Abstract
The carotene cis-trans isomerase CRTISO is a constituent of the carotene desaturation pathway as evolved in cyanobacteria and prevailing in plants, in which a tetra-cis-lycopene species, termed prolycopene, is formed. CRTISO, an evolutionary descendant of the bacterial carotene desaturase CRTI, catalyzes the cis-to-trans isomerization reactions leading to all-trans-lycopene, the substrate for the subsequent lycopene cyclization to form all-trans-α/β-carotene. CRTISO and CRTI share a dinucleotide binding motif at the N terminus. Here we report that this site is occupied by FAD in CRTISO. The reduced form of this cofactor catalyzes a reaction not involving net redox changes. Results obtained with C(1)- and C(5)-deaza-FAD suggest mechanistic similarities with type II isopentenyl diphosphate: dimethylallyl diphosphate isomerase (IDI-2). CRTISO, together with lycopene cyclase CRTY and IDI-2, thus represents the third enzyme in isoprenoid metabolism belonging to the class of non-redox enzymes depending on reduced flavin for activity. The regional specificity and the kinetics of the isomerization reaction were investigated in vitro using purified enzyme and biphasic liposome-based systems carrying specific cis-configured lycopene species as substrates. The reaction proceeded from cis to trans, recognizing half-sides of the symmetrical prolycopene and was accompanied by one trans-to-cis isomerization step specific for the C(5)-C(6) double bond. Rice lycopene β-cyclase (OsLCY-b), when additionally introduced into the biphasic in vitro system used, was found to be stereospecific for all-trans-lycopene and allowed the CRTISO reaction to proceed toward completion by modifying the thermodynamics of the overall reaction.
Keywords: Carotenoid, Flavoproteins, Isoprenoid, Membrane Enzymes, Plant, Lycopene
Introduction
Carotenoids belong to a large isoprenoid family, some members of which are indispensible in all photosynthetic organisms; however, they can also be formed by some heterotrophic bacteria and fungi. In photosynthesis, carotenoids play an important role in light harvesting and in the protection of cells from photo damage caused by reactive oxygen species. In addition, some carotenoids are precursors of phytohormones such as abscisic acid (1) and the strigolactones (2, 3). In human nutrition, carotenoids containing at least one unsubstituted β-ionone ring serve as provitamin A carotenoids (for review, see Ref. 4). In recent years, a wealth of molecular information on carotenogenesis in plants and microorganisms has become available (see Refs. 5, 6 for reviews on plant carotenogenesis), however, information on the enzymology and biochemistry of the involved reactions is comparatively scarce.
In the carotenoid biosynthetic pathway, phytoene is the first carotene formed. Phytoene is a colorless C40 triene prenyl hydrocarbon, which undergoes four desaturation steps during which double bonds are introduced to finally form the fully conjugated undecaene chromophore of the red-colored lycopene (Fig. 1). There are two divergent classes of carotene desaturases catalyzing the formation of the identical product (all-trans-lycopene) from the same precursor (15-cis-phytoene). The CRTI-type carotene desaturases present in archaea, bacteria, and fungi are capable of catalyzing the entire reaction sequence toward trans-lycopene (and beyond in some cases, forming di- and tetra-dehydrolycopene), additionally mediating a single cis-to-trans isomerization step at the central C15-C15′ double bond. Although the reaction mechanism of CRTI-type desaturases has not been resolved, all currently available evidence suggests that no additional protein components are required. This is contrasted by the complex carotene desaturation system in cyanobacteria and plants, where at least four gene products are required to achieve the formation of all-trans-lycopene (Fig. 1). In plants, these are the two carotene desaturases named phytoene desaturase (PDS)2 and ζ-carotene desaturase (ZDS) which introduce two double bonds, each. The PDS and ZDS polypeptides share 27% amino acid sequence identity and 41% similarity but both are unrelated to CRTI (7, 8). In addition, PDS is a constituent of more or less extended redox chain and requires additional protein and non-protein components (9 - 12). The plant desaturase system is complemented by two cis-trans isomerases, represented by Z-ISO (13) and the carotene cis-trans isomerase CRTISO, which is the subject of this work. CRTISO isomerizes all cis double bonds formed by the action of PDS and ZDS (14) yielding 7,9,9′,7′-tetra-cis-lycopene3 (commonly referred to as prolycopene; 15) to finally form all-trans-lycopene. The latter is a substrate for the subsequent introduction of cyclic β- and/or ϵ-ionone end groups to form α- and β-carotene through the catalysis of the respective lycopene cyclases. It is interesting to note that CRTISO shares sequence homology with CRTI, as discussed above. Hence, it appears plausible that CRTISO originated from CRTI (7, 16, 17).
FIGURE 1.
Carotene desaturation and isomerization reactions. The reaction sequence starts with 15-cis-phytoene. With bacterial CRTI, a direct conversion into all-trans-lycopene occurs. This involves four desaturation reactions corresponding to 8 e− equivalents (right hand side) and one isomerization reaction at the C15-C15′ double bond. In the case of cyanobacteria and plants, PDS carries out 2 desaturation steps (4 e−) to generate the 9,15,9′-tri-cis-ζ-carotene shown. Z-ISO then catalyzes the isomerization of the central double bond to form 9,9′-di-cis-ζ-carotene. Subsequently, the desaturase (ZDS), forms two cis double bonds at the 7 and 7′ positions (4 e−) to yield 7,9,9′,7′-tetra-cis-lycopene. CRTISO isomerizes 7,9,9′-tri-cis-neurosporene (proneurosporene, not shown) and 7,9,9′,7′- tetra-cis-lycopene (prolycopene) into the all-trans form. LCY-b/e: lycopene beta/epsilon-cyclase; CRTY: bacterial lycopene beta-cyclase. CRTISO and CRTI are given in bold to indicate their homology.
One feature of CRTISO, which it shares with carotene desaturases of both types, is the presence of a GXGXXG-bearing dinucleotide-binding motif in proximity of the N terminus of the polypeptide (18). Whereas the presence of a redox-active cofactor is a necessity in desaturases catalyzing net electron transfer, such a requirement is not evident for the cis-trans isomerase CRTISO, because the reaction it catalyzes does not involve redox changes. A cryptic electron transfer might be envisaged to occur transiently. However, in the absence of information on the nature of the cofactor mechanistic considerations are just speculative. Earlier attempts to shed light on the reaction mechanism showed CRTISO to be dependent on dialyzable cytoplasmatic components as well as on plasma membranes of Escherichia coli. Furthermore, CRTISO was shown to depend on the redox state of the respiratory chain. In particular, the isomerization reaction accelerated in the presence of respiratory chain substrates indicating a requirement for reducing conditions (19).
Exactly such conditions were recently found to be required for lycopene cyclization to proceed. This conversion, as catalyzed by the bacterial CRTY, can also be viewed as a carotene isomerization reaction. Therefore, the lines of thinking developed for CRTY might also apply for CRTISO: It was shown that lycopene cyclization depended on reduced FAD involved in the stabilization of a transition state. A specific glutamate was also needed that is believed to be involved in acid/base catalysis (20, 21). Based on this, it was suggested that the sole function of respiratory chain activities in vitro was to ensure reducing conditions (anaerobiosis), i.e. to prevent (re)oxidation of FADred (20).
