E., Aiello R., Gesiot L., Bonetto G., Bellanda M., Battistutta R. (2010) J. Mol. Biol. 400, 448–462 [DOI] [PubMed] [Google Scholar]
- 32. Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 33. Kraulis P. J., Domaille P. J., Campbell-Burk S. L., Van Aken T., Laue E. D. (1994) Biochemistry 33, 3515–3531 [DOI] [PubMed] [Google Scholar]
- 34. Cavanagh J., Fairbrother W. J., Palmer A. G., Skelton N. J. (1996) Protein NMR Spectroscopy: Principles and Practice, Academic Press Inc., San Diego, CA [Google Scholar]
- 35. Bax A., Grzesiek S. (1993) Acc. Chem. Res. 26, 131–138 [Google Scholar]
- 36. Muhandiram D. R., Farrow N. A., Xu G. Y., Smallcombe S., Kay L. E. (1993) J. Magn. Reson. Ser. B 102, 317–321 [Google Scholar]
- 37. Güntert P., Mumenthaler C., Wüthrich K. (1997) J. Mol. Biol. 273, 283–298 [DOI] [PubMed] [Google Scholar]
- 38. Cornilescu G., Delaglio F., Bax A. (1999) J. Biomol. NMR 13, 289–302 [DOI] [PubMed] [Google Scholar]
- 39. Sharma A. K., Sharma S. K., Surolia A., Surolia N., Sarma S. P. (2006) Biochemistry 45, 6904–6916 [DOI] [PubMed] [Google Scholar]
- 40. Linge J. P., Williams M. A., Spronk C. A., Bonvin A. M., Nilges M. (2003) Proteins 50, 496–506 [DOI] [PubMed] [Google Scholar]
- 41. Laskowski R. A., Rullmannn J. A., MacArthur M. W., Kaptein R., Thornton J. M. (1996) J. Biomol. NMR 8, 477–486 [DOI] [PubMed] [Google Scholar]
- 42. Koradi R., Billeter M., Wuthrich K. (1996) J. Mol. Graph. 14, 51–55, 29–32 [DOI] [PubMed] [Google Scholar]
- 43. Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 44. Trott O., Olson A. J. (2010) J. Comput. Chem. 31, 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rao A., Martin P., Reithmeier R. A., Cantley L. C. (1979) Biochemistry 18, 4505–4516 [DOI] [PubMed] [Google Scholar]
- 46. Greenstein A. E., MacGurn J. A., Baer C. E., Falick A. M., Cox J. S., Alber T. (2007) PLoS Pathog. 3, e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buttani V., Losi A., Polverini E., Gärtner W. (2006) FEBS Lett. 580, 3818–3822 [DOI] [PubMed] [Google Scholar]
- 48. Tang Y., Cao Z., Livoti E., Krauss U., Jaeger K. E., Gärtner W., Losi A. (2010) Photochem. Photobiol. Sci. 9, 47–56 [DOI] [PubMed] [Google Scholar]
- 49. Mazhab-Jafari M. T., Marshall C. B., Smith M., Gasmi-Seabrook G. M., Stambolic V., Rottapel R., Neel B. G., Ikura M. (2010) J. Biol. Chem. 285, 5132–5136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Avila-Pérez M., Vreede J., Tang Y., Bende O., Losi A., Gärtner W., Hellingwerf K. (2009) J. Biol. Chem. 284, 24958–24964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bergbrede T., Chuky N., Schoebel S., Blankenfeldt W., Geyer M., Fuchs E., Goody R. S., Barr F., Alexandrov K. (2009) J. Biol. Chem. 284, 2628–2635 [DOI] [PubMed] [Google Scholar]
- 52. Bergbrede T., Pylypenko O., Rak A., Alexandrov K. (2005) J. Struct. Biol. 152, 235–238 [DOI] [PubMed] [Google Scholar]
- 53. Chang M. H., Plata C., Sindic A., Ranatunga W. K., Chen A. P., Zandi-Nejad K., Chan K. W., Thompson J., Mount D. B., Romero M. F. (2009) J. Biol. Chem. 284, 28306–28318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alvarez B. V., Vilas G. L., Casey J. R. (2005) EMBO J. 24, 2499–2511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hassan H. A., Mentone S., Karniski L. P., Rajendran V. M., Aronson P. S. (2007) Am. J. Physiol. Cell Physiol. 292, C1485–1492 [DOI] [PubMed] [Google Scholar]
- 56. Wooff E., Michell S. L., Gordon S. V., Chambers M. A., Bardarov S., Jacobs W. R., Jr., Hewinson R. G., Wheeler P. R. (2002) Mol. Microbiol 43, 653–663 [DOI] [PubMed] [Google Scholar]
- 57. Niederweis M. (2008) Microbiology 154, 679–692 [DOI] [PubMed] [Google Scholar]
- 58. Murphy D. J., Brown J. R. (2007) BMC Infect. Dis. 7, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Riordan J. T., Tietjen J. A., Walsh C. W., Gustafson J. E., Whittam T. S. (2010) Microbiology 156, 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu A., Tran L., Becket E., Lee K., Chinn L., Park E., Tran K., Miller J. H. (2010) Antimicrob. Agents Chemother. 54, 1393–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dong T., Schellhorn H. E. (2009) BMC Genomics 10, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Byers D. M., Gong H. (2007) Biochem. Cell Biol. 85, 649–662 [DOI] [PubMed] [Google Scholar]
- 63. Shibagaki N., Grossman A. R. (2010) J. Biol. Chem. 285, 25094–25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.