Such evident similarities in the reaction conditions required by both, CRTISO and CRTY, prompted the research on CRTISO presented in this report. We show that this enzyme is FADred-dependent and give details on the regional specificity and the kinetic properties of the cis-trans isomerization reaction.
EXPERIMENTAL PROCEDURES
Chemicals Used
Flavin cofactors modified at various positions have been detailed in a compiled report (22). It contains references to sources or synthetic methods and to methods for conversion of riboflavin analogs into the corresponding FAD derivatives as well as their redox potentials. Flavin cofactor analogs were purified by HPLC, and their identity was verified by UV/Vis spectroscopy and LC-MS as described (20). Ni2+-NTA-agarose resin for protein purification was from Qiagen. 2H2O was obtained from Euriso-top. 5-cis-lycopene, 5,5′di-cis-lycopene and 9-cis-β-carotene were from CaroteNature (Switzerland); all-trans-γ-carotene, was a gift from BASF (Germany). Prolycopene (7,9,9′,7′-tetra-cis-lycopene) was extracted with acetone from fruits of the tangerine mutant of tomato, being defective in CRTISO (16). All other fine chemicals employed were purchased from Sigma-Aldrich.
Construction of the CRTISO Expression Vector
The cDNA of CRTISO from tomato (16), from which the first 79 codons of the transit peptide were truncated, was inserted into the BamHI site behind a His6 tag, an 83 amino acids lipoyl domain and a TEV protease cleavage site (HLT domain) (23) in the plasmid vector pHis-Parallel2 (24). The resulting plasmid, termed pHLT-CRTISO, was transfected into E. coli cells of the strain BL21(DE3) harboring the plasmid pGro7 (Takara), which encodes the groES-groEL-chaperone system under the control of an arabinose-inducible promoter.
Expression and Purification of CRTISO
Bacteria transformed with pHLT-CRTISO were grown in 2*YT-medium under continuous shaking at 37 °C till an A600 of 0.6 and then induced by adding arabinose (8 mm) and IPTG (0.2 mm). The induction took place over night at 16 °C. Cultures were harvested and frozen at −20 °C for further use.
Protein purification was carried out on ice. Cell pellets were resuspended in buffer A (25 mm phosphate-sodium buffer, pH 6.2; MgCl2, 2.5 mm; NaCl, 300 mm; glycerol 15%, 1.5 ml buffer A/g cell mass) and disintegrated by two passages through a French Press Cell, operated at 18,000 psi. After the removal of cell debris by centrifugation at 17,000 × g for 15 min, the supernatant was solubilized with Tween 20 at a final concentration of 10× CMC (0.067%) for 30 min. The solubilized protein was then allowed to bind Ni2+-NTA-agarose (Qiagen) equilibrated with buffer A containing 0.067% Tween 20. The binding step was performed for 45 min under shaking at 37 rpm and followed by three washing steps with buffer A containing 0.02% Tween 20 and 4 mm imidazole. Elution of the bound protein was accomplished with buffer A containing 100 mm imidazole and 0.02% Tween 20. The preparation was used to record the UV/Vis spectra (Shimadzu, UV-2501PC) or dialyzed against buffer A to remove imidazole before freezing aliquots at −80 °C.
Protein concentrations were determined with the Bradford method. Protein purity was checked by SDS-PAGE using 10% polyacrylamide gels. Proteins were detected using Coomassie Brilliant Blue G250 (Sigma-Aldrich). For the identification of protein-bound cofactors, CRTISO was purified essentially following the procedures described above, however, glycerol and Tween 20 were omitted from the elution buffer. 10 mg of such protein was heat-denatured the protein removed by centrifugation. The supernatant was lyophilized and the residue dissolved in 50 μl of water of which 4 μl were used for LC-MS analysis.
Apo-CRTISO Preparation and Reconstitution
The CRTISO apo-enzyme was prepared according to published procedures (20) with some modifications. IMAC- purified protein (2 mg/ml) was dialyzed for 16 h at 4 °C against buffer A containing 2 m KBr. The KBr was removed by dialysis against buffer A and eventually occurring precipitates were removed by centrifugation at 21,000 × g for 15 min. Absence of cofactors was confirmed by UV/Vis spectroscopy, fluorescence measurement, and by the absence of enzymatic activity.
Cofactor reconstitution of the apo-protein was carried out under a N2 atmosphere. 100 μl of 200 μm flavin analogs were first reduced with 4 μl of freshly prepared 0.1 m Na-dithionite solution of which 50-μl aliquots were then transferred to 115 μl of buffer A to which 200 pmol of apo-enzyme was added (around 5 μl, MWCRTISO = 67 kDa). The mixtures were incubated at room temperature for 1 h; then the reaction was initiated by adding 30 μl of prolycopene liposomes which led to the final prolycopene concentration of 5 μm. Enzyme kinetic studies to determine the Km of FAD were performed analogously using an incubation time of 15 min. The final concentrations of FAD were 0.2, 0.25, 0.4, 1, 2, 5, and 10 μm.
Enzymatic Assays
Protein-free liposomes containing carotene substrates were prepared with soybean lecithin (Sigma-Aldrich) in buffer A according to previously published methods (20). The incorporation of carotenes into the lipid bilayer was assessed by extracting the carotenes from the liposomes formed with chloroform/methanol 2:1 (v/v) followed by spectrophotometric estimation (Shimadzu, UV-2501PC) using ϵ470 nm = 187,000 liters mol−1 cm−1 for all-trans-lycopene, ϵ470 nm = 184,000 liters mol−1 cm−1 for 5-cis- lycopene and ϵ439 nm = 105,000 liters mol−1 cm−1 for prolycopene. Additional molar extinction coefficients used were ϵ468 nm = 154,000 liters mol−1 cm−1 for 7-cis- lycopene, ϵ465 nm = 169,000 liters mol−1 cm−1 for 9-cis- lycopene, ϵ444 nm = 115,000 liters mol−1 cm−1 for 7,9-di-cis-lycopene. The extinction coefficient for 9-cis-lycopene was also used for the “unknown” peak (e) since they share similar spectra with a trans-like fine structure (25).
The standard CRTISO assay mixture (final volume 200 μl) consisted of 163 μl of buffer A which was supplemented with typically 30 μl of the substrate-containing liposome suspension to result in a final carotene concentration of 5 μm. The final concentration of FAD was 50 μm. CRTISO was added at a 1 μm final concentration typically added to the assay in 5 μl, leading to a final Tween 20 concentration of ∼0.0005% (1× CMC = 0.0067%). Reducing conditions were obtained by supplementing the assay with 2 μl of freshly prepared 0.1 m Na-dithionite solution. All solutions were equilibrated with N2 before use and the reactions carried out in a glove box under an N2 atmosphere in very dim daylight at 37 °C for 15 min, unless otherwise indicated. Time course experiments were performed under standard conditions. The reactions were terminated at various intervals by mixing with an equal volume of chloroform/methanol 2:1 (v/v).
Cyclization reactions of carotenes were carried out using lycopene β-cyclase from Oryza sativa (OsLCY-b). Because the reaction conditions of OsLCY-b are essentially identical with those of CRTY including anaerobosis (20), 5 μg of IMAC-purified OsLCY-b protein was added to the standard CRTISO assays.
For deuteration experiments, all buffers, the Na-dithionite and FAD stock solutions as well as the lycopene-liposome suspension were prepared, as described above, but in 2H2O. The protein, added in very small volume (around 2.5 vol %) was in H2O.
Extraction and Analytical Methods
Enzymatic assays were extracted twice with chloroform/methanol 2:1 (v/v). The organic phases containing substrate and products were combined and dried under reduced pressure. The residue was dissolved in chloroform and analyzed by HPLC-System 1 (Waters, Alliance 2695) on a C30 reversed phase column (YMC-Europe) as detailed earlier (20) or dissolved in hexane and analyzed by HPLC-System 2 (UFLC, Shimadzu, Prominence) using a direct phase column (Nucleosil 300–5, Macherey & Nagel) with dry hexane/N-ethyldiisopropylamine 2000:1 (v/v) as the mobile phase at a flow-rate of 1.5 ml/min (25). Equilibration of this direct phase system can take several days. Both HPLC systems were equipped with a photodiode array detector (PDA).
NADP(H), NAD(H), FAD, FMN, and flavin analogs were analyzed and purified using HPLC-System 3, and identified by LC-MS-MS using ESI ionization, as previously described (20).
Prolycopene was extracted from the “Tangerine” variety of tomato fruit with acetone. After partition against petroleum ether/diethyl ether 2:1 (v/v), the organic phase was dried. After dissolving in CHCl3, the extract was applied to a self-packed silica gel column, which was developed with n-hexane to isolate the hydrocarbon fraction. Prolycopene was then purified on a 3 μm C18 RP column (Hypersil Gold, Thermo-Fisher Scientific) using acetonitrile isocratically at a flow-rate of 1.4 ml/min (HPLC-System 4).
For LC-MS of carotenes in the deuteration experiment we utilized HPLC-System 5, as described earlier (20) coupled to a Thermo-Fisher Scientific LTQ instrument. Carotenes were separated on a C30 RP (Thermo-Fisher Scientific) column, APCI-ionized using N2 as reagent gas and analyzed in the positive ion mode. Separated carotenes were identified by their spectra, retention times in comparison with authentic references and by their quasi-molecular (M+1) ions.
Procedures Used for Kinetic Simulations
The time dependence of concentrations of lycopene species (a)–(g) was simulated using the global analysis program Specfit®, which solves the series of differential equations representing the reaction sequences shown in Fig. 8. Because the conditions of catalysis in the membrane system used cannot be defined, the simplifying assumption was made that the interaction constants for enzyme and the various lycopene species are similar. Consequently, steps that lead to the formation of the corresponding Michaelis-Menten complexes were not included in the simulation. The simulation/fitting procedure consists in a gradual approach in which values for the various steps are first fixed in a range compatible with the measured kobs values; the system then fits the experimental data representing the disappearance of species (a) in a first step. Subsequently, the rates of steps that affect the time dependent concentrations of single, specific species ((a)–(g) are optimized while the others are held “fixed”). Finally a fit of the data (curves) obtained from the simulation and of the experimental data points is done with the application KaleidaGraph using the same mono- or bi-exponential equation (of the type: y = A·e−k1t + B·e−k2t + C, where A and B are amplitudes and C the initial value). For more detailed information, refer to Gradinaru et al. (26).
FIGURE 8.
Stereospecific course of reactions catalyzed by CRTISO and specific rates of conversion. The numbers refer to the positions of the cis-double bonds in the lycopenes shown. (a): 7,9,7′,9′-tetra-cis-lycopene; (b): 7,9-di-cis-lycopene; (c): 9-cis-lycopene; (d): 7-cis-lycopene; (e): position of cis double bond(s) unknown; (f): 5-cis-lycopene; (g): all-trans-lycopene. (h): β-carotene; LCY-b: lycopene cyclase that introduces β-ionone ring groups into the all-trans-lycopene precursor (g). The k values at the arrows are the rate constants (min−1) for a given step as obtained from the simulation procedures. Steps identified by (?) represent possible, though probably not relevant reaction sequences. The ratios of forward/reverse steps are (a)→(b) = 2; (b) → (c) = 0.5; (b) → (d) = 0.2; (b) → (e) = 0.15; (b) → (f) = 40; (c) → (g) = 40; (d) → (g) = 60; (e) → (g) = 50; (f) → (g) = 100.
RESULTS
CRTISO Requires Anaerobic Conditions for Activity
The carotene cis-trans isomerase CRTISO was cloned from tomato (16) and expressed in E. coli in the form of a N-terminal His6 fusion. Differential centrifugation and IMAC produced a near homogeneous protein preparation which was used in the investigations (supplemental Fig. S1). Inspired by results obtained with two other isomerases involved in isoprenoid metabolism, namely type II isopentenyl diphosphate: dimethylallyl diphosphate isomerase (IDI-2; see Ref. 27 and citations therein) and lycopene β-cyclase (CRTY/LCY-b; 20, 21) both catalyzing FADred-dependent non-redox reactions, we hypothesized a similar mechanism operating with CRTISO. In fact, under a nitrogen atmosphere used to maintain FADred, the 7,9,9′,7′-tetra-cis-lycopene (prolycopene) substrate present in the assays embedded into phosphatidylcholine liposomal membranes was readily converted into mainly all-trans-lycopene at a high velocity. The reaction can be monitored visually by the ∼40 nm bathochromic shift of the UV/Vis spectrum upon cis-to-trans isomerization of prolycopene (Fig. 2, A and B). This reflects the enhanced electron delocalization in the system consisting of eleven double bonds (see structure in Fig. 1). Analysis of the reaction on a C30 RP column revealed all-trans-lycopene as the main lycopene species formed, accompanied by certain amounts of newly formed cis isomers (Fig. 2C). CRTISO activity did not exhibit a pH dependence in the range of 5.8–8.0.
FIGURE 2.
In vitro assay of CRTISO isomerization reaction with 7,9,9′,7′-tetra-cis-lycopene (prolycopene) as a substrate. A, CRTISO isomerizes yellow prolycopene into pink-colored products, shown in the liposome-containing assay (left) and after carotenoid extraction in organic solution (right). A 30-min assay (standard conditions) is shown. Con, control incubation in the absence of CRTISO. B, UV/Vis spectra (in hexane) of the extracted assays shown above. Showing the bathochromic shift of the spectra. C, HPLC separation of the assays shown above on 3 μm C30 RP column (HPLC-system 1). Prolycopene (peak (a)) was isomerized into mainly all-trans-lycopene (peak (g)) by CRTISO.
CRTISO Catalysis Requires Reduced FAD (FADred)
The IMAC-eluted protein had a very faint yellow color, suggesting the presence of some small amounts of protein-bound flavin, i.e. of holo-protein. The UV/Vis spectrum obtained with a concentrated protein sample shown in Fig. 3D (left panel) is compatible with the presence of a flavin, FAD or FMN in the oxidized state. Gel filtration removed bound cofactor beyond the detection limit. To reveal its nature, the IMAC-purified protein was heat-denatured and the resulting soluble fraction subjected to HPLC-MS-MS analysis, optimized to unequivocally identify dinucleotide cofactors based on retention time, UV/Vis spectra, molecular ions, and Single Reaction Ion Monitoring employing the specific MS2 daughter ions as the diagnostic tool (Fig. 3, A–C). Based on retention time and UV/Vis spectrum, FAD was the only detectable cofactor, while mass spectrometry revealed the additional presence of some FMN and NAD, however too small in amount to be photometrically detectable.
FIGURE 3.
Identification of cofactors bound to CRTISO. 10 mg of IMAC-purified CRTISO was heat-denatured. After centrifugation and concentration, 4 μl of supernatant was applied to LC (system 3)-MS-MS. A, the UV/VIS trace recorded at 450 nm and the corresponding spectrum of peak2 (D, right panel) demonstrates flavin presence. B, SRM, using the MS2 daughter ions (in brackets) previously identified with cofactor standards (shown in C) was employed to detect the presence of NAD+ (peak 1), FAD (peak 2), and FMN (peak 3). NAD+ was present in trace amounts only since it was undetectable in the UV. FMN was present in trace amounts as seen in the accompanying UV/Vis trace (A). No MS-SRM signal was observed for NADP+ (trace not shown). D, left: UV/Vis absorption spectrum of IMAC-purified and concentrated CRTISO.
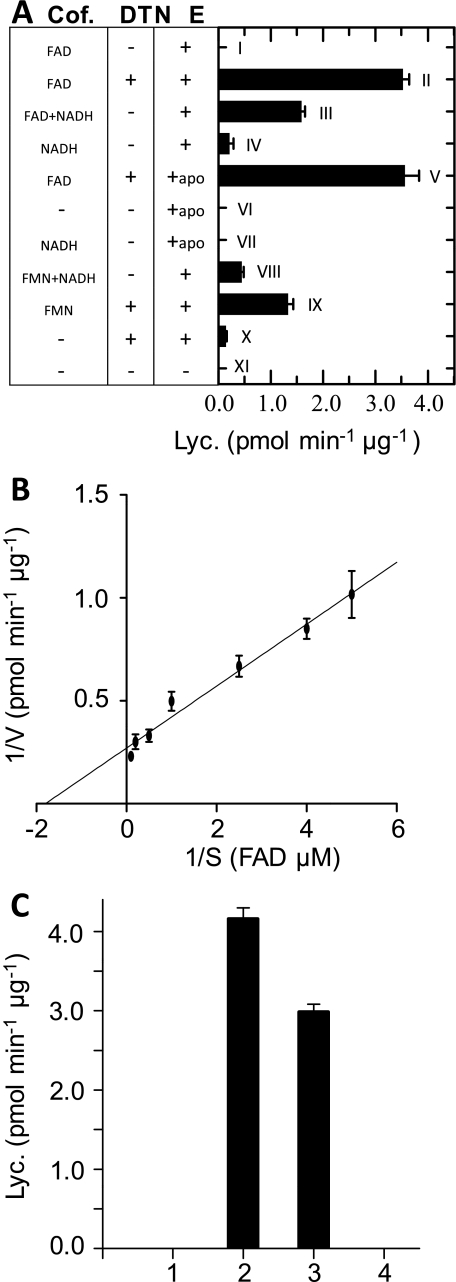
The nature of the effective cofactor, being either FAD or FMN, was further assessed with enzymatic assays. Both, IMAC-purified protein and apo-CRTISO were incubated in a N2 atmosphere with either of the cofactors and in the presence/absence of Na-dithionite (Fig. 4A). Whereas oxidized FAD (in the absence of the reductant) was ineffective (I), FADred was able to drive the reaction at a rate of 3.6 pmol min−1 μg−1 (II). NADH H+, at high (2 mm) concentrations was able to partially substitute for Na-dithionite as an FAD reductant (III), but did not support catalysis as the sole cofactor (IV). In our interpretation, this residual activity is due to an NADH dependent reduction of a small fraction of the enzyme that contains FAD (holo-protein). In agreement with this, IMAC-purified CRTISO that was subjected to KBr-treatment to remove all FAD (apo-protein) had no activity. Subsequent addition of reduced FAD led to CRTISO reconstitution (V) as shown by specific conversion rates similar to those obtained in (II). No reactivation of the apo-protein was obtained in the absence of cofactors (VI) and in the presence of NADH H+ as the sole cofactor (VII). Moreover, IMAC-purified protein exhibited only little activity in the presence of reductant when free FAD was not added (X). FMNred was able to substitute for FADred but was about 60% less effective (IX). As with FAD, reduction of FMN was possible in the presence of high concentrations of NADH H+ (VIII). In conclusion, FADred appears to be the cofactor of choice while FMNred is only partially active. Under the conditions detailed under “Experimental Procedures,” the Km for FADred was ≈ 0.55 μm (Fig. 4B).
FIGURE 4.
A, cofactor requirement of CRTISO. Enzymatic assays were carried out under an N2 atmosphere with 200 pmol of purified CRTISO (E) and prolycopene liposome (final concentration of prolycopene, 5 μm) in 200 μl. The assays were incubated for 15 min at 37 °C. I. 100 μm FAD + IMAC-purified CRTISO; II. 100 μm FAD + Na-dithionite + IMAC-purified CRTISO; III. 100 μm FAD + 2 mm NADH + IMAC-purified CRTISO; IV. 2 mm NADH + IMAC-purified CRTISO; V. 100 μm FAD + dithionite + apo-CRTISO; VI. apo-CRTISO; VII. 2 mm NADH + apo-CRISO; VIII. 100 μm FMN + 2 mm NADH + IMAC-purified CRTISO; IX. 100 μm FMN + dithionite + IMAC-purified CRTISO; X. IMAC-purified CRTISO + dithionite; XI. Control, no CRTISO and cofactors added. B, Lineweaver-Burk plot for FAD (0.2, 0.25, 0.4, 1, 2, 5, 10 μm) carried out with apo-CRTISO. Reflavinylation of CRTISO was allowed to take place at room temperature for 1 h (y = 0.15x + 0.27; Km ≈555 nm). C, reconstitution of apo-CRTISO with deaza-flavins. The assays were performed according to the standard procedures given under “Experimental Procedures.” 1. apo-CRTISO; 2. apo-CRTISO + FADred; 3. apo-CRTISO + C(1)-deaza-FADred; 4. apo-CRTISO + C(5)-deaza-FADred. Lyc.: lycopene, DTN: Na-dithionite.
Flavin Analogs Used as Mechanistic Probes
Flavin analogs are instrumental for the study of mechanism of flavin-dependent catalysis. In the present work we used C(5)-deaza- and C(1)-deaza-FAD to discriminate between 1e− and 2e− redox chemistry (28). Using C(1)-deaza-FADred, a conversion rate of 3 pmol min−1 μg−1 was found while the system was fully inactive with C(5)-deaza-FADred (Fig. 4C).
A series of FAD analogs substituted at position C(8) was then used to assess a possible dependence of the conversion rate on the flavin redox potential (Linear Free Energy Relationship, LFER, Ref. 29) in analogy to the study carried out recently with bacterial lycopene cyclase CRTY (20). With C(8)-O-CH3, C(8)-CH3 (FAD), C(8)-Cl, and C(8)-SO2-CH3 (in decreasing order of their midpoint potential; see supplemental Fig. S2 for structures) conversion rates of 2.94 ± 0.23, 3.62 ± 0.31, 4.16 ± 0.28, and 0.3 ± 0.08 pmol min−1 μg−1 (mean ± S.E.), respectively, were found. Thus, in contrast to the case of CRTY, a LFER relationship was not found (see “Discussion”).
The mechanism of isomerization reactions relying on acid-base catalysis can be identified by carrying out the reaction in labeled solvent, when the sites of proton attack and abstraction are not identical. The lycopene cyclase CRTY is an example (see Ref. 20 and citations therein). Prolycopene isomerization experiments were therefore carried out in 2H2O otherwise maintaining the standard assay conditions. Analysis of the reaction products by LC-MS showed the absence of mass gain, i.e. of deuterium incorporation (supplemental Fig. S3).
Characterization of the Catalytic cis-to-trans Isomerization Sequences
Assigning regional specificities of cis-trans isomerization is not trivial with lycopene isolated in small amounts from biological samples because NMR is the only applicable methodology to elucidate the resulting structures. Only few lycopene cis isomers possess unique UV/Vis spectral features such as prolycopene (Fig. 2B) or central cis isomers such as 13-cis or 15-cis-lycopene exhibiting a strong “cis-peak” at 360 nm. We therefore resorted to work published by Hengartner et al. (25) describing the chemical synthesis and NMR-spectroscopic characterization of a series of specific lycopene cis isomers, some of which represent members of the eight possible intermediates existing between prolycopene (7,9,9′,7′-tetra-cis-lycopene (a); Fig. 8) and all-trans-lycopene (g), the species with the lowest ground state of energy. Where applicable, the authors provided to us the corresponding unpublished original UV spectra of this work (supplemental Fig. S4). In addition, we adopted the direct phase HPLC system they used (HPLC-System 2) which allowed structural assignments by comparison of the elution order and time normalized relative to the elution time of all-trans-lycopene. Using this HPLC system, five isomers were resolved which were formed enzymatically at the expense of prolycopene (peak (a) in Fig. 5, A and B). The main product formed was all-trans-lycopene (peak (g)) accompanied by smaller amounts of 5-cis-lycopene (peak (f), both identified by internal standardization with authentic references). Following the logic of an ordered cis-to-trans isomerization reaction, the formation of 5-cis-lycopene (f) is somewhat unexpected since this double bond is trans-configured in prolycopene. Peak (b) unequivocally, represents, 7,9-di-cis-lycopene, in which only one half-side of the symmetrical prolycopene molecule is isomerized to trans. The C(7) and C(9) mono-cis species (c, d) eluted at their expected position and showed absorption maxima as well as spectral fine structures that matched the reference spectra (for UV/Vis reference spectra and spectra observed see supplemental Figs. S4 and S5, respectively). One additional lycopene species (e) was enzymatically formed but could not be structurally assigned.
FIGURE 5.
Time course of the CRTISO reaction and identification of intermediates. A, separation of lycopene isomers formed in a 15-min CRTISO standard reaction (direct phase HPLC-system 2). Arrows indicate the expected position of the di-cis isomers given (as explained in the text). Asterisks denote cis isomers that were artifactually formed. Letters denote enzymatic products as given in Fig. 8. (a), 7,9,9′,7′-tetra-cis-lycopene (prolycopene); (b) 7,9-di-cis-lycopene; (c) 9-cis-lycopene; (d) 7-cis-lycopene; (e) unidentified cis-lycopene; (f) 5-cis-lycopene; (g) all-trans-lycopene. See supplemental Fig. S5 for UV/VIS spectra. B, chromatographic runs of a time course (HPLC-system 2). C and D, time courses of species involved in prolycopene isomerization under standard assay conditions. C, disappearance of the substrate and formation of the final product; D, time courses of intermediate lycopene species (for structures see Fig. 8); note the different scales of the y-axes. The symbols denote experimental data points and are the average of two independent measurements, bars represent data scatter. The lines through the data points are fits. E, symbols and color codes are as in C and D. The lines through the data points are the results of the simulations described in the text. Data points for (c) and (e) have very low values between 0 and 2%, the corresponding profiles are similar to those shown in Fig. 5D; they are not shown for clarity. See Table 1 for kobs values.
It is relevant to note that some of the expected lycopene cis-species were not formed (supplemental Fig. S6). These were the symmetrical di-cis-lycopenes (9,9′-di-cis-lycopene and 7,7′-di-cis-lycopene). Similarly, the identified 5-cis-lycopene (f) was not accompanied by the respective di-cis species (arrows in Fig. 5A indicate their expected elution). In addition, there was no indication for the occurrence of tri-cis-lycopenes, i.e. 7,9,9′-tri-cis-lycopene and 7,7′9′-tri-cis-lycopene.
Kinetic Course of Chemical Transformations Catalyzed by CRTISO
The time courses of the formation and disappearance of species occurring upon incubation of 7,9,9′,7′-tetra-cis-lycopene with CRTISO was analyzed as shown in Fig. 5, C and D. The disappearance of prolycopene (a), the appearances of the intermediates (e, f) and of the final product all-trans-lycopene (g) were analyzed using a monoexponential fitting routine; the appearance and decay of the intermediate species (b), (c), and (d) were analyzed using a bi-exponential fit. The obtained apparent rates (k1-obs) are listed in Table 1. They reflect an approach to equilibria and thus reflect all the intrinsic rates of formation and decay of a given species (30). In accordance with this, the equilibrium situation was disrupted experimentally by the addition of lycopene cyclase from rice OsLCY-b (20), an enzyme that converts all-trans-lycopene (g) essentially irreversibly into β-carotene (h; Fig. 6), This is evidenced by the rapid disappearance of all lycopene species (a)-(g). In an attempt to verify this interpretation we simulated the time course of concentration changes of relevant species in a system assumed to represent the experimental set-up described in Fig. 5, C and D. The simulation was based on the conversion scheme depicted in Fig. 8 and includes all species shown and the steps k1 to k18 with their rate constants. As shown in Fig. 5E, there is satisfactory coincidence of experimental data points with the corresponding simulation curves. Importantly, “plateaus” reflecting equilibrium concentrations of lycopene species were attained at the end of the time axis which were similar to those determined experimentally.
TABLE 1.
Apparent rate constants for the formation and decay of species in comparison with the simulated model
For structures of lycopene species see Fig. 8. Experimental (Fig. 5, C and D) and simulation (Fig. 5E) data points were fitted using the same algorithms, i.e. a mono-exponential equation for (a),(e),(f),(g) and a bi-exponential one for (b),(c),(d).
| Lycopene isomer | Formation/ decay | k1-obs (min−1) experimental (simulation) | ±S.E. | k2-obs (min−1) experimental (simulation) | ±S.E. |
|---|---|---|---|---|---|
| (a) 7,9,9́,7́-tetra-cis | d | 0.068 | 0.002 | – | |
| (0.073) | (0.001) | ||||
| (b) 7,9-di-cis | f/d | 0.4 | 0.06 | 0.057 | 0.01 |
| (0.29) | (0.002) | (0.065) | (0.004) | ||
| (c) 9-cis | f/d | 0.12 | 0.04 | 0.014 | 0.02 |
| (0.021) | (0.006) | (0.007) | (0.002) | ||
| (d) 7-cis | f/d | 0.27 | 0.09 | 0.001 | 0.001 |
| (0.22) | (0.006) | (0.07) | (0.002) | ||
| (e) “unknown” | f | 0.11 | 0.01 | a | |
| (0.52) | (0.01) | ||||
| (f) 5-cis | f | 0.067 | 0.003 | a | |
| (0.042) | (0.001) | ||||
| (g) all-trans | f | 0.050 | 0.003 | – | |
| (0.060) | (0.001) |
a For species (e), formed at low concentrations, both types of fit could be applied yielding very similar k1-obs values, while the apparent rate of decay k2-obs obtained from a bi-exponential fit is very low (k1 ≥ 100 × k2). For species (f) a bi-exponential fit did not yield meaningful results.
FIGURE 6.
Addition of lycopene β-cyclase disrupts the chemical equilibrium in standard CRTISO assays. A, negative control (no enzyme added). The equilibrium between substrate and products in the CRTISO assay carried out under standard conditions was established after an incubation time of 60 min (B, comp. Fig. 5), because it remained unchanged after 90 min of incubation (C). The addition of lycopene cyclase OsLCY-b at the 60th minute led to the complete conversion of all lycopenes into mainly all-trans-β-carotene (peak h) after additional 30 min of incubation time (D). (a) Prolycopene; (b) 7,9-di-cis-lycopene; (c) 9-cis-lycopene; (d) 7-cis-lycopene; (g) all-trans-lycopene; (f) 5-cis-lycopene.
In these simulations, the magnitude of steps k1/k2 can only be varied within a narrow range (± 20% of the listed rates) without losing coincidence with the experimental data. This suggests a good approximation to the intrinsic values. Only narrow variation is also allowed with respect to the sum of the rates of steps leading to (g) or (f). In contrast, the time course simulation for the formation of all-trans-lycopene allows considerable variation, provided the sum of k-values remains constant. This is due to the presence of four parallel single pathways originating from (c), (d), (e), and (f) leading to all-trans-lycopene (g),. We selected the values for k12 to k16 to best fit the “equilibrium abundances” (plateaus) of species (c), (d), and (e).
The ratios of “forward” and “reverse” steps (see legend of Fig. 8) are in agreement with the presence of equilibria. For species (a) and (b) the ratio is 2, while for the transformation of (b) into (c), (d), and (e) the equilibrium appears to be in favor of (b). This contrasts with the equilibria linking species (c), (d), and (e) to (f) and (g), where ratios of 40–60 in favor of (g) are found. A high ratio of ≈100 for k17/k18 is necessary for simulating the observed plateau concentration of species (f). These latter high ratios might be related to the experimental difficulty observed in driving the isomerization reaction “backwards” starting from all-trans-lycopene ((g), see supplemental Fig. S8). It is also noted that the abundance of the various lycopene species at equilibrium corresponds roughly to their relative theoretical energy levels calculated by Guo et al. (31).
The simulations were validated by fitting both the experimental and the simulation data using the same algorithms. A comparison of the results listed in Table 1 shows that in the case of the most abundant lycopene species (a), (b), (f), and (g) the coincidence of kobs for both sets of data is good. With the species (c), (d), and (e), the abundance of which was ≤ 2%, the scatter of experimental data were large and the match correspondingly less pronounced.
The results of the simulations provide arguments to assess the possible sequences and courses of isomerizations depicted in Fig. 8. Several alternative pathways are represented, which can all lead to the final product all-trans-lycopene (g). Steps denoted with (?) link the species in (c), (d), and (e) in the middle row. Thus formation of (g) from (b) might be envisaged to pass in sequence e.g. via (e), (c), and (d). However, the results of the simulations suggest the absence of rapid steps interlinking (c), (d), and (e). Such rapid steps would lead to similar (observed) rates of formation for these species, which is not observed (Fig. 5D, Table 1). Fig. 8 represents a minimal scheme, it does not exclude the occurrence of further species and combinations of steps.
Lycopene cis Isomers in Lycopene Cyclization
The introduction of lycopene cyclase OsLCY-b into the CRTISO-containing system, as discussed above (Fig. 6) led to the formation of all-trans-β-carotene. When 5-cis-lycopene was used as a substrate, with CRTISO plus OsLCY-b the monocyclic γ-carotene formed, in addition to all-trans-β-carotene (Fig. 7B, traces III, IV). This monocyclic carotene was hypothesized to derive from 5-cis-lycopene (with the 5-cis-end not being amenable to cyclization). At longer (30 min) incubation time, this end was isomerized to trans by CRTISO, allowing the accumulation of all-trans-β-carotene (Fig. 7B, trace V). To prove this assumption, we incubated 5-cis-lycopene with OsLCY-b (in the absence of CRTISO, Fig. 7C, trace VI). This yielded a γ-carotene species of which it appeared reasonable to assume that it carried the 5-cis double bond in the aliphatic chain. This is because the simultaneous presence of CRTISO was required to achieve a 100% conversion into all-trans-β-carotene without any notable formation of γ-carotene (Fig. 7C, trace VII). This shows that CRTISO not only acts on the aliphatic lycopene chain (Fig. 7A) but also on monocyclic carotenoids. These results also exclude for a potential cis-trans isomerization side activity of OsLCY-b (being itself a FADred-dependent isomerase; 20).
FIGURE 7.
Stereoselectivity of lycopene β-cyclase (OsLCY-b). A, CRTISO can isomerize 5-cis-lycopene (f) into all-trans-lycopene (g), but not in the reverse direction (see supplemental Fig. S8). (I) Control (incubated in the absence of CRTISO), (b) upon 30 min of incubation in the presence of CRTISO. B, this cis-to-trans isomerization process is accelerated when CRTISO and OsLCY-b are combined. OsLCY-b was added after 30 min of CRTISO reaction (as in (II)) and the reaction was analyzed after 5 (III), 10 (IV), and 30 min (V). Monocyclic 5-cis-γ-carotene formed from 5-cis-lycopene and all-trans-β-carotene formed from all-trans-lycopene and/or from all-trans-γ-carotene. This is inferred from C, where 5-cis-lycopene (f) was incubated with OsLCY-b in the absence of CRTISO (VI) yielding only 5-cis-γ-carotene, while all-trans-β-carotene was only formed when CRTISO was added simultaneously (VII). D, 7- and 9-cis isomers were collected (peaks (d) and (c) in Fig. 5). They do not separate on HPLC-System 1 used here (VIII); (IX) shows the elution of an all-trans-γ-carotene standard. Upon incubation with OsLCY-b (in the absence of CRTISO) the two cis isomers were converted within 30 min into 7-cis- and 9-cis-γ-carotene (peaks 1 and 2, see spectra in supplemental Fig. S4). The elution profiles in A, B, and C were obtained with HPLC-system 2. lyc., lycopene; car., carotene.
The 5-cis configuration of lycopene may forbid cyclization because this C(5)-C(6) double bond is mechanistically involved in lycopene cyclization (20, 21) while double bonds in a more central position are not. 9-cis-configured double bonds are especially interesting since 9-cis configured xanthophylls represent a molecular “earmark” to direct them into the biosynthesis of abscisic acid (ABA) through the action of 9-mono-cis epoxycarotenoid oxygenases (NCEDs). With the suitable in vitro system at hand, the question arose whether CRTISO in combination with OsLCY-b can decide on the proportion of 9-cis configured β-carotene and derived xanthophylls thus playing a regulatory role. To test, we isolated 7-cis and 9-cis-lycopene from isomerization assays (peaks (c) and (d) in Fig. 5A), incorporated them into liposomes to run lycopene cyclization assays with OsLCY-b. Again, no β-carotene was formed; instead, two monocyclic cis-configured γ-carotene species accumulated which are presumed to represent the 7- and 9-mono-cis forms, respectively (Fig. 7D).
DISCUSSION
Role and Redox State of FAD
CRTISO is the cis-trans carotene isomerase acting at the stage of tri-cis-neurosporene and tetra-cis-lycopene (prolycopene, 19), both representing canonical desaturation intermediates in the carotenogenesis of cyanobacteria and plants (14). CRTISO is required to drive carotenogenesis in non-green tissues, while there is no strict requirement in green tissues. For instance, developing leaves of the “Tangerine” variety of tomato show bleaching because they are defective in CRTISO, but mature leaves are phenotypically unaffected (16). CRTISO requirement is also pronounced in developing seedlings, facilitating chloroplast development during seedling germination and photomorphogenesis (17). With the photosynthetic apparatus once established, the isomerization reaction is thought to be substituted by photo-isomerization (17, 32, 33), which, however, requires a hitherto unknown sensitizer (16).
CRTISO possesses a dinucleotide binding domain and shares this property with the bacterial carotene desaturase CRTI, to which it is evolutionarily, related. The nature of the cofactor needed was unknown. Based on our data, FAD is the cofactor required by CRTISO; trace amounts of FMN and NAD might occur by cofactor exchange in E. coli since FAD is not tightly bound. We can state further that it is the reduced form of FAD, partially replaceable by FMNred, which drives enzymatic activity of CRTISO. This calls for revisiting the previous findings mentioned (see Introduction). Oxygen depletion through respiration apparently prevents FADred oxidation in the complex E. coli system used (19). Taken together, the findings presented place CRTISO into a novel group of flavoproteins catalyzing non-redox reactions with the help of reduced flavins.
CRTI, thus indirectly CRTISO, and CrtY are thought to have evolved during the anoxygenic period of life on Earth (7). It would therefore appear that such anoxygenic mechanisms of flavinred catalysis in carotenogenesis are ancient as well. It is, however, unclear to date how such mechanisms persisted in an oxygenic atmosphere, i.e. what protective molecular mechanisms co-evolved allowing the use of reduced flavins in aerobic bacterial cells (CRTY) or in plastids (CRTISO) in close vicinity of oxygenic photosynthesis.
Similarities and Dissimilarities between CRTISO, CRTY, and IDI-2
There are two additional enzymes involved in isoprenoid metabolism that belong to this class of FADred-dependent non-redox enzymes, both representing non-homologous isomerases, namely isopentenyl-diphosphate isomerase type 2 (IDI-2) and lycopene β-cyclase (LCY-b in plants, CRTY in bacteria). Modified flavins, in particular C(1)- and C(5)-deaza-flavins have been used in all three cases as probes to unravel possible reaction mechanisms. CRTISO is similar to IDI-2 (34) because both were inactive when reconstituted with C(5)-deaza-flavins, whereas they were active with C(1)-deaza-flavins. This is contrasted by CRTY where “normal” activity was found with C(5)-deaza-FAD (20). The fact that C(5)-deaza-flavins do not support 1e− transfer (28) together with the finding of an LFER led us to propose a CRTY reaction mechanism not involving transient (cryptic) electron transfer. In the present case, the absence of an observable LFER (with flavin analogs carrying substituents at position C(8)), may be explained by the occurrence of a rate-limiting step that is not linked to the chemical event.
In view of the similarities of the catalyzed reactions and the requirement for reduced flavin it would appear reasonable to assume a similar basic chemical mechanism in all three cases (IDI-2, CRTY, CRTISO). However, this conflicts with C(5)-deaza-flavin being active with CRTY but not with CRTISO and IDI-2. The question therefore arises whether the mechanisms of these enzymes differ in a fundamental aspect. There are various possibilities to rationalize the absence of activity of CRTISO/IDI-2 with C(5)-deaza-flavins. The flavin N(5) position could be involved in the formation of a crucial H-bridge or play an active role in acid/base catalysis (as suggested for IDI-2; 27). Alternatively, in the case of a charge transfer mechanism, such as suggested for CRTY, it is conceivable that the absence of the specific N(5) HOMO orbital of reduced flavin in CRTISO/IDI-2 precludes a productive overlap interaction with the transition state. Obviously, a reversible transfer of a single e− in a transient state cannot be excluded either for CRTISO/IDI-2 at the current state of our knowledge. Although it is possible to distinguish the different reduced flavin species through UV/Vis and fluorescence spectroscopy (35, 36) such measurements are illusory with CRTISO due to sample turbidity caused by liposomes and to the absorption spectra of substrate and products that lie in the same spectral range (see Fig. 2A).
It is thus not possible as yet to formulate a distinction between the catalytic mechanisms of CRTY and CRTISO. Efforts are currently underway to resolve the three-dimensional structure of both enzymes which will hopefully lead to a clearer picture.
Regional Specificities and Time Course of the cis-to-trans Isomerization Sequence
Separation and identification of specific cis-lycopenes formed during catalysis as well as the capability of converting monocyclic cis-γ-carotene species are in favor of a “half-side” recognition of the symmetrical tetra-cis-lycopene substrate. This is mirrored by the predominant formation of the asymmetrically cis-configured intermediate, 7,9-di-cis-lycopene (peak (b), Fig. 8) that carries cis double bonds in one-half-side and all-trans double bonds in the other. Onwards from this intermediate (energetically downhill toward the all-trans form) the 9-mono-cis (c) and 7-mono-cis (d) intermediates can be derived (supplemental Fig. S7). Corroborating this principle of half-side recognition, the symmetric intermediates 7,7′-di-cis- and 9,9′-di-cis-lycopene or the respective tri-cis-lycopenes were not found (supplemental Fig. S6).
The occurrence of 5-cis-lycopene (f) was unexpected because this cis double bond is derived from a trans-configured one in the precursor lycopene species indicating the involvement of a trans-to-cis isomerization step at this position. Quantum chemistry calculations indicate that the 5-cis isomer of lycopene (f) has the lowest energy among the lycopene cis isomers considered in this investigation (31). However, its formation from all-trans-lycopene (step g > f, k18 in Fig. 8) is assumed to occur at a very low rate due to the predicted presence of a large rotational energy barrier (31). In the presence of CRTISO, this isomer (f) was found to be formed from poly-cis configured precursors but not from all-trans-lycopene (supplemental Fig. S8). The biological role of this reaction remains enigmatic. There may be significance with respect to the lycopene ϵ-cyclase reaction based on a consideration by Eugster (37, 38) according to which the cis configuration of the C(5)-C(6) double bond of lycopene together with the mode of folding of the ring precursor (chair or boat) determine the feasibility of axial proton abstraction and thus the formation of the ϵ-ionone ring.
CRTISO establishes an equilibrium between cis isomers (Figs. 5 and 8). In accordance with this, the addition of LCY-b disrupts the equilibrium by catalyzing the essentially irreversible cyclization of all-trans-lycopene (g) to β-carotene (h). Consequently, all cis isomers (a)-(f) are rapidly converted into all-trans lycopene (Fig. 6). However, upon incubation of CRTISO with all-trans-lycopene (g), the expected reversibility of the reaction could not be detected (supplemental Fig. S8). This might be attributed to hitherto unknown secondary parameters, for instance to an altered enzyme-substrate accessibility at the enzyme/membrane interface.
On the Source of 9-cis Carotenoid Isomers with Altered Biological Significance
Carotenoids carrying a cis-configured double bond at position C(9) are frequently found in plant tissues (39). The presence of 9-cis configured xanthophylls has received special attention since the discovery of the 9-cis epoxycarotenoid oxygenases (NCEDs, 1, see Ref. 40 for review) catalyzing the initial step in the biosynthesis of abscisic acid (ABA). It is therefore tempting to hypothesize that the 9-cis double bond of violaxanthin and neoxanthin may be a reminiscence of their (9-cis bearing) prolycopene origin, in which case a regulatory role might be attributed to CRTISO. By using isolated 9-cis and 7-cis-lycopene produced by CRTISO, as well as commercially available 5-cis-lycopene, we show that LCY-b from rice, encoded by a single gene, acts as a non-permissive selectivity filter for cis isomers incapable of producing β-carotene with any of these mono-cis-lycopenes (Fig. 7, C and D). Under these circumstances only monocylic carotenes accumulate. This finding, if generally applicable, calls for the presence of a yet unidentified β-xanthophyll trans-to-cis isomerase acting at the C(9) double bond.
Supplementary Material
This work was supported by The German-Israel Foundation and by the Excellence Initiative of the German Federal and State Governments (EXC 294).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
The C-atom numbering of carotenes is given according to IUPAC nomenclature rules for carotenoids.
- PDS
- phytoene desaturase
- ZDS
- ζ-carotene desaturase
- HOMO
- highest occupied molecular orbital
- IMAC
- immobilized metal affinity chromatography
- LFER
- linear free energy relationship
- SRM
- single reaction monitoring.
REFERENCES
- 1. Schwartz S. H., Tan B. C., Gage D. A., Zeevaart J. A., McCarty D. R. (1997) Science 276, 1872–1874 [DOI] [PubMed] [Google Scholar]
- 2. Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., Kyozuka J., Yamaguchi S. (2008) Nature 455, 195–200 [DOI] [PubMed] [Google Scholar]
- 3. Gomez-Roldan V., Fermas S., Brewer P. B., Puech-Pagès V., Dun E. A., Pillot J. P., Letisse F., Matusova R., Danoun S., Portais J. C., Bouwmeester H., Bécard G., Beveridge C. A., Rameau C., Rochange S. F. (2008) Nature 455, 189–194 [DOI] [PubMed] [Google Scholar]
- 4. Von Lintig J. (2010) Annu. Rev. Nutr. 30, 35–56 [DOI] [PubMed] [Google Scholar]
- 5. DellaPenna D., Pogson B. J. (2006) Annu. Rev. Plant Biol. 57, 711–738 [DOI] [PubMed] [Google Scholar]
- 6. Fraser P. D., Bramey P. (2004) Prog. Lip. Res. 43, 228–265 [DOI] [PubMed] [Google Scholar]
- 7. Klassen J. L. (2010) PLoS ONE 5, e11257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sandmann G. (2009) Arch. Biochem. Biophys. 483, 169–174 [DOI] [PubMed] [Google Scholar]
- 9. Beyer P., Mayer M., Kleinig H. (1989) Eur. J. Biochem. 184, 141–150 [DOI] [PubMed] [Google Scholar]
- 10. Mayer M. P., Beyer P., Kleinig H. (1990) Eur. J. Biochem. 191, 359–363 [DOI] [PubMed] [Google Scholar]
- 11. Norris S. R., Barrette T. R., DellaPenna D. (1995) Plant Cell 7, 2139–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carol P., Stevenson D., Bisanz C., Breitenbach J., Sandmann G., Mache R., Coupland G., Kuntz M. (1999) Plant Cell 11, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y., Li F., Wurtzel E. T. (2010) Plant Physiol. 153, 66–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartley G., Scolnik P., Beyer P. (1999) Eur. J. Biochem. 259, 396–403 [DOI] [PubMed] [Google Scholar]
- 15. Clough J. M., Pattenden G. (1979) J. Chem. Soc. Chem. Commun. 14, 616–619 [Google Scholar]
- 16. Isaacson T., Ronen G., Zamir D., Hirschberg J. (2002) Plant Cell 14, 333–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Park H., Kreunen S. S., Cuttriss A. J., DellaPenna D., Pogson B. J. (2002) Plant Cell 14, 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schulz C. E. (1992) Curr. Opin. Struct. Biol. 2, 61–67 [Google Scholar]
- 19. Isaacson T., Ohad I., Beyer P., Hirschberg J. (2004) Plant Physiol. 136, 4246–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu Q., Schaub P., Ghisla S., Al-Babili S., Krieger-Liszkay A., Beyer P. (2010) J. Biol. Chem. 285, 12109–12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mialoundama A. S., Heintz D., Jadid N., Nkeng P., Rahier A., Deli J., Camara B., Bouvier F. (2010) Plant Physiol. 153, 970–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edmondson D., Ghisla S. (1999) Methods Mol. Biol. 131, 157–179 [DOI] [PubMed] [Google Scholar]
- 23. Veprintsev D. B., Freund S. M., Andreeva A., Rutledge S. E., Tidow H., Cañadillas J. M., Blair C. M., Fersht A. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 2115–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheffield P., Garrard S., Derewenda Z. (1999) Protein Express. Purif. 15, 34–39 [DOI] [PubMed] [Google Scholar]
- 25. Hengartner U., Bernhard K., Meyer K., Englert G., Glinz E. (1992) Helv. Chim. Acta 75, 1848–1865 [Google Scholar]
- 26. Gradinaru R., Schowen R., Ghisla S. (2007) Biochemistry 46, 2497–2509 [DOI] [PubMed] [Google Scholar]
- 27. Unno H., Yamashita S., Ikeda Y., Sekiguchi S. Y., Yoshida N., Yoshimura T., Kusunoki M., Nakayama T., Nishino T., Hemmi H. (2009) J. Biol. Chem. 284, 9160–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hersh L. B., Walsh C. (1980) Methods Enzymol. 66, 277–287 [DOI] [PubMed] [Google Scholar]
- 29. Eckstein J. W., Hastings J. W., Ghisla S. (1993) Biochemistry 19, 404–411 [DOI] [PubMed] [Google Scholar]
- 30. Strickland S., Palmer G., Massey V. (1975) J. Biol. Chem. 250, 4048–4052 [PubMed] [Google Scholar]
- 31. Guo W. H., Tu C. Y., Hu C. H. (2008) J. Phys. Chem. B 112, 12158–12167 [DOI] [PubMed] [Google Scholar]
- 32. Römer S., Humbeck K., Senger H. (1991) Photobiology 53, 535–538 [Google Scholar]
- 33. Sandmann G. (1991) Arch. Microbiol. 155, 229–233 [Google Scholar]
- 34. Kittleman W., Thibodeaux C. J., Liu Y. N., Zhang H., Liu H. W. (2007) Biochemistry 46, 8401–8413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghisla S., Massey V., Lhoste J. M., Mayhew S. G. (1974) Biochemistry 13, 589–597 [DOI] [PubMed] [Google Scholar]
- 36. Kao Y. T., Saxena C., He T. F., Guo L., Wang L., Sancar A., Zhong D. (2008) J. Am. Chem. Soc. 130, 13132–13139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eugster C. H. (1979) Pure Appl. Chem. 51, 463–506 [Google Scholar]
- 38. Zumbrunn A., Uebelhart P., Eugster C. H. (1985) Helv. Chim. Acta 68, 1519–1539 [Google Scholar]
- 39. Ben-Amot A., Fishler R. (1997) Food Chem. 62, 515–520 [Google Scholar]
- 40. Nambara E., Marion-Poll A. (2005) Annu. Rev. Plant Biol. 56, 165–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